Abstract
Heart failure is characterized by the loss of sympathetic innervation to the ventricles, contributing to impaired cardiac function and arrhythmogenesis. We hypothesized that renal denervation (RDx) would reverse this loss. Male Wistar rats underwent myocardial infarction (MI) or sham surgery and progressed into heart failure for 4 wk before receiving bilateral RDx or sham RDx. After additional 3 wk, left ventricular (LV) function was assessed, and ventricular sympathetic nerve fiber density was determined via histology. Post-MI heart failure rats displayed significant reductions in ventricular sympathetic innervation and tissue norepinephrine content (nerve fiber density in the LV of MI+sham RDx hearts was 0.31 ± 0.05% vs. 1.00 ± 0.10% in sham MI+sham RDx group, P < 0.05), and RDx significantly increased ventricular sympathetic innervation (0.76 ± 0.14%, P < 0.05) and tissue norepinephrine content. MI was associated with an increase in fibrosis of the noninfarcted ventricular myocardium, which was attenuated by RDx. RDx improved LV ejection fraction and end-systolic and -diastolic areas when compared with pre-RDx levels. This is the first study to show an interaction between renal nerve activity and cardiac sympathetic nerve innervation in heart failure. Our findings show denervating the renal nerves improves cardiac sympathetic innervation and function in the post-MI failing heart.
Keywords: heart failure, renal nerve, sympathetic nerve activity, cardiac innervation
the occurrence of heart failure is increasing throughout the industrialized world, in part, due to the increased survival rates following myocardial infarction (MI) (29). Mortality and morbidity following the diagnosis of heart failure are high, with 50% of newly diagnosed patients dying within 5 years (49).
Renal norepinephrine (NE) spillover in human heart failure patients is elevated (24), indicative of increased renal sympathetic nerve activity (RSNA), and is associated with increased morbidity and mortality (24, 47). As such, renal denervation has received attention as a therapeutic intervention to treat heart failure (16). Chronic elevations in renal sympathetic nerve activity (SNA) are known to increase sodium and fluid retention and activate the renin-angiotensin system, all hallmarks of heart failure (17, 45). However, the predictive value of renal NE spillover in heart failure patients is independent of kidney function (47). Therefore, in heart failure, elevated renal nerve activity appears to drive adverse changes, at least in part, via pathways independent of fluid and sodium retention.
In heart failure, there is an increase in cardiac sympathetic neural tone, which is associated with reduced NE reuptake (4), depletion of NE in the myocardium, downregulation of β-adrenergic signal transmission (3, 8), and impaired inotropic responses to adrenergic stimulation (21). These changes cause myocardial damage, reduced cardiac function, and lethal arrhythmias (9, 12, 44). In conjunction with changes in cardiac SNA in heart failure, the distribution of the cardiac sympathetic nerves in the ventricles may also be altered in a pathophysiological manner. The cardiac ventricles are abundantly innervated by sympathetic nerves, which act to increase conduction velocity and myocardial contraction and relaxation. Regional disturbances in the sympathetic innervation of the cardiac ventricles have been associated with disturbed heart function and arrhythmia generation (2, 13, 50, 57). As chronic heart failure develops, sympathetic innervation throughout the functioning myocardium of both left and right ventricles is reduced (28), and this reduction is a stronger predictor of cardiac death than ejection fraction in patients (41, 43). When combined with elevated cardiac SNA, altered distribution of cardiac sympathetic nerves may have particularly significant impacts on ventricular function and arrhythmogenesis. Therefore, available evidence advocates the potential benefit of preserving appropriate cardiac innervation in post-MI heart failure.
In experimental heart failure, renal denervation can improve cardiac function, which is associated with improved cardiac adrenergic receptor expression (27, 62). Therefore, it has been proposed that the beneficial action of renal denervation in heart failure occurs via attenuating adverse changes in the cardiac SNS. However, no one has investigated the effects of renal denervation on the cardiac autonomic nerve innervation in heart failure. Given the positive effects of renal denervation on cardiac function in heart failure, we evaluated whether the renal nerves influence the cardiac autonomic innervation, and function, of the ventricles in a rat model of established MI-induced heart failure. We hypothesized that renal denervation in established post-MI heart failure would normalize the cardiac sympathetic innervation of the intact ventricular myocardium and be associated with improved cardiac function.
METHODS
Ethical approval.
Experiments were conducted in male Wistar rats and were approved by and carried out following the guidelines by the Animal Ethics Committee of the University of Auckland. The following experiments were conducted in four groups: sham MI and sham renal denervation (RDx), MI+sham RDx, sham MI+RDx, MI, and MI+RDx.
Surgical procedures.
For all recovery surgeries, animals were anesthetized with isoflurane anesthesia (2% in oxygen) and given prophylactic antibiotics (12.5 mg/kg enrofloxacin, Baytril; Bayer, Auckland, New Zealand) and analgesia (20 µg/kg buprenorphine and again 24 h later, Temgesic; Reckitt Benckiser, Auckland, New Zealand). After surgery, rats were returned to their home cages. A heating pad was placed under the cage for 24 h postsurgery. All rats were housed 2-4 per cage with water and food ad libitum in a room of constant temperature (22°C) with a 12:12-h light-dark cycle.
MI or sham MI surgery was performed, as previously described (48). Briefly, with the rat under isoflurane anesthesia, MI was induced by tying off the left anterior descending coronary artery 2–3 mm from origin using a 6-0 silk suture. In the sham groups, a suture was passed through the heart wall, but the LAD was not tied off. At the conclusion of the surgery the lungs were reinflated, and the chest was sutured closed.
Bilateral RDx or sham-RDx surgery was performed 4 wk after MI or sham MI surgery. The renal nerves were exposed via a retroperitoneal incision that revealed the left and right kidney. To ensure complete denervation of both kidneys, all visible nerves were stripped from the arteries followed by application of 10% phenol in saline on and around the renal artery using a cotton swab. Sham RDx followed the same procedure as for denervation without stripping and phenol application. Once the procedures were complete, the retroperitoneal cavity was sutured. No deaths occurred following the RDx surgery.
Echocardiography.
Echocardiography was performed at 4 and 7 wk post-MI surgery. In brief, the rat was anesthetized (5% isoflurane in oxygen) until it could be handled easily and did not move during the imaging. The rat was then removed from the anesthesia, and the echocardiograph was obtained to assess left ventricular (LV) function. Echocardiography at 4 wk post-MI surgery was performed just before renal denervation surgery, and at 7 wk post-MI, surgery was performed before beginning the terminal experiment. Analysis was performed by an observer blinded to the treatment groups.
Left ventricular pressure measurements.
Hemodynamic measurements were performed 7 wk post-MI or sham-MI. On the day of the experiment, animals were anesthetized “to effect” over the course of an hour with α-chloralose (120 mg/kg body wt) and urethane (1.5 g/kg body wt), as described previously (48). Body temperature was maintained at 37°C by a heating pad and heating lamp. Once sufficient level of anesthesia was obtained, the trachea was cannulated, and the rat was artificially ventilated (model 680; Harvard Apparatus, Holliston, MA) with inspirate gas enriched with O2 (~50% O2), a tidal volume of ~3–4 ml and a breathing rate of ~70 breaths/min. The femoral artery and vein were cannulated to monitor arterial pressure and for administration of drugs, respectively. A pressure catheter (model SPR-838, Millar Instruments, Houston, TX) was then inserted into the right carotid artery and advanced into the LV for continuous pressure measurements. Once recordings had stabilized, a period of at least 10 min of stable recordings were obtained. Data were acquired for computer analysis (PVAN software, Millar Instruments) using LabChart 7 software system (PowerLab, ADInstruments, Bella Vista, NSW, Australia).
Tissue preparation and estimation of myocardial infarct size.
At completion of the terminal experiment, both left and right renal arteries and veins were clamped, and the left and right kidneys were removed from all animals, before euthanizing the animal, and were stored in −80°C for later measurement of NE content. In animals to be used for immunoblotting and catecholamine content, hearts were removed, transversely sectioned, and then the midventricular section apical to the ligation was photographed for later measurement of MI size. A portion of the noninfarcted myocardium of the LV, apical to the occlusion, including both the LV free wall and septum, was then flash frozen in liquid nitrogen before being stored in −80°C until processing. Animals to be used for immunohistochemistry had a cannula inserted into the descending aorta for perfusion with heparinized saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). The perfused hearts were removed, transversely sectioned, and then the midventricular section apical to the ligation was photographed for later measurement of MI size. The sections were then stored in PFA overnight at 4°C and then dehydrated using a series of graded alcohols before being embedded in paraffin wax. MI size was calculated, as previously described by our group (48).
Norepinephrine and ACh tissue content.
NE content in the LV and kidneys was measured using HPLC with electrochemical detection as previously described (38). Detection limits were 0.05 pmol with recoveries from the alumina extraction 60%. ACh content in the LV was measured using mass spectrometry.
Determination of tyrosine hydroxylase and PGP 9.5 in the cardiac ventricles.
All immunohistochemistry was carried out on slide-adhered 10-µm sections cut from ventricular tissue apical to the ligation (Fig. 1). All sections were at least 150 µm apart to minimize duplicate labeling of the same neuron. To expose antigenic sites, heat-mediated antigen retrieval was performed using the 2100 Retriever (Pick-Cell Laboratories, Amsterdam, The Netherlands) and an EDTA buffer (pH 8.0). Sections were then incubated in sodium borohydride-PBS solution 2× for 10 min. Sections were blocked in 10% normal goat serum and then incubated overnight at 4°C with polyclonal rabbit anti-tyrosine hydroxylase (1:1,000, cat. no. AB152, EMD Millipore, Temecula, CA) to visualize sympathetic nerve fibers or with polyclonal rabbit anti-PGP 9.5 (1:1,000, cat. no. AB1761-I, EMD Millipore) to visualize total nerve fibers.
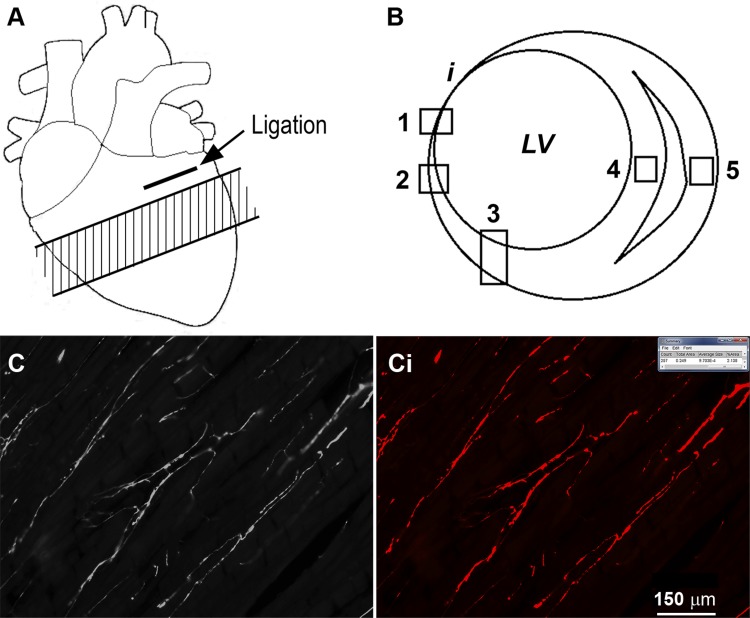
Fig. 1.
A: approximate area of the cardiac ventricles that was processed for immunohistochemistry analysis apical to the ligation (shaded area). B: areas within each individual section that were imaged. i, infarct; LV, left ventricle; 1, infarct border zone; 2, peri-infarct; 3, left ventricle (LV) free wall; 4, septum; 5, right ventricle free wall. C: sample image of tyrosine hydroxylase immunostaining obtained from the intact myocardium of the LV. Ci: image of the result from threshold analysis using ImageJ software, as performed on the image in C, in which the areas above the set threshold are shown in red. In sham myocardial infarction (MI) animals, the areas of the heart tissue that were imaged were similar to MI animals; however, there was no infarct. Scale bar in Ci applies also to C.
The following day, the sections were incubated with biotinylated antirabbit secondary antibody (1:200, cat. no. BA-1000, Vector Laboratories, Burlingame, CA) and then incubated with Alexa Fluor 594 streptavidin (1:400, cat. no. A11008, Molecular Probes, Eugene, OR) and Alexa Fluor-488 conjugated wheat germ agglutinin (WGA; 1:200, cat. no. W11261, Molecular Probes). Sections were rinsed, coverslipped with Citifluor mounting medium (Citifluor, London, UK), and sealed using fast-dry nail enamel.
The antibodies used are all commercial antibodies subject to routine quality assurance. Where positive results were obtained, the pattern of reactivity was found to be distinctive to that particular antibody, with specific structures consistently labeled on repeated assays. Specificity of the primary and secondary antibodies was confirmed by including appropriate control sections for each assay, and no labeling was observed in each control section.
Image acquisition and analysis.
For each animal and immunoreactive (IR) label to be analyzed, images were obtained using a ×20 objective (Carl Zeiss Microscopy, Jena, Germany) under epifluorescence. Digital images were captured using an Olympus DP-72 color camera (Olympus, Tokyo, Japan) and MetaMorph imaging software (version 7.8.3; Universal Imaging Corporation, Downingtown, PA). From each section, two or three representative images were obtained from the following regions: epicardium, endocardium, and myocardium of the LV; myocardium of the interventricular septum; and myocardium of the right ventricle (RV) (Fig. 1). In MI hearts, images were also acquired of the peri-infarct region and infarct border-zone region (Fig. 1). In MI hearts, the infarcted tissue typically penetrated close to the septum on the anterior wall; therefore, for comparative purposes, all of the LV images, regardless of treatment, were obtained from the posterior wall.
Tyrosine hydroxylase (TH)- and PGP-positive IR were quantified by threshold discrimination using ImageJ software, as previously described (37). All images were treated in an identical manner, and no images underwent any form of manipulation subsequent to acquisition. Color photos were opened in ImageJ, and the threshold tool was manually adjusted to ensure only TH- or PGP-positive staining was identified. Specific criteria for the size and shape of neuronal fibers were not used in our analysis. Innervation density was expressed as percent area that was above the threshold (TH- and PGP-positive fibers). Figure 1, C and Ci demonstrates the typical result obtained from the threshold analysis. For each IR label, six sections from each heart were analyzed, and the mean results were presented for analysis. To determine spatial variability of sympathetic innervation, the SD of TH innervation density taken from images of the noninfarcted epicardium, myocardium, and endocardium of the LV within each heart was determined, and the within-animal SDs were subsequently grouped.
Immunoblotting to determine protein expression levels of tyrosine hydroxylase and choline transporter relative to PGP in the left ventricle.
LV cardiac tissue apical to the occlusion was homogenized in lysis buffer [150 mM sucrose, 15 mM HEPES (pH 7.9), 60 mM KCl, 5 mM EDTA, 1 mM EGTA, protease inhibitor cocktail tablet (complete Mini, Roche)]. Protein concentration of the samples was quantified using a Bio-Rad Dc protein assay. The optimal loading concentration was determined to be 20 µg of total cardiac protein per sample. TH, choline transporter (CHT), and protein gene product 9.5 (PGP 9.5) were fractionated and then transferred to membrane. After transfer, membranes were blocked with Tris-buffered saline with 0.01% Tween 20 (TBST) containing 5% nonfat milk powder for 2 h at RT. Membranes were cut at ~40 kDa. The upper parts of the membrane (40–250 kDa) containing TH and CHT protein were incubated overnight at 4°C with polyclonal rabbit anti-TH (1:1000, cat. no. AB152; EMD Millipore, Temecula, CA), or polyclonal rabbit anti-CHT (1:1000, no. ABN458; EMD Millipore). The lower parts of the membranes (0–40 kDa) containing PGP 9.5 protein were incubated separately overnight at 4°C with rabbit polyclonal anti-PGP 9.5 (1:1,000, cat. no. AB1761-I; EMD Millipore). Membranes were then washed with TBST buffer and incubated for 2 h at RT with goat antirabbit horseradish peroxidase conjugate (1:2,000, sc 2004; Santa Cruz Biotechnology, Santa Cruz, CA) to visualize TH, CHT, and PGP 9.5. IR bands were then visualized by chemiluminescence (Pierce ECL Plus, ThermoFisher, Rockford, IL). Band intensity was recorded using a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA) and was quantified using ImageJ software. TH and CHT band densities were normalized to PGP 9.5 from the same sample. Immunoblots were run in triplicate, and mean results were presented for analysis.
Histology.
Midventricular sections (5 µM, each section taken at least 100 µM apart) were stained using hematoxylin and eosin (H&E) or Masson trichrome stain (MTS). Image acquisition was performed using Leica DMR upright microscope and NIS Elements software. For measurement of cardiomyocyte cross-sectional area, images were obtained using ×60 objective. Vertically oriented, H&E-stained cardiomyocytes were then measured using ImageJ software. For measurement of collagen content, images were obtained using ×20 objective and then were analyzed using a combination of Adobe Photoshop and ImageJ software. Collagen content is presented as a percentage (%) of total area. All analysis was performed by an observer blinded to treatments.
Data analysis.
For the anesthetized experiments, all data were sampled at 1,000 Hz using the LabChart 7 software system (PowerLab; ADInstruments). All subsequent data analysis was performed using LabChart 7. A two-way ANOVA with Bonferroni post hoc tests was used to analyze between-group data. Repeated measures two-way ANOVA with Bonferroni post-hoc test was used to analyze the serial echo data. Post-hoc comparisons were made with appropriate controls; therefore, MI+RDx and sham MI+sham RDx or MI+sham RDx and sham MI+RDx were not compared. Data are shown as means ± SE. P < 0.05 were considered significant.
RESULTS
Renal denervation improves LV function after chronic myocardial infarction.
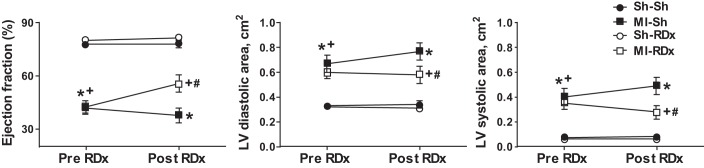
Before renal denervation, echocardiography revealed a similar level of LV impairment in both MI groups, including a significantly enlarged LV chamber and reduced ejection fraction (EF) (Fig. 2). Renal denervation at 4 wk post-MI significantly improved LV end-systolic area and EF (LV EF pre-RDx was 43 ± 3% vs. 56 ± 5% post-RDx, P < 0.05), and did not affect LV function in sham MI rats (Fig. 2). In comparison, sham renal denervation surgery did not alter LV end-diastolic and systolic areas or EF in MI rats (LV EF pre-sham RDx was 42 ± 4% vs. 38 ± 4% post-sham RDx, P > 0.05) and sham MI rats (78 ± 1% vs. 78 ± 2%, P > 0.05, Fig. 2). Thus, at 7 wk post-MI, MI+RDx group had better LV function than the MI+sham RDx group, with significantly greater EF (P < 0.05, Fig. 2) and reduced LV end-systolic and end-diastolic areas (Fig. 2).
Fig. 2.
LV ejection fraction (left), diastolic area (middle), and systolic area (right) before renal denervation (RDx) or sham RDx surgery [pre-RDx, 4 wk postmyocardial infarction (MI) or sham MI surgery] and at 3 wk post-RDx or sham RDx surgery (post-RDx, 7 wk post-MI or sham MI surgery), as measured using echocardiography in the four groups. *Significant difference between MI-Sh group and sham MI groups, P < 0.05. +Significant difference between MI-RDx group and sham MI groups, P < 0.05. #Significant difference between MI-RDx group and MI-Sh group, P < 0.05. Values are expressed as means ± SE (n = 8–13). Sh-Sh, sham myocardial MI + sham RDx; MI-Sh, MI + sham RDx; Sh-RDx, sham MI + RDx; MI-RDx – MI + RDx.
The mean hemodynamic and LV function values as assessed by cardiac catheterization under anesthetic are shown in Table 1. RDx rats displayed a slightly lower resting arterial pressure compared with renal nerve intact rats. Resting heart rate was similar in all groups. As expected, MI resulted in a significant impairment of LV function at baseline, including reduced maximal rate of left ventricular pressure change during systole (dP/dtmax), maximal rate of left ventricular pressure change during diastole (dP/dtmin), and increased left ventricular end-diastolic pressure (LVEDP) and τ (Table 1). RDx attenuated the decrease in LV contractility and relaxation, such that there were no statistically significant differences in dP/dtm, dP/dtmin, τ, and LVEDP, when compared with sham MI+RDx group (Table 1). In sham MI animals, RDx resulted in a lower maximum LV systolic pressure and reduced dP/dtmin, but did not alter the other determinants of LV function (Table 1). Post mortem analysis confirmed the average infarct size did not differ between the two MI groups (40 ± 3% in MI+sham RDx vs. 37 ± 3% in MI+RDx). No infarcts were observed in any of the sham MI rats.
Table 1.
Left ventricular function in four groups of animals that underwent either sham MI or MI and sham RDx or RDx
| Sh-Sh | MI-Sh | Sh-RDx | MI-RDx | |
|---|---|---|---|---|
| n | 11 | 7 | 11 | 9 |
| Mean arterial pressure, mmHg | 92 ± 5 | 89 ± 5 | 72 ± 2* | 78 ± 5 |
| Heart rate, bpm | 364 ± 11 | 340 ± 15 | 372 ± 15 | 375 ± 13 |
| LV systolic max, mmHg | 122 ± 3 | 113 ± 5 | 104 ± 5* | 107 ± 6 |
| LVEDP, mmHg | 3.8 ± 0.3 | 8.1 ± 1.7* | 3.9 ± 0.7 | 7.1 ± 1.4 |
| LV dP/dtmax, mmHg/s | 10,395 ± 650 | 6,465 ± 632* | 8,837 ± 1079 | 7,714 ± 845 |
| LV dP/dtmin, mmHg/s | −9,247 ± 865 | −5,365 ± 804* | −6,799 ± 544* | −6,092 ± 676 |
| τ, ms | 9.5 ± 1.3 | 12.9 ± 1.1* | 8.4 ± 0.4 | 9.0 ± 0.9 |
| Infarct size, % of LV | 40 ± 3 | 37 ± 3 |
Values are expressed as means ± SE. bpm, beats per minute; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; BW, body weight; dP/dtmax, maximal left ventricular rate of pressure over time; dP/dtmin, minimal left ventricular rate of pressure over time; MI, myocardial infarction; RDx, renal denervation; Sh-Sh, sham myocardial MI+sham RDx; MI-Sh, MI+sham RDx; Sh-RDx, sham MI+RDx; MI-RDx, MI+RDx.
Significant difference from Sh-Sh group, P < 0.05.
Renal denervation reduces norepinephrine content in the kidneys.
Tissue NE content in the left and right kidneys of renal denervated groups was significantly reduced when compared with renal nerve-intact groups, confirming successful bilateral renal denervation. Tissue NE content in the left and right kidney of sham MI+RDx group was 0.02 ± 0.01 pmol/mg and 0.03 ± 0.01 pmol/mg, respectively, and in MI+RDx group was 0.00 ± 0.00 pmol/mg and 0.01 ± 0.01 pmol/mg, respectively. In sham RDx rats, MI was associated with significantly elevated tissue NE content in the left kidney (MI+sham RDx 1.39 ± 0.11 pmol/mg vs. sham MI+sham RDx 1.11 ± 0.10 pmol/mg, P < 0.05), but not in the right kidney (MI+sham RDx 1.33 ± 0.16 pmol/mg vs. sham MI+sham RDx 1.12 ± 0.12 pmol/mg, P > 0.05).
Characterizing the changes in cardiac sympathetic innervation after chronic myocardial infarction.
The distribution of sympathetic and total nerve fibers in hearts of sham-MI and MI rats was examined by visualizing the enzyme tyrosine hydroxylase and the pan-neuronal marker PGP 9.5, respectively. Sympathetic nerve fibers and total nerve fibers displayed similar distribution patterns and were located running alongside myocytes. In sham-MI hearts, numerous sympathetic and total nerve fibers were detected throughout the left and right ventricles. Renal denervation did not alter the distribution of sympathetic and total nerve fibers in the hearts of sham-MI rats.
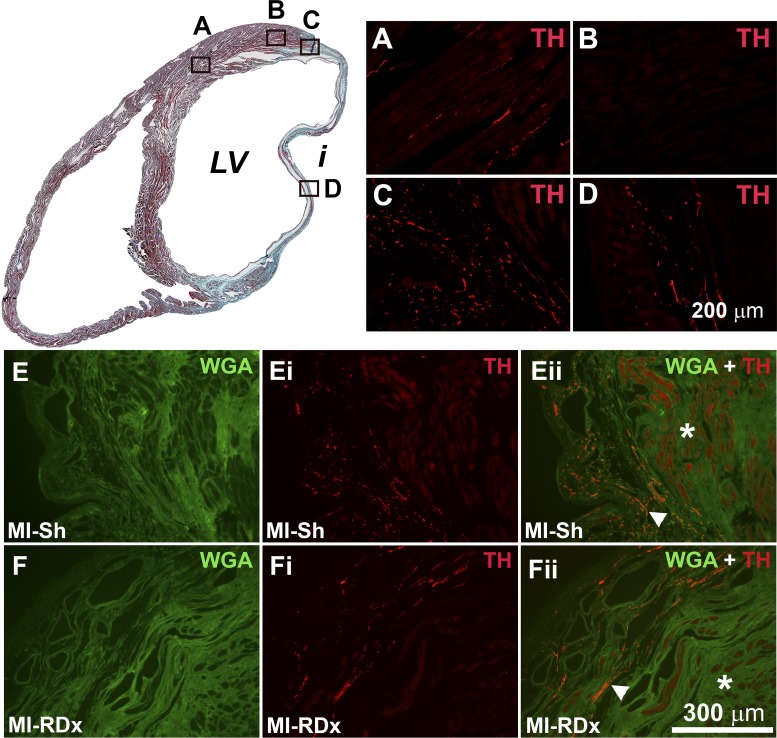
MI hearts displayed distinct regional variations in sympathetic nerve fiber distribution (Fig. 3). In all MI hearts, sympathetic nerve fiber density was significantly reduced in the noninfarcted myocardium of the peri-infarct zone when compared with the noninfarcted myocardium remote from the infarct (in MI+sham RDx group, the sympathetic nerve fiber density of the peri-infarct zone was 0.03 ± 0.01% vs. 0.31 ± 0.05% in the LV myocardium remote from the infarct, P < 0.05, and in MI+RDx group: 0.06 ± 0.02% vs. 0.76 ± 0.14%, P < 0.05). In MI hearts there were regions in the border-zone of the infarcted myocardium that displayed a high density of sympathetic innervation (Fig. 3C) and regions that were devoid of innervation (not shown). Thus, the apparent hyperinnervation of the infarct border zone was regionally specific and selectively located in scar tissue, as has been noted by others (11, 63). The hyperinnervation of the infarct border zone was greater in the posterior wall of the LV, with the least innervation seen in anterior wall infarct tissue. Furthermore, we were also able to visualize sympathetic nerve fibers deep within the infarct (Fig. 3D). RDx did not alter the regionally specific distribution patterns of sympathetic innervation in MI hearts (Fig. 3). The distribution and density of PGP IR and, thus, total nerve fibers in MI hearts were similar to the observed patterns of sympathetic innervation (not shown).
Fig. 3.
Top left: Masson’s trichrome stained (MTS) section from a rat heart that underwent a MI and subsequent sham renal denervation (RDx); viable myocardium is stained red, and infarct (indicated by i) is stained blue. Top right: images of tyrosine hydroxylase (TH) immunostaining (red) in regions as indicated by A, B, C and D shown on the MTS section. A: sympathetic nerve fibers travel parallel to cardiomyocytes in the normal myocardium distant from the infarct. B: sympathetic fibers are minimally expressed in the normal myocardium of the peri-infarct region. C: sympathetic hyperinnervation was observed in the infarcted tissue of the infarct border-zone; D: sympathetic fibers were observed to penetrate deep into the infarct. E and F: wheat germ agglutinin (WGA) immunostaining (green) in the peri-infarct region of MI + sham RDx heart (E) and MI + RDx heart (F). Ei and Fi: TH immunostaining (red) in the peri-infarct region of MI + sham RDx heart (Ei) and MI + RDx heart (Fi). Eii and Fii: WGA and TH double labeling of peri-infarct region of MI + sham RDx heart (Ei) and MI + RDx heart (Fi). *Intact myocardium of the peri-infarct region; arrowhead indicates sympathetic hyperinnervation in the infarcted myocardium of the infarct border-zone. Scale bar in D applies also to A, B, and C. Scale bar in Fii applies to E, Ei, Eii, F and Fi. LV indicates left ventricle.
Renal denervation recovers sympathetic and total innervation in the intact myocardium of the heart after chronic myocardial infarction.
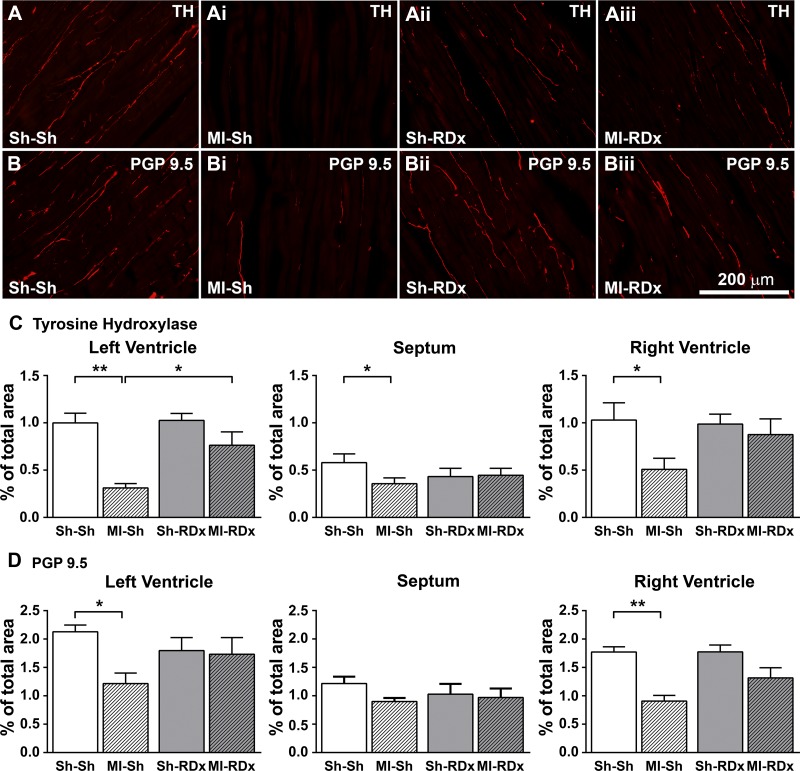
In sham RDx rats, MI hearts displayed reduced density of sympathetic nerve fibers in the noninfarcted myocardium of the LV, septum, and RV remote from the infarct (nerve fiber density in the LV of MI+sham RDx hearts was 0.31 ± 0.05% vs. 1.00 ± 0.10% in sham MI+sham RDx hearts, P < 0.05) (Fig. 4). In MI+sham RDx hearts, total nerve fiber density, as measured from PGP 9.5 IR, was also significantly reduced throughout the noninfarcted myocardium of the cardiac ventricles when compared with sham MI+sham RDx hearts (Fig. 4). Concurrently, the spatial variation of sympathetic fibers throughout the noninfarcted endocardium, myocardium, and epicardium of the LV was significantly diminished in MI+sham RDx hearts (0.195 ± 0.01 SD) compared with sham MI+sham RDx hearts (0.429 ± 0.04 SD, P < 0.05). The reduced variability of sympathetic innervation throughout the noninfarcted LV of MI+sham RDx hearts was consistent with reduced sympathetic innervation density in areas with usually higher innervation densities, for example, in the epicardium and myocardium of the LV when compared with the normally low level of sympathetic innervation density in the endocardium.
Fig. 4.
Sample images of TH (A–Aiii) and PGP 9.5 (B–Biii) immunostaining in the intact myocardium of the LV free wall remote from the infarct in rats that underwent a sham MI or MI and RDx or RDx. Sample images for sham MI + sham RDx (Sh-Sh: A and B), MI + sham RDx (MI-Sh: Ai and Bi), sham MI + RDx (Sh-RDx: Aii and Bii) and MI + RDx (MI-RDx: Aiii and Biii). Sympathetic innervation (TH; C) and total innervation (PGP 9.5; D) was quantified in the intact myocardium remote from the infarct in the left-ventricle (LV) free wall, septum, and right ventricle free wall as a percentage (%) of total area. Values are expressed as means ± SE. *Significantly different between groups, P = 0.01 < 0.05. **Significant difference between groups, P < 0.01. Scale bar: Biii applies to A, Ai, Aii, Aiii, B, Bi, and Bii.
Renal denervation post-MI increased the sympathetic nerve fiber density in the noninfarcted myocardium of the LV, so that sympathetic nerve fiber density was significantly greater in MI+RDx group (0.76 ± 0.14%) compared with MI+sham RDx group (0.31 ± 0.05%, P < 0.05), and to a level similar in sham MI+RDx group (1.02 ± 0.07%, P > 0.05) (Fig. 4). RDx post-MI also recovered the sympathetic nerve fiber density in the noninfarcted myocardium of the septum and RV free-wall, resulting in similar levels as sham MI+RDx group (Fig. 4). However, the effect of RDx on the sympathetic nerve fiber density in the septum and RV in MI hearts was not statistically significant when compared with MI group with intact renal nerves (sympathetic nerve fiber density in the RV of MI+RDx was 0.88 ± 0.17% vs. 0.51 ± 0.12% in MI+sham RDx group, P = 0.189) (Fig. 4). RDx post-MI effected similar changes in total cardiac innervation as the sympathetic innervation, improving total cardiac nerve fiber density to be similar as sham MI hearts (Fig. 4). Concurrently, RDx post-MI recovered the spatial variation of sympathetic fibers throughout the LV (SD of sympathetic nerve fiber density in the LV of MI+RDx hearts was 0.34 ± 0.05 vs. 0.195 ± 0.01 SD in MI+sham RDx hearts, P < 0.05), so that there was no longer a significant difference when comparing MI+RDx hearts to sham MI+RDx hearts (0.45 ± 0.04 SD, P > 0.05).
In sham MI hearts, sympathetic and total nerve fiber density in the myocardium of the cardiac ventricles was unaffected by RDx (Fig. 4). Sympathetic and total nerve fiber density in the myocardium of the RV was similar when compared with the LV, but reduced in the septum (Fig. 4).
Relative changes of sympathetic and parasympathetic innervation compared with total innervation in the left ventricle.
In renal innervated rats, the relative expression of TH protein levels when normalized to the expression of PGP 9.5 protein levels in MI hearts tended to be reduced compared with sham-MI hearts (MI+sham RDx group: 0.66 ± 0.05 vs. sham MI+sham RDx: 0.98 ± 0.11), although the difference did not reach statistical significance (P = 0.17). In renal denervated animals, the relative expression of TH-to-PGP protein levels was similar when comparing MI hearts with sham MI hearts (TH/PGP relative mean band density in MI+RDx group was 0.73 ± 0.16 vs. 0.78 ± 0.16 in sham MI+RDx group, P > 0.05). There were no effects of MI or RDx observed on the relative expression of CHT in the LV when normalized to PGP protein levels.
Renal denervation attenuates the decrease in norepinephrine content in the LV after chronic myocardial infarction.
Tissue NE content in the noninfarcted myocardium of the LV in MI hearts from renal nerve-intact rats (2.70 ± 0.44 pmol/mg) was significantly decreased compared with sham MI hearts (4.55 ± 0.20 pmol/mg, P < 0.05). RDx in MI rats improved the tissue NE content in the LV, such that there was no statistical significance between the MI+RDx (3.28 ± 0.68 pmol/mg) and sham MI+RDx group (4.44 ± 0.32 pmol/mg, P < 0.05). Tissue ACh content in the noninfarcted myocardium of the LV was similar in all groups.
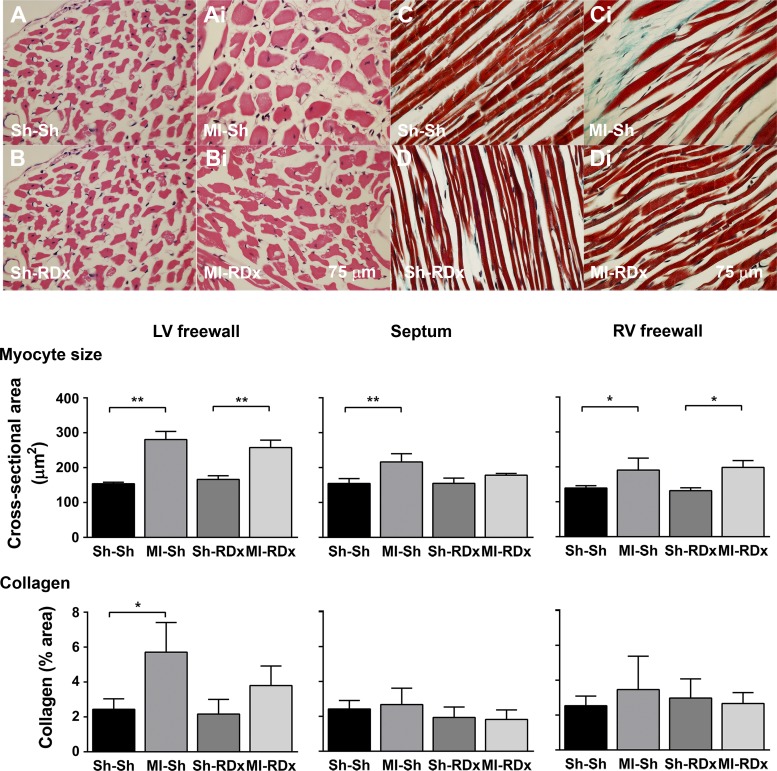
Renal denervation attenuates cardiac remodeling.
As seen in Fig. 5, MI resulted in elevated cardiomyocyte size in the LV and RV free walls of rats regardless of the state of renal innervation. RDx in MI animals reduced cardiomyocyte size in the septum, resulting in no significant differences when compared with sham MI+RDx group (Fig. 5). In renal innervated rats, MI increased the level of fibrosis in the noninfarcted LV free-wall but not in the septum and RV free-wall (Fig. 5). In MI hearts, RDx attenuated the level of fibrosis in the LV free wall, so that the degree of collagen deposition was no longer significantly greater when compared with sham MI+RDx group (Fig. 5).
Fig. 5.
Cardiomyocyte size and collagen content in the left ventricle (LV) in relation to treatment group. Hematoxylin and eosin (H&E)-stained cardiomyocytes (top left) and MTS-stained LV tissue (top right) for animals that underwent either sham MI or MI and sham RDx or RDx. Sample images for H&E staining for sham MI + sham RDx (Sh-Sh: A), MI + sham RDx (MI-Sh: Ai) sham MI + RDx (Sh-RDx: B) and MI + RDx (MI-RDx: Bi). Example images for MTS staining for sham MI + sham RDx (Sh-Sh: C), MI + sham RDx (MI-Sh: Ci) sham MI + RDx (Sh-RDx: D) and MI + RDx (MI-RDx: Di). Mean cardiomyocyte size and collagen content, as a percentage (%) of total area, in the free wall of the LV, septum, and right ventricle across experimental groups are shown in bar graphs. *Significant difference between groups, P = 0.01 < 0.05. **Significant difference between groups, P < 0.01. Values are expressed as means ± SE (n = 4–6). Scale bar in Bi applies also to A, Ai, and B, and scale bar in Di applies also to C, Ci, and D.
DISCUSSION
This is the first study to show an interaction between renal nerve activity and cardiac sympathetic innervation in heart failure. We found that renal denervation normalized sympathetic nerve density in the ventricular myocardium, which was associated with improved LV tissue catecholamine content. Furthermore, we showed that the renal denervation-mediated improvements in sympathetic innervation in heart failure were associated with a recovery of LV function. These findings support the notion that renal denervation in established heart failure is able to improve cardiac function by attenuating adverse changes in the cardiac sympathetic innervation.
The experimental coronary ligation model in the rat, as used in the current study, successfully replicates the clinical situation of heart failure with LV systolic dysfunction (23). In heart failure, cardiac adrenergic drive is typically increased and is associated with disease progression (24). In addition, a decrease in the sympathetic innervation density in the failing myocardium, combined with heterogeneous nerve distribution in ischemic tissue, is an anatomical substrate contributing to the adverse effects of adrenergic stimulation (12, 15, 22, 37). For example, under conditions of enhanced adrenergic tone, such as in heart failure, sympathetically mediated heterogeneities in refractory periods throughout the myocardium can cause a loss of coordinated myocardial contraction and generation of arrhythmia (42), thereby attenuating LV function and exacerbating heart failure. Clinically, blocking the sympathetic drive to the heart using β-blockers improves LV systolic function and ejection fraction in heart failure patients (20), which is associated with increased cardiac adrenergic innervation (15), a similar result to that observed in response to renal denervation in the current study. The current findings support our hypothesis that renal denervation in postinfarction heart failure can improve the cardiac sympathetic innervation and may be one pathway by which renal denervation is effective in improving cardiac function.
In the current study, surgical denervation of the renal efferent and afferent nerves was performed, thereby, ensuring complete and consistent denervation, as evidenced by the lack of NE in the kidney tissue. This is in contrast to the radio-ablation denervation technique that is used clinically, which typically results in inconsistent and/or incomplete denervation (5). Renal efferent nerves respond to autonomic reflex input (e.g., baroreflex) and central stimuli (e.g., ANG II) and act to increase fluid and sodium retention and stimulate the release of renin. In contrast, renal afferent nerves respond to mechanosensory and chemosensory stimuli within the kidney and potentially contribute to changes in sympathetic nerve activity (6). In heart failure rats, renal denervation has been shown to improve fluid and sodium balance and also the circulating levels of renin and ANG II, presumably via the denervation of the renal efferent nerves specifically (27, 45). The effects of the renal afferents in heart failure are poorly studied and, therefore, less clear (6). Renal afferents may become activated in heart failure, possibly in response to reduced renal blood flow and, therefore, disturbed nutrient supply/demand balance, resulting in increased sympathetic outflow (6). Therefore, renal denervation in heart failure may affect changes in the cardiac innervation via relieving mechanical strain in the ventricular myocardium, attenuating the actions of the renin-angiotensin system or attenuating cardiac adrenergic stimulation. However, it is not possible to comment further on the pathway by which renal denervation may improve cardiac sympathetic innervation in heart failure, and this requires further research.
After MI, axonal damage has the potential to alter the neurochemical properties of the sympathetic axons, which could result in diminished production of TH and NE (54, 60, 64). However, our findings show that changes in total neuronal density, as measured using the pan-neuronal marker PGP 9.5, were consistent with changes in sympathetic neuronal density. Total neuronal density was significantly decreased in the ventricular myocardium of heart failure rats with intact renal innervation and was increased nearer to sham levels following renal denervation. PGP 9.5 has been demonstrated to be resilient to the molecular and chemical changes occurring in the failing heart and, therefore, provides a robust marker of neuronal axons in the disease state (51). Therefore, the current results suggest the changes in sympathetic innervation both in response to heart failure and renal denervation were due to altered neuronal density. It should be noted that it is not possible to comment on the functioning of the nerve fibers and, therefore, these may have been affected independently of a change in neuronal density.
Renal denervation in rats with heart failure improved the levels of NE in the intact myocardium. Reduced levels of NE in the cardiac ventricular tissue is typically observed in severe heart failure (14) and is likely due to increased NE release (53) and reduced reuptake (4) by the cardiac sympathetic neurons. Furthermore, the reduced expression of TH in the failing myocardium, as visualized in heart failure rats with intact renal innervation, likely contributes to decreased levels of tissue NE, as tyrosine hydroxylase is the rate-limiting enzyme involved in NE biosynthesis (32). In heart failure, the reduced levels of NE stored in the ventricular myocardium are associated with increased levels of NE in the synaptic cleft and plasma, and therefore, the ventricular tissue is exposed to greater adrenergic stimulation (1). Therefore, the improved levels of tissue NE in the ventricular myocardium of renal denervation rats with heart failure is supportive of increased expression of TH and suggestive of attenuated cardiac adrenergic drive.
In the current study, we used Western blot analysis to determine protein levels of both TH and CHT in the LV myocardium, as indices for sympathetic and parasympathetic innervation, respectively. CHT is selectively expressed in cholinergic nerves and has been used as a robust marker for cardiac parasympathetic fibers (26). Our findings suggest that TH, compared with CHT, is particularly reduced in the noninfarcted ventricular myocardium of heart failure rats. This observation is consistent with those in humans where the sympathetic nerve fibers are selectively reduced throughout the myocardium of heart failure patients (39). In heart failure, it has been suggested that sympathetic fibers may alter their phenotype to become cholinergic, which may help to explain the selective reduction in cardiac sympathetic neurons and reduced levels of NE in the myocardium (31). However, we did not observe evidence to suggest a selective effect of MI or renal denervation on the expression of CHT relative to total neuronal density, or ACh in the cardiac ventricles of heart failure animals. Therefore, either the phenotypic transdifferentiation from sympathetic to parasympathetic nerve fiber did not occur in our model of heart failure, or it occurred on a scale that was outside our detection limit (46). Furthermore, the ratio of parasympathetic fibers to total fibers remained the same whether in heart failure or not, suggesting there is also a reduction in parasympathetic fibers in the cardiac ventricles in the MI hearts that is relative to the reduction in total neuronal density.
Our findings build on previous research by showing that male rats in heart failure 7 wk following a large, chronic MI display distinct regional variations in sympathetic nerve fiber density. These changes include an overall reduction in nerve density throughout the intact myocardium of the ventricles, as well as areas of hyperinnervation in the infarcted tissue. Evidence in both animals and humans agree with these findings (28, 33, 57, 59, 61). An association between the degree of hyperinnervation of the infarct and occurrence of arrhythmia has been demonstrated (11, 12) and may be related to the super sensitivity to catecholamine in the neighboring denervated intact myocardium (30). In the current study, renal denervation increased the cardiac sympathetic innervation of the viable myocardium remote from the infarct and may have helped to normalize, at least in part, the sensitivity of the myocardium to catecholamine stimulation. Indeed, recent evidence in humans and animals supports the concept that renal denervation can attenuate atrial and ventricular arrhythmia (34). For example, in goats with pacing-induced atrial arrhythmia, renal denervation reduced nerve sprouting and attenuated the increase in sympathetic nerve density in the atria, while concurrently reducing the complexity and occurrence of arrhythmic events (34). Therefore, it appears that renal denervation in heart failure may attenuate changes in cardiac sympathetic nerve innervation that may otherwise provide a substrate for adversely affecting the pump function of the LV and driving arrythmia.
In the current study, collagen content in the intact myocardium of the LV was assessed as an indicator of fibrosis. Collagen content in the noninfarcted myocardium was increased in the renal nerve-intact heart failure group, consistent with previous studies reporting elevated cardiac fibrosis in the rat MI model of heart failure and in human patients (18, 56). In the current study, renal denervation in heart failure rats attenuated the amount of interstitial collagen in the LV. These results are consistent with recent experimental data in which the renal denervation was performed 4 wk post-MI or after 5 wk of isoproterenol injections; cardiac fibrosis was attenuated in both disease models (36, 62). Clinically, renal denervation has been shown to reduce cardiac remodeling in patients with hypertension-induced heart failure (10, 19, 40). Potentially, the beneficial effects of renal denervation on cardiac fibrosis may be mediated, at least in part, by a decrease in circulating ANG II (58). ANG II can drive the formation of fibrosis (58) and a reduction in the actions of ANG II using either ACE inhibitors or AT1R antagonists can reduce cardiac fibrosis in humans and rats with heart failure (7, 18, 56). However, the precise mechanisms by which renal denervation can attenuate cardiac remodeling in heart failure is poorly understood.
Limitations.
Cardiac function was measured under anesthetic and, therefore, the results must be interpreted accordingly. During the LV pressure recordings, taken under chloralose-urethane anesthetic, the resting mean arterial pressure in renal denervated rats was lower when compared with renal innervated rats. Renal nerve activity increases in rats in response to chloralose-urethane anesthetic and likely contributes to the maintenance of arterial pressure (55). Both preload and afterload significantly impact on cardiac function parameters, such as rate and force of contraction, with higher cardiac loads resulting in increased rate and force of contraction. Therefore, the finding that renal denervated sham MI rats displayed lower dP/dt minimum compared with sham-MI renal innervated rats may be due to the reduced cardiac loads, and therefore, it is difficult to interpret this result. This also highlights the possibility that the benefit of renal denervation on LV pressure parameters in heart failure rats may be underestimated in the current study as arterial pressure; therefore, afterload, was significantly lower compared with renal innervated heart failure rats.
Perspectives and Significance
We provide new evidence that renal denervation in established heart failure can restore the sympathetic innervation of the cardiac ventricles, which may contribute to improved cardiac function. Altered cardiac sympathetic innervation in postinfarction heart failure may mediate reduced contractile function and also generation of arrhythmias, and possibly fibrillation (2, 13, 50, 57). Recent reports have suggested that renal denervation can successfully treat patients with ventricular electrical instability (25, 52), suggesting that elevated renal nerve activity can contribute to the generation of arrhythmia (35). Although these studies demonstrated a rapid improvement in the occurrence of arrhythmia and fibrillation, there were also long-term improvements that can potentially be explained by alterations in the cardiac innervation (52). Therefore, the current results suggest that the interaction between renal nerve activity and cardiac innervation may be, at least in part, responsible for mediating the beneficial actions of renal denervation on cardiac function in heart failure, and possibly arrhythmogenesis. However, it remains to be determi ned whether renal denervation can improve cardiac electrical stability and long-term survival in heart failure and what role the cardiac autonomic innervation may have in this process. Furthermore, future research is required to better elucidate the mechanisms by which renal denervation can alter the cardiac sympathetic innervation in post-MI heart failure.
GRANTS
This work was supported by the University of Auckland Faculty Research and Development Fund, the Royal Society of New Zealand International Research Staff Exchange Scheme, the National Heart Foundation of New Zealand (1617), and the National Institutes of Health R01 HL-068231 (to B. A. Habecker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.I.P., M.T.L., S.A., W.R.W., B.A.H., and C.J.B. performed experiments; M.I.P., M.T.L., S.A., G.Q., W.R.W., and B.A.H. analyzed data; M.I.P., M.T.L., S.A., S.-J.G., G.Q., B.A.H., and C.J.B. interpreted results of experiments; M.I.P. prepared figures; M.I.P. and C.J.B. drafted manuscript; M.I.P., S.-J.G., and C.J.B. edited and revised manuscript; M.I.P., M.T.L., S.A., S.-J.G., G.Q., W.R.W., B.A.H., and C.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical expertise of Stephanie Lindsay, who helped with animal care and data analysis.
REFERENCES
- 1.Abraham WT, Hensen J, Schrier RW. Elevated plasma noradrenaline concentrations in patients with low-output cardiac failure: dependence on increased noradrenaline secretion rates. Clin Sci (Lond) 79: 429–435, 1990. doi: 10.1042/cs0790429. [DOI] [PubMed] [Google Scholar]
- 2.Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med 345: 731–738, 2001. doi: 10.1056/NEJMoa010519. [DOI] [PubMed] [Google Scholar]
- 3.Böhm M, Beuckelmann D, Brown L, Feiler G, Lorenz B, Näbauer M, Kemkes B, Erdmann E. Reduction of β-adrenoceptor density and evaluation of positive inotropic responses in isolated, diseased human myocardium. Eur Heart J 9: 844–852, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Böhm M, La Rosée K, Schwinger RH, Erdmann E. Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll Cardiol 25: 146–153, 1995. doi: 10.1016/0735-1097(94)00353-R. [DOI] [PubMed] [Google Scholar]
- 5.Böhm M, Linz D, Ukena C, Esler M, Mahfoud F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ Res 115: 400–409, 2014. doi: 10.1161/CIRCRESAHA.115.302522. [DOI] [PubMed] [Google Scholar]
- 6.Booth LC, May CN, Yao ST. The role of the renal afferent and efferent nerve fibers in heart failure. Front Physiol 6: 270, 2015. doi: 10.3389/fphys.2015.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388–1393, 2000. doi: 10.1161/01.CIR.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. β1- and β2-Adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986. doi: 10.1161/01.RES.59.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV. β-Adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 89: 803–815, 1992. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruno RM, Taddei S. Renal denervation and regression of left ventricular hypertrophy. Eur Heart J 35: 2205–2207, 2014. doi: 10.1093/eurheartj/ehu127. [DOI] [PubMed] [Google Scholar]
- 11.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res 86: 816–821, 2000. doi: 10.1161/01.RES.86.7.816. [DOI] [PubMed] [Google Scholar]
- 12.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960–1969, 2000. doi: 10.1161/01.CIR.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 13.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 50: 409–416, 2001. doi: 10.1016/S0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 14.Chidsey CA, Braunwald E, Morrow AG. Catecholamine excretion and cardiac stores of norepinephrine in congestive heart failure. Am J Med 39: 442–451, 1965. doi: 10.1016/0002-9343(65)90211-1. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, Merlet P. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med 46: 1796–1803, 2005. [PubMed] [Google Scholar]
- 16.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol 162: 189–192, 2013. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105: 2512–2517, 2002. doi: 10.1161/01.CIR.0000017264.66561.3D. [DOI] [PubMed] [Google Scholar]
- 19.Doltra A, Messroghli D, Stawowy P, Hassel JH, Gebker R, Leppänen O, Gräfe M, Schneeweis C, Schnackenburg B, Fleck E, Kelle S. Potential reduction of interstitial myocardial fibrosis with renal denervation. J Am Heart Assoc 3: e001353, 2014. doi: 10.1161/JAHA.114.001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. A new era in the treatment of heart failure. Circulation 94: 2285–2296, 1996. doi: 10.1161/01.CIR.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 21.Fowler MB, Laser JA, Hopkins GL, Minobe W, Bristow MR. Assessment of the β-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation 74: 1290–1302, 1986. doi: 10.1161/01.CIR.74.6.1290. [DOI] [PubMed] [Google Scholar]
- 22.Gardner RT, Wang L, Lang BT, Cregg JM, Dunbar CL, Woodward WR, Silver J, Ripplinger CM, Habecker BA. Targeting protein tyrosine phosphatase σ after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nat Commun 6: 6235, 2015. doi: 10.1038/ncomms7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman S, Raya TE. Rat infarct model of myocardial infarction and heart failure. J Card Fail 1: 169–177, 1995. doi: 10.1016/1071-9164(95)90019-5. [DOI] [PubMed] [Google Scholar]
- 24.Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 73: 615–621, 1986. doi: 10.1161/01.CIR.73.4.615. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann BA, Steven D, Willems S, Sydow K. Renal sympathetic denervation as an adjunct to catheter ablation for the treatment of ventricular electrical storm in the setting of acute myocardial infarction. J Cardiovasc Electrophysiol 24: 1175–1178, 2013. doi: 10.1111/jce.12282. [DOI] [PubMed] [Google Scholar]
- 26.Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res 62: 112–121, 2004. doi: 10.1016/j.cardiores.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Yan Y, Zhou Q, Ji M, Niu C, Hou Y, Ge J. Effects of renal denervation on the development of post-myocardial infarction heart failure and cardiac autonomic nervous system in rats. Int J Cardiol 172: e414–e416, 2014. doi: 10.1016/j.ijcard.2013.12.254. [DOI] [PubMed] [Google Scholar]
- 28.Igawa A, Nozawa T, Yoshida N, Fujii N, Inoue M, Tazawa S, Asanoi H, Inoue H. Heterogeneous cardiac sympathetic innervation in heart failure after myocardial infarction of rats. Am J Physiol Heart Circ Physiol 278: H1134–H1141, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Jhund PS, McMurray JJ. Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation 118: 2019–2021, 2008. doi: 10.1161/CIRCULATIONAHA.108.813493. [DOI] [PubMed] [Google Scholar]
- 30.Kammerling JJ, Green FJ, Watanabe AM, Inoue H, Barber MJ, Henry DP, Zipes DP. Denervation supersensitivity of refractoriness in noninfarcted areas apical to transmural myocardial infarction. Circulation 76: 383–393, 1987. doi: 10.1161/01.CIR.76.2.383. [DOI] [PubMed] [Google Scholar]
- 31.Kanazawa H, Ieda M, Kimura K, Arai T, Kawaguchi-Manabe H, Matsuhashi T, Endo J, Sano M, Kawakami T, Kimura T, Monkawa T, Hayashi M, Iwanami A, Okano H, Okada Y, Ishibashi-Ueda H, Ogawa S, Fukuda K. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest 120: 408–421, 2010. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther 148: 1–8, 1965. [PubMed] [Google Scholar]
- 33.Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol 286: H2229–H2236, 2004. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- 34.Linz D, van Hunnik A, Hohl M, Mahfoud F, Wolf M, Neuberger HR, Casadei B, Reilly SN, Verheule S, Böhm M, Schotten U. Catheter-based renal denervation reduces atrial nerve sprouting and complexity of atrial fibrillation in goats. Circ Arrhythm Electrophysiol 8: 466–474, 2015. doi: 10.1161/CIRCEP.114.002453. [DOI] [PubMed] [Google Scholar]
- 35.Linz D, Wirth K, Ukena C, Mahfoud F, Pöss J, Linz B, Böhm M, Neuberger HR. Renal denervation suppresses ventricular arrhythmias during acute ventricular ischemia in pigs. Heart Rhythm 10: 1525–1530, 2013. doi: 10.1016/j.hrthm.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Zhang Q, Wang K, Wang S, Lu D, Li Z, Geng J, Fang P, Wang Y, Shan Q. Renal denervation findings on cardiac and renal fibrosis in rats with isoproterenol-induced cardiomyopathy. Sci Rep 5: 18582, 2015. doi: 10.1038/srep18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorentz CU, Alston EN, Belcik T, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered β-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol 298: H1652–H1660, 2010. doi: 10.1152/ajpheart.01128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorentz CU, Woodward WR, Tharp K, Habecker BA. Altered norepinephrine content and ventricular function in p75NTR−/− mice after myocardial infarction. Auton Neurosci 164: 13–19, 2011. doi: 10.1016/j.autneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado CR, Camargos ER, Guerra LB, Moreira MC. Cardiac autonomic denervation in congestive heart failure: comparison of Chagas’ heart disease with other dilated cardiomyopathy. Hum Pathol 31: 3–10, 2000. doi: 10.1016/S0046-8177(00)80191-4. [DOI] [PubMed] [Google Scholar]
- 40.Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, Buecker A, Schneeweis C, Doltra A, Schlaich MP, Esler MD, Fleck E, Böhm M, Kelle S. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J 35: 2224–2231, 2014. doi: 10.1093/eurheartj/ehu093. [DOI] [PubMed] [Google Scholar]
- 41.Merlet P, Valette H, Dubois-Randé JL, Moyse D, Duboc D, Dove P, Bourguignon MH, Benvenuti C, Duval AM, Agostini D, Loisance D, Castaigne A, Syrota A. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 33: 471–477, 1992. [PubMed] [Google Scholar]
- 42.Misier AR, Opthof T, van Hemel NM, Vermeulen JT, de Bakker JM, Defauw JJ, van Capelle FJ, Janse MJ. Dispersion of “refractoriness” in noninfarcted myocardium of patients with ventricular tachycardia or ventricular fibrillation after myocardial infarction. Circulation 91: 2566–2572, 1995. doi: 10.1161/01.CIR.91.10.2566. [DOI] [PubMed] [Google Scholar]
- 43.Nakata T, Miyamoto K, Doi A, Sasao H, Wakabayashi T, Kobayashi H, Tsuchihashi K, Shimamoto K. Cardiac death prediction and impaired cardiac sympathetic innervation assessed by MIBG in patients with failing and nonfailing hearts. J Nucl Cardiol 5: 579–590, 1998. doi: 10.1016/S1071-3581(98)90112-X. [DOI] [PubMed] [Google Scholar]
- 44.Nolan J, Flapan AD, Capewell S, MacDonald TM, Neilson JM, Ewing DJ. Decreased cardiac parasympathetic activity in chronic heart failure and its relation to left ventricular function. Br Heart J 67: 482–485, 1992. doi: 10.1136/hrt.67.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels 16: 51–56, 2002. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- 46.Olivas A, Gardner RT, Wang L, Ripplinger CM, Woodward WR, Habecker BA. Myocardial infarction causes transient cholinergic transdifferentiation of cardiac sympathetic nerves via gp130. J Neurosci 36: 479–488, 2016. doi: 10.1523/JNEUROSCI.3556-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J 26: 906–913, 2005. doi: 10.1093/eurheartj/ehi184. [DOI] [PubMed] [Google Scholar]
- 48.Pinkham MI, Whalley G, Guild SJ, Malpas SC, Barrett CJ. Arterial baroreceptor reflex control of renal sympathetic nerve activity following chronic myocardial infarction in male, female and ovariectomized female rats. Am J Physiol Regul Integr Comp Physiol 309: R169–R178, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA 292: 344–350, 2004. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 50.Sasano T, Abraham MR, Chang KC, Ashikaga H, Mills KJ, Holt DP, Hilton J, Nekolla SG, Dong J, Lardo AC, Halperin H, Dannals RF, Marbán E, Bengel FM. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 51: 2266–2275, 2008. doi: 10.1016/j.jacc.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 51.Satoh JI, Kuroda Y. Ubiquitin C-terminal hydrolase-L1 (PGP9.5) expression in human neural cell lines following induction of neuronal differentiation and exposure to cytokines, neurotrophic factors or heat stress. Neuropathol Appl Neurobiol 27: 95–104, 2001. doi: 10.1046/j.1365-2990.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- 52.Scholz EP, Raake P, Thomas D, Vogel B, Katus HA, Blessing E. Rescue renal sympathetic denervation in a patient with ventricular electrical storm refractory to endo- and epicardial catheter ablation. Clin Res Cardiol 104: 79–84, 2015. doi: 10.1007/s00392-014-0749-4. [DOI] [PubMed] [Google Scholar]
- 53.Schömig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J 12 Suppl F: 38–47, 1991. [DOI] [PubMed] [Google Scholar]
- 54.Shadiack AM, Sun Y, Zigmond RE. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci 21: 363–371, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst 72: 46–54, 1998. doi: 10.1016/S0165-1838(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 56.Silvestre JS, Heymes C, Oubénaïssa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation 99: 2694–2701, 1999. doi: 10.1161/01.CIR.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 57.Simões MV, Barthel P, Matsunari I, Nekolla SG, Schömig A, Schwaiger M, Schmidt G, Bengel FM. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J 25: 551–557, 2004. doi: 10.1016/j.ehj.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Sopel MJ, Rosin NL, Lee TD, Légaré JF. Myocardial fibrosis in response to Angiotensin II is preceded by the recruitment of mesenchymal progenitor cells. Lab Invest 91: 565–578, 2011. doi: 10.1038/labinvest.2010.190. [DOI] [PubMed] [Google Scholar]
- 59.Stanton MS, Tuli MM, Radtke NL, Heger JJ, Miles WM, Mock BH, Burt RW, Wellman HN, Zipes DP. Regional sympathetic denervation after myocardial infarction in humans detected noninvasively using I-123-metaiodobenzylguanidine. J Am Coll Cardiol 14: 1519–1526, 1989. doi: 10.1016/0735-1097(89)90391-4. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem 67: 1751–1760, 1996. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- 61.Wernli G, Hasan W, Bhattacherjee A, van Rooijen N, Smith PG. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res Cardiol 104: 681–693, 2009. doi: 10.1007/s00395-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H, Liu X, Sharma NM, Patel KP. Renal denervation improves cardiac function in rats with heart failure: effects on expression of beta-adrenoceptors. Am J Physiol Heart Circ Physiol, 311: H337–H346, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95: 76–83, 2004. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 64.Zigmond R, Mohney R, Schreiber R, Shadiack A, Sun Y, Vaccaroniello YS, Zhou Y. Changes in gene expression in adult sympathetic neurons after axonal injury. Adv Pharmacol 42: 899–903, 1997. doi: 10.1016/S1054-3589(08)60892-3. [DOI] [PubMed] [Google Scholar]