Abstract
The low O2 experienced at high altitude is a significant challenge to effective aerobic locomotion, as it requires sustained tissue O2 delivery in addition to the appropriate allocation of metabolic substrates. Here, we tested whether high- and low-altitude deer mice (Peromyscus maniculatus) have evolved different acclimation responses to hypoxia with respect to muscle metabolism and fuel use during submaximal exercise. Using F1 generation high- and low-altitude deer mice that were born and raised in common conditions, we assessed 1) fuel use during exercise, 2) metabolic enzyme activities, and 3) gene expression for key transporters and enzymes in the gastrocnemius. After hypoxia acclimation, highland mice showed a significant increase in carbohydrate oxidation and higher relative reliance on this fuel during exercise at 75% maximal O2 consumption. Compared with lowland mice, highland mice had consistently higher activities of oxidative and fatty acid oxidation enzymes in the gastrocnemius. In contrast, only after hypoxia acclimation did activities of hexokinase increase significantly in the muscle of highland mice to levels greater than lowland mice. Highland mice also responded to acclimation with increases in muscle gene expression for hexokinase 1 and 2 genes, whereas both populations increased mRNA expression for glucose transporters. Changes in skeletal muscle with acclimation suggest that highland mice had an increased capacity for the uptake and oxidation of circulatory glucose. Our results demonstrate that highland mice have evolved a distinct mode of hypoxia acclimation that involves an increase in carbohydrate use during exercise.
Keywords: hypoxia, exercise, carbohydrates, lipids, lactate, hexokinase, glucose transporter
the low O2 at high altitude poses a significant challenge to effective aerobic locomotion. Animals living in these environments must be able to transport sufficient O2 to support exercise but also allocate the appropriate metabolic substrates to sustain aerobic ATP production rates. The pattern of metabolic substrate use during exercise has been characterized in a number of mammals (10, 41, 45, 55). In general, as metabolic demand increases with higher work rates, there is a predictable increase in the reliance on carbohydrates to support aerobic ATP production. This pattern is remarkably conserved in low-altitude native mammals that vary in body size and aerobic capacity, suggesting the basis for a predictive model of exercise fuel use (45). Although exercise fuel use demonstrates phenotypic plasticity to chronic energetic (e.g., exercise training) and environmental (e.g., hypoxia) stress, the observed changes in rates of metabolic substrate oxidation are correlated with changes in aerobic capacity (e.g., Refs. 6, 9, and 34). One of the only documented deviations from this conserved fuel use pattern was observed in mice from the genus Phyllotis that are native to the high Andes of Peru. An increased reliance on carbohydrates to power aerobic exercise, independent of aerobic capacity, was observed in two species native to high altitude compared with two low-altitude congeneric species (44).
Substrate allocation is regulated, in part, by the mobilization and transport of circulating fuels for uptake by activated muscle fibers. However, the best-understood mechanisms involve the selective recruitment of intramuscular metabolic pathways. Although membrane transport in the muscle has been proposed to exert a high level of control on substrate utilization, recent evidence suggests that glucose phosphorylation in the cytosol by the enzyme hexokinase (HK) is an important bottleneck for muscle carbohydrate oxidation during exercise (54). Thus, it was surprising that no significant differences in skeletal muscle metabolic phenotype were found between highland and lowland Phyllotis mice that could provide a mechanistic explanation for differences in substrate use during exercise (44).
Acclimatization is important for adult animals to adjust on a physiological timescale to changing environments, but these changes are not always in a direction that is considered beneficial (15, 50). Past research has shown that chronic hypoxia can have varying effects on the skeletal metabolic phenotype of lowland mammals, including humans (1, 28). For example, a number of studies on lowland humans have suggested that chronic hypobaric hypoxia reduces muscle aerobic capacity (12, 20), contrary to the predicted benefit of a high mitochondrial content in low-O2 environments (25). Few studies have examined whether high- and low-altitude natives differ in their acclimation responses to chronic hypoxia, either with regard to whole animal performance or the metabolic phenotype of skeletal muscle. In a previous study on high- and low-altitude deer mice (Peromyscus maniculatus) that were born and reared under common conditions, we showed that differences in aerobic capacity under hypoxia were attributable to differences in acclimation responses as well as evolved, genetically based changes. However, capillary surface density, tissue oxidative capacity, and transverse areas of oxidative fiber types (type I and IIa) were genetically fixed at higher levels in the gastrocnemius muscle in highland mice compared with lowland mice, and these traits were unaffected by hypoxia acclimation (31). Furthermore, these traits did not vary between wild deer mice and those raised in captivity (47). While a fixed trait in muscle aerobic capacity may reflect the stable low-O2 conditions at high altitude (50), muscle capacities for substrate oxidation may be more flexible to match appropriate substrate use with changing energetic demands.
Deer mice are well suited to examine mechanisms of local adaptation since they can be found from below sea level to above 4,300 m (36). Estimates of field metabolic rates suggest that highland deer mice expend significantly more energy per day than mice from a lowland population (22), which would require correspondingly high rates of O2 and substrate consumption. As a likely result, aerobic capacity in hypoxia is under strong directional selection at high altitude (23). Indeed, highland deer mice have higher maximal O2 consumption (V̇o2max) rates in hypoxia than lowland mice for thermogenesis and locomotion (14–16, 31). Highland deer mice have also been found to have greater capacities for lipid oxidation in muscle, presumably to support higher rates of sustained shivering thermogenesis (14). However, these mice may be expected to rely to a greater extent on carbohydrates to power exercise due to a superior yield of ATP per mole of O2 consumed (8, 44).
The objective of the present study was to test whether the hypoxia acclimation responses of highland and lowland deer mice differ with respect to muscle metabolic phenotype and fuel use during submaximal exercise. We hypothesized that highland deer mice would have a greater reliance on carbohydrates during submaximal exercise, similar to high-altitude Phyllotis mice in the Andes (44). Since hypoxia acclimation does not affect exercise fuel use in laboratory rodents (34), we predicted that population differences in exercise metabolism would be fixed and would not be affected by chronic hypoxia. We assessed fuel use during submaximal exercise, measured metabolic enzyme activities, and gene expression for key membrane transporters and enzymes in the gastrocnemius muscle. We used F1 generation mice born and raised in common-garden conditions to distinguish between population differences that reflect evolved, genetically based changes and those that reflect differences in acclimation to hypobaric hypoxia.
MATERIALS AND METHODS
Animals and acclimation conditions.
All procedures were approved by the McMaster University Animal Research Ethics Board in accordance with guidelines from the Canadian Council on Animal Care. We used F1 generation deer mice from a captive breeding colony that were offspring of wild-caught parents trapped at 4,350 m above sea level on the summit of Mount Evans, CO (highland mice, P. m. rufinus) and at an elevation of 430 m above sea level in Lancaster County, NE (lowland mice, P. m. nebracensis), as previously described (14, 31). F1 mice born and raised at the University of Nebraska were shipped to McMaster University (~100 m above sea level) and kept at ~23°C with a 12:12-h light-dark cycle and provided standard rodent chow and water ad libitum for at least 4 wk before experiments started. This common-garden design helps control for both developmental and adult phenotypic plasticity, but we cannot rule out the possibility that changes in the examined traits are partly attributable to transgenerational epigenetic effects.
Mice from each population were randomly divided into a control (unacclimated) group kept in normoxic laboratory conditions and a hypoxia-acclimated group held in hypobaric chambers at the equivalent of 4,300 m (450 mmHg). These mice were returned briefly to normobaria 1–2 times/wk for cage cleaning and replenishment of food and water. The acclimation period was for a minimum of 6–8 wk, as previously described (4, 31).
Indirect calorimetry.
An open-flow respirometry system (Sable Systems, Las Vegas, NV) was used to determined V̇o2max at ~23°C in mice running on a Plexiglas-enclosed motorized treadmill with a volume of ~800 ml (44). Incurrent air (or 12% O2-balance N2) that had been stripped of CO2 and H2O was flowed into the treadmill chamber at an average rate of 1,940 ml/min. A subsample of excurrent air was dried using prebaked Drierite (56) and flowed through an O2 and CO2 analyzer at ~200 ml/min using a subsampling pump (Sable Systems). The accuracy of the system was periodically verified by burning methanol in a chamber and comparing theoretical and measured V̇o2 and CO2 consumption (V̇co2) as previously described (44, 51). The V̇o2max values presented here are from previously published data (31), with the addition of four unacclimated lowland, five acclimated lowland, and eight acclimated highland mice. V̇o2max was defined as when at least two of the following three criteria were fulfilled: 1) V̇o2 did not increase with an increase in treadmill speed, 2) the respiratory exchange ratio (RER; V̇co2/V̇o2) was ≥1.0, and 3) the mouse demonstrated behavioral signs of exhaustion and could not maintain its position on the treadmill. We have used these same criteria in previous studies (31, 44, 51). To control for the effect of exercise intensity on fuel use (see Ref. 45), mice were exercised at a speed equivalent to ~75% of V̇o2max. For a few mice, we lacked V̇o2max data and used average treadmill speeds for their acclimation group to perform submaximal running tests on these individuals. Mice were run in the postabsorptive state by fasting for 4–6 h and under their respective acclimation conditions as we have previously done (34, 35). To calculate V̇o2 and V̇co2, we used the following equations obtained from Withers (57):
| (1) |
| (2) |
where VIstp is the rate of inflow into the chamber, is the fraction of expired CO2, is the fraction of inspired CO2, is the fraction of inspired O2, and is the fraction of expired O2. Cost of transport was calculated by dividing V̇o2 (in ml/min) by treadmill speed (in m/min) to obtain the metabolic cost of moving a meter distance (in ml O2/m). Rates of carbohydrate and lipid oxidation were calculated according to Frayn (17) assuming that the contribution of protein to overall exercise energy expenditure is minimal in the postabsorptive state (11, 40), but, to our knowledge, this has not been confirmed for mice. Relative rates of carbohydrate and lipid oxidation were calculated by dividing absolute oxidation rates by V̇o2 and expressed as a percentage of total V̇o2. Individual values over 100% or under 0% were set to 100% and 0% (one individual acclimated highland mouse).
Gastrocnemius and liver enzyme activities.
Mice were anaesthetized in a small chamber using an isofluorance-soaked cotton ball and euthanized by cervical dislocation. Gastrocnemius muscle and liver samples were collected at rest, crushed between liquid N2-cooled aluminum plates, and then stored at −80°C until analysis. All enzymes measurements were performed using a Spectromax Plus 384, 96-well microplate reader (Molecular Devices, Sunnyvale, CA). Approximately 30 mg of gastrocnemius and liver samples were powdered in a liquid N2-cooled mortar and homogenized on ice using a glass on glass homogenizer in 20 volumes of homogenizing buffer containing (in mM) 100 potassium phosphate (pH 7.2), 5 EDTA, and 0.1% Triton X-100. Assays were performed at 37°C in triplicate, and background activities were determined for each assay by omitting substrate.
The apparent Vmax of cytochrome c oxidase, phosphofructokinase (PFK), β-hydroxyacyl CoA dehydrogenase (HOAD), isocitrate dehydrogenase (IDH), and HK were measured on fresh homogenates. The activities of pyruvate kinase (PK) and citrate synthase (CS) were measured after the homogenates had been frozen and thawed once and three times, respectively. Assay conditions were PFK: 10 mM fructose-6-phosphate (omitted in control), 4 mM ATP, 0.28 mM NADH, 2 mM AMP, 5 mM MgCl2, 50 mM KCl, 5 mM DTT, 1 unit aldolase, 50 units triose phosphate isomerase, and 8 units α-glycerophosphate dehydrogenase in 50 mM triethanolamine (TEA)·HCl (pH 7.6); HOAD: 0.1 mM acetoacetyl-CoA (omitted in control), 0.28 mM NADH, and 5 mM EDTA in 100 mM TEA·HCl (pH 7.0); IDH: 1.5 mM isocitrate (omitted in control), 2 mM ADP, 0.5 mM NADP, 1 mM MnCl2, and 8 mM MgCl2 in 40 Tris·HCl (pH 7.4); HK: 5 mM d-glucose (omitted in control), 8 mM ATP, 8 mM MgCl2, 0.5 mM NADP, and 4 units glucose-6-phosphate dehydrogenase in 50 mM HEPES (pH 7.6); PK: 5 mM phosphoenol pyruvate (PEP; omitted in control), 5 mM ADP, 10 mM MgCl2, 0.15 mM NADH, 10 mM fructose-1,6-phosphate, 100 mM KCl, and 9.25 units lactate dehydrogenase (LDH) in 50 mM imidazole (pH 7.4); CS: 0.5 mM oxaloacetate (omitted in control), 0.22 mM acetyl-CoA, and 0.1 mM dithiobisnitrobenzoic acid in 40 Tris·HCl (pH 8.0); phosphoenolpyruvate carboxykinase (PEPCK) in the liver: 1 mM MnCl2, 0.15 mM NADH, 20 units malate dehydrogenase, 20 mM NaHCO3, 0.2 mM GDP, and 0.5 mM PEP (omitted in control) in 20 mM imidazole (pH 7.0).
Blood and tissue metabolites.
Resting blood samples were obtained from a subset of unstressed mice by placing a lid containing an isofluorane-soaked cotton ball carefully over their home cage. Once lightly anesthetized, mice were quickly removed from the cage and blood was obtained from the facial vein by pricking with a lancet and collecting in a heparinized capillary tube. Mice were then returned to their cage to recover for a minimum of 48 h. Postexercise blood samples were obtained immediately after 15 min of exercise in unanesthetized mice. Whole blood lactate was determined using a lactate analyzer (Lactate Pro LT, Arkray, Kyoto, Japan), and glucose was determined using a handheld glucose meter (Accu-Chek Aviva, Roche Diagnostics) as previously described in mice (7, 30).
Gastrocnemius muscle and liver samples were collected at rest as described above and immediately after 15 min of exercise at ~75% V̇o2max by cervical dislocation. Tissues were quickly removed, crushed between liquid N2-cooled aluminum plates, and then stored at −80°C until analysis. A separate group of unacclimated mice from each population was sampled for skeletal muscle at rest as described above for blood sampling. These values were used to compare with postexercise results from all groups. Metabolites were determined by standard methods (5) adapted for a 96-well format using a Spectramax Plus 384 microplate reader (Molecular Devices). Briefly, ~30–50 mg of powdered tissues were homogenized (PowerGen 125, Fisher Scientific, Whitby, ON, Canada) in 600 µl of 6% perchloric acid on ice. A portion of this homogenate (100 µl) was removed and stored at −80°C for glycogen determination. The remaining homogenate was centrifuged at 10,000 g for 10 min at 4°C. The supernatant was removed and subsequently neutralized with K2CO3 (3 M). Samples were then centrifuged again at 10,000 g for 10 min at 4°C, and the resulting supernatant was used for metabolite quantification. Assay conditions for lactate determinations were 2 mM NAD+ in glycine/hydrazine buffer (pH 9.2, Sigma), and the reaction was started using 8 units LDH (Roche Diagnostics). For glycogen determinations, 50 µl of 1 M K2HCO3 and 100 µl of 400 mM acetate buffer (pH 4.8) were added to the crude homogenate (see above). Half of this homogenate was removed for determinations of free glucose, and the remaining homogenate was combined with 7 µl (4 U/µl) of amyloglucosidase (Roche Diagnostics). All samples were incubated for 2 h at 40°C and then neutralized with 1 M K2CO3. Neutralized samples were assayed for glucose under the following conditions: 20 mM imidazole, 1 mM ATP, 0.5 mM NADP, 5 mM MgCl2, and 1 unit glucose-6-phosphate dehydrogenase (Roche Diagnostics) in buffer containing 300 mM TEA·HCl and 4.05 mM MgSO4 (pH 7.5) (Roche Diagnostics). The reaction was initiated by adding 1 unit HK (in TRA buffer, Roche Diagnostics).
Gene expression.
Gastrocnemius muscles that were sampled from resting animals (with the exception of one unacclimated highland mouse sampled postexercise) were powdered under liquid N2 as described above, and total RNA was extracted from 30−40 mg tissue using TRIzol reagent (Invitrogen, Carlsbad, CA) based on the acid guanidinium thiocyanate-phenol-chloroform extraction method. One microgram of DNase I-treated total RNA was used to synthesize cDNA using SuperScript II RNase H- reverse transcriptase (Invitrogen). Specific primers (Table 1) were designed by Primer 3 software (43) using available deer mouse sequences from the National Center for Biotechnology Information Sequence Read Archive (Accession Nos. SRA051883 and SRA091630) (14, 15). Each transcript was amplified in duplicate using 4 µl of cDNA diluted fivefold in RNase and DNase-free water (Invitrogen), 5 µl of SsoFast EvaGreen Supermix (Bio-Rad, Mississauga, ON, Canada), 10 µM of forward and reverse primers in 0.4 µl, and 0.2 µl of RNAase and DNase-free water. Real-time PCRs were run in the 96-well format using a CFX Connect Real-Time System (Bio-Rad) with a thermal program of 30 s of initial denaturation at 95°C, 40 cycles of 5 s at 95°C, and finally 30 s at 60°C. For each plate, a no-template control was included. Standard curves using one sample were generated for each primer set and used to determine the relative expression of all other samples. Expression levels were normalized to 12S rRNA expression, as previously described (31).
Table 1.
Primer sequences used for real-time PCR analysis of mRNA expression in deer mice gastrocnemius muscle
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Glut4 | 5′-GCTTTGTGGCCTTCTTTGAG-3′ | 5′-TCAGGCACTTTGAGGAAGGT-3′ |
| Slc2a1 | 5′-CATCCCAGCCCTGATACAGT-3′ | 5′-CAACTCCAGGATGGTGACCT-3′ |
| Hk1 | 5′-AAACCCCAGAGAACATCGTG-3′ | 5′-TTCAGCAGCTTGACCACATC-3′ |
| Hk2 | 5′-GGTGGAGATGGAGAACCAGA-3′ | 5′-CCTTCCACACCACTGGACTT-3′ |
| Ndfuc2 | 5′-ACGAGCCCTTCACATTTCTG-3′ | 5′-AATAGAAGCTGGCGATGCAG-3′ |
| Pck1 | 5′-CAGGATTGAAGGGGAAGACA-3′ | 5′-TCTGTCTCAGGGCTTGGAGT-3′ |
| 12S | 5′-CTGGCCATCGCTTAAAACTC-3′ | 5′-TTGCTTCCCACCTCATAAGC-3′ |
Glut, glucose transporter; Hk, hexokinase. Ndfuc encodes a NADH dehydrogenase (ubiquinone) subunit; Pck1 encodes phosphoenolpyruvate carboxykinase.
Calculations and statistics.
Statistical analyses were performed using GraphPad Prism and SPSS (IBM) software packages. V̇o2max values were corrected for body mass using allometric regressions, as previously described (31). We also analyzed V̇o2max data using analysis of covariance (ANCOVA) with body mass as a covariate and obtained similar results as the allometric analysis. ANCOVA using mass as a covariate was also used to analyze cost of transport (COT) and exercise intensity as a covariate for RER (51). All other data were analyzed using two-way ANOVA with population and acclimation as main factors. Exercise was also included as a main factor for metabolite data. Data are presented at means ± SE.
RESULTS
Aerobic capacity and submaximal exercise intensities.
There is a strong relationship between exercise intensity relative to maximum aerobic capacity and the proportional use of carbohydrates and lipids (45). Thus, we standardized exercise intensity by first determining running V̇o2max for animals in their respective acclimation condition. The results show that hypoxic V̇o2max was ~14% higher in acclimated highland versus lowland mice (3.47 ± 0.05 vs. 3.05 ± 0.20 ml/min, P = 0.023). However, highland mice had a significantly higher body mass, and there were no significant population differences in mass-corrected V̇o2max detected for unacclimated mice tested in normoxia or for acclimated mice tested in hypoxia (ANCOVA, F1,51 = 0.62, P = 0.44; Table 2).
Table 2.
Average body mass, V̇o2max, V̇o2, respiratory exchange ratio, and cost of transport at a target intensity of 75% V̇o2max in normoxia ( = 0.2095) for unacclimated F1 deer mice and in hypoxia ( = 0.12) for hypoxia-acclimated F1 lowland and highland deer mice
| Normoxia |
Hypoxia |
|||
|---|---|---|---|---|
| Lowland mice | Highland mice | Lowland mice | Highland mice | |
| Body mass, g | 20.5 ± 0.9 (12) | 21.6 ± 1.2 (13)† | 19.0 ± 0.9 (12) | 23.8 ± 1.4 (13)† |
| V̇o2max, ml/min | 3.91 ± 0.18 | 3.79 ± 0.20 | 3.05 ± 0.20* | 3.47 ± 0.05* |
| Submaximal running | ||||
| V̇o2, ml/min | 2.99 ± 0.21 (12) | 2.88 ± 0.15 (20)† | 2.60 ± 0.08 (13) | 2.75 ± 0.11 (18) |
| %V̇o2max | 76 ± 4 | 72 ± 2 | 82 ± 2 | 79 ± 3 |
| Speed, m/min | 18.9 ± 1.0 | 23.4 ± 1.4† | 17.0 ± 1.2 | 20.3 ± 0.8* |
| Respiratory exchange ratio (V̇co2/V̇o2) | 0.854 ± 0.011 | 0.827 ± 0.009 | 0.849 ± 0.016 | 0.884 ± 0.018* |
| Cost of transport, ml/m | 0.17 ± 0.02 | 0.13 ± 0.01† | 0.16 ± 0.02 | 0.14 ± 0.01 |
Numbers in parentheses are sample sizes for each group. V̇o2max, maximal O2 consumption (V̇o2); V̇co2, CO2 production; , fraction of inspired O2.
Significant effect of acclimation within a population;
significant effect of population within an acclimation.
Mice were then run at a target submaximal intensity of 75% V̇o2max. There were no significant differences between populations in relative exercise intensity calculated after the run. However, highland mice were running at a significantly higher absolute treadmill speed, and they also had a significantly lower V̇o2 in normoxia (F1,30 = 4.26, P = 0.049; Table 2). The result of a higher running speed and lower metabolic cost resulted in a significantly lower COT for unacclimated highland mice compared with unacclimated lowland mice (F1,31 = 7.19, P = 0.012; Table 2). A similar difference in COT was seen between populations after acclimation and approached statistical significance (F1,29 = 3.86, P = 0.06).
We determined RERs from V̇o2 and V̇co2 during submaximal running as an index of metabolic fuel use. There was a significant effect of acclimation on RER during submaximal running (F1,56 = 6.68, P = 0.013) that was driven primarily by an increase in exercise RER values in highland mice from 0.827 ± 0.009 when unacclimated to 0.884 ± 0.018 after hypoxia acclimation (Table 2).
Fuel oxidation with submaximal exercise.
To determine rates of whole animal substrate oxidation, we used V̇o2 and V̇co2 in the oxidation of carbohydrates and lipids (17) to calculate absolute rates of use during exercise. We found that there was a significant interaction between population and acclimation for the absolute rates of carbohydrate oxidation during submaximal running (F1,63 = 4.11, P = 0.047; Fig. 1). Rates of carbohydrate oxidation were significantly lower in unacclimated highland mice running in normoxia compared with lowland mice (P = 0.027). However, acclimation led to a significant 34% increase in carbohydrate oxidation in highland mice (P = 0.031; Fig. 1A). There was also a significant interaction effect of acclimation and population for lipid oxidation (F1,63 = 4.14, P = 0.041), which showed a corresponding decline in acclimated highland mice to rates significantly lower than lowland mice (P = 0.016; Fig. 1B). To account for any differences in metabolic rate during submaximal exercise, absolute rates of fuel oxidation were divided by total V̇o2. The results show that relative rates of carbohydrate oxidation in highland mice significantly increased with acclimation (P = 0.004) to a higher level than rates in acclimated lowland mice (population × acclimation, F1,63 = 4.35, P = 0.041; Fig. 1C). There was no significant effect of acclimation on either absolute rates or the relative proportion of substrate oxidation in lowland mice (Fig. 1). These results demonstrate that high- and low-altitude mice have different acclimation responses with regard to substrate use during exercise, such that only highland mice respond to chronic hypoxia with an increased reliance on carbohydrates to power submaximal exercise.
Fig. 1.
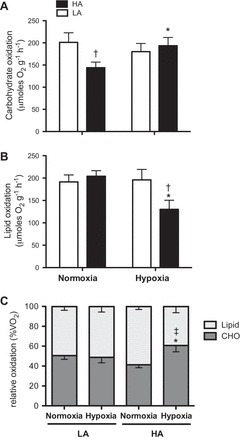
Absolute rates of carbohydrate (CHO; A) and lipid (B) oxidation at a target intensity of 75% maximal O2 consumption (V̇o2max) (actual intensities appear in Table 2) in lowland (LA) and highland (HA) mice exercising in their acclimation conditions {normoxia [fraction of inspired O2 (): 0.2095] for unacclimated and hypoxia (: 0.12) for hypoxia acclimated}. The proportional contribution of carbohydrates and lipids as relative oxidation (percent V̇o2; C) was calculated by dividing absolute oxidation rates by V̇o2 and multiplying by 100. Results are presented as means ± SE. *Significant effect of acclimation within a population; †significant effect of population within an acclimation group; ‡significant interaction of population × acclimation.
Blood and tissue metabolites.
To determine if exercise substrate use was reflected in changes in blood and tissue metabolite concentrations, we determined the levels of circulating glucose and lactate at rest and immediately after submaximal exercise. We found a significant effect of exercise on blood glucose (F1,49 = 12.21, P = 0.001) and a significant interaction between population and exercise (F1,49 = 6.08, P = 0.018; Fig. 2A). At rest, glucose levels were significantly lower in highland mice compared with lowland mice but only after acclimation (P = 0.08 before acclimation and P = 0.009 after acclimation). Fifteen minutes of submaximal exercise led to a significant decline in blood glucose in lowland mice in normoxia (P = 0.016) and hypoxia (P = 0.001). In contrast, highland mice maintained stable glucose levels with exercise in both conditions (P > 0.05).
Fig. 2.
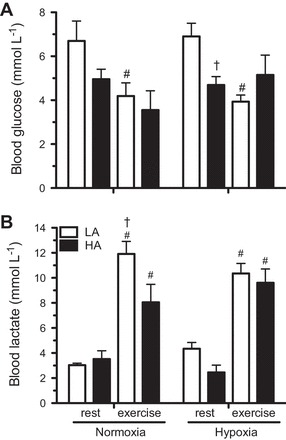
Blood glucose and lactate concentrations in LA and HA mice at rest and after 15 min of exercise at 75% V̇o2max in their acclimation conditions [normoxia (: 0.2095) for unacclimated and hypoxia (: 0.12) for hypoxia acclimated]. Results are presented as means ± SE. *Significant effect of acclimation within a population; †significant effect of population within an acclimation group; #significant effect of exercise within a population for each acclimation.
Blood lactate also showed a main effect of exercise (F1,49 = 95.54, P = 0.00) and an effect of population approached statistical significance (F1,50 = 3.50, P = 0.068; Fig. 2B). Both populations showed a significant accumulation of blood lactate with exercise but to a higher concentration in unacclimated lowland mice in normoxia (P = 0.006). However, neither population showed any significant effect of acclimation, but blood lactate concentrations reached equivalent levels in lowland and highland mice exercising in hypoxia (Fig. 2B).
Muscle lactate and glycogen and liver glycogen.
There was a significant effect of exercise on muscle glycogen, which declined in all groups compared with rest (F1,30 = 65.67, P = 0.00; Fig. 3B). Similarly, muscle lactate increased significantly with exercise in all groups (F1,37 = 10.72, P = 0.003, Fig. 3). However, for both muscle glycogen and lactate, there were no significant main effects of population or acclimation, and there were no significant interactions between population, acclimation, and exercise. Liver glycogen is an important extramuscular carbohydrate source for locomotion. Resting levels of liver glycogen were not significantly different between the populations and did not change with acclimation (Fig. 4). However, there was a significant reduction in liver glycogen after exercise (F1,37 = 10.72, P = 0.003), but there were no main effects of population or acclimation, and there were no significant interactions between population, acclimation, and exercise.
Fig. 3.
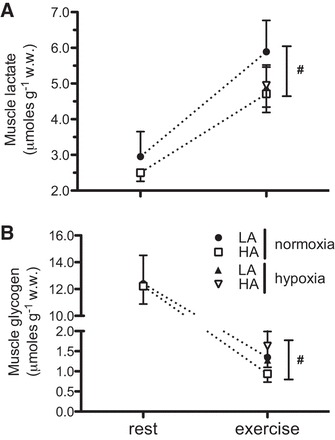
Gastrocnemius muscle lactate (A) and glycogen concentrations (B) in LA and HA mice at rest and after 15 min of exercise at 75% V̇o2max in their acclimation conditions [normoxia (: 0.2095) for unacclimated and hypoxia (: 0.12) for hypoxia acclimated]. Results are presented as means ± SE. #Significant effect of exercise in all groups.
Fig. 4.
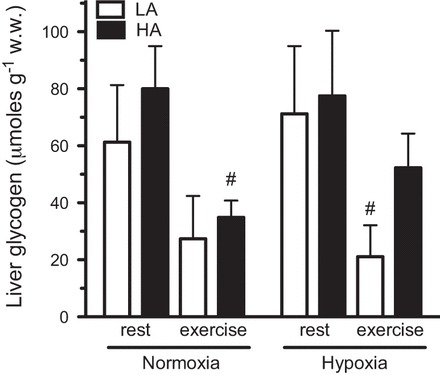
Liver glycogen concentrations in LA and HA mice at rest and after 15 min of exercise at 75% V̇o2max in their acclimation conditions [normoxia (: 0.2095) for unacclimated and hypoxia (: 0.12) for hypoxia acclimated]. Results are presented as means ± SE. #Significant effect of exercise.
Gastrocnemius and liver enzyme activities.
We previously showed that the gastrocnemius of highland mice has greater oxidative capacity than that of lowland mice, and this trait was not affected by hypoxia acclimation (31). Our results confirm these findings and showed a significant main effect of population for the enzymes HOAD (F1,34 = 8.02, P = 0.008) and IDH (F1,34 = 17.55, P = 0.00), which was higher in highland mice with normoxia (P = 0.022) and hypoxia acclimation (P = 0.01; Fig. 5, D and E). However, the differences in fuel use in lowland and highland mice after acclimation (Fig. 1) suggest that representative enzymes in glycolysis may be affected by chronic hypoxia. Indeed, there was a significant effect of acclimation for HK activity (F1,34 = 4.15, P = 0.05). Muscle HK activity increased by 35% in highland mice after hypoxia acclimation to activities higher than those in lowland mice (P = 0.022; Fig. 5A). Similarly, PK activity showed a significant effect of population (F1,34 = 4.12, P = 0.051) and was higher in lowland mice, but only after acclimation (P = 0.015; Fig. 5C). While there was an overall significant population effect for PFK (F1,20 = 5.42, P = 0.033), the slight increase of ~20% in both populations after hypoxia acclimation did not constitute a statistically significant change (Fig. 5B).
Fig. 5.
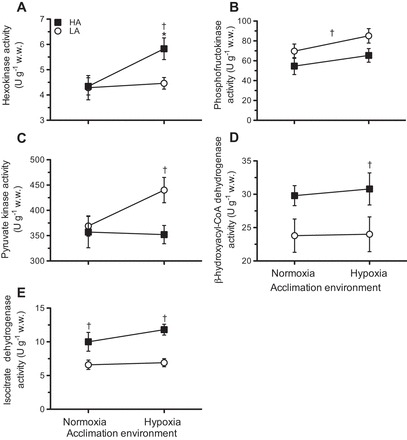
Apparent Vmax (in U/g tissue wet wt) in gastrocnemius for the enzymes hexokinase (A), phosphofructokinase (B), pyruvate kinase (C), β-hydroxyacyl-CoA dehydrogenase (D), and isocitrate dehydrogenase (E) for LA and HA deer mice acclimated to normoxia or hypoxia. Results are presented as means ± SE. *Significant effect of acclimation within a population; †significant effect of population within an acclimation group.
Gene expression in gastrocnemius muscle.
We tested whether high- and low-altitude mice exhibited differences in the expression of six candidate genes involved in muscle metabolism. Specifically, we tested for differences in constitutive gene expression as well as differences in the acclimation response to hypoxia. Relative transcript levels of Slc2a1 [glucose transporter (GLUT)1] and GLUT4 showed a significant effect of acclimation (F1,22 = 11.51, P = 0.003, and F1,21 = 23.18, P = 0.00, respectively). Slc2a1 significantly increased but only in highland mice (P = 0.005), and Glut4 increased in both lowland and highland populations (P = 0.003 and P = 0.004; Fig. 6, A and B). There was a significant acclimation effect in the expression of the Hk I transcript (F1,20 = 5.42, P = 0.033) and a significant acclimation effect (F1,22 = 4.81, P = 0.042) and nearly statistically significant interaction effect of population and acclimation (F1,22 = 4.07, P = 0.059) for the Hk II expression (Fig. 6, C and D). In unacclimated mice, lowland mice had higher HK II expression (P = 0.016), but highland mice showed a significant increase in the expression of this gene with acclimation (P = 0.007) to a level equivalent to that in lowland mice (Fig. 6D). Ndfuc2, which encodes a NADH dehydrogenase (ubiquinone) subunit, showed a significant acclimation effect (F1,22 = 8.95, P = 0.008), with acclimation increasing transcript levels in lowland mice to a value greater than highland mice (P = 0.045; Fig. 6E). Finally, Pck1, which encodes the PEPCK enzyme, a component of the gluconeogenic pathway (Fig. 6F), showed a significant effect of population (F1,22 = 5.65, P = 0.029) and tended to be higher in highland mice (P = 0.065).
Fig. 6.
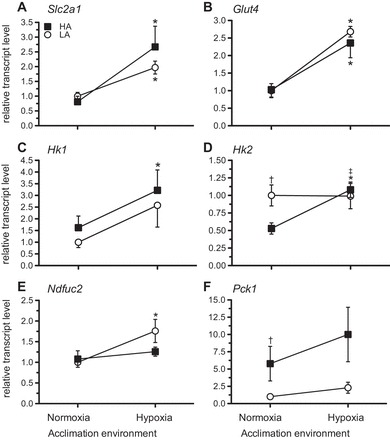
Relative transcript abundance in gastrocnemius muscle for the following genes involved in carbohydrate metabolism: Slc2a1 (A), glucose transporter (Glut)4 (B), hexokinase (Hk)1 (C), Hk2 (D), Ndfuc2 [which encodes for a NADH dehydrogenase (ubiquinone) subunit; E], and Pck1 (which encodes phosphoenolpyruvate carboxykinase; F) in LA and HA mice acclimated to normoxia or hypoxia. Results are presented as means ± SE. *Significant effect of acclimation within a population; †significant effect of population within an acclimation group; ‡significant interaction of population × acclimation.
Liver enzyme Vmax.
Glycogenolysis and gluconeogenesis in the liver are major sources of circulatory glucose for working muscle. We examined the Vmax of PEPCK as an index of gluconeogenic capacity of the liver and CS and cytochrome c oxidase for the capacity to supply ATP for liver metabolism. We found that although mean PEPCK activity tended to be higher in highland mice by ~8–18%, there was no statistically significant effect of population (F1,41 = 2.42, P = 0.14; Fig. 7B). However, activities of cytochrome c oxidase showed a significant population effect (F1,42 = 5.30, P = 0.027) and was greater in unacclimated highland mice (P = 0.04). There was a significant main effect of population for HOAD activity (F1,42 = 7.22, P = 0.011), which was also higher in highland mice in the unacclimated state (P = 0.049; Fig. 7, A and D). Thus, capacities for electron transport and fatty acid oxidation were higher in the livers of unacclimated highland versus lowland mice. Although none of the enzymes measured responded to acclimation, activities of cytochrome c oxidase and HOAD were not significantly different between highland and lowland mice in this condition (P = 0.27 and P = 0.085, respectively).
Fig. 7.
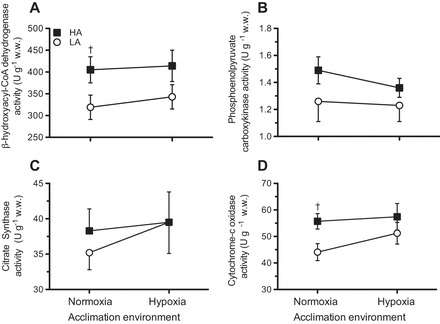
Apparent Vmax (in U/g tissue wet wt) in the liver for the following enzymes: β-hydroxyacyl-CoA dehydrogenase (A), phosphoenolpyruvate carboxykinase (B), citrate synthase (C), and cytochrome c oxidase (D) in LA and HA mice acclimated to normoxia or hypoxia. Results are presented as means ± SE. †Significant effect of population within an acclimation group.
DISCUSSION
The primary goal of the present study was to characterize differences between highland and lowland mice in the phenotypic plasticity of substrate oxidation during submaximal exercise. Our results show that F1 generation high-altitude deer mice have increased rates of carbohydrate oxidation after hypoxia acclimation, resulting in a higher proportional contribution of this fuel to total exercise energy expenditure. Highland mice had elevated activities of the mitochondrial enzyme IDH in gastrocnemius muscle, which was unaffected by acclimation. These data confirmed our previous findings that high-altitude mice have evolved a more aerobic skeletal muscle phenotype (31, 47). Similarly, high-altitude mice have a higher capacity for lipid oxidation in skeletal muscle. In contrast, we found that the activities of enzymes involved in glycolysis showed significant phenotypic plasticity in muscle with hypoxia acclimation. In particular, chronic hypoxia increased the activity of HK in highland mice, suggesting an enhanced capacity for circulatory glucose uptake by working muscle during exercise.
Fuel use during submaximal exercise.
Our previous studies demonstrated that high- and low-altitude deer mice did not exhibit a significant change in running V̇o2max in normoxia in response to hypoxia acclimation (31). However, in this study, F1 generation high-altitude deer mice exhibited increased rates of carbohydrate oxidation during submaximal exercise with hypoxia acclimation (Fig. 1). Thus, highland and lowland mice exhibited independent differences in the phenotypic plasticity of exercise fuel use and aerobic capacity. These results are consistent with cardiac data from highland native humans (27) and whole animal data from other highland mouse lineages (44). High-altitude species of Phyllotis mice were found to have a greater reliance on carbohydrates for exercise in hypoxia than did low-altitude species from the same genus (44). It is interesting to note that these mice were wild caught but kept in laboratory conditions for several weeks before being tested. These results suggest a fixed genetic adaptation in the high-altitude Phyllotis species is principally responsible for the observed phenotype. In contrast, we found that, in the unacclimated state, lowland and highland populations of deer mice showed no significant differences in proportional fuel use. Lowland deer mice also showed no change in exercise fuel use with hypoxia acclimation, consistent with data from laboratory rats (34, 35) and lowland humans (9, 32). The differences in acclimation responses documented here demonstrate that high-altitude mice have greater phenotypic plasticity for proportional fuel use than do their low-altitude counterparts. Moreover, these and other data (44) suggest that an increased reliance on carbohydrates to power exercise is a trait unique to highland natives, presumably to optimize the energy yield per unit O2 in a hypoxic environment (8). The present study also suggests that both genetic adaptation and phenotypic plasticity can affect this trait, but their relative influence is likely a factor of the degree of gene flow with lowland populations or length of colonization at altitude.
Highland mice had a lower COT when tested in normoxia and a similar difference that approached statistical significance when tested in hypoxia after hypoxia acclimation (Table 2). Mechanisms that may lower the cost of moving a unit distance can be either biochemical or biomechanical in nature. Increasing the use of carbohydrates decreases the O2 cost of ATP production, whereas increased efficiency of generating force decreases ATP consumption at a given work rate. Although the former may contribute to differences in COT in the acclimated state, the lack of variation in exercise fuel use in the unacclimated state does not support this biochemical mechanism in lowering COT (Fig. 1). Highland mice must therefore lower COT by increasing the efficiency of producing muscle force by a yet unknown biomechanical mechanism.
Muscle metabolic phenotype.
The gastrocnemius muscle of highland mice showed both higher aerobic capacities and higher capacities for fatty acid oxidation (Fig. 5). Our previous studies have shown that differences in muscle aerobic capacity can be largely attributed to the higher aerial densities of aerobic fibers in highland mice, and these population differences are robust to acclimation conditions (31, 47). The more aerobic phenotype of highland muscle is associated with higher exercise (31) and thermogenic (15) capacities in hypoxia. In fact, there is strong directional selection for increased aerobic capacity for thermogenesis in highland deer mice (14, 23). Lipids are an essential fuel for sustained aerobic heat production in rodents (52), and in deer mice, elevated capacities for thermogenesis in the highland population are associated with elevated capacities for fatty acid oxidation in muscle (14). These data suggest aerobic capacity and lipid metabolism are strongly linked in this species. However, capacities for muscle uptake and cytosolic transport of fatty acids, two traits associated with increased lipid oxidation in laboratory mice (51), have not been assessed in high-altitude natives.
In contrast to lipid metabolism, the results of this study demonstrate that the capacity for muscle glucose uptake and glycolysis exhibits substantial phenotypic plasticity in response to hypoxia in deer mice. The significant increase in HK activity in highland mice suggests that the capacity for glucose uptake was enhanced in this population. Phosphorylation of glucose by HK has been shown to play an essential role in determining muscle glucose uptake during exercise (54), and elevated muscle HK activity is associated with increased endurance in mice (18). The importance of circulatory fuel to total V̇o2 can be low in humans (42), but it varies across species and with exercise intensity. Rodents exercising at equivalent work rates to those reported here have been shown to support up to 25% of V̇o2 using circulatory glucose (34). In addition, mice lacking the muscle isoform of glycogen synthase showed no decline in endurance (38), suggesting that the ability to use circulating glucose independent of muscle glycogen breakdown is very beneficial. We also found that activities of the glycolytic enzymes PFK and PK were higher after acclimation but in lowland mice. Hypoxia is known to stimulate the increase in glycolytic enzymes including LDH, which was also higher in lowland mice (31). However, after acclimation, both populations had an equivalent capacity of aerobic relative to anaerobic glucose use (PK/LDH: ~0.75). These data suggest that the skeletal muscle of hypoxia-acclimated highland mice likely has a uniquely enhanced capacity for using circulatory glucose compared with lowland mice. This would have the combined advantage of yielding more ATP per mole of O2 and also conserving valuable intramuscular carbohydrate stores. It is important to note that our measurements of enzyme activity indicate the capacity at specific reaction steps but not the in vivo rates of pathway flux.
Muscle gene expression.
The change in muscle capacity for glucose use with acclimation was associated with increases in mRNA for the genes Hk I and Hk II in highland mice. Expression levels of membrane glucose transporters (Slc2a1 and Glut4) also increased with acclimation but in mice from both populations. However, these data suggest that membrane transport and glucose phosphorylation capacity are both increased in highland mice. Surprisingly, highland mice had higher relative gene expression for Pck1, which encodes the enzyme PEPCK, a component of the gluconeogenic pathway. While skeletal muscles generally exhibit negligible rates of gluconeogenesis, PEPCK has been implicated in enhancing exercise endurance (21, 37). Mice overexpressing this enzyme in locomotory muscles had higher levels of aerobic activity, perhaps by reducing the concentrations of inhibitory tricarboxylic acid cycle end products or by enhancing glycerol synthesis in this nongluconeogenic tissue (21, 58). Previously, we found that expression levels of transcription factors that regulate genes involved in angiogenesis and various metabolic pathways were either unchanged or decreased with hypoxia acclimation regardless of altitude ancestry. The exception was peroxisome proliferator-activated receptor (PPAR)-γ, which was higher in highland mice muscle at mRNA and protein levels (31). Elevated PPAR-γ may play a role in stimulating Pck1 expression in muscle, as it does in adipocytes. Elevated PPAR-γ has also been associated with increased muscle glucose uptake and insulin sensitivity (2). Alternatively, various other metabolic genes are more highly expressed in the muscle of highland mice (14, 15, 47) and could contribute to the observed differences in metabolic phenotype and exercise fuel use.
Blood and skeletal muscle metabolites.
Chronic hypoxia did not significantly alter resting blood glucose levels in either population (Fig. 2). This contrasts with some (19) but not all studies (34) on hypoxia-exposed laboratory rodents and suggests a possible distinct hypoxia response in deer mice. Highland mice maintained constant blood glucose concentrations between rest and immediately postexercise (Fig. 2). This suggests rapid adjustments of hepatic supply and muscle uptake to match changing energetic demands. In contrast, lowland mice showed significant declines in blood glucose with exercise, suggesting a substantial mismatch between substrate supply and demand. Population differences in blood glucose were not associated with muscle or liver glycogen depletion with exercise, which was not significantly different between high- and low-altitude mice or with acclimation (Figs. 3 and 4). The lower blood glucose levels seen in highland mice are consistent with other highland populations, including humans (39), and may be related to elevated PPAR-γ protein expression in skeletal muscle of this population (31). This would be consistent with studies of laboratory mice that demonstrated that overexpression of PPAR-γ conferred an increased insulin sensitivity (2, 3, 24).
Postexercise accumulation of blood lactate was higher in lowland mice in the unacclimated condition but had similar blood concentrations to those in highland mice after hypoxia acclimation. Since muscle lactate was not different between the populations postexercise, these data suggest that lowland mice may have reduced lactate production with exercise after acclimation, possibly by relying to a lesser extent on anaerobic metabolism. It is also possible that reductions in blood lactate reflect increase uptake by tissues, such as the liver, to serve as a gluconeogenic precursor.
Liver enzymes and glycogen.
Neither glycogen nor enzyme activities in the liver were significantly altered by hypoxia acclimation (Figs. 4 and 7). Both populations showed a decline in liver glycogen with exercise but to equivalent levels, suggesting that hepatic glucose production from glycogenolysis did not differ. However, the higher HOAD and cytochrome c oxidase activities in the livers of highland mice suggest a greater capacity for energy supply to support processes such as gluconeogenesis. An elevated capacity to generate ATP combined with a trend for higher PEPCK activities is suggestive of enhanced hepatic glucose production (29) in highland mice. A greater capacity for hepatic glucose production and muscle uptake would lead to higher glucose availability for exercise and tighter regulation of blood glucose levels.
Substrate and oxygen transport.
Aerobic metabolism involves transport steps that are shared by both substrates and O2. Adaptations for enhanced O2 transport observed in highland mice (31) could also enhance substrate transport rates. Highland mice respond to acclimation with a blunted increase in hematocrit compared with lowland mice (31). Under hypoxia, they are able to sustain tissue O2 delivery by virtue of an increased hemoglobin-O2 affinity, which safeguards arterial O2 saturation, thereby enhancing the O2 capacitance of the blood (the total amount of O2 unloaded for a given arteriovenous difference in O2 tension) (48, 49). Circulatory substrate transport should thus be elevated in highland mice, because a lower hematocrit would 1) lead to higher rates of plasma flow at equivalent cardiac outputs and 2) avoid viscosity-related declines in cardiac output that can reduce tissue blood flow (46). As a result, highland mice may be able to avoid depleting valuable intramuscular substrate stores (33). Capillary surface area also plays an essential role in determining net substrate flux from the circulation into muscle (53). Highland mice have significantly higher muscle capillary surface densities (31, 47), which should considerably increase substrate conductance compared with lowland mice. When combined with the capacity for an increased transendothelial glucose concentration difference via increased glucose phosphorylation potential, this increased substrate conductance likely facilitates carbohydrate oxidation at high altitude.
Conclusions and implications.
A potential downside to increased carbohydrate use is the exhaustion of limited glycogen stores. We estimate that high-altitude mice could sustain 75% V̇o2max for ~20 min on available glycogen stores estimated from our muscle and liver measurements. These estimates assume that all of the stored glycogen is available for working muscle and that gluconeogenesis in the liver plays no role in supplying glucose. However, our data suggest that high-altitude mice may have enhanced capacities from gluconeogenesis and circulatory glucose uptake that may extend this estimated running time. When voluntary locomotion on running wheels was examined in a colony of laboratory-reared deer mice, they preferred to run at speeds equivalent to ~70% V̇o2max but for durations much shorter than 20 min (13), suggesting glucose availability is not limiting to locomotion. Previous work has demonstrated that some traits in the O2 delivery pathway exhibit a greater acclimation response to hypoxia in low-altitude mice than in high-altitude mice (31). We show here that given the same environmental acclimation conditions, aspects of substrate delivery and oxidation show greater flexibility in high-altitude mice than low-altitude mice. This suggests that adaptive variation in phenotypic plasticity can be retained for specific subordinate traits while other traits become fixed in a population.
GRANTS
This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery grants to G. B. McClelland and G. R. Scott. Additional funding was provided by a National Heart, Lung, and Blood Institute Grant HL-087216 (to J. F. Storz) and National Science Foundation grants IOS-1354390 and IOS-1354934 (to Z. A. Cheviron and J. F. Storz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.S.L., A.D.C., S.M., and G.B.M. performed experiments; D.S.L., A.D.C., S.M., N.W., Z.A.C., J.F.S., G.R.S., and G.B.M. edited and revised manuscript; D.S.L., A.D.C., S.M., N.W., Z.A.C., J.F.S., G.R.S., and G.B.M. approved final version of manuscript; N.W. and G.B.M. analyzed data; Z.A.C., J.F.S., G.R.S., and G.B.M. interpreted results of experiments; G.B.M. prepared figures; G.B.M. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Danielle Tufts and Mikaela Lui for help with running trials and blood sampling collection.
REFERENCES
- 1.Abdelmalki A, Fimbel S, Mayet-Sornay MH, Sempore B, Favier R. Aerobic capacity and skeletal muscle properties of normoxic and hypoxic rats in response to training. Pflügers Arch 431: 671–679, 1996. doi: 10.1007/BF02253829. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 19: 557–566, 2013. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPARγ in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab 298: E28–E37, 2010. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 4.Beaudry JL, McClelland GB. Thermogenesis in CD-1 mice after combined chronic hypoxia and cold acclimation. Comp Biochem Physiol B Biochem Mol Biol 157: 301–309, 2010. doi: 10.1016/j.cbpb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Bergmeyer HU, Gawehn K. Methods of Enzymatic Analysis. Weinheim: Verlag Chemie, Academic, 1974. [Google Scholar]
- 6.Bergman BC, Brooks GA. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J Appl Physiol 86: 479–487, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol 98: 1258–1263, 2005. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- 8.Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 33: 897–904, 2005. doi: 10.1042/BST0330897. [DOI] [PubMed] [Google Scholar]
- 9.Braun B, Mawson JT, Muza SR, Dominick SB, Brooks GA, Horning MA, Rock PB, Moore LG, Mazzeo RS, Ezeji-Okoye SC, Butterfield GE. Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol 88: 246–256, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76: 2253–2261, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Carraro F, Naldini A, Weber JM, Wolfe RR. Alanine kinetics in humans during low-intensity exercise. Med Sci Sports Exerc 26: 348–353, 1994. doi: 10.1249/00005768-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Cerretelli P, Hoppeler H.. Morphologic and metabolic response to chronic hypoxia: the muscle system. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. II, chapt. 50, p. 1155–1181. [Google Scholar]
- 13.Chappell MA, Garland T Jr, Rezende EL, Gomes FR. Voluntary running in deer mice: speed, distance, energy costs and temperature effects. J Exp Biol 207: 3839–3854, 2004. doi: 10.1242/jeb.01213. [DOI] [PubMed] [Google Scholar]
- 14.Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc Natl Acad Sci USA 109: 8635–8640, 2012. doi: 10.1073/pnas.1120523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheviron ZA, Connaty AD, McClelland GB, Storz JF. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68: 48–62, 2014. doi: 10.1111/evo.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheviron ZA, Bachman GC, Storz JF. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J Exp Biol 216: 1160–1166, 2013. doi: 10.1242/jeb.075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Fueger PT, Shearer J, Krueger TM, Posey KA, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. J Physiol 566: 533–541, 2005. doi: 10.1113/jphysiol.2005.085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamboa JL, Garcia-Cazarin ML, Andrade FH. Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol 300: R85–R91, 2011. doi: 10.1152/ajpregu.00078.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green HJ, Sutton JR, Cymerman A, Young PM, Houston CS. Operation Everest II: adaptations in human skeletal muscle. J Appl Physiol 66: 2454–2461, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem 282: 32844–32855, 2007. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes JP. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol Zool 159: 453–459, 1989. [Google Scholar]
- 23.Hayes JP, O’Connor CS. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53: 1280–1287, 1999. doi: 10.2307/2640830. [DOI] [PubMed] [Google Scholar]
- 24.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 9: 1491–1497, 2003. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 25.Hochachka PW, Stanley C, Merkt J, Sumar-Kalinowski J. Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: an interpretive hypothesis. Respir Physiol 52: 303–313, 1983. doi: 10.1016/0034-5687(83)90087-7. [DOI] [PubMed] [Google Scholar]
- 26.Hock RJ. Physiological responses of deer mice to various native altitudes. In: The Physiological Effects of High Altitude, edited by Weihe WH. Oxford: Pergamon, 1964, p. 59–72. doi: 10.1016/B978-1-4831-6699-5.50013-9. [DOI] [Google Scholar]
- 27.Holden JE, Stone CK, Clark CM, Brown WD, Nickles RJ, Stanley C, Hochachka PW. Enhanced cardiac metabolism of plasma glucose in high-altitude natives: adaptation against chronic hypoxia. J Appl Physiol 79: 222–228, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Hoppeler H, Mueller M, Vogt M. Skeletal muscle tissue changes with hypoxia. In: High Altitude: Human Adaptation to Hypoxia, edited by Swenson ER, Bärtsch P. New York: Springer, 2013, p. 191–202. [Google Scholar]
- 29.Knudsen JG, Biensø RS, Hassing HA, Jakobsen AH, Pilegaard H. Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol Cell Biochem 403: 209–217, 2015. doi: 10.1007/s11010-015-2351-0. [DOI] [PubMed] [Google Scholar]
- 30.Le Moine CMR, Morash AJ, McClelland GB. Changes in HIF-1α protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 301: R1098–R1104, 2011. doi: 10.1152/ajpregu.00070.2011. [DOI] [PubMed] [Google Scholar]
- 31.Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol 308: R779–R791, 2015. doi: 10.1152/ajpregu.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundby C, Van Hall G. Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand 176: 195–201, 2002. doi: 10.1046/j.1365-201X.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- 33.McClelland G, Zwingelstein G, Taylor CR, Weber JM. Increased capacity for circulatory fatty acid transport in a highly aerobic mammal. Am J Physiol Regul Integr Comp Physiol 266: R1280–R1286, 1994. [DOI] [PubMed] [Google Scholar]
- 34.McClelland GB, Hochachka PW, Weber JM. Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc Natl Acad Sci USA 95: 10288–10293, 1998. doi: 10.1073/pnas.95.17.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClelland GB, Hochachka PW, Weber JM. Effect of high-altitude acclimation on NEFA turnover and lipid utilization during exercise in rats. Am J Physiol Endocrinol Metab 277: E1095–E1102, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol 32: 978–997, 2015. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, Koch LG, Levine JA. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One 4: e5869, 2009. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pederson BA, Cope CR, Schroeder JM, Smith MW, Irimia JM, Thurberg BL, DePaoli-Roach AA, Roach PJ. Exercise capacity of mice genetically lacking muscle glycogen synthase: in mice, muscle glycogen is not essential for exercise. J Biol Chem 280: 17260–17265, 2005. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- 39.Picon-Reategui E, Buskirk ER, Baker PT. Blood glucose in high-altitude natives and during acclimatization to altitude. J Appl Physiol 29: 560–563, 1970. [DOI] [PubMed] [Google Scholar]
- 40.Rennie MJ, Edwards RH, Krywawych S, Davies CT, Halliday D, Waterlow JC, Millward DJ. Effect of exercise on protein turnover in man. Clin Sci (Lond) 61: 627–639, 1981. doi: 10.1042/cs0610627. [DOI] [PubMed] [Google Scholar]
- 41.Roberts TJ, Weber JM, Hoppeler H, Weibel ER, Taylor CR. Design of the oxygen and substrate pathways. II. Defining the upper limits of carbohydrate and fat oxidation. J Exp Biol 199: 1651–1658, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265: E380–E391, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologists programmers. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by Krawetz S, Misener S. Totowa, NJ: Humana, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Schippers M-P, Ramirez O, Arana M, Pinedo-Bernal P, McClelland GB. Increase in carbohydrate utilization in high-altitude Andean mice. Curr Biol 22: 2350–2354, 2012. doi: 10.1016/j.cub.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Schippers M-P, LeMoine CMR, McClelland GB. Patterns of fuel use during locomotion in mammals revisited: the importance of aerobic scope. J Exp Biol 217: 3193–3196, 2014. doi: 10.1242/jeb.099432. [DOI] [PubMed] [Google Scholar]
- 46.Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, Gassmann M. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci USA 107: 419–423, 2010. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol 32: 1962–1976, 2015. doi: 10.1093/molbev/msv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA 106: 14450–14455, 2009. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol 213: 2565–2574, 2010. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storz JF, Scott GR, Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol 213: 4125–4136, 2010. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Templeman NM, Schutz H, Garland T Jr, McClelland GB. Do mice bred selectively for high locomotor activity have a greater reliance on lipids to power submaximal aerobic exercise? Am J Physiol Regul Integr Comp Physiol 303: R101–R111, 2012. doi: 10.1152/ajpregu.00511.2011. [DOI] [PubMed] [Google Scholar]
- 52.Vaillancourt E, Haman F, Weber J-M. Fuel selection in Wistar rats exposed to cold: shivering thermogenesis diverts fatty acids from re-esterification to oxidation. J Physiol 587: 4349–4359, 2009. doi: 10.1113/jphysiol.2009.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vock R, Weibel ER, Hoppeler H, Ordway G, Weber JM, Taylor CR. Design of the oxygen and substrate pathways. V. Structural basis of vascular substrate supply to muscle cells. J Exp Biol 199: 1675–1688, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Wasserman DH, Kang L, Ayala JE, Fueger PT, Lee-Young RS. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 214: 254–262, 2011. doi: 10.1242/jeb.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber JM. Metabolic fuels: regulating fluxes to select mix. J Exp Biol 214: 286–294, 2011. doi: 10.1242/jeb.047050. [DOI] [PubMed] [Google Scholar]
- 56.White CR, Portugal SJ, Martin GR, Butler PJ. Respirometry: anhydrous drierite equilibrates with carbon dioxide and increases washout times. Physiol Biochem Zool 79: 977–980, 2006. doi: 10.1086/505994. [DOI] [PubMed] [Google Scholar]
- 57.Withers PC. Measurement of V̇o2, V̇co2, and evaporative water loss with a flow-through mask. J Appl Physiol Respir Environ Exerc Physiol 42: 120–123, 1977. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem 284: 27025–27029, 2009. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]


