Abstract
Endotoxin injection has been widely used to study the acute inflammatory response. In this study, we directly compared the inflammatory responses to endotoxin in mice and humans. Escherichia coli type O113 endotoxin was prepared under identical conditions, verified to be of equal biological potency, and used for both mice and humans. The dose of endotoxin needed to induce an interleukin-6 (IL-6) concentration in plasma of ∼1,000 pg/ml 2 h after injection was 2 ng/kg of body weight in humans and 500 ng/kg in mice. Healthy adult volunteers were injected intravenously with endotoxin, and male C57BL/6 mice (n = 4 to 12) were injected intraperitoneally with endotoxin. Physiological, hematological, and cytokine responses were determined. Endotoxin induced a rapid physiological response in humans (fever, tachycardia, and slight hypotension) but not in mice. Both mice and humans exhibited lymphopenia with a nadir at 4 h and recovery by 24 h. The levels of tumor necrosis factor (TNF) and IL-6 in plasma peaked at 2 h and returned to baseline levels by 4 to 6 h. IL-1 receptor antagonist RA and TNF soluble receptor I were upregulated in both mice and humans but were upregulated more strongly in humans. Mice produced greater levels of CXC chemokines, and both mice and humans exhibited peak production at 2 h. These studies demonstrate that although differences exist and a higher endotoxin challenge is necessary in mice, there are several similarities in the inflammatory response to endotoxin in mice and humans.
Administration of specific pathogens has proven to be a powerful tool for investigating the host's inflammatory response. Infusion of endotoxin, a cell wall component of gram-negative bacteria, is a well-established model for studying the acute inflammatory response (11, 17, 35). The murine endotoxin model has provided basic information resulting in dramatic improvements in understanding the inflammatory response. As a specific example, injecting endotoxin into mice led to the discovery of tumor necrosis factor (TNF) (6). Further investigation of this critical mediator of inflammation demonstrated that TNF also played a role in chronic inflammatory conditions, such as arthritis and inflammatory bowel disease. At this time, TNF inhibitors are therapies for the treatment of patients with rheumatoid arthritis (32) and Crohn's disease (23) approved by the Food and Drug Administration.
Complementary studies of endotoxin administration in humans are often limited due to the risk of toxicity. On the basis of the pyrogenic response to endotoxin, humans are considered the most sensitive of all models to the effects of endotoxin (38). Despite the differences in sensitivity, the human model is useful in measuring the responses that are common to the acute inflammatory response and those responses that are specific to the endotoxin (17). Linking the murine and human models into one definitive comparison proves to be more difficult, if not impossible. A recent review article has highlighted the differences between murine and human immunology (19).
The purpose of this study was to provide a direct comparison of the acute inflammatory response to endotoxin in mice and humans. By injecting both healthy human volunteers and mice with identical endotoxin, we are providing the missing commonality between these models. For these studies, we injected the same serotype of endotoxin prepared in the same manner into male mice and healthy male and female human volunteers to determine the magnitude and kinetics of the in vivo inflammatory response. The parallel parameters measured include heart rate, temperature, blood pressure, complete cell count, and cytokine concentration.
MATERIALS AND METHODS
Experimental subjects. (i) Mice.
Male C57BL/6 mice (6 to 8 weeks old) were obtained from Jackson Laboratory (Bar Harbor, Maine) and kept under standard laboratory conditions. Mice were housed in a temperature-controlled room with a 12-h light/dark cycle and allowed food and water ad libitum. The University of Michigan Committee on the Use and Care of Animals approved all experiments.
(ii) Humans.
Thirteen adult (10 males and 3 females), paid volunteers were recruited by public advertisement for entry into the protocol approved by the Institutional Review Board of UMDNJ-Robert Wood Johnson Medical School. Inclusion criteria were as follows: (i) good general health, as demonstrated by medical history, physical examination, and laboratory tests within 6 weeks of the study; (ii) age between 18 and 40 years; and (iii) written informed consent prior to the performance of any study-related procedure. Exclusion criteria were as follows: (i) history of cancer, rheumatoid arthritis, heart disease, hypertension, or immunological, renal, hepatic, endocrine, neurological dysfunction; (ii) recent history of alcohol or drug abuse; (iii) mental capacity limited to the extent that the subject could not provide written informed consent or information regarding treatment-emergent adverse events and/or tolerance of study medication and/or procedures; (iv) exposure to any experimental agent or procedure within 30 days of study; (v) pregnancy or breast-feeding; and (vi) prior intravenous endotoxin administration.
Endotoxin.
The mice were injected with endotoxin derived from Escherichia coli O113, lot 5 (28). A frozen aliquot of the endotoxin was prepared in normal saline on the day of use. A limulus amebocyte lysate (LAL; Associates of Cape Cod, Inc., East Falmouth, Mass.) test was performed on each lot of endotoxin to validate its concentration (data not shown). Results from the LAL test were used to standardize the concentrations of the different lots administered to the human subjects (lot 6) and mice (lot 5). Human volunteers were injected with National Institutes of Health (NIH) Clinical Center Reference Endotoxin, which is the same strain of E. coli prepared under similar conditions but a different lot (lot 6).
Whole-blood stimulation.
Although the endotoxin was derived from the same serotype and was prepared in the same manner, we wished to determine whether the biological activities of the two different lots were equivalent. In keeping with the in vivo studies described below, the experiments focused on inducing interleukin-6 (IL-6) via endotoxin. To perform the studies, healthy volunteers donated whole blood, which was stimulated with endotoxin (from either source) using our previous protocols (8, 9, 39). Blood samples from six healthy donors were drawn into a syringe containing heparin (10 U/ml). One milliliter of whole blood was aliquoted into a microcentrifuge tube and individually stimulated with endotoxin in a dose-dependent manner with 3, 1, or 0.1 ng/ml of either lot 5 or lot 6 endotoxin. All tubes were incubated (37°C) on a rotator for 6 h. After incubation, tubes were centrifuged at 2,000 × g for 5 min, and plasma was collected. Plasma was frozen at −70°C until IL-6 measurement via an enzyme-linked immunosorbent assay (ELISA).
Dose response.
Five mice were used for optimization of the endotoxin concentration to achieve a concentration of IL-6 at 2 h equal to approximately 1,000 pg/ml. All mice were injected intraperitoneally (i.p.) and sacrificed at 2 h, plasma was harvested, and IL-6 was measured via an ELISA.
Route of administration (i.p. versus i.v.).
We compared the responses of male C57BL/6 mice to injection of lipopolysaccharide (LPS) via either the intravenous (i.v.) or intraperitoneal (i.p.) route. Vasodilation was achieved by the topical application of methyl salicylate to the tail (Sigma, St. Louis, Mo.). Ten nanograms of endotoxin was diluted in normal saline and injected in a 100-μl volume either i.p. or i.v. Mice were sacrificed 2, 4, or 6 h after injection. Blood samples were collected via cardiac puncture of the mice anesthetized with isoflurane and treated with EDTA, an anticoagulant. In a separate experiment, rabbit immunoglobulin G (IgG) was injected i.v. and i.p. to determine the distribution of a relatively inert, large molecule and our ability to perform reproducible i.v. injections. Serial blood samples were drawn from the same mouse every 2, 6, 9, and 24 h.
Human endotoxin model. (i) Procedure.
On study day 1 by 1500 h, the volunteer was admitted to the hospital and underwent a physical examination, and blood and urine tests were performed. An i.v. catheter was placed in one upper extremity, and a continuous i.v. drip of dextrose 5% water-0.5 normal saline (100 ml/h) was begun and continued until the subject tolerated a regular meal after completion of the acute part of the study on the following day. On the morning of study day 2, a radial artery was cannulated percutaneously with a 20-gauge catheter and connected to a pressure transducer. Hemodynamic and respiratory parameters, rectal temperature, and subjective symptoms of distress (chills, muscle aches, headache, nausea, perceived fever, sensitivity to light, and arterial line discomfort) were monitored every 30 min for the next 6 h. After baseline monitoring, subjects were administered NIH Clinical Center Reference Endotoxin at a dose of 2 ng/kg of body weight over a 5-min period through the i.v. line. In our studies, this endotoxin is referred to as lot 6. Blood samples were collected before endotoxin infusion (0 h) and at postinfusion times of 2, 4, 6, 9, and 24 h. On study day 3, after the 24-h blood sample and a urine sample were obtained, the subject was discharged.
(ii) Blood samples for plasma.
Blood samples treated with EDTA, an anticoagulant, were immediately placed on ice and processed within 1 h. After centrifugation at 1,000 × g for 10 min, plasma was collected, aliquoted, and stored at −70°C until assayed for cytokine concentrations.
(iii) Blood samples for CBC and differential WBC count.
Blood samples treated with EDTA, an anticoagulant, were transported to the Robert Wood Johnson University Hospital Clinical Laboratory where automated complete blood count (CBC) and differential white blood cell (WBC) counts were performed. Differential WBC counts flagged as being outside the normal range by the automated count were then assessed by manual cell counting per the clinical laboratory procedures.
Mouse endotoxin model.
The total dose of endotoxin for all subsequent experiments was 10 ng into an approximately 20-g mouse to yield a dose of 500 ng/kg.
(i) Part I. Physiology.
This section focuses on the changes in blood pressure (systolic, diastolic, and mean), heart rate, and body temperature after endotoxin is injected into mice.
(a) Blood pressure and heart rate protocol.
Blood pressure and heart rate were determined by the method of Krege (16) using a Visitech BP-2000 blood pressure analysis system (Apex, N.C.). All mice were “trained” to become acclimated to the physiology equipment and to allow proper collection of blood pressure and heart rate. The mice were acclimated by placing the mice in the equipment for 5 to 7 days before the endotoxin injection. After a baseline measurement, data were collected every hour for the first 8 h.
(b) Temperature protocol.
In a separate group of mice, implantable radiotransmitters (Mini Mitter Co.; SunRiver, Oreg.) were used to continuously monitor the internal body temperature. For subcutaneous implantation of the radiotransmitters, mice were anesthetized using isoflurane (AErrane; Baxter, Deerfield, Ill.), and the skin was closed with 9-mm-long wound clips (MikRon Autoclip). This surgery occurred 5 days before endotoxin injection to allow sufficient time for recovery from surgery. Baseline temperatures were recorded the day before injection. For controls, two mice were injected (i.p.) with saline at the same time as the mice injected with endotoxin.
(ii) Part II. Hematology and cytokines.
For these studies, the mice were injected with endotoxin, and groups of mice were sacrificed to harvest blood. Mice were randomized into groups for each time point (2, 4, 6, 9, and 24 h after injection). An additional group of eight mice were sacrificed at time zero before any i.p. injections. At the time of sacrifice, the mice were anesthetized with isoflurane. Blood was harvested by cardiac puncture using a 1-ml syringe with a 25-gauge 5/8 in. needle that contained 0.05 ml of 169 mM EDTA (Sigma) solution. Blood was transferred to a 1.5-ml microcentrifuge tube.
(a) Hematology.
The whole blood harvested from the cardiac puncture was used for a CBC and automated differential performed by the Hemavet instrument (CDC Technologies, Oxford, Conn.).
(b) Cytokine levels.
After Hemavet analysis, all blood was centrifuged (1,500 × g for 5 min), and plasma was removed and stored at −70°C until cytokines were analyzed by ELISAs.
ELISA.
All cytokine ELISAs were measured using matched antibody pairs (R&D Systems, Inc., Minneapolis, Minn.) in 96-well plates (Nunc Immunoplate Maxisorb; Nunc, Neptune, N.J.) as previously described (21). Briefly, capture antibodies were placed on the plates and allowed to incubate overnight. The next morning, the plates were blocked at room temperature for 1 h on a rotator. After the plates were washed, plasma samples were added (50 μl per well) in duplicate and incubated for 1 or 2 h at room temperature on a rotator. The samples were diluted at least 1:10 before the assay and in some situations up to 1:500 in order to keep the results on the scale of the standard curve. Biotinylated antibodies were added (50 μl per well), and plates were incubated for 1 or 2 h at room temperature on a rotator. The plates were incubated for 0.5 h with streptavidin-horseradish peroxidase at a dilution of 1:20,000, and the antibodies were detected with TMB (3,3′,5,5′-tetramethylbenzidine) (Sigma catalog no. T-2885) dissolved in dimethyl sulfoxide (Sigma catalog no. D-2650) to a concentration of 1% in a solution containing 0.1 M citric acid, 0.1 M sodium acetate (pH 6), and 0.016% H2O2 for 30 min. The reaction was stopped by the addition of 1.5 M H2SO4. Plates were read using two wavelengths (465 and 590 nm) on a Biotek microplate reader (Biotek Instruments, Inc., Winooski, Vt.), and total cytokine concentrations were calculated using the standard curve prepared from recombinant cytokines. The lower limit of detection for the cytokines based on the standard curves ranged from 1 to 3 pg/ml.
Statistical analysis.
Values were expressed as means ± standard errors of the means (SEMs) of the experiment. Data were analyzed using a Student's t test, and P values of <0.05 were considered statistically significant.
RESULTS
Endotoxin.
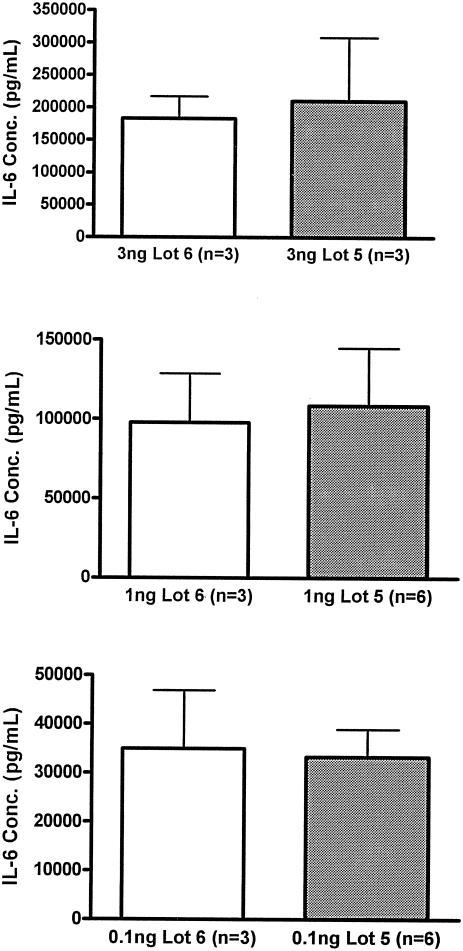
While the two lots of endotoxin had similar compositions, it was necessary to evaluate their relative potency. The activities of both lots of endotoxin were first evaluated in the limulus assay which showed similar activities (data not shown). We further tested the biological activity by determining the potency with regards to inducing cytokine production. Although many different parameters could be used, the concentration of IL-6 in plasma was used as the point of reference, both for this study and in vivo comparisons. Whole-blood samples from healthy human volunteers were stimulated with the different lots of endotoxin in a dose-response manner. Figure 1 shows three representative doses of endotoxin. Both lot 5 and 6, for all endotoxin doses represented, induced nearly identical concentrations of IL-6. These data indicated that the endotoxin used for the human studies (lot 6) had the same activity as that used for the murine studies (lot 5).
FIG. 1.
Ex vivo IL-6 production. Human whole blood was stimulated with the indicated doses of the E. coli O113 endotoxin that was used in the human studies (lot 6) or murine studies (lot 5). Plasma was harvested after 6 h of stimulation, and IL-6 concentrations (Conc.) were determined. At each dose of endotoxin, both lots induced statistically similar amounts of IL-6. Each value is the mean ± SEM (error bar) for the indicated number of donors.
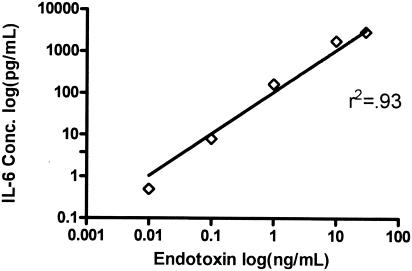
Dose response.
A decision was made to focus on one parameter to align the human and mouse models. The concentration of IL-6 in plasma was chosen as the omnibus marker to find an equivalent, inflammation-inducing endotoxin dose. Previous studies have shown that an infusion of endotoxin (E. coli O113) in humans produces, on average, 1,200 pg of IL-6 per ml (2, 18, 31). Initial evaluation of the plasma from the present studies indicated that the endotoxin induced approximately 1,000 pg of IL-6 per ml 2 h after endotoxin infusion. For determination of the endotoxin dose in mice, a dose-response experiment was performed (Fig. 2), which demonstrated increasing plasma IL-6 levels with increasing endotoxin doses. A dose of 10 ng/mouse approximately achieved the desired IL-6 levels in the mice. Collectively, the results indicated that 2 ng of endotoxin per kg in humans and 500 ng of endotoxin per kg in mice would produce the desired response of 1,000 pg of IL-6 per ml. This dose of endotoxin was used for all ensuing studies.
FIG. 2.
IL-6 production with increasing doses of endotoxin. Mice were injected with the indicated doses of endotoxin, and plasma was harvested 2 h later. Increasing doses of endotoxin resulted in increasing plasma IL-6 levels. Note that both the IL-6 concentration (Conc.) and endotoxin dose are plotted on a log scale. The line indicates the regression through the data.
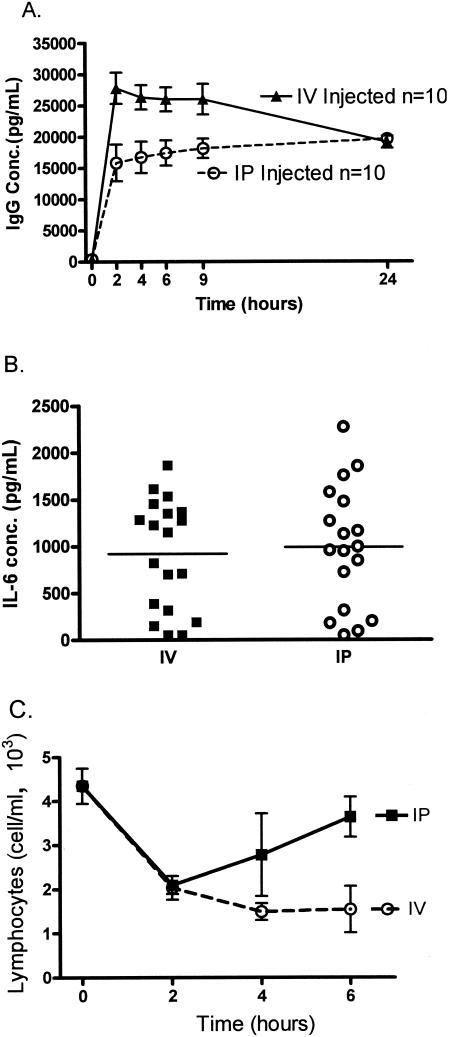
Route of administration.
A significant difference between the mouse and human studies was the route of injection, i.p. in the mice and i.v. in the mouse. Subsequent differences in the response may not reflect species differences but merely differences in the routes of injection. A series of experiments were designed to carefully test the equivalency of the i.v. versus i.p. injection. Injecting i.v. in mice, without prior warming, can be technically difficult. In order to ensure reproducible technical injections, we gave the mice rabbit immunoglobulin either i.v. or i.p., and serial blood samples were taken. Figure 3A shows that either route of injection results in reproducible levels of immunoglobulin, although higher levels were obtained when compounds were administered i.v. Next, we evaluated the levels of IL-6 in plasma in response to endotoxin when it was injected i.v. or i.p. Identical levels of IL-6 were induced via either method. The range of the response to the endotoxin was surprising; it ranged from nearly undetectable to 2,000 pg/ml (Fig. 3B). Based on the data shown in Fig. 3A, the differences in the IL-6 response were not due to variations in the adequacy of the injection. We also evaluated another response, the lymphopenia which develops after endotoxin, because this response persists longer than the production of IL-6. Either route of injection induced rapid lymphopenia, but the response persisted longer after i.v. injection. These data demonstrate that injection of endotoxin by either the i.p. or i.v. route induces a similar inflammatory response, although certain aspects continue longer after i.v. injection.
FIG. 3.
Effect of route of administration (i.v. versus i.p.). (A) Rabbit IgG was injected i.v. and i.p. to determine the distribution of a relatively inert, large molecule. Serial blood samples were drawn from the same mouse every 2, 6, 9, and 24 h. Each value is the mean ± SEM (error bar) for 10 mice. Conc., concentration. (B and C) Ten nanograms of endotoxin was diluted in normal saline and injected in a 100-μl volume either i.p. or i.v. Mice were sacrificed at 2, 4, or 6 h after injections. (B) IL-6 production from individual mice at 2 h. (C) Changes in lymphocyte counts over time. Each value is the mean ± SEM (error bar) for eight mice.
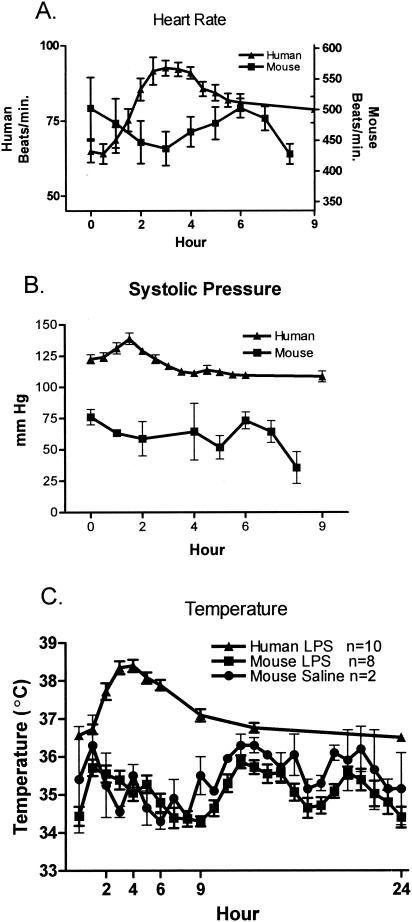
Physiology.
The monitoring of temperature, systolic pressure, and heart rate in the human volunteers after the infusion of endotoxin revealed an increase in all parameters measured, whereas the mice showed no significant alteration over the same time period. Figure 4A shows the increase in heart rate in humans over a 9-h period, while there is essentially no alteration in the mice. Monitoring of systolic pressure (Fig. 4B) in the humans after endotoxin infusion showed a slight increase in blood pressure at 2 h, but no changes were observed in the mice. Figure 4C shows the results of monitoring the core body temperature for 24 h; endotoxin induced a sharp increase in the core temperature of humans that was not observed in the mice. As expected, endotoxin elicited a marked physiological response in the human volunteers; however, the physical response in the mice was not significantly altered. There is some variability in the temperature of the mice, so the temperatures of two mice injected with saline are also included in Fig. 4C which demonstrates that the variation is not due to the endotoxin. These data show that at this dose of endotoxin, humans have a physiological response, while the mice do not.
FIG. 4.
Physiological changes. (A) Heart rate increased in humans, while no changes were observed in the mice after endotoxin administration. (B) Systolic blood pressure also increased slightly with no real change in the mice. In panels A and B, each value is the mean ± SEM (error bar) for 10 humans or 6 to 10 mice. (C) Body temperature increased in response to endotoxin in humans but no discernible pattern was seen in the mice. Data from two mice injected with saline are included to demonstrate that the changes in body temperature are not due to the endotoxin but to normal diurnal variations.
Hematology and cytokine concentrations.
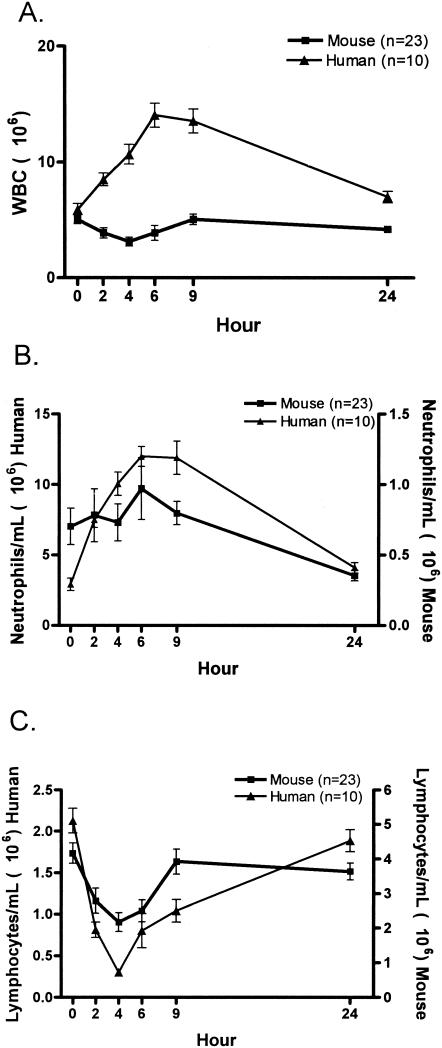
The endotoxin infusion induced significant alterations in many of the parameters measured for humans and mice. Figure 5A demonstrates the changes in peripheral WBC after the infusion of endotoxin for humans and mice. Humans showed a gradual increase in the total WBC count over the 24-h period, but the mice had no significant alterations. However, differential cell counts revealed similar changes in the peripheral neutrophils and lymphocytes for humans and mice. It must be noted that in healthy humans the majority of the circulating WBCs are neutrophils, while in mice the majority of the circulating cells are lymphocytes. Neutrophils (Fig. 5B) demonstrated an increase and recovery over a 24-h period in humans and mice. Note that the data are graphed on a different scale for the mice and humans. While the shape of the curve is similar, the increase in absolute numbers of circulating neutrophils is much greater in humans. This greater increase in neutrophils accounts for the increase in the total WBC count observed in the humans relative to mice. Lymphocytes (Fig. 5C) demonstrated a significant decrease and recovery over a 24-h period for humans and mice. Although overall cell numbers from humans and mice are statistically different, the patterns of change caused by the infusion of endotoxin are similar.
FIG. 5.
Hematological alterations after endotoxin injection. (A) Humans have an overall increase in circulating WBCs, while the murine response is virtually unchanged. Neutrophilia (B) and lymphopenia (C) develop in mice and humans. In panels B and C, the human and mouse data are plotted on different scales, since the number of circulating cells differs in mice and humans. Each value is the mean ± SEM (error bar) for 10 humans or 23 mice.
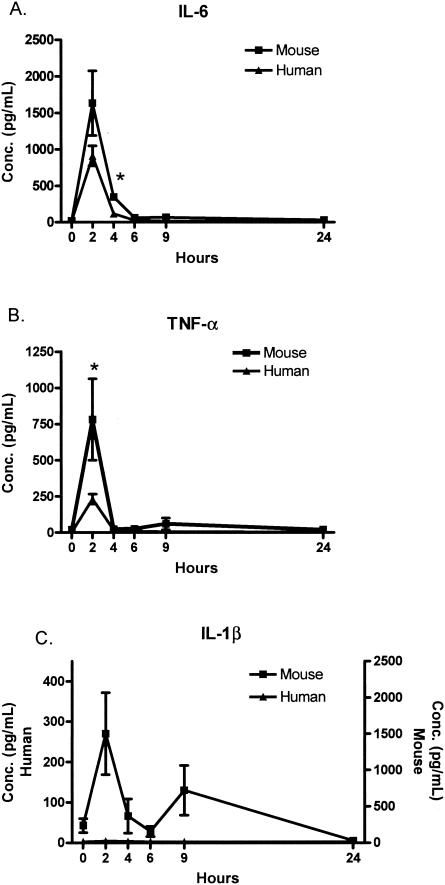
As expected, the cytokines demonstrated a time-dependent increase and return to baseline levels over the 24-h period after infusion of endotoxin for humans and mice. Figure 6A demonstrates the peak in IL-6 at the 2-h time point and the return to the baseline level at 6 h for humans and mice. Pivotal for the entire study, the IL-6 concentration for mice and humans was approximately 1,000 pg/ml at the 2-h time point. At the dose of endotoxin used, the mice had a greater induction of IL-6, which was statistically significant at the 4-h time point. Figure 6B demonstrates the brisk rise in TNF alpha (TNF-α) levels in mice and humans at 2 h and return to baseline levels by 4 h. Similar to IL-6, the levels of TNF-α in mice (782 ± 281 pg/ml) were higher than those in humans (231 ± 35 pg/ml) at the 2-h time point. IL-1β was readily detected at multiple time points in mice but was not present at a detectable level at any time point in humans.
FIG. 6.
Rapid induction of proinflammatory cytokines after endotoxin injection. Both IL-6 and TNF quickly appeared in the plasma and returned to baseline levels (not detected) by 6 h. IL-1β was not detected in the human samples at any time point. Note the different axes in panel C. Each value is the mean ± SEM (error bar) for 4 to 13 humans or 5 to 11 mice. Values that were significantly different (P < 0.05) in mice and humans are indicated by an asterisk. Conc., concentration.
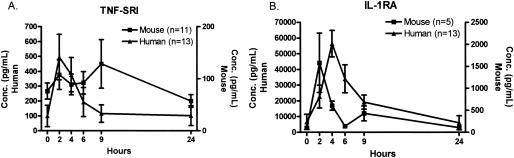
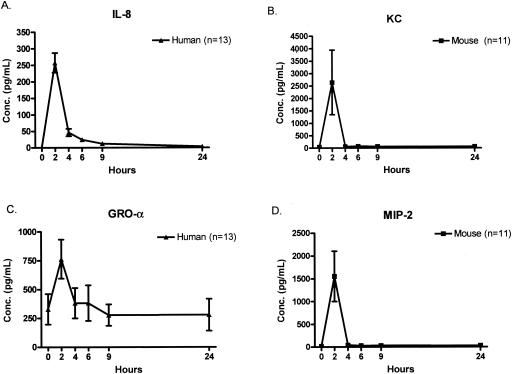
TNF soluble receptor I (TNF-SRI) was strongly induced in humans, reaching peak levels within 2 h, while in mice there was little variation in the levels of TNF-SRI in plasma (Fig. 7A). Figure 7B demonstrates the common pattern of IL-1 receptor antagonist RA (IL-1RA) levels in humans and mice, but there were some important differences. IL-1RA peaked at 2 h in mice but peaked at 4 h in humans. The levels of IL-1RA were also much higher in the humans than in the mice. Figure 8 depicts the kinetics of CXC chemokine appearance in humans and mice. Each of the CXC chemokines examined demonstrated a nearly identical pattern, with a sharp peak at 2 h after endotoxin injection, followed by a rapid return to the baseline level.
FIG. 7.
Profiles of anti-inflammatory cytokines after endotoxin injection. Similar kinetics of appearance of TNF-SRI and IL-1RA were observed in mice and humans, although the levels of the anti-inflammatory cytokines were substantially greater in humans than in mice. Each value is the mean ± SEM (error bar) for 13 humans or 5 to 11 mice. Conc., concentration.
FIG. 8.
Kinetics of CXC chemokine appearance after endotoxin injection. Each of the measured CXC chemokines peaked within 2 h of endotoxin injection. Note that the values of KC and macrophage inflammatory protein 2 in the mouse were significantly higher than IL-8 or growth-related oncogene-α in humans. Each value is the mean ± SEM (error bar) for 13 humans or 11 mice. Conc., concentration.
DISCUSSION
The many parameters measured in this set of studies demonstrated the similarities and differences between the human and mouse models of endotoxin challenge. The most striking difference observed was the difference in dose between humans and mice. Although the lots of endotoxin were similar in potency, the final dose given to the mice to achieve the targeted concentration of 1,000 pg of IL-6 per ml was 250 times greater than that given to humans. The IL-6 target was chosen in response to the large body of evidence identifying this key cytokine in endotoxin (13) and sepsis (7, 25) work for example. These findings are also consistent with previous studies that found humans to be the most sensitive of all models after an endotoxin challenge (17, 38). Historically, human endotoxin studies have found an effective dose at 4 ng/kg; however, this study achieved a profound physiological response at 2 ng/kg (2, 5, 11, 17, 18, 29, 31). A previous report indicates that the lot 5 previously used for the human studies had lost some potency (30), but our studies showed that both lots (lots 5 and 6) induced similar levels of IL-6 in human whole blood. Additionally, the different routes of administration (i.p. in mice, i.v. in humans) did not affect the magnitude of the response to endotoxin. This lack of difference in the response to the route of administration is supported by other work, including fever production after endotoxin administration (3), antibody treatment in a tumor model (33), and clondronate administration and deposition in bone (20).
The physiological response (i.e., blood pressure, heart rate, and temperature) in humans in our present work is comparable to those of previous reports. The elevation in core temperature has been documented and confirmed by this study to peak around 3 h after endotoxin infusion and to stabilize an average of 5 h after endotoxin infusion (2, 10, 11, 17, 18, 22, 29, 36). On the other hand, although no difference was seen in mice in this study, previous reports have noted a decrease in temperature with an endotoxin challenge (15, 24). These previous studies used substantially higher concentrations of endotoxin; at the low dose used for our studies, no alterations in temperature were observed. This mouse model is also in keeping with other studies utilizing C57BL/6 mice for studies of endotoxin and thrombotic disease (37), atherosclerosis (14), and cell kinetics (12). Cardiac function in humans, as measured by heart rate and systolic pressure, in response to an endotoxin challenge is consistent with previous studies that found an elevated heart rate and increased blood pressure over an approximately 2-h period after infusion of endotoxin (1, 2, 5, 10, 11, 17, 22, 36, 38). Unfortunately, no previous reports were found to align with this study's findings of the lack of effect on the cardiac function after endotoxin infusion in mice. Given the lack of temperature response to endotoxin in the mice, it is not surprising that the cardiac parameters also did not change.
The hematological response in humans and mice can be best described as mixed, with different alterations in lymphocytes and neutrophils. Starting with the dramatic decrease in lymphocytes over 4 h after endotoxin infusion, the number of lymphocytes gradually returns to normal. The neutrophil response is the exact opposite, with a rapid rise in the total number of circulating neutrophils, followed by a return to normal levels by 24 h. Since one major constituent of the peripheral blood is rising while the other is decreasing, the overall picture of WBC is a gentle rise in humans over 6 to 9 h after endotoxin infusion and virtually no change in mice. These findings compare favorably with previous reports in which the human hematological profile consisted of initial leukopenia followed by leukocytosis, with most of the dramatic changes observed in the neutrophil counts (2, 4, 5, 27, 29, 34, 36, 38).
The overall cytokine pictures for humans and mice were strikingly similar. With the exception of IL-1β in mice, all proinflammatory cytokines peaked around 2 h after endotoxin infusion and returned to baseline levels by 4 to 6 h after endotoxin administration. The same pattern was observed with the anti-inflammatory cytokines and chemokines, with the exception of IL-1RA in humans, which peaked at 4 h. Similar findings have been reported in numerous studies in humans and mice (1, 2, 5, 17, 18, 24, 26, 31, 34, 35).
Collectively, these studies create a profile for humans and mice for a nonlethal endotoxin challenge over a 24-h period. These studies provide the link between these common models for investigating the host inflammatory response. Humans clearly demonstrated a physiological response which was not evident in mice. The cytokine induction patterns in mice and humans were similar, although greater concentrations of endotoxin were needed to induce the response in mice.
Acknowledgments
This work was supported in part by NIH grant U54 GM 62119.
The participating members of the Inflammation and the Host Response to Injury Investigators are Paul E. Bankey, Timothy R. Billiar, Steven E. Calvano, Irshad H. Chaudry, Chuck Cooper, Bradley Freeman, Richard L. Gamelli, Nicole S. Gibran, Brian G. Harbrecht, Wyrta Heagy, David M. Heimbach, David N. Herndon, Jureta W. Horton, John Lee Hunt, Jeffrey Johnson, James A. Lederer, Tanya Logvinenko, Stephen F. Lowry, John A. Mannick, Bruce A. McKinley, Joseph P. Minei, Ernest E. Moore, Frederick A. Moore, Avery B. Nathens, Grant E. O’Keefe, Laurence G. Rahme, Daniel G. Remick, Jr., Michael B. Shapiro, Robert L. Sheridan, Geoffrey M. Silver, Richard D. Smith, Scott Somers, Gregory Stephanopoulos, Mehmet Toner, H. Shaw Warren, Michael A. West, Steven E. Wolf, Martin Yarmush, and Vernon R. Young.
REFERENCES
- 1.Barber, A. E., S. M. Coyle, M. A. Marano, E. Fischer, S. E. Calvano, Y. Fong, L. L. Moldawer, and S. F. Lowry. 1993. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 150:1999-2006. [PubMed] [Google Scholar]
- 2.Boujoukos, A. J., G. D. Martich, E. Supinski, and A. F. Suffredini. 1993. Compartmentalization of the acute cytokine response in humans after intravenous endotoxin administration. J. Appl. Physiol. 74:3027-3033. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell, F. T., Jr., D. B. Graves, and B. H. Wallace. 1999. Humoral versus neural pathways for fever production in rats after administration of lipopolysaccharide. J. Trauma 47:120-129. [DOI] [PubMed] [Google Scholar]
- 4.Calvano, S. E., W. A. Thompson, M. N. Marra, S. M. Coyle, H. F. de Riesthal, R. K. Trousdale, P. S. Barie, R. W. Scott, L. L. Moldawer, and S. F. Lowry. 1994. Changes in polymorphonuclear leukocyte surface and plasma bactericidal/permeability-increasing protein and plasma lipopolysaccharide binding protein during endotoxemia or sepsis. Arch. Surg. 129:220-226. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, B. Chernow, et al. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 6.Carswell, E. A., L. J. Old, R. L. Kassel, S. Green, N. Fiore, and B. Williamson. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 72:3666-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coopersmith, C. M., D. M. Amiot, Jr., P. E. Stromberg, W. M. Dunne, C. G. Davis, D. F. Osborne, K. D. Husain, I. R. Turnbull, I. E. Karl, R. S. Hotchkiss, and T. G. Buchman. 2003. Antibiotics improve survival and alter the inflammatory profile in a murine model of sepsis from Pseudomonas aeruginosa pneumonia. Shock 19:408-414. [DOI] [PubMed] [Google Scholar]
- 8.DeForge, L. E., J. C. Fantone, J. S. Kenney, and D. G. Remick. 1992. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J. Clin. Investig. 90:2123-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeForge, L. E., J. S. Kenney, M. L. Jones, J. S. Warren, and D. G. Remick. 1992. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 148:2133-2141. [PubMed] [Google Scholar]
- 10.Fong, Y. M., M. A. Marano, L. L. Moldawer, H. Wei, S. E. Calvano, J. S. Kenney, A. C. Allison, A. Cerami, G. T. Shires, and S. F. Lowry. 1990. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J. Clin. Investig. 85:1896-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godin, P. J., L. A. Fleisher, A. Eidsath, R. W. Vandivier, H. L. Preas, S. M. Banks, T. G. Buchman, and A. F. Suffredini. 1996. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit. Care Med. 24:1117-1124. [DOI] [PubMed] [Google Scholar]
- 12.Hosoya, K., T. Takashima, K. Matsuda, and T. Terasaki. 2002. Spleen lymphocyte kinetics in mice under normal and inflammatory conditions: an application of the transgenic mouse expressing beta-galactosidase (ROSA 26). Biol. Pharm. Bull. 25:1378-1380. [DOI] [PubMed] [Google Scholar]
- 13.Husain, K. D., P. E. Stromberg, P. Javadi, T. G. Buchman, I. E. Karl, R. S. Hotchkiss, and C. M. Coopersmith. 2003. Bcl-2 inhibits gut epithelial apoptosis induced by acute lung injury in mice but has no effect on survival. Shock 20:437-443. [DOI] [PubMed] [Google Scholar]
- 14.Kadl, A., J. Huber, F. Gruber, V. N. Bochkov, B. R. Binder, and N. Leitinger. 2002. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul. Pharmacol. 38:219-227. [DOI] [PubMed] [Google Scholar]
- 15.Kozak, W., C. A. Conn, and M. J. Kluger. 1994. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am. J. Physiol. 266:R125-R135. [DOI] [PubMed] [Google Scholar]
- 16.Krege, J. H., J. B. Hodgin, J. R. Hagaman, and O. Smithies. 1995. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25:1111-1115. [DOI] [PubMed] [Google Scholar]
- 17.Martich, G. D., A. J. Boujoukos, and A. F. Suffredini. 1993. Response of man to endotoxin. Immunobiology 187:403-416. [DOI] [PubMed] [Google Scholar]
- 18.Martich, G. D., R. L. Danner, M. Ceska, and A. F. Suffredini. 1991. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J. Exp. Med. 173:1021-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestas, J., and C. C. W. Hughes. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172:2731-2738. [DOI] [PubMed] [Google Scholar]
- 20.Monkkonen, J., and P. Ylitalo. 1990. The tissue distribution of clodronate (dichloromethylene bisphosphonate) in mice. The effects of vehicle and the route of administration. Eur. J. Drug Metab. Pharmacokinet. 15:239-243. [DOI] [PubMed] [Google Scholar]
- 21.Nemzek, J. A., J. Siddiqui, and D. G. Remick. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J. Immunol. Methods 255:149-157. [DOI] [PubMed] [Google Scholar]
- 22.Parrillo, J. E., M. M. Parker, C. Natanson, A. F. Suffredini, R. L. Danner, R. E. Cunnion, and F. P. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113:227-242. [DOI] [PubMed] [Google Scholar]
- 23.Raza, A. 2000. Anti-TNF therapies in rheumatoid arthritis, Crohn's disease, sepsis, and myelodysplastic syndromes. Microsc. Res. Technique 50:229-235. [DOI] [PubMed] [Google Scholar]
- 24.Remick, D., P. Manohar, G. Bolgos, J. Rodriguez, L. Moldawer, and G. Wollenberg. 1995. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 4:89-95. [DOI] [PubMed] [Google Scholar]
- 25.Remick, D. G., G. R. Bolgos, J. Siddiqui, J. Shin, and J. A. Nemzek. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463-467. [DOI] [PubMed] [Google Scholar]
- 26.Remick, D. G., R. M. Strieter, M. K. Eskandari, D. T. Nguyen, M. A. Genord, C. L. Raiford, and S. L. Kunkel. 1990. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am. J. Pathol. 136:49-60. [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson, R. P., C. D. Rhyne, Y. Fong, D. G. Hesse, K. J. Tracey, M. A. Marano, S. F. Lowry, A. C. Antonacci, and S. E. Calvano. 1989. Peripheral blood leukocyte kinetics following in vivo lipopolysaccharide (LPS) administration to normal human subjects. Influence of elicited hormones and cytokines. Ann. Surg. 210:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudbach, J. A., F. I. Akiya, R. J. Elin, H. D. Hochstein, M. K. Luoma, E. C. Milner, K. C. Milner, and K. R. Thomas. 1976. Preparation and properties of a national reference endotoxin. J. Clin. Microbiol. 3:21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, P. D., A. F. Suffredini, J. B. Allen, L. M. Wahl, J. E. Parrillo, and S. M. Wahl. 1994. Endotoxin administration to humans primes alveolar macrophages for increased production of inflammatory mediators. J. Clin. Immunol. 14:141-148. [DOI] [PubMed] [Google Scholar]
- 30.Suffredini, A. F., H. D. Hochstein, and F. G. McMahon. 1999. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J. Infect. Dis. 179:1278-1282. [DOI] [PubMed] [Google Scholar]
- 31.Suffredini, A. F., D. Reda, S. M. Banks, M. Tropea, J. M. Agosti, and R. Miller. 1995. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J. Immunol. 155:5038-5045. [PubMed] [Google Scholar]
- 32.Taylor, P. C. 2003. Anti-TNFα therapy for rheumatoid arthritis: an update. Intern. Med. 42:15-20. [PubMed] [Google Scholar]
- 33.Tibben, J. G., L. F. Massuger, O. C. Boerman, G. F. Borm, R. A. Claessens, and F. H. Corstens. 1994. Effect of the route of administration on the biodistribution of radioiodinated OV-TL 3 F(ab′)2 in experimental ovarian cancer. Eur. J. Nucl. Med. 21:1183-1190. [DOI] [PubMed] [Google Scholar]
- 34.van der Poll, T., C. C. Braxton, S. M. Coyle, M. A. Boermeester, J. C. Wang, P. M. Jansen, W. J. Montegut, S. E. Calvano, C. E. Hack, and S. F. Lowry. 1995. Effect of hypertriglyceridemia on endotoxin responsiveness in humans. Infect. Immun. 63:3396-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Poll, T., S. E. Calvano, A. Kumar, C. C. Braxton, S. M. Coyle, K. Barbosa, L. L. Moldawer, and S. F. Lowry. 1995. Endotoxin induces downregulation of tumor necrosis factor receptors on circulating monocytes and granulocytes in humans. Blood 86:2754-2759. [PubMed] [Google Scholar]
- 36.Van Zee, K. J., S. M. Coyle, S. E. Calvano, H. S. Oldenburg, D. M. Stiles, J. Pribble, M. Catalano, L. L. Moldawer, and S. F. Lowry. 1995. Influence of IL-1 receptor blockade on the human response to endotoxemia. J. Immunol. 154:1499-1507. [PubMed] [Google Scholar]
- 37.Weiler, H., V. Lindner, B. Kerlin, B. H. Isermann, S. B. Hendrickson, B. C. Cooley, D. A. Meh, M. W. Mosesson, N. W. Shworak, M. J. Post, E. M. Conway, L. H. Ulfman, U. H. von Andrian, and J. I. Weitz. 2001. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler. Thromb. Vasc. Biol. 21:1531-1537. [DOI] [PubMed] [Google Scholar]
- 38.Wolff, S. M. 1973. Biological effects of bacterial endotoxins in man. J. Infect. Dis. 128(Suppl.):259-264. [DOI] [PubMed] [Google Scholar]
- 39.Xing, L., and D. G. Remick. 2003. Relative cytokine and cytokine inhibitor production by mononuclear cells and neutrophils. Shock 20:10-16. [DOI] [PubMed] [Google Scholar]