Abstract
The brain networks connected to the sympathetic motor and sensory innervations of brown (BAT) and white (WAT) adipose tissues were originally described using two transneuronally transported viruses: the retrogradely transported pseudorabies virus (PRV), and the anterogradely transported H129 strain of herpes simplex virus-1 (HSV-1 H129). Further complexity was added to this network organization when combined injections of PRV and HSV-1 H129 into either BAT or WAT of the same animal generated sets of coinfected neurons in the brain, spinal cord, and sympathetic and dorsal root ganglia. These neurons are well positioned to act as sensorimotor links in the feedback circuits that control each fat pad. We have now determined the extent of sensorimotor crosstalk between interscapular BAT (IBAT) and inguinal WAT (IWAT). PRV152 and HSV-1 H129 were each injected into IBAT or IWAT of the same animal: H129 into IBAT and PRV152 into IWAT. The reverse configuration was applied in a different set of animals. We found single-labeled neurons together with H129+PRV152 coinfected neurons in multiple brain sites, with lesser numbers in the sympathetic and dorsal root ganglia that innervate IBAT and IWAT. We propose that these coinfected neurons mediate sensory-sympathetic motor crosstalk between IBAT and IWAT. Comparing the relative numbers of coinfected neurons between the two injection configurations showed a bias toward IBAT-sensory and IWAT-sympathetic motor feedback loops. These coinfected neurons provide a neuroanatomical framework for functional interactions between IBAT thermogenesis and IWAT lipolysis that occurs with cold exposure, food restriction/deprivation, exercise, and more generally with alterations in adiposity.
Keywords: herpes simplex virus, pseudorabies virus, Siberian hamsters
brown (BAT) and white (WAT) adipose tissues are controlled by a complex interplay of humoral and neural factors. Although humoral influences have long been recognized as major controllers, the impact of the brain on adipose tissue function has emerged more recently (for review, see Ref. 4). We now know that sympathetic motor (SNS) and sensory systems (SS) innervate BAT and WAT (1, 7, 13, 21, 42, 46, 49, 54) to help enable their functions (4, 36, 48, 50, 53, 55). The SNS input is important for activating lipolysis in inguinal (IWAT) and epididymal (EWAT) WAT (4), whereas BAT is completely dependent on its SNS innervation for virtually all of its functions, including lipolysis and thermogenesis (for reviews, see Refs. 7, 13, 46).
BAT and WAT also have SS innervations. Both contain two SS nerve-associated peptides, substance P, and calcitonin gene-related peptide (20, 42). We and others have shown the necessity of the intact SS innervation of BAT for functional cold exposure-evoked thermogenesis (19, 30, 59).
The SNS innervation of IWAT and EWAT was first identified with conventional tract tracers (65). The full extent of this innervation was later revealed using the polysynaptic and retrogradely transported Bartha strain of the pseudorabies virus (PRV152), which identified neurons in the brain that contribute to the WAT premotor neural network. These experiments produced infected neurons in a number of forebrain, midbrain, and hindbrain regions (48). Similarly, PRV152 injections into BAT produced a pattern of labeling in the brain that was comparable but not identical to that seen with PRV152 injections into WAT (8). The distinct but overlapping nature of these patterns was further highlighted when two PRVs capable of driving different fluorescent proteins were injected into the WAT and BAT of the different animals (48, 49). This experiment produced both single- and double-labeled neurons in the brain and spinal cord, thereby showing the divergent nature of adipose tissue premotor SNS control networks.
Transneuronal virus-tracing techniques have also shown which parts of the brain receive sensory information from BAT and WAT. Injecting the anterogradely transported herpes simplex virus-1 H129 (HSV-1 H129) into BAT (49, 59) or WAT (48, 53) produces infected neurons in the forebrain, midbrain, and hindbrain. The fact that many of these brain regions also receive sensory information from WAT and BAT has raised the possibility that some neurons could act as links in sensorimotor control loops.
When we combined H129 and PRV152 injections into the WAT or BAT of the same animal, we found that in addition to single-labeled neurons in the brain and spinal cord, there were significant numbers of double-labeled neurons in both locations (48, 49). These neurons are, therefore, positioned to receive sensory information from each type of adipose tissue (53) and then influence the appropriate SNS efferent outflow. In this way, they contribute to short- (in the spinal cord) and long- (in more rostral parts of the brain) SS-SNS feedback loops. The function of these WAT/BAT feedback circuits remains unclear. But because SNS innervation of WAT is the principal regulator of lipolysis (for review, see Ref. 4), these putative feedback circuits may monitor the degree of lipolysis in a way that can then influence SNS outflow to WAT.
Those parts of the brain containing double-labeled IBAT SNS-SS neurons are the same as those with double-labeled IWAT SNS-SS neurons (47, 49), thereby, raising the possibility of crosstalk between BAT and IWAT control networks. This function would mean that BAT thermogenesis could be influenced by IWAT SNS-norepinephrine (NE)-induced lipolysis and vice versa. Therefore, we now use combined H129 and PRV152 injections to determine whether short and long feedback connections exist to enable functional crosstalk between interscapular BAT (IBAT) and IWAT.1
METHODS
All animal procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with Public Health Service and United States Department of Agriculture Guidelines.
Animals
Eighteen male Siberian hamsters (Phodopus sungorus; ~3–4 mo old) from our breeding colony were singly housed in a temperature-controlled vivarium (21 ± 2°C, ~50 ± 10% humidity) with ad libitum access to food (LabDiet Rodent Chow 5001, St. Louis, MO) and tap water. Hamsters were maintained with a 12-h light/dark schedule and habituated to vivarium conditions for 1 wk before viral inoculation.
Viral Injections
All virus injections were performed according to Biosafety Level 2 standards. Hamsters were anesthetized via isoflurane (2–3% in oxygen; Baxter Healthcare, Deerfield, IL) inhalation and placed in dorsolateral recumbency; the skin around the inguinal region was wiped with 10% povidone iodine (Ricca Chemical, Arlington, TX) and 70% ethanol, and finished with povidone iodine.
In nine hamsters, an incision was made to reveal the right IWAT, whereupon a series of PRV152 (a gift from Dr. Lynn Enquist, Princeton University) microinjections [4.5 × 108 plaque-forming units (PFU/ml); 150 nl/locus] were made directly into seven loci across the IWAT pad 6 days before euthanasia. PRV152 contains the cytomegalovirus-enhanced green fluorescent protein (CMV-EGFP) reporter gene cassette inserted into the gG locus of the viral genome. The syringe was held in place for at least 60 s to prevent efflux of virus after each injection. The incision was closed with sterile sutures and wound clips. Nitrofurozone powder (NFZ Puffer; Hess & Clark, Lexington, KY) was applied topically as adjunctive therapy to minimize the risk of bacterial infection, and ketofen (5 mg/kg sc; Fort Dodge Animal Health, Fort Dodge, IA) was administered for three consecutive days postinjection to alleviate postoperative discomfort. Forty eight hours after PRV152 microinjections, the right IBAT of the same hamsters were exposed for a series of H129 (a gift from Dr. Richard Dix, Georgia State University) microinjections (7.5 × 107 PFU/ml; 150 nl/locus) into five loci evenly distributed across the IBAT depot.
In the second set of hamsters (n = 9), we reversed the viruses; H129 was inoculated intra-right IWAT (150 nl/locus; 7 loci) and then PRV152 was inoculated intra-right IBAT (150 nl/locus; 5 loci). Both microinjections were conducted the same day to match the rate of progression of each virus across the neuroaxis.
All animals remained asymptomatic until day 5 after viral inoculation with PRV152 and day 4 after H129 microinjections, whereafter most began to display symptoms of infection. These included occasional body weight loss, attenuated immobility, but mostly an ungroomed coat. Animals were euthanized immediately when such symptoms became apparent.
Control Injections
To control for possible viral diffusion away from the target region, we placed the same virus titer and volume of H129 or PRV152 on the surface of the each fat pad. For each virus at this locus, there was no infection in the IWAT- or IBAT-associated SNS ganglia, dorsal root ganglia (DRG), intermediolateral cell column (IML) of the spinal cord, and brain (Ryu V and Bartness TJ, unpublished observations). Moreover, we have previously demonstrated that surgical isolation of the fat pads from the surrounding tissues before the viral inoculation led to a pattern of infection indistinguishable from that of fat pads inoculated in their natural in situ position, favoring the specific neural routes of infections originating from either fat pad (Ryu V and Bartness TJ, unpublished observations).
Tissue Treatment
Hamsters were euthanized 6 days after PRV152 intra-IWAT and 4 days after H129 intra-IBAT injections. These times were based on our preliminary studies that optimized progression of both viruses to and within the brain (Ryu V and Bartness TJ, unpublished observations). When we switched the virus injections (H129-IWAT/PRV152-IBAT), we optimized rates of infection across the neuroaxis to obtain approximately equal percentages for both viruses. Therefore, all hamsters were euthanized 5 days after either PRV152 intra-IBAT or H129 intra-IWAT injections. Because it is not possible to predict precise survival times, so that all brain regions are infected equally with both viruses at the same time, we used infections within the nucleus of the solitary tract (NTS), periaqueductal gray (PAG), paraventricular hypothalamic nucleus (PVH), and medial preoptic area (MPA), in particular, as well as a general survey across the neuroaxis to determine these times.
Animals were overdosed with pentobarbital sodium (300 mg/kg) and transcardially perfused with 0.9% heparinized saline followed by 4% paraformaldehyde in 0.1 M PBS, pH 7.4. The extracted brains were immediately post-fixed in the same fixative for 3–4 h and then transferred to a 30% sucrose solution in 0.1 M PBS containing 0.1% sodium azide at 4°C overnight. Thirty-micrometer coronal sections were cut through the entire brain using a freezing, sliding microtome (HM440E; Microm, Walldorf, Germany).
Section Preparation
IBAT-associated SNS and DRG at vertebral levels T1–T3 and IWAT-associated T12-L3 were carefully harvested, peeled from the epineurium, and transferred to an 18% sucrose solution in 0.1 M PBS containing 0.1% sodium azide at 4°C. All ganglia were then sectioned longitudinally at 20-µm-thick sections using a cryostat. They were directly mounted onto slides (Superfrost Plus; VWR International, West Chester, PA) in three series with every fourth section on the same slide. Typically, this procedure yielded ~21 sections for the SNS ganglion and 24 sections for DRG with each slide containing seven to eight ganglia, respectively. Because the number of neurons was averaged across each ganglion, multiplying the number per section by 21 for the SNS ganglia and 24 for the DRGs will represent an estimated total number of neurons per ganglion.
After the sections were dried on the slides, they were rehydrated and then processed for immunodetection of the selected antigen.
Immunohistochemistry
Double-label fluorescence immunohistochemistry for HSV-1 and green fluorescent protein (GFP) was performed on brain, SNS ganglia, and DRG sections, as previously described (47). Briefly, free-floating brain sections were incubated in a mixture of primary antibodies for rabbit anti-HSV-1 (1:2,000; DakoCytomation, Carpinteria, CA) and mouse anti-GFP (1:700; Abcam, Cambridge, MA). These were followed by incubating sections in an appropriate mixture of the secondary goat anti-rabbit Cy3 (1:700; Jackson Immunoresearch, West Grove, PA) and goat anti-mouse Alexa Fluor 488 (1:700; Jackson Immunoresearch) antibodies. Immunohistochemistry for HSV-1 and PRV152 in SNS ganglia and DRG was conducted directly on the slides using primary rabbit anti-HSV-1 (1:100; DakoCytomation) and mouse anti-GFP (1:500; Abcam) antibodies.
Immunohistochemical controls consisted of either omitting the primary antibody or preadsorbing it with the immunizing peptide overnight at 4°C. No specific immunostaining was observed with either control.
After the immunohistochemistry was completed, all slides were coverslipped using ProLong Gold antifade reagent (Life Technologies, Grand Island, NY).
Quantitative and Statistical Analysis
Twelve of 18 animals were equally infected by both viruses within the brain and were, therefore, included in the analyses. The remaining six animals were excluded from the study because they exhibited clear PRV152 and/or H129 overinfection in the CNS. This was characterized by widespread “cloudy plaques” surrounding overinfected cells.
Images were viewed and captured using ×10 and ×20 objective lens with a DP73 digital camera attached to an Olympus BX41 microscope (Tokyo, Japan), equipped with appropriate Cy3 and Alexa Fluor 488 excitation and emission filters. Single- and double-labeled PRV152 and H129 images were evaluated and overlaid using CellSens (Olympus) and the Adobe Photoshop CS5 (Adobe Systems, San Jose, CA) software. We also used Adobe Photoshop CS5 to adjust the brightness and contrast and to eliminate section artifacts (i.e., obscuring bubbles) from the composite microscopic illustrations. No other manipulations or adjustments were made. Because there is no Siberian hamster brain atlas currently available, we used a mouse brain atlas (43) to identify brain sites. As we have noted previously (49), there is significant similarity in size and shape of the majority of brain nuclei in mice and Siberian hamsters.
Exhaustive counts of single PRV152- or H129-immunoreactive (-IR) neurons and double SNS PRV152 + SS H129-IR neurons in every sixth brain section were performed using the manual tag feature of the Adobe Photoshop CS5, thereby, precluding the likelihood of counting the same cells twice. Neurons were considered positively immunostained on the basis of the fluorescent intensity, cell size, and shape. The number and percentage of neurons in all ganglia, as well as the percentage of neurons in the brain, were averaged across each sample site from all hamsters.
The statistical significance of differences between group means was determined using the National Council for the Social Studies statistical software (version 2007, Kaysville, UT). Student’s t-test and one-way ANOVA followed by post hoc Bonferroni’s and Holm-Sidak’s tests were used as appropriate. Significance was set at P < 0.05. For simplicity and clarity, values with P < 0.05, P < 0.01, and P < 0.001 were all indicated with a single asterisk. All values are presented as means ± SE.
RESULTS
Two combinations of virus injections were used to determine whether short and long feedback loops exist to enable functional crosstalk between IBAT and IWAT. First, combined IBAT-H129 and IWAT-PRV152 injections were used to identify neurons that receive SS input from IBAT, and those that provide SNS drive to IWAT, as well as any that contributed to both systems. Second, combined IBAT-PRV152 injections and IWAT-H129 injections were used to identify neurons that provide SNS drive to IBAT, those that receive SS from IWAT, together with ones that contributed to both systems. The resulting anatomical distributions and numbers of single- and double-labeled neurons from each injection set were recorded from the sympathetic and dorsal root ganglia, and throughout the brain. The results for each region are presented as numbers of single- or double-labeled H129- and PRV152-IR neurons, as well as the percentage of each type compared with the total number of labeled neurons within that region.
PRV152 and H129 Infections in Sympathetic Ganglia
IBAT-H129 injections and IWAT-PRV152 injections.
With this combination, we found H129- and PRV152-IR neurons were distributed unevenly across two vertebral levels. In T1–T3 SNS ganglia (Figs. 1A, 2, A and B), which are associated with the IBAT innervation, the numbers of H129-IR neurons were significantly greater than PRV152-IR neurons (P < 0.05). Hardly any double-labeled PRV152/H129-IR cells were observed at these thoracic three spinal levels (Fig. 2, A and B). In contrast, in T12-L3 SNS ganglia, which are associated with the SNS innervation of IWAT, the absolute neuronal numbers and percentages of PRV152-IR cells were significantly higher compared with H129-IR (P < 0.05). Although the number of PRV152-IR cells at L3 appeared higher than H129-IR neurons, this was not statistically significant (Fig. 2A). A small number of double-labeled PRV152/H129-IR were seen at T13-L1 (Fig. 1A), which comprised ~10% (Fig. 2B).
Fig. 1.
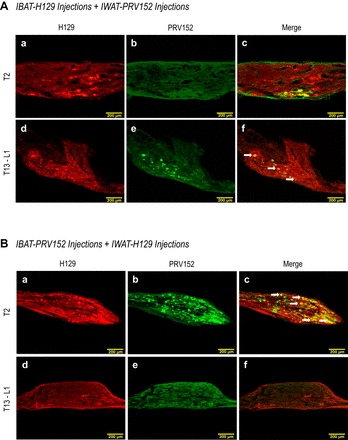
A: sensory H129 (red) and sympathetic PRV152 (green) immunostaining in the T13-L1 and T2 sympathetic ganglia after H129 microinjections intra-IBAT and PRV152 intra-IWAT. B: equitable viral immunostaining after the viruses were switched, i.e., IWAT received H129 and IBAT received PRV152 microinjections. Double-labeled H129 + PRV152 neurons are indicated by arrows. Scale bar = 200 µm.
Fig. 2.

Total neuronal (A, C) and percentile neuronal (B, D) distribution within the T1–L3 sympathetic ganglia after H129 and PRV152 microinjections intra-IWAT or IBAT. *P < 0.05 vs. H129. #P < 0.05 vs. H129 + PRV152.
IBAT-PRV152 injections and IWAT-H129 injections.
In T1 and T2 SNS ganglia, the numbers and percentages of PRV152-IR neurons were significantly increased (P < 0.05) compared with single-labeled H129-IR and double-labeled PRV152 + H129-IR neurons (Fig. 2, C and D). The numbers of PRV152-IR neurons at T3 only tended toward significance (Fig. 2C). This pattern was reversed in T13-L1 SNS ganglia where numbers of H129-IR neurons and their percentages at T13-L1 were significantly elevated (P < 0.05) compared with single PRV152-IR and colocalized PRV152 + H129-IR neurons (Fig. 2, C and D). However, it should be noted that the absolute numbers of H129-IR neurons at these vertebral levels were dramatically lower than the numbers of PRV152-IR neurons seen at T1–T3. The highest number of doubly infected H129/PRV152-IR neurons at T1–T3 SNS ganglia comprised ~20% (Fig. 2D).
PRV152 and H129 Infections in Dorsal Root Ganglia
IBAT H129 injections and IWAT-PRV152 injections.
In a manner similar to the virus infections seen in the SNS chain, PRV152- and H129-IR pseudounipolar neurons were observed across the T1–T3 DRG corresponding to the SS innervation of IBAT, and T12-L3 DRG associated with the innervation of IWAT (see Fig. 4, A–D). Figures 3A and 4A show that injections of intra-IWAT PRV152 and intra-IBAT H129 resulted in profound increase (P < 0.05) in H129-IR neurons relative to single PRV152-IR and double-labeled PRV152 + H129-IR at T1–T3 (Fig. 4, A and B). However, only the percentage of PRV152-IR was significantly higher (P < 0.05) than that of double-labeled cells at T1 and T3 DRG (Fig. 4B). The numbers of PRV152-IR neurons in DRG innervating IWAT were significantly increased (P < 0.05) compared with single-labeled H129- and double-labeled PRV152 + H129-IR at T13-L1, whereas their percentage values also reached statistical significance (P < 0.05) at L2-L3 (Fig. 4, A and B). As with sympathetic ganglia infections, the absolute numbers of double-labeled neurons in DRG was small, and the percentage of doubly labeled PRV152 + H129-IR was significantly lower (P < 0.05) relative to singly labeled PRV152-IR at T12 (Fig. 4B).
Fig. 4.
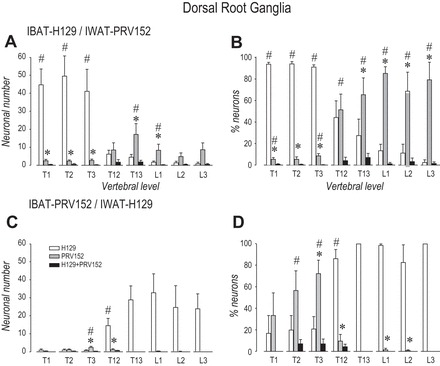
Total neuronal (A and C) and percentile neuronal (B and D) distribution within the T1–L3 DRG after H129 and PRV152 microinjections intra-IWAT or IBAT. *P < 0.05 vs. H129. #P < 0.05 vs. H129 + PRV152.
Fig. 3.
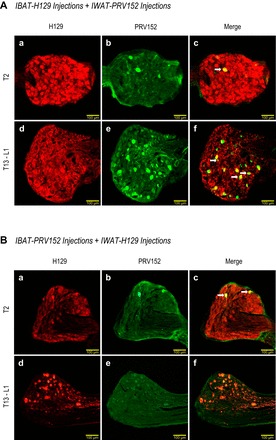
A: sensory H129 (red) and sympathetic PRV152 (green) immunostaining in the T13-L1 and T2 dorsal root ganglia (DRG) after H129 microinjections intra-IBAT and PRV152 intra-IWAT. B: equitable immunostaining after the viruses were switched, i.e., IWAT received H129, and IBAT received PRV152 microinjections. Double-labeled H129 + PRV152 neurons are indicated by arrows. Scale bar = 100 µm.
IBAT-PRV152 injections and IWAT-H129 injections.
With this combination of virus injections, immunolabeling in DRG innervating IBAT and IWAT was markedly different comparing with the IBAT H129 injections and IWAT-PRV152 injections. The number PRV152-IR neurons and their percentage were significantly elevated (P < 0.05) at T3 compared with H129-IR neurons, with their percentage value markedly higher (P < 0.05) than PRV152 + H129-IR at T2 (Fig. 4, C and D). In contrast, the number of H129-IR neurons was significantly greater (P < 0.05) than that of other groups in all DRG innervating IWAT (Fig. 4, C and D).
Viral Infections in the Brain
Although both sets of IBAT/IWAT injections produced a primarily bilateral infection pattern throughout the brain, there was a slight ipsilateral preference to the side of injections for both viruses, which is consistent with our previous findings (49). The IBAT-H129/IWAT-PRV152 injection combination also produced somewhat larger numbers of neurons than the IBAT-PRV152/IWAT-H129 combination (Supplemental Tables S1 and S2).
Not surprisingly, there was a striking similarity in the labeling pattern in brain regions receiving SS information from IWAT or IBAT (i.e., contained H129-IR neurons) compared with the pattern we have previously reported using PRV152 to identify those neurons that contribute to the SNS outflow from the brain to each fat pad (47, 49). Indeed, we found ~70% of all neurons in most brain sites colocalized PRV152 + H129 after the IBAT-H129/IWAT-PRV152 injection combination (Supplemental Table S1).
Hindbrain
IBAT-H129 injections and IWAT-PRV152 injections.
With this combination of virus injections, among the hindbrain sites with highest numbers of infected neurons for both viruses were the NTS, gigantocellular reticular nucleus (Gi), and intermediate reticular nucleus (IRt) in the medulla, and the locus coeruleus (LC), pontine reticular nucleus, oral part (PnO), and pontine reticular nucleus, caudal part (PnC) in the pons (Supplemental Table S1; Fig. 5). The numbers of double-labeled PRV152 + H129-IR neurons and their percentages (~66% in average) were both significantly increased in these particular regions (P < 0.05) compared with either single PRV152- or single H129-IR neurons (Supplemental Table S1). For the most part, hindbrain regions showed mainly similar patterns of SNS and SS labeling. However, several brain regions had divergent SNS output/SS inputs. Thus, consistent with our previous findings from WAT (47), the percentage of PRV152-IR neurons in the lateral paragigantocellular nucleus (LPGi), parvicellular reticular nucleus (PCRt), and ventral spinocerebellar tract (vsc) was significantly decreased (P < 0.05) compared with the number of the SS H129-labeled neurons (Supplemental Table S1).
IBAT-PRV152 injections and IWAT-H129 injections.
With this combination of virus injections, the hindbrain sites with the highest numbers of infected neurons were again the NTS, IRt, and vsc in the medulla (Fig. 5). However, in the pons, the A5 region was also heavily labeled along with the PnO and LC (Supplemental Table S2). Overall, ~46% in all labeled neurons in the hindbrain colocalized PRV152 and H129.
Among the regions with a significantly greater number of PRV152-IR than SS H129-IR neurons (P < 0.05) were the facial nucleus (7N), dorsal paragigantocellular nucleus (DPGi), laterodorsal tegmental nucleus (LDTg), and medial longitudinal fasciculus (mlf). Therefore, these regions contain neurons that are associated predominantly with influencing SNS output. On the other hand, the LPGi, PnC, and PnO had significantly greater numbers of SS H129-IR than PRV152-IR neurons (Supplemental Table S2; P < 0.05), indicating that they are regions that preferentially receive SS information.
Midbrain
IBAT-H129 injections and IWAT-PRV152 injections.
In the midbrain, regions such as the PAG and deep mesencephalic nucleus (DpMe) contained the highest numbers of both single- or double-labeled viruses (Supplemental Table S1; Fig. 5). There was a greater percentage of double-labeled neurons (~72%) than single PRV152- or H129-IR neurons in many parts of the midbrain. However, the percentage of double-labeled cells was significantly higher (P < 0.05) than single PRV152-IR cells in the lateral parabrachial nucleus, central part, medial parabrachial nucleus, and higher (P < 0.05) than single H129-IR cells in the dorsal raphe nucleus, dorsal part (Supplemental Table S1).
IBAT-PRV152 injections and IWAT-H129 injections.
Although containing fewer labeled neurons than the IBAT-H129/ IWAT-PRV152 combination, the PAG and DpMe were again among the sites with the highest number of coinfected neurons with the IBAT-PRV152/IWAT-H129 combination (Supplemental Table S2; Fig. 5). The overall percentage of PRV152 + H129 colocalization in the midbrain was ~50%. One of the midbrain parts with a predominant (P < 0.05) SNS output to IBAT compared with its SS input from IWAT was the DMPAG (Supplemental Table S2). As we have previously reported (47), this PAG subregion also contained a predominantly SNS output to the IWAT compared with its SS input from the same fat pad.
Forebrain
IBAT-H129 injections and IWAT-PRV152 injections.
The highest numbers of singly H129-IR, PRV152-IR, and coinfected forebrain neurons were in the PVH, lateral hypothalamic area (LH), and MPA (Supplemental Table S1; Fig. 6). The percentage numbers of double-labeled neurons, totaling ~69%, were significantly higher (P < 0.05) compared with those of single PRV152- or H129-IR neurons in most of the forebrain regions (Supplemental Table S1). Several areas, such as the LA and MPA, were represented by a markedly higher (P < 0.05) SNS output to the IWAT compared with their SS input from the IBAT (Supplemental Table S1).
IBAT-PRV152 injections and IWAT-H129 injections.
With this combination of viruses, the PVH, LH, and MPA again contained the highest numbers of singly or coinfected neurons, with ~54% of infected neurons being colabeled (Supplemental Table S2; Fig. 6). The LPO and MPOL of the preoptic area displayed significantly increased SNS outflow to the IBAT relative to the SS inflow from the IWAT (Supplemental Table S2; P < 0.05).
Fig. 5.
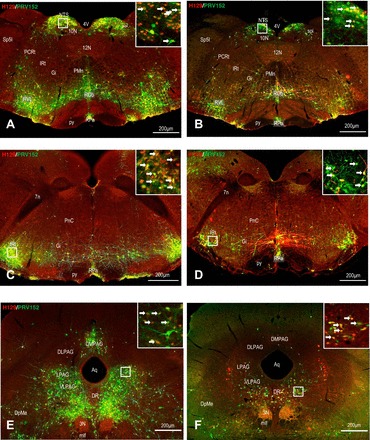
Left: low and high (inset) magnification of the photomicrographs, illustrating single H129 (red), single PRV152 (green), and colocalized H129 + PRV152 immunolabeling (yellow) cells in the NTS (A), IRt (C) in the medulla and PAG (D) in the midbrain following H129 microinjections intra-IBAT and PRV152 intra-IWAT. Right: equitable immunolabeling in the NTS (B), IRt (D) and PAG (E) after the viruses were switched. Arrows indicate double-labeled neurons. Scale bar = 200 µm. 3N, oculomotor nucleus; 4V, fourth ventricle; 7n, facial nucleus; 10N, dorsal motor nucleus of vagus; 12N, hypoglossal nucleus; Aq, aqueduct; DLPAG, dorsolateral periaqueductal gray; DMPAG, dorsomedial periaqueductal gray; DpMe, deep mesencephalic nucleus; DR, dorsal raphe nucleus; Gi, gigantocellular reticular nucleus; IRt, intermediate reticular nucleus; LPAG, lateral periaqueductal gray; mlf, medial longitudinal fasciculus; NTS, nucleus of the solitary tract; PCRt, parvicellular reticular nucleus; PMn, paramedian reticular nucleus; PnC, pontine reticular nucleus, caudal part; py, pyramidal tract; ROb, raphe obscurus nucleus; RPa, raphe pallidus nucleus; RVL, rostroventrolateral reticular nucleus; sol, solitary tract; Sp5I, spinal trigeminal nucleus, interpolar part; VLPAG, ventrolateral periaqueductal gray.
Fig. 6.
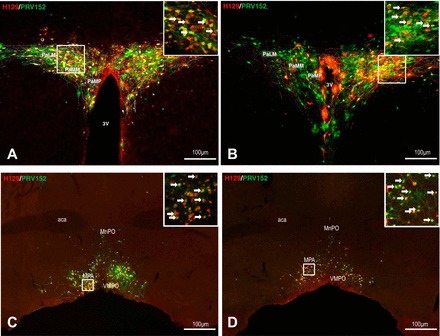
Left: low and high (inset) magnification of the photomicrographs illustrating single H129 (red), single PRV152 (green), and colocalized H129 + PRV152 immunolabeling (yellow) cells in the PVH (A) and MPA (C) in the forebrain following H129 microinjections intra-IBAT and PRV152 intra-IWAT. Right: equitable immunolabeling in the PVH (B) and MPA (D) after the viruses were switched. Arrows indicate double-labeled neurons. Scale bar = 100 µm. 3V, third ventricle; aca, anterior commissure; MnPO, median preoptic nucleus; MPA, medial preoptic area; PaLM, paraventricular hypothalamic nucleus, lateral magnocellular part; PaMM, paraventricular hypothalamic nucleus, medial magnocellular part; PaMP, paraventricular hypothalamic nucleus, medial parvicellular part; VMPO, ventromedial preoptic nucleus.
DISCUSSION
We first proposed the existence of neural feedback loops between different fats pads when we used H129 to identify the brain targets of sensory information from WAT (48). Later, we identified clear overlaps in the brain locations containing neurons that receive SS input from IBAT and IWAT, and neurons positioned to control their SNS motor inputs (47, 49). Therefore, we hypothesized that any IBAT-SS/IWAT-SNS and IWAT-SS/IBAT-SNS feedback circuits coexisting in the same brain regions would be obvious substrates to enable IWAT-SS regulation of IBAT thermogenesis, and IBAT-SS control of IWAT lipolysis, particularly under conditions of cold exposure, food restriction/deprivation, exercise, and more general alterations in adiposity. We investigated this idea in the current study by injecting H129 into IBAT and PRV152 into IWAT together with the reverse configuration.
The fact that we found many brain regions contain either IBAT-H129/IWAT-PRV152 or IBAT-PRV152/IWAT-H129 coinfected neurons indicates the existence of bidirectional SNS-SS interactions (long feedback loops) between IBAT and IWAT (Fig. 7A). These same PRV152/H129 injections also produced coinfected neurons in the SNS ganglia and DRG innervating these fat pads, which is consistent with short feedback loops (Fig. 7A). However, their numbers were far fewer than those in the brain, suggesting that the extent and possible functional significance of these short feedback loops is much less than the long feedback loops in the brain. Collectively, these colocalized H129/PRV152 neurons are strategically positioned to act as the links that enable sensorimotor integration between IWAT and IBAT.
Fig. 7.
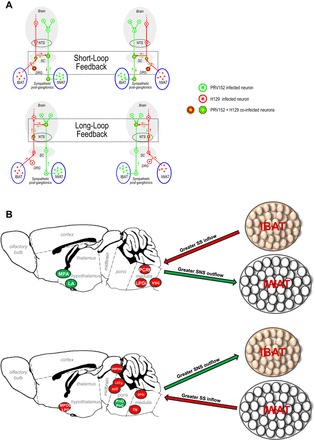
A: neuroanatomical model for the long and short neural feedback loops between IBAT sensory and IWAT sympathetic innervations defined by using viral H129 (red) and PRV152 (green) transneuronal tract tracing. B: a diaphragmatic representation of divergent sympathetic outflow (green arrows)/sensory inflow (red arrows) to the brain/from IBAT-IWAT pads comprising reciprocal neural feedback circuit. NTS, nucleus of the solitary tract; SC, spinal cord; DRG, dorsal root ganglia; IBAT, interscapular brown adipose tissue; IWAT, inguinal white adipose tissue; PRV, pseudorabies virus; HSV, herpes simplex virus; SS, sensory systems; SNS, sympathetic nervous system; MPOL, medial preoptic nucleus, lateral part; LPO, lateral preoptic area; DMPAG, dorsomedial periaqueductal gray; LDTg, laterodorsal tegmental nucleus; mlf, medial longitudinal fasciculus; DPGi, dorsal paragigantocellular nucleus; PnO, pontine reticular nucleus, oral part; PnC, pontine reticular nucleus, caudal part; 7N, facial nucleus.
A caveat worth mentioning at this point is that it is possible that we underestimated the numbers of coinfected neurons as a consequence of superinfection resistance. This can occur when two different viral strains arrive at a neuron from separate sites of inoculation, and infection with the first virus can suppress infection by the second (12). This means that, despite our attempts to optimize postinjection survival times for equal infection rates, coinfected neurons may exist, but remain undetected. However, because the two experiments were organized so that both viruses were injected into both fat pads, superinfection should not influence our overall conclusions.
In agreement with our previous findings (49), although neurons that were labeled after unilateral H129 intra-IBAT and PRV152 intra-IWAT injections (together with the reverse configuration) appeared mostly bilaterally in the brain, their distributions did have a slight ipsilateral preference to the side of injection for both viruses. The only site where we found a primarily unilateral infection was the dorsal motor nucleus of the vagus (Ryu V and Bartness TJ, unpublished observations), which is consistent with the unilateral distribution of the vagal efferents that innervate peripheral tissues in rodents (41, 44).
Although most brain sites contained more or less equal numbers of PRV152 and H129 coinfected neurons, several had asymmetric numbers, indicating an unequal distribution of SS input and SNS output (Fig. 7B). This was manifest as greater numbers of IBAT-H129/IWAT-PRV152 coinfected neurons compared with IBAT-PRV152/IWAT-H129 coinfected neurons. These results identify regions with neurons that can mediate interactions between SS input from IWAT and the SNS outflow to IBAT. Thus, asymmetric labeling patterns provide important insights about the functional interactions between IWAT and IBAT.
We have previously shown with H129 that multiple brain sites receive SS information from both IBAT and IWAT (48, 49). These findings have been recently confirmed and extended using dual injections of intra-IBAT H129-772 and intra-IWAT HSV-424 injections, and vice versa (Ryu V, Watts AG, Xue B, and Bartness TJ, unpublished data), suggesting that the SS in IBAT and IWAT is mediated via the same nature of neurons. Comparing the numbers of coinfected neurons between the two sets of injections, the LPGi, PCRt, and vsc in the hindbrain were regions with a somewhat greater SS inflow from IBAT compared with the SNS outflow to IWAT. These regions have already been identified as contributing nodes in the network that controls IBAT thermogenesis (45, 59). The LPGi, in particular, appears to be a key region in this regard because it contributes with the MPA, the ventromedial PAG, and Kölliker-Fuse nucleus (KF) to respiratory and/or SNS control by way of the raphe pallidus nucleus (RPa) (2, 16, 26, 31, 57). Moreover, the close proximity of LPGi neurons to those RPa SNS premotor neurons that control thermoregulatory and metabolic effectors and the fact that it also receives and sends a SS input/SNS output to IWAT (47), places the LPGi in a pivotal position to integrate the thermogenic and/or adiposity status of specific fat pads, and then appropriately coordinate their return SNS drives.
Importance of Feedback Loops
We found substantial numbers of H129/PRV152 colocalized neurons, particularly in the NTS, Gi, and IRt (medulla); the LC, PnO, and PnC (pons); the PAG and DpMe (midbrain); and the PVH, LH, and MPA (forebrain). We (49) and others (33, 34, 45, 58) have previously implicated these brain sites in the control of IBAT thermoregulation (for review, see Ref. 7). Moreover, all of these brain regions have already been identified as key constituents of IWAT SS-SNS feedback loops that can monitor the lipolytic capacity of WAT [(47), for review, see Refs. 3, 5, 6].
Regarding IWAT-SS and IBAT-SNS feedback loops within the hindbrain, sites with significantly larger SNS output to the IBAT were the 7N, DPGi, LDTg, and mlf. We have previously identified these sites as being significant contributors to the SNS output to BAT and WAT (38, 47, 49). Among these regions, the LDTg was reported to be within the central melanocortin circuitry regulating IBAT thermogenesis (61). Furthermore, the cluster of hindbrain and midbrain nuclei, including the PnC, PnO, LPGi, caudal raphe, PAG, and LC was noted to be likely involved in SNS-mediated thermogenesis (1, 14, 34), because all of them relay information from the forebrain to the IML SNS preganglionic neurons implicated in the control of IBAT thermogenesis.
Interestingly, the thermogenic contribution of the PnC and PnO extending to the midbrain and receiving mostly the SS input from the IWAT is contrary to the hindbrain. The midpontine neurons were reported to tonically inhibit IBAT thermogenesis (32), and inactivation of neurons in the vicinity of this area is capable of stimulating IBAT thermogenic response (52). As with the midbrain PAG, only the DMPAG subregion contained mostly the SNS output to the IBAT. In this regard, we previously reported that some PAG subregions send predominantly SNS outflow to IWAT (47) and IBAT (49) compared with the SS input from the same fat pads, suggesting the PAG could be an integral node guiding differential SNS drives to IBAT and IWAT. Finally, in the forebrain the LA and MPA had predominantly IWAT SNS output compared with the SS input from IBAT, while the LPO and MPOL had markedly higher SNS output to IBAT compared with the SS input from the IWAT. Most of these regions are implicated in the IBAT thermoregulatory SNS responses to thermosensory cold signals from skin (34, 37) and contribute to a prominent efferent signaling for IBAT thermogenesis (18, 56).
Functional Implications
The existence of bidirectional functional feedback circuits between IBAT and IWAT is consistent with the presence of central and peripheral nodes involved in a so-called “adipose afferent reflex” (AAR) first discovered by Niijima (39, 40) and later replicated by us (23, 24, 35). Notably, the large numbers of double-labeled neurons within the PVH (51), NTS, and PAG are well placed to enable long SNS-SS feedback loops involved in the AAR. In support of this notion, lesioning the PVH with kainic acid blocks the ability of low-dose capsaicin when applied intra-IWAT to trigger the AAR (51). Additional evidence for the existence of bidirectional links between IBAT and IWAT comes from the direct dependence of IBAT thermogenesis on WAT lipolysis. Whereas SNS/NE-triggered lipolysis in IBAT intracellular lipids fuel within-fat pad thermogenesis (13, 17, 49), stimulated lipolysis in WAT provides the main source of fatty acids for IBAT thermogenesis in Siberian hamsters (10, 22, 60) and humans (9). Furthermore, the necessity of WAT lipolysis for normal SNS/NE-induced IBAT thermogenic functioning has been shown in murine brown adipocytes. Here ablating all protein kinase A phosphorylation sites on adipocyte intracellular perilipin A abolishes NE-stimulated lipolysis. This is accompanied by an ~70% decrease in NE-induced thermogenesis (55), which shows that BAT thermogenesis is strongly dependent on WAT lipid reserves.
Various metabolic factors can differentially affect lipolysis-governed thermogenesis. For example, after food deprivation or 2-deoxy-d-glucose (2DG)-driven glucoprivation, BAT thermogenesis declines at same time as WAT lipid reserves decrease (11, 27, 28, 62). This can be explained by the ability of 2DG to increase the SNS drive to adipose tissues (35), thus depleting lipid reserves by inducing lipolysis (11). Importantly, we previously found in hamsters that micro-intra-WAT injections of 2DG profoundly increased SS nerve multiunit activity in decentralized nerve fibers innervating IWAT (35). By contrast, cold exposure increases BAT thermogenesis (11, 25, 29), despite decreases in WAT lipid reserves. These include the dorsomedial hypothalamic nucleus, the RPa, as well as other sites, that are part of the neural circuity excitatory projections to the SNS preganglionic neurons in the IML. Together, these mechanisms are well positioned to support body temperature homeostasis during changes in ambient temperature.
About 27% of SNS ganglia neurons and ~14% of the DRG pseudounipolar neurons participated in both the IWAT-SS and IBAT-SNS feedback circuitries. The presence of these coinfected neurons suggests peripheral interaction between the IBAT/IWAT SS and SNS neural feedback loops, presumably to monitor the status of the energy reserves and thermogenesis. However, the presence of H129-infected neurons in these ganglia should be interpreted cautiously because of a potential contribution from a retrograde transport component exhibited by this virus. This component is restricted to first-order projections and occurs much more slowly than the predominant anterograde component (63). Nevertheless, it is a consideration when interpreting H129 labeling in these ganglia. Furthermore, SS afferent neurons are found in the superior cervical SNS ganglion (64) and the thoracolumbar DRG, the latter considered to be non-nociceptive; i.e., nonpain visceral SS neurons involved in the reflexive control and/or homeostasis (15).
Perspectives and Significance
Our results show that neurons in many parts of the hindbrain, midbrain, and forebrain, as well as sympathetic ganglia and DRGs, are double-labeled after HSV-1 H129 and PRV152 injections into IBAT and IWAT, and vice versa. These neurons provide the structural foundation for the long feedback circuits in the brain, and to a lesser extent short feedback circuits in the spinal cord that enable a bidirectional SS-SNS crosstalk between IBAT and IWAT (Fig. 7A). These feedback loops emphasize the importance of SS control over SNS functions both within IWAT or IBAT, and also between IWAT and IBAT. Our data are consistent with an arrangement, whereby IBAT-SS has greater control over the SNS drive to IWAT than vice versa (Fig. 7B). Therefore, these results provide the structural basis for how IBAT-SS can orchestrate IWAT lipolysis during a variety of metabolic challenges (e.g., cold exposure, food deprivation/restriction, glucoprivation, and exercise), and perhaps also more generally during changes in adiposity. Conversely, IWAT-SS could sense changes in temperature, blood flow, or lipolytic activity in IBAT. Going forward, further studies are needed to clarify our understanding of the neuroanatomical and functional bases underlying the SS-SNS interaction between IBAT and IWAT both in the brain and in peripheral neural nodes. Greater understanding of these arrangements may also provide important pointers toward improving pharmacological interventions for controlling adiposity and body weight gain.
GRANTS
This work was funded by National Institutes of Health Grants R37 DK-35254 to T. J. Bartness, RO1 DK-35254 to B. Xue, and NS029728 to A. G. Watts.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.R. and T.J.B. conceived and designed research; V.R. performed experiments; V.R., A.G.W., and T.J.B. analyzed data; V.R., A.G.W., B.X., and T.J.B. interpreted results of experiments; V.R. and A.G.W. prepared figures; V.R. and T.J.B. drafted manuscript; V.R., A.G.W., B.X., and T.J.B. edited and revised manuscript; V.R., A.G.W., B.X., and T.J.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Lynn Enquist at Princeton University and the NIH-funded Center for Neuroanatomy with Neurotropic Viruses (P40RR018604) for the generous gift of PRV152 and to Dr. Richard Dix at Georgia State University for the generous gift of H129.
Glossary
- 2DG
2-deoxy-d-glucose
- AAR
adipose afferent reflex
- BAT
brown adipose tissue
- EWAT
epididymal white adipose tissue
- GFP
green fluorescent protein
- H129
strain of herpes simplex virus-1
- HSV-1
herpes simplex virus-1
- IBAT
interscapular brown adipose tissue
- IR
immunoreactive
- IWAT
inguinal white adipose tissue
- NE
norepinephrine
- PBS
phosphate-buffered saline
- PRV
pseudorabies virus
- SNS
sympathetic nervous system
- SS
sensory system
- WAT
white adipose tissue
Glossary
- 3N
oculomotor nucleus
- 3V
third ventricle
- 4V
fourth ventricle
- 7N
facial nucleus
- 10N
dorsal motor nucleus of vagus
- 12N
hypoglossal nucleus
- A5
norepinephrine neurons in the ventrolateral pons
- aca
anterior commissure
- Aq
aqueduct
- DLPAG
dorsolateral periaqueductal gray
- DMPAG
dorsomedial periaqueductal gray
- DPGi
dorsal paragigantocellular nucleus
- DpMe
deep mesencephalic nucleus
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRG
dorsal root ganglia
- Gi
gigantocellular reticular nucleus
- IML
intermediolateral cell column
- IRt
intermediate reticular nucleus
- KF
Kölliker-Fuse nucleus
- LA
lateroanterior hypothalamic nucleus
- LC
locus coeruleus
- LDTg
laterodorsal tegmental nucleus
- LH
lateral hypothalamic area
- LPAG
lateral periaqueductal gray
- LPBC
lateral parabrachial nucleus, central part
- LPGi
lateral paragigantocellular nucleus
- LPO
lateral preoptic area
- mlf
medial longitudinal fasciculus
- MnPO
median preoptic nucleus
- MPA
medial preoptic area
- MPB
medial parabrachial nucleus
- MPOL
medial preoptic nucleus, lateral part
- NTS
nucleus of the solitary tract
- PAG
periaqueductal gray
- PaLM
paraventricular hypothalamic nucleus, lateral magnocellular part
- PaMM
paraventricular hypothalamic nucleus, medial magnocellular part
- PaMP
paraventricular hypothalamic nucleus, medial parvicellular part
- PCRt
parvicellular reticular nucleus
- PMn
paramedian reticular nucleus
- PnC
pontine reticular nucleus, caudal part
- PnO
pontine reticular nucleus, oral part
- PVH
paraventricular hypothalamic nucleus
- py
pyramidal tract
- ROb
raphe obscurus nucleus
- RPa
raphe pallidus nucleus
- sol
solitary tract
- Sp5I
spinal trigeminal nucleus, interpolar part
- VLPAG
ventrolateral periaqueductal gray
- VMPO
ventromedial preoptic nucleus
- vsc
ventral spinocerebellar tract
Footnotes
Glossary appears at the end of the article.
REFERENCES
- 1.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci 17: 379–389, 1994. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol Reul Integr Comp Physiol 275: R1399–R1411, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35: 473–493, 2014. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34, Suppl 1: S36–S42, 2010. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol 593: 701–714, 2015. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–5347, 2007. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 11.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 12.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol 62: 159–167, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460: 303–326, 2003. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 15.Cervero F, Foreman RD. Sensory innervation of the viscera. In: Central Regulation of Autonomic Functions, edited by Loewy AD and Spyer KM. New York: Oxford University, 1990, p. 104–125. [Google Scholar]
- 16.Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci 14: 6500–6510, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhry A, Granneman JG. Differential regulation of functional responses by β-adrenergic receptor subtypes in brown adipocytes. Am J Physiol Regul Integr Comp Physiol 277: R147–R153, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol 512: 883–892, 1998. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J, Zaror-Behrens G, Himms-Hagen J. Capsaicin desensitization induces atrophy of brown adipose tissue in rats. Am J Physiol Regul Integr Comp Physiol 259: R324–R332, 1990. [DOI] [PubMed] [Google Scholar]
- 20.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol 27: 877–886, 1998. doi: 10.1023/A:1006996922657. [DOI] [PubMed] [Google Scholar]
- 22.Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab 5: 626–634, 2016. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14, Suppl 5: 216S–221S, 2006. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 24.Grill HJ, Kaplan J, Kaplan JM. Caudal brainstem participates in the distributed neural control of feeding. In: Neurobiology of Food and Fluid Intake, edited by Stricker EM. New York: Plenum, 1990, p. 125–149. doi: 10.1007/978-1-4613-0577-4_6. [DOI] [Google Scholar]
- 25.Habara Y. Effects of cold exposure on cyclic AMP concentration in plasma, liver, and brown and white adipose tissues in cold-acclimated rats. Int J Biometeorol 33: 95–100, 1989. doi: 10.1007/BF01686285. [DOI] [PubMed] [Google Scholar]
- 26.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13: 1–21, 1997. doi: 10.1016/S0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 27.Himms-Hagen J. Brown adipose tissue metabolism and thermogenesis. Annu Rev Nutr 5: 69–94, 1985. doi: 10.1146/annurev.nu.05.070185.000441. [DOI] [PubMed] [Google Scholar]
- 28.Himms-Hagen J, Cui J, Danforth E Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic β3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol Regul Integr Comp Physiol 266: R1371–R1382, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Hogan S, Himms-Hagen J. Brown adipose tissue of mice with gold thioglucose-induced obesity: effect of cold and diet. Am J Physiol Endocrinol Metab 244: E581–E588, 1983. [DOI] [PubMed] [Google Scholar]
- 30.Jancsó G, Király E, Joó F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett 59: 209–214, 1985. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- 31.Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 683: 1–24, 1995. doi: 10.1016/0006-8993(95)00276-V. [DOI] [PubMed] [Google Scholar]
- 32.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3: 00005, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 93: 773–797, 2008. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab 304: E1338–E1347, 2013. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol 306: R375–R386, 2014. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst 73: 19–25, 1998. doi: 10.1016/S0165-1838(98)00109-X. [DOI] [PubMed] [Google Scholar]
- 40.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett 262: 125–128, 1999. doi: 10.1016/S0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 41.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 42.Norman D, Mukherjee S, Symons D, Jung RT, Lever JD. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J Neurocytol 17: 305–311, 1988. doi: 10.1007/BF01187853. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic, 2007. [Google Scholar]
- 44.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Richard D, Carpentier AC, Doré G, Ouellet V, Picard F. Determinants of brown adipocyte development and thermogenesis. Int J Obes 34: Suppl 2: S59–S66, 2010. doi: 10.1038/ijo.2010.241. [DOI] [PubMed] [Google Scholar]
- 46.Richard D, Picard F. Brown fat biology and thermogenesis. Front Biosci (Landmark Ed) 16: 1233–1260, 2011. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 47.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol 306: R886–R900, 2014. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci 35: 2181–2190, 2015. doi: 10.1523/JNEUROSCI.3306-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol 289: R514–R520, 2005. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 51.Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol (1985) 112: 1008–1014, 2012. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 52.Shibata M, Uno T, Hashimoto M. Disinhibition of lower midbrain neurons enhances non-shivering thermogenesis in anesthetized rats. Brain Res 833: 242–250, 1999. doi: 10.1016/S0006-8993(99)01532-2. [DOI] [PubMed] [Google Scholar]
- 53.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295: R417–R428, 2008. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souza SC, Christoffolete MA, Ribeiro MO, Miyoshi H, Strissel KJ, Stancheva ZS, Rogers NH, D’Eon TM, Perfield JW II, Imachi H, Obin MS, Bianco AC, Greenberg AS. Perilipin regulates the thermogenic actions of norepinephrine in brown adipose tissue. J Lipid Res 48: 1273–1279, 2007. doi: 10.1194/jlr.M700047-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Szymusiak R, Satinoff E. Acute thermoregulatory effects of unilateral electrolytic lesions of the medial and lateral preoptic area in rats. Physiol Behav 28: 161–170, 1982. doi: 10.1016/0031-9384(82)90118-4. [DOI] [PubMed] [Google Scholar]
- 57.Travis KA, Johnson AK. In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure, and temperature. Am J Physiol Regul Integr Comp Physiol 264: R1200–R1205, 1993. [DOI] [PubMed] [Google Scholar]
- 58.Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Front Neurosci 8: 14, 2014. doi: 10.3389/fnins.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol 302: R1049–R1058, 2012. doi: 10.1152/ajpregu.00640.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 537: 199–225, 2014. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148: 1550–1560, 2007. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 62.Welle SL, Thompson DA, Campbell RG. β-Adrenergic blockade inhibits thermogenesis and lipolysis during glucoprivation in humans. Am J Physiol Regul Integr Comp Physiol 243: R379–R382, 1982. [DOI] [PubMed] [Google Scholar]
- 63.Wojaczynski GJ, Engel EA, Steren KE, Enquist LW, Patrick Card J. The neuroinvasive profiles of H129 (herpes simplex virus type 1) recombinants with putative anterograde-only transneuronal spread properties. Brain Struct Funct 220: 1395–1420, 2015. doi: 10.1007/s00429-014-0733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto K, Senba E, Matsunaga T, Tohyama M. Calcitonin gene-related peptide containing sympathetic preganglionic and sensory neurons projecting to the superior cervical ganglion of the rat. Brain Res 487: 158–164, 1989. doi: 10.1016/0006-8993(89)90952-9. [DOI] [PubMed] [Google Scholar]
- 65.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 268: R744–R751, 1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


