Abstract
The purpose of this investigation was to examine the influence of short-term intense endurance training on cycling performance, along with the acute and chronic signaling responses of skeletal muscle stress and stability markers. Ten recreationally active subjects (25 ± 2 yr, 79 ± 3 kg, 47 ± 2 ml·kg−1·min−1) were studied before and after a 12-day cycling protocol to examine the effects of short-term intense (70–100% V̇o2max) exercise training on resting and exercise-induced regulation of molecular factors related to skeletal muscle cellular stress and protein stability. Skeletal muscle biopsies were taken at rest and 3 h following a 20-km cycle time trial on days 1 and 12 to measure mRNA expression and protein content. Training improved (P < 0.05) cycling performance by 5 ± 1%. Protein oxidation was unaltered on day 12, while resting SAPK/JNK phosphorylation was reduced (P < 0.05), suggesting a reduction in cellular stress. The maintenance in the myocellular environment may be due to synthesis of cytoprotective markers, along with enhanced degradation of damage proteins, as training tended (P < 0.10) to increase resting protein content of manganese superoxide dismutase and heat shock protein 70 (HSP70), while mRNA expression of MuRF-1 was elevated (P < 0.05). Following training (day 12), the acute exercise-induced transcriptional response of TNF-α, NF-κB, MuRF-1, and PGC1α was attenuated (P < 0.05) compared with day 1. Collectively, these data suggest that short-term intense training enhances protein stability, creating a cellular environment capable of resistance to exercise-induced stress, which may be favorable for adaptation.
Keywords: SAPK/JNK, protein oxidation, MuRF-1, myostatin, proliferator-activated receptor gamma coactivator 1-α
endurance exercise training induces various physiological adaptations that aid in enhancing exercise performance, including an increase in maximal exercise capacity, changes in substrate utilization, and increases in mitochondrial number, size, and function. These positive benefits are evident in as short as 3 wk, as indicated by improved time trial performance (3, 8) and V̇o2max (34, 40). Similarly, short-term (~1–3 wk) training has been shown to improve cardiovascular function (40), spare skeletal muscle glycogen during exercise (2, 3), and enhance oxidative metabolism (i.e., mitochondrial enzyme activity and oxidative capacity (3, 8, 34).
The myocellular stress response brought upon by repeated skeletal muscle contraction is critical for the positive adaptations in response to exercise training. However, a chronic increase in myocellular stress as a result of skeletal muscle perturbations (i.e., damage to myofibers, low muscle glycogen, inflammation, and/or production of reactive oxygen species) can result in severe implications on skeletal muscle health, including myofiber atrophy and impaired function (31). To combat these deleterious effects, skeletal muscle cells are capable of synthesizing and activating proteins that aid in the stability of the myocellular milieu, most notably antioxidants and heat shock proteins (HSP) (14, 15, 27, 38). Interestingly, short-term training has been shown to elicit a systemic increase in stress (22, 23, 35, 36); however, the response of skeletal muscle is unclear.
Along with the myocellular stress response, an acute bout of exercise induces transient and varied changes in gene expression that, with cumulative bouts of exercise, can lead to specific training adaptations. Interestingly, previous research has shown in response to a similar bout of exercise before and after training, exercise-trained skeletal muscle is less transcriptionally responsive (25, 33), potentially due to a refined response in skeletal muscle to respond earlier following the acute bout of exercise. Surprisingly, the transcriptional response to an acute bout of exercise following short-term exercise training is incompletely understood.
The purpose of this study was to examine the influence of short-term intense endurance training on cycling performance, along with the acute and chronic signaling responses of skeletal muscle stress and stability markers. To accomplish this aim, we examined a 20-km time trial, as well as resting and postexercise skeletal muscle biopsy samples from 10 recreationally active subjects before and after 12 days of intense (70–100% V̇o2max) training on a cycle ergometer.
MATERIALS AND METHODS
Subjects.
Ten subjects (8 males, 2 females) participated in this investigation. Subjects (25 ± 2 yr, 179 ± 2 cm, and 79 ± 3 kg) were recreationally active, defined as exercising >3 days per week with no history of competitive cycling. Participants were excluded from the study if they had any acute or chronic illness; cardiac, pulmonary, liver, or kidney abnormalities; uncontrolled hypertension, Type 1 or Type 2 diabetes or other metabolic disorders; arthritis; a history of neuromuscular problems; or tobacco use. Subjects provided written informed consent before completing any data collection procedures. All experimental procedures were submitted to, and approved by, the Institutional Review Board of Ball State University.
Experimental design.
Each subject completed the experimental protocol over a period of ~4 wk, including 15 visits to the laboratory. The visits included an initial interview regarding informed consent and medical history, aerobic capacity testing, a time trial familiarization session, and 12 days of exercise training, which included two experimental trials during the training period (days 1 and 12). Details of the 12-day study design are displayed in Table 1.
Table 1.
Twelve-day cycling protocol
| Day | Exercise Type | Duration,min | Intensity,% V̇o2max | Workload, W |
|---|---|---|---|---|
| 1 | Time Trial | 37.0 ± 0.6 | N/A | 192 ± 8 |
| 2 | Steady-State | 45 | 78 ± 1 | 175 ± 7 |
| 3 | Intervals | 6 × 5 | 89 ± 1 | 226 ± 8 |
| 4 | Steady-State | 45 | 78 ± 1 | 176 ± 6 |
| 5 | Intervals | 6 × 5 | 90 ± 1 | 229 ± 8 |
| 6 | Steady-State | 45 | 80 ± 1 | 178 ± 7 |
| 7 | Steady-State | 60 | 77 ± 1 | 174 ± 7 |
| 8 | Intervals | 6 × 5 | 92 ± 1 | 234 ± 8 |
| 9 | Steady-State | 60 | 78 ± 1 | 177 ± 7 |
| 10 | Intervals | 6 × 5 | 93 ± 2 | 235 ± 8 |
| 11 | Steady-State | 60 | 79 ± 1 | 180 ± 8 |
| 12 | Time Trial | 35.4 ± 0.6* | N/A | 212 ± 10* |
Data are expressed as means ± SE. N/A, not available.
P < 0.05, before vs. after.
Aerobic capacity.
A graded exercise test using a cycle ergometer (Velotron, RacerMate) was performed before training and ~2 days following the posttraining experimental trial to determine maximum aerobic capacity (V̇o2max). The test began with the subject performing a warm-up, consisting of 2 min at 75–100 W and 4 min at 125–150 W. After completion of the warm-up, the workload increased every 2 min in increments of 25–50 W until exhaustion. Heart rate and perceived exertion were recorded during the test, and respiratory gases were measured using a gas analyzer (Ameteck, S3A; Applied Electrochemistry, Pittsburgh, PA).
Experimental trials.
On the first (day 1) and last (day 12) exercise sessions, subjects participated in an experimental trial. Subjects reported to the laboratory in the fasted state following a standardized meal (Ensure Plus; Abbott Laboratories, Abbot Park, IL) the evening before. Following a resting muscle biopsy from the vastus lateralis, subjects performed a 20-km time trial on a cycle ergometer (Velotron, RacerMate). Subjects were encouraged to complete the course in the shortest time possible and were only allowed access to the distance during the time trial while being blinded to the time and power variables. After a 3-h rest period, a second muscle biopsy was taken from the opposite leg. The timing of these biopsies was based on the time course of myogenic, proteolytic, and cytokine mRNA expression, as previously described (20, 39).
Muscle biopsy.
Percutaneous muscle biopsies were obtained from the vastus lateralis before and 3 h following the time trial. Immediately following the biopsies, the samples were dried of excess blood, and any visible adipose tissue and/or connective tissue were removed. A ~15-mg piece was placed in RNAlater and stored at −20°C until RNA extraction and later analysis of gene expression by real-time RT-PCR. The remaining tissue was quickly frozen and stored in liquid nitrogen (−190°C).
Training.
Steady-state cycling and interval training were performed on a cycle ergometer (Velotron, RacerMate) during days 2–11 of the study. A similar protocol has been used previously to examine cell signaling following short-term exercise training (2). The steady-state training consisted of cycling for 45–60 min at 76.8 ± 0.4% of the subject’s V̇o2max. Exercise intensity was confirmed using indirect calorimetry 15 and 35 min into each exercise bout. The interval training consisted of six, 5-min intervals with subjects cycling at 91.0 ± 0.6% of his/her predetermined V̇o2max. Rest periods were provided between each interval during which subjects cycled at 50 W for 2 min with 1 min of passive rest. During the first and fourth intervals, exercise intensity was confirmed using indirect calorimetry. Table 1 provides details of each exercise session.
Total RNA extraction and RNA quality check.
Total RNA extraction and RNA quality check were performed by methods previously described (39). Briefly, total RNA was extracted in TRI Reagent and 2 µl of polyAcryl carrier, according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, OH). The quality and integrity of total RNA extracted were evaluated by using the RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Reverse transcription and quantitative real-time PCR.
Oligo(dT) primed first-strand cDNA was synthesized using SuperScript II RT (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Quantification of mRNA content for each sample was performed in duplicate using a 72-well Rotor-Gene 3000 centrifugal real-time cycler (Corbett Research, Mortlake, NSW, Australia). GAPDH, which previous research has shown not to be influenced by prior exercise (13), was used as a housekeeping gene. All primers used in this study were mRNA-specific (on different exons and/or over introns) and designed (Vector NTI Advance 9 Software, Invitrogen) for RT-qPCR analysis by using SYBR Green chemistry. Primer sequences for IL-6, TNF-α, citrate synthase (CS), peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α), MuRF-1, and myostatin have been reported (5, 10, 20). Forward and reverse primer sequences for manganese superoxide dismutase (MnSOD) (NM_000636), nuclear factor-κB (NF-κB) (NM_003998), and eukaryotic initiation factor 4E binding protein (4EBP1) (NM_004095) were MnSOD forward: CAGGAGGCGTTGGCCAAGGGAGATGTTAC, MnSOD reverse: CCGTTAGGGCTGAGGTTTGTCCAGAAAA, NF-κB forward: GGCAGCTGGGAGATCGGGAAAA, NF-κB reverse: ACCACGCTGAGGTCCATCTCCTTGG, 4EBP1 forward: AAGATAAGCGGGCGGGCGGTGAAGA, and 4EBP1 reverse: TTCATAAGGCCTGGCTGGTGGGACTCCTC. Details regarding the generation of a melting curve, as well as reaction parameters for RT and PCR has been previously described (20, 30, 39).
Relative qPCR data analysis.
The effects of the short-term training period on resting gene expression was analyzed by using 2−(ΔCT) method [Δ = (CT target gene – CT reference gene)time 0, expressed in arbitrary units (AU)] (19). Exercise-induced gene expression comparing day 1 (preexercise vs. postexercise levels) to day 12 (preexercise vs. postexercise) was analyzed by the 2−(ΔΔCT) method [ΔΔCT = (CT target gene – CT reference gene)time x – (CT target gene – CT reference gene)time 0, expressed as a fold change of postexercise to preexercise level] (29, 39).
Western blot analysis.
For each sample, a ~20-mg piece of muscle was homogenized using an electrically powered glass pestle (Polyscience, Niles, IL) in 30 vol of cold RIPA buffer (25 mM Tris·HCL, pH 7.6, 150 mM NaCl, 1% sodium deoxycholate, and 0.1% SDS) (Pierce, Rockford, IL) with Halt protease Inhibitor Cocktail, Halt Phosphatase Inhibitor Cocktail, and 0.5 M EDTA (Pierce), and centrifuged at 1,000 g for 5 min at 4°C. Total protein concentration was determined (BCA assay, Pierce), and aliquots of homogenate were diluted in SDS sample buffer. Western blot analysis was assessed by methods described previously (11, 16). Primary antibodies used for this investigation were MnSOD (no. 2299–1; Epitomics, Burlingame, CA), CS (no. 96600; Abcam, Cambridge, MA), total stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK) (no. 9252; Cell Signaling, Danvers, MA), phospho-SAPK/JNK (no. 9251; Cell Signaling), HSP70 (no. 4872; Cell Signaling), total 4EBP1 (no. 9644; Cell Signaling), and phospho 4EBP1 (no. 2855; Cell Signaling). Verification of equal protein loading was accomplished by use of Pan-actin (no. 4968; Cell Signaling), a housekeeping protein. Sizes of the immunodetected proteins were confirmed by molecular weight markers (See Blue, Invitrogen). Day 1 and day 12 training samples for each subject were analyzed on the same blot to control for intra-assay variability.
Protein oxidation.
Protein oxidation was assessed by using a commercially available kit, according to the manufacturer’s protocol (Oxyblot Protein Oxidation Kit S7150; Millipore, Billerica, MA). Briefly, 5 µl of muscle homogenate was added to 5 µl of 12% SDS and 10 µl of 2, 4-dinitrophenylhydrazine. The samples were then vortexed and incubated for 15 min at room temperature. Neutralizing solution (7.5 µl) was added to the samples and vortexed, followed by the addition of 1.5 µl of β-mercaptoethanol. Samples were stored at 4°C until analysis. On the day of the experiment, samples were subjected to gel electrophoresis, and transferred to polyvinylidene difluoride membrane. After 1 h of blocking with 5% milk, membranes were incubated for 1 h at room temperature in primary antibody for DNP (1:150 dilution with 5% milk-TBST solution). Blots were identified by incubation with horseradish peroxidase-conjugated-secondary antibody (1:300 dilution with 5% milk-TBST solution). The blots were then exposed to an enhanced chemiluminescent substrate (ECL Plus Western Blotting Detection System, GE; Amersham, Buckinghamshire, UK). Digital images were captured using a chemiluminescent imaging system (FluorChem SP; Alpha Innotech, San Leandro, CA). Verification of equal protein loading was accomplished by use of Pan-actin (no. 4968, Cell Signaling, Danvers, MA). Sizes of the immunodetected proteins were confirmed by molecular weight markers (See Blue, Invitrogen). Day 1 and day 12 training samples for each subject were analyzed on the same blot to control for intra-assay variability. Two distinct bands visible at 28 and 43 kDa were analyzed. The 28-kDa band has been previously examined to assess protein oxidation after exercise training in lean and obese women (6), as well as being associated with amyotrophic lateral sclerosis pathology in a mouse model (21).
Data analysis.
The effects of training on cycling performance, V̇o2max, and baseline protein expression were analyzed using a paired Student’s t-test. Performance (power output, time) during the time trial was assessed using a two-way ANOVA with repeated measures on trial (day 1 vs. day 12) and condition (0–5 km vs. 15–20 km). For each gene, data were checked for normality and equality of variance, and transformations were applied where necessary. When normality was present, a paired t-test was used to compare the relative change in resting mRNA levels (expressed as AU) between trials (day 1 vs. day 12). Exercise-induced gene response (expressed as fold change) was compared using a two-way ANOVA with repeated measures on trial (day 1 vs. day 12) and condition (rest vs. postexercise). For MuRF-1 mRNA expression, Friedman test and Sign test were used in place of repeated-measures ANOVA and paired t-tests, respectively. Significance for all analysis was set at P < 0.05. Data are presented as means ± SE.
RESULTS
Cycling performance.
Performance in a 20-km time trial improved 5% (P < 0.05) following training (Table 1). Average absolute workload increased 11% (P < 0.05) after the 12-day cycling protocol (Table 1), while there was no change (P > 0.05) in average pedal cadence (91 ± 3 vs. 95 ± 2 rpm). Table 2 provides an assessment of performance during the first and last 5 km of the time trial on day 1 and day 12 of training. During the day 1 time trial, there was a significant (P < 0.05) reduction in average power output (9%) from the first to the last 5-km segments, which resulted in a slower (4%) time (P < 0.05). After training (day 12), a higher power output and a faster time were maintained throughout the entire 20-km time trial.
Table 2.
Fatigue assessment during the 20-km time trial before (Day 1) and after (Day 12) training
| Time, min |
Average Workload, W |
|||
|---|---|---|---|---|
| First 5 km (0–5 km) | Last 5 km (15–20 km) | First 5 km (0–5 km) | Last 5 km (15–20 km) | |
| Before | 8.94 ± 0.18 | 9.31 ± 0.13# | 209 ± 11 | 187 ± 7# |
| After | 8.73 ± 0.16* | 8.73 ± 0.15* | 219 ± 10* | 219 ± 10* |
Data are expressed as means ± SE. Before, before training; After, after training.
P < 0.05, first 5 km vs. last 5 km.
P < 0.05, before vs. after.
Aerobic capacity.
Absolute (l/min) and relative (ml·kg−1·min−1) maximum oxygen consumption were both increased (P < 0.05) by 8% following training (Table 3). Both peak (Wmax) and relative (Wmax·kg−1) workload also improved (P ≤ 0.05) by 5% following the training program, while maximum heart rate tended to decrease (P = 0.07). Body weight was maintained (P > 0.05) during the training period.
Table 3.
Effects of training on body weight, maximal oxygen consumption, peak workload, and maximum heart rate
| Time Point | Weight, kg | V̇o2max, l/min | V̇o2max, ml·kg−1·min−1 | Peak Workload, W | Normalized Workload, W/kg | HRmax, bpm |
|---|---|---|---|---|---|---|
| Before | 79.4 ± 3.5 | 3.7 ± 0.1 | 47.1 ± 1.9 | 283 ± 11 | 3.6 ± 0.2 | 190 ± 3 |
| After | 79.3 ± 3.5 | 4.0 ± 0.1* | 50.8 ± 2.3* | 298 ± 13* | 3.8 ± 0.2* | 187 ± 4** |
Data are expressed as means ± SE. HRmax, maximum heart rate.
P ≤ 0.05, before vs. after.
P = 0.07, Pre vs. Post.
Myocellular stress response.
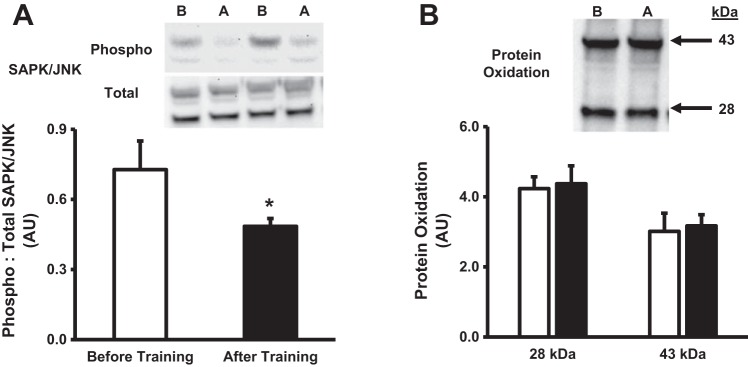
Figure 1 provides the influence of short-term exercise training on the myocellular stress response under basal conditions. Phosphorylation status of SAPK/JNK was significantly (P < 0.05) reduced following training (Fig. 1A), while basal protein oxidation was unaltered (Fig. 1B). Collectively, these results suggest short-term exercise training prevents the exercise-induced increase in myocellular stress.
Fig. 1.
Myocellular stress following short-term exercise training. Quantification and representative images of phosphorylated to total SAPK/JNK (A) and protein oxidation (B). White bars denote before training, while black bars denote after training. In representative blots, B denotes before training, while A denotes after training. Values are expressed as means ± SE. *P < 0.05, significant difference compared with before training.
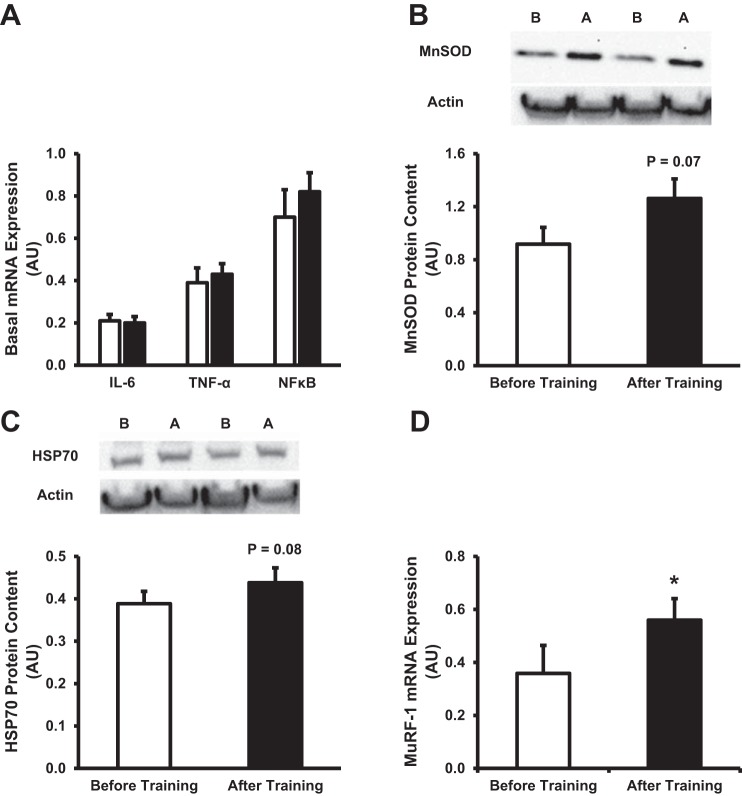
Figure 2 details the intracellular inflammatory response in skeletal muscle under basal conditions, along with the expression of markers shown to minimize myocellular stress. As shown in Fig. 2A, basal intracellular inflammation was unaltered, as indicated by a lack of change in the mRNA expression of IL-6, TNF-α, and NF-κB. Interestingly, MnSOD (Fig. 2B) and HSP70 (Fig. 2C), markers involved in protein stability and in the prevention of myocellular stress, tended (P < 0.10) to increase following training. Furthermore, maintenance of the myocellular milieu may be due to an increase in the turnover of damaged proteins after training, perhaps mediated by MuRF-1, a critical marker that targets that degradation of oxidatively damaged proteins (9). In accordance with this, we observed an elevation of basal MuRF-1 mRNA expression following intense short-term training (Fig. 2D).
Fig. 2.
Effect of short-term training on intracellular inflammation and protein stability. A: basal mRNA expression of IL-6, TNF-α, and NF-κB. Data are expressed as arbitrary units (AU), in which each gene of interest is normalized to GAPDH (2−ΔCT × 104). B and C: quantification and representative images of manganese superoxide dismutase (MnSOD; B), and heat shock protein 70 (HSP70; C). D: basal mRNA expression of MuRF-1 before and after training. White bars denote before training, while black bars denote after training. In representative blots, B denotes before training, while A denotes after training. Values are expressed as means ± SE. *P < 0.05 compared with before training.
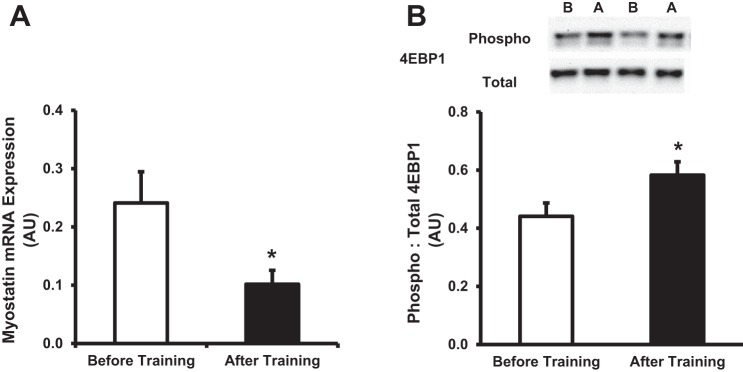
Previously, utilizing a similar exercise training protocol described in this study, Benziane et al. (2) observed an increase in the Akt/PKB-mammalian/mechanistic target of rapamycin (mTOR) signaling cascade, suggesting a possible mechanism in which short-term exercise training may elicit an increase in protein stability markers (Fig. 2, B and C). In line with these results, we observed that basal mRNA expression of myostatin, a negative regulator of Akt/PKB signaling (1, 17), was significantly reduced (P < 0.05) following training (Fig. 3A). Furthermore, similar to Benziane et al. (2), we observed that basal phosphorylation of 4EBP1, a downstream marker of the Akt/PKB-mTOR signaling cascade, was increased (P < 0.05) following the training protocol (Fig. 3B).
Fig. 3.
Cellular markers of protein synthesis following short-term exercise training. A: basal mRNA expression of myostatin before and after short-term training. B: quantification and representative images of phosphorylated to total eukaryotic initiation factor 4E binding protein (4EBP1). In representative blots, B denotes before training, while A denotes after training. Values are expressed as means ± SE. White bars denote before training, while black bars denote after training. *P ≤ 0.05 compared with before training.
Mitochondrial content.
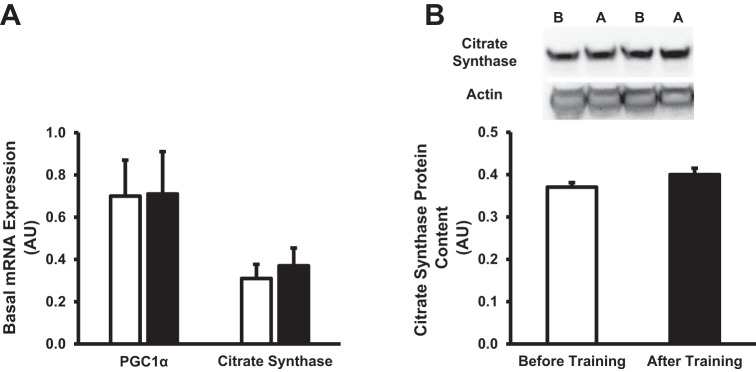
Figure 4 provides the expression of biomarkers associated with mitochondrial content following training. Interestingly, basal mRNA expression of PGC1α, a major transcriptional factor associated with mitochondrial biogenesis (18), was unaltered following training (Fig. 4A). Furthermore, mRNA expression (Fig. 4A) and protein content (Fig. 4B) of citrate synthase, a biomarker of mitochondrial content, were unaltered. Collectively, it appears that the exercise protocol utilized in the current study did not elicit an increase in mitochondrial biogenesis in skeletal muscle.
Fig. 4.
Effect of short-term exercise training on markers of mitochondrial biogenesis. A: basal mRNA expression of proliferator-activated receptor gamma coactivator 1-α (PGC1α) and citrate synthase before and after short-term training. B: quantification and representative images of citrate synthase. In representative blots, B denotes before training, while A denotes after training. Values are expressed as means ± SE. White bars denote before training, while black bars denote after training.
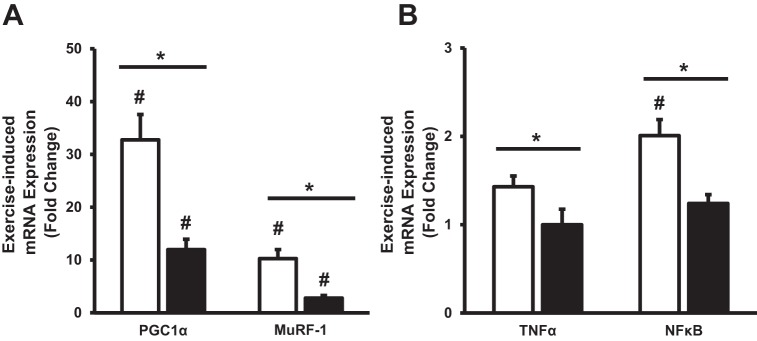
Exercise-induced gene expression.
Figure 5 provides the transcriptional response of target genes following a 20-km time trial before and after the 12-day training period. PGC1α and MuRF-1 were elevated (P < 0.05) following exercise in both conditions, with an attenuated (P < 0.05) response after training (Fig. 5A). NF-κB was elevated (P < 0.05) following the day 1 time trial, whereas it did not change with exercise after training (Fig. 5B). A significant interaction (P < 0.05) was found for exercise-induced gene expression of myostatin and TNF-α, suggesting a greater response in TNF-α during the day 1 time trial (Fig. 5B) and a greater reduction in myostatin (0.9-fold vs. 0.5-fold) during the day 12 trial. IL-6 (1.3-fold vs. 1.1-fold), citrate synthase (1.4-fold vs. 1.4-fold), MnSOD (1.7-fold vs. 1.5-fold), and 4EBP1 (1.1-fold vs. 1.2-fold) mRNA expression did not change following the 20-km time trial in either condition.
Fig. 5.
Exercise-induced gene expression for PGC1α and MuRF-1 (A), and TNF-α and NF-κB (B) before and after short-term exercise training. White bars denote before training, while black bars denote after training. Values are reported as means ± SE. #P < 0.05, significant acute response to exercise compared with preexercise. *P < 0.05 denotes a significant interaction between trials.
DISCUSSION
The purpose of this investigation was to examine the influence of short-term intense endurance exercise training on cycling performance, the acute and chronic signaling responses of skeletal muscle stress response, and stability markers. To accomplish this aim, 10 recreationally active subjects were examined on the first (day 1) and last (day 12) exercise sessions of a 12-day study protocol. The major findings from this study demonstrate that intense short-term exercise training 1) enhanced cycling time trial performance; 2) reduced resting phosphorylation of SAPK/JNK protein; 3) did not alter protein oxidation, suggesting an enhanced ability to scavenge damaged protein, as evidenced by an increase in MnSOD and HSP70 protein content along with enhanced MuRF-1 mRNA; and 4) attenuated the exercise-induced transcriptional response of TNF-α, NF-κB, MuRF-1, and PGC1α. These data provide unique insight into the ability of skeletal muscle to rapidly enhance the management of the stress of intense exercise training. Globally, it appears that these changes favor a less stressful myocellular environment that is capable of withstanding the homeostatic challenge generated by intense skeletal muscle contraction.
The myocellular response to repeated stress associated with muscle contraction is critical for the activation of pathways involved in skeletal muscle adaptations. To assess the myocellular stress response following the 12 consecutive days of intense cycling, we examined resting SAPK/JNK phosphorylation and protein oxidation. In the current study, the phosphorylation status of SAPK/JNK was reduced on day 12. Similarly, Schenk et al. (32) observed a decrease in phosphorylation of JNK in obese women after 12 wk of a combined weight loss and exercise intervention, suggesting a less stressful cellular environment after exercise training. Furthermore, we observed no change in protein oxidation after the training protocol. Although an acute rise in oxidant production is required for myocellular adaptations to exercise (28), a chronic increase in protein oxidation can have severe consequences, including the rapid promotion of muscle fatigue (7). In line with this, we observed, along with maintained protein oxidation of skeletal muscle following short-term training, subjects were less susceptible to muscle fatigue, as shown by a higher power output that was maintained throughout the posttraining 20-km time trial.
Maintenance of myocellular stress is dependent on the degradation of damaged cellular proteins, along with synthesis of cytoprotective markers. Accordingly, the gene expression of MuRF-1, a marker that targets damaged proteins for the 26S proteasome to degrade (9), was elevated at rest after intense short-term training, suggesting a possible marker for degradation of damaged proteins. Similarly, Johnson et al. (14) observed an increase in skeletal muscle proteasome activity in young adults following endurance exercise training, which appeared to minimize oxidative damage. Furthermore, short-term exercise appears to activate the major protein synthetic pathways in skeletal muscle, as Benziane et al. (2) observed an increase in the Akt/PKB-mTOR signaling cascade. In accordance with this, we observed an increase in Akt/PKB-mTOR signaling, as indicated by decreased mRNA expression of the Akt inhibitor myostatin, along with an increase in 4EBP1 phosphorylation, a downstream target of mTOR signaling. The activation of anabolic signaling resulted in an increase in cytoprotective markers MnSOD and HSP70 in the current study. This observation is in line with the results from Parise et al. (24), who observed an increase in antioxidant enzyme activity after 12 wk of resistance training in older individuals, which resulted in maintained protein oxidation levels. Collectively, these data suggest that 12 days of intense training stabilizes the myocellular milieu by enhancing the ability to combat stress.
Previous studies have used nonexhaustive bouts of exercise to assess the transcriptional response of genes involved in skeletal muscle adaptation (10, 12, 25); however, the transcriptional response following a competitive cycling event with short-term training is unknown. Therefore, muscle biopsies were taken before and 3 h following an exhaustive 20-km cycling time trial to examine the influence of short-term intense training on the exercise-induced transcriptional response of skeletal muscle. Of the nine genes of interest, three (MuRF-1, PGC1α, and NF-κB) were responsive to the first exercise bout. After completion of training, MuRF-1 and PGC1α transcripts increased in response to exercise; however, the magnitude of the transcriptional response was attenuated. The attenuated transcriptional response is intriguing, considering subjects were cycling at a higher workload (~11%) in comparison to pretraining conditions. Similarly, despite an 18% increase in workload, Perry et al. (25) observed a decreased transcriptional response of PGC1α in skeletal muscle following a short-term, high-intensity interval training protocol (seven sessions over 2 wk). Collectively, these findings reveal that trained skeletal muscle is less transcriptionally responsive across a range of exercise intensities. Although the timing of the postexercise muscle biopsy was insufficient in determining posttranslational modifications of proteins (i.e., phosphorylation status), our mRNA results provide novel insight into how rapidly the transcriptional response can be altered in skeletal muscle with exercise training.
The mechanism for the attenuated transcriptional response in skeletal muscle following short-term training is unclear. Schmutz et al. (33) suggest the decreased response is primarily due to an increase in resting gene expression. However, in the current study, only one transcript (MuRF-1) was elevated at rest following the 12-day training protocol; thus, the attenuated response is most likely due to another mechanism. Utilizing a similar exercise protocol described in the current study, Benziane et al. (2) observed that muscle glycogen levels were elevated following training. An elevation in muscle glycogen has been shown to attenuate the transcriptional response in skeletal muscle following acute exercise (26). Although not examined in the current study, it is possible that the attenuated transcriptional response following training may be due to increased resting glycogen levels. Furthermore, Wilkinson et al. (37) suggest that the myocellular signaling response following exercise training is more efficient, as the activation of key signaling proteins occurs rapidly in the trained state.
An interesting finding from this study was the exaggerated response in PGC1α following the day 1 time trial. To our knowledge, the 33-fold increase is the most robust response reported after an acute exercise bout. The exaggerated response can potentially be due to the nature of the exercise bout. In the current study, we used a high-intensity, maximal-effort cycling time trial to examine the transcriptional response to exercise. Previous research groups have observed only a modest increase in PGC1α after a controlled, nonexhaustive exercise bout (5, 10, 12). Further, exercise-induced PGC1α expression appears to be intensity-dependent, as Perry et al. (25) observed after an acute bout of high-intensity interval training, skeletal muscle PGC1α mRNA expression increased ~12-fold postexercise. Therefore, the combined effects of high-intensity muscle contraction, along with the maximal nature of the exercise bout, may have induced the robust increased in PGC1α expression. Similar to the current study, Cartoni et al. (4) examined PGC1α transcription 2 h following a competitive 10-km time trial; however, only a three-fold increase in PGC1α was observed in trained cyclists, a value far less than that observed in the current study. We likely observed a more pronounced increase in PGC1α mRNA due to the training status of our subjects. As previously mentioned, trained individuals have an attenuated transcriptional response to an acute bout of exercise (25, 33); therefore, the exaggerated response of PGC1α observed before training could potentially be due to the nature of the exercise bout and/or the training status of the participant.
Perspective and Significance
The current study suggests that 12 days of intense training alters the myocellular milieu, resulting in a more stable environment that is resistant to the stress of a single intense exercise bout and the accumulated stress of repeated exercise bouts. Specifically, the exercise-induced transcriptional responses of TNF-α, NF-κB, MuRF-1, and PGC1α were attenuated; suggesting a refined response in skeletal muscle to respond to contractile activity. Additionally, training appeared to minimize resting levels of stress in skeletal muscle, potentially due to an enhancement in degradation of damaged proteins, while synthesizing new proteins to aid in stability of skeletal muscle following training. These data suggest that short-term training creates a less stressful environment that is likely conducive to protein stability and efficient skeletal muscle adaptation. Collectively, the alterations in the molecular regulation of skeletal muscle may provide unique adaptations that can aid in improving whole body performance after only 12 days of training.
GRANTS
This research was funded by National Institutes of Health Grant AG032127 (to M. P. Harber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
JMH, ARK, and MPH conception and design research. JMH, ARK, MKS, and MPH performed experiments. JMH and MPH analyzed data. JMH, ARK, MKS, and MPH interpreted results of experiments. JMH drafted the manuscript. JMH, ARK, MKS, and MPH edited and revised the manuscript. JMH, ARK, MKS, and MPH approved the final version of the manuscript.
ACKNOWLEDGMENTS
Current address for J. M. Hinkley is the Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL 32607. Current address for A. R. Konopka is the Department of Health and Exercise Science, Colorado State University, Fort Collins, CO 80523.
REFERENCES
- 1.Amirouche A, Durieux A-C, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 150: 286–294, 2009. doi: 10.1210/en.2008-0959. [DOI] [PubMed] [Google Scholar]
- 2.Benziane B, Burton TJ, Scanlan B, Galuska D, Canny BJ, Chibalin AV, Zierath JR, Stepto NK. Divergent cell signaling after short-term intensified endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 295: E1427–E1438, 2008. doi: 10.1152/ajpendo.90428.2008. [DOI] [PubMed] [Google Scholar]
- 3.Burgomaster KA, Heigenhauser GJF, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol (1985) 100: 2041–2047, 2006. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cartoni R, Léger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener J-L, Luthi F, Dériaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol 567: 349–358, 2005. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey VG, Jemiolo B, Edge J, Garnham AP, Trappe SW, Hawley JA. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297: R1441–R1451, 2009. doi: 10.1152/ajpregu.00351.2009. [DOI] [PubMed] [Google Scholar]
- 6.Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med 45: 503–511, 2008. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol (1985) 104: 853–860, 2008. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 8.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899, 2003. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 10.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 11.Harber MP, Crane JD, Douglass MD, Weindel KD, Trappe TA, Trappe SW, Fink WF. Resistance exercise reduces muscular substrates in women. Int J Sports Med 29: 719–725, 2008. doi: 10.1055/s-2007-989442. [DOI] [PubMed] [Google Scholar]
- 12.Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010. doi: 10.1152/ajpregu.00348.2010. [DOI] [PubMed] [Google Scholar]
- 13.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 14.Johnson ML, Irving BA, Lanza IR, Vendelbo MH, Konopka AR, Robinson MM, Henderson GC, Klaus KA, Morse DM, Heppelmann C, Bergen HR III, Dasari S, Schimke JM, Jakaitis DR, Nair KS. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol A Biol Sci Med Sci 70: 1386–1393, 2015. doi: 10.1093/gerona/glu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Dalla Man C, Cobelli C, Amols MH, Irving BA, Nair KS. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes 64: 2104–2115, 2015. doi: 10.2337/db14-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S, Harber MP. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci 65A: 1201–1207, 2010. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res 11: 163–175B, 2008. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 18.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299: E145–E161, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney DJ, Kaczor JJ, Bourgeois J, Yasuda N, Tarnopolsky MA. Oxidative stress and antioxidant enzyme upregulation in SOD1-G93A mouse skeletal muscle. Muscle Nerve 33: 809–816, 2006. doi: 10.1002/mus.20542. [DOI] [PubMed] [Google Scholar]
- 22.Margonis K, Fatouros IG, Jamurtas AZ, Nikolaidis MG, Douroudos I, Chatzinikolaou A, Mitrakou A, Mastorakos G, Papassotiriou I, Taxildaris K, Kouretas D. Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radic Biol Med 43: 901–910, 2007. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Palazzetti S, Richard M-J, Favier A, Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can J Appl Physiol 28: 588–604, 2003. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- 24.Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med 39: 289–295, 2005. doi: 10.1016/j.freeradbiomed.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541: 261–271, 2002. doi: 10.1113/jphysiol.2002.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589: 2129–2138, 2011. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol (1985) 101: 53–59, 2006. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- 30.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 31.Reid MB. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288: R1423–R1431, 2005. doi: 10.1152/ajpregu.00545.2004. [DOI] [PubMed] [Google Scholar]
- 32.Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol 587: 4949–4961, 2009. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmutz S, Däpp C, Wittwer M, Vogt M, Hoppeler H, Flück M. Endurance training modulates the muscular transcriptome response to acute exercise. Pflügers Arch 451: 678–687, 2006. doi: 10.1007/s00424-005-1497-0. [DOI] [PubMed] [Google Scholar]
- 34.Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7-10 days of cycle exercise. J Appl Physiol (1985) 80: 2250–2254, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Tanskanen M, Atalay M, Uusitalo A. Altered oxidative stress in overtrained athletes. J Sports Sci 28: 309–317, 2010. doi: 10.1080/02640410903473844. [DOI] [PubMed] [Google Scholar]
- 36.Vollaard NBJ, Cooper CE, Shearman JP. Exercise-induced oxidative stress in overload training and tapering. Med Sci Sports Exerc 38: 1335–1341, 2006. doi: 10.1249/01.mss.0000227320.23847.80. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada P, Amorim F, Moseley P, Schneider S. Heat shock protein 72 response to exercise in humans. Sports Med 38: 715–733, 2008. doi: 10.2165/00007256-200838090-00002. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 40.Ziemba AW, Chwalbińska-Moneta J, Kaciuba-Uścilko H, Kruk B, Krzeminski K, Cybulski G, Nazar K. Early effects of short-term aerobic training. Physiological responses to graded exercise. J Sports Med Phys Fitness 43: 57–63, 2003. [PubMed] [Google Scholar]