Abstract
Adipose tissue is an important energy depot and endocrine organ, and the degree of adiposity impacts the host response to infection. However, little is known regarding the mechanisms by which white adipose tissue (WAT) is lost acutely and then restored after the resolution of sepsis. Therefore, the signaling pathways governing protein synthesis, autophagy, apoptosis, and the ubiquitin-proteasome were investigated to identify potential mechanisms mediating the acute (24 h) loss of WAT after cecal ligation and puncture as well as the failure to replenish WAT during recovery (day 10). While whole body fat mass was decreased equally in pair-fed control and septic mice at 5 days after cecal ligation and puncture, fat mass remained 35% lower in septic mice at day 10. During sepsis-recovery, protein synthesis in epididymal WAT was increased compared with control values, and this increase was associated with an elevation in eukaryotic translation initiation factor (eIF)2Bε but no change in mammalian target of rapamycin complex 1 activity (eIF4E-binding protein-1 or S6 kinase 1 phosphorylation). Protein breakdown was increased during sepsis-recovery, as evidenced by the elevation in ubiquitin-proteasome activity. Moreover, indexes of autophagy (light chain 3B-II, autophagy-related protein 5/12, and beclin) were increased during sepsis-recovery and associated with increased AMP-activated kinase-dependent Ser555-phosphorylated Unc-51-like autophagy activating kinase-1. Apoptosis was increased, as suggested by the increased cleavage of caspase-3 and poly(ADP-ribose) polymerase. These changes were associated with increased inflammasome activity (increased NLR family, pyrin domain containing 3; TMS1; and caspase-1 cleavage) and the endoplasmic reticulum stress response (increased eIF2α and activating transcription factor-4) and browning (uncoupling protein-1) in epididymal WAT. Our data suggest that WAT stores remain depleted during recovery from sepsis due to sustained inflammation and elevations in protein and cellular degradation, despite the increase in protein synthesis.
Keywords: inflammasome, apoptosis, autophagy, protein synthesis, infection, adipose tissue
the catabolic state produced by sustained sepsis is characterized by a reduction in body weight, lean body mass (LBM), and fat mass (19, 39). While the erosion of LBM and atrophic response in skeletal muscle have been the subject of considerable research interest (32), sepsis-induced changes in fat mass and adipocyte biology have been less thoroughly studied. In murine models, depletion of whole body fat mass is detected 5 days after the induction of abdominal sepsis using cecal ligation and puncture (CLP) and associated with a reduction in the median size of adipocytes in subcutaneous, epididymal, and renal adipose tissue depots (39). Emerging evidence suggests that the metabolic activity of white adipose tissue (WAT) governs sepsis-induced disturbances in the metabolism and function of distance organs through increased release of hormones, inflammatory cytokines, and fatty acids (16, 29, 41, 43, 45). These mediators are capable of producing immunosuppression, ectopic fat deposition, insulin resistance, and prolonged wound healing that can increase morbidity and mortality (1). Accordingly, it has been reported that mild obesity may be protective against the chaotic inflammatory responses to sepsis or burns and the associated mortality (25, 57). This beneficial outcome associated with increased fat mass, known as the “obesity paradox,” has been observed in diverse forms of acute and chronic illness (11, 61). Enthusiasm for the concept has tempered, however, by the recognition that outcomes of critical illness may not be favorable in some forms of obesity or in specific animal models of obesity (40, 56). Reconciliation of these conflicting perspectives depends on a clearer mechanistic understanding by which WAT is acutely depleted and subsequently restored in response to illness, such as abdominal sepsis.
Although originally recognized as a central regulator of protein synthesis, the mammalian target of rapamycin (mTOR) also governs lipid metabolism in adipocytes and other cell types (34). Notably, mTOR is important in the differentiation and maintenance of adipocytes, adipose tissue morphogenesis, and growth as well as adipokine secretion (36). Changes in autophagy are also inversely related activity of mTOR (27). The mTOR kinase activity of tissue is also responsive to nutrients and growth factors, as evidenced by the hyperphosphorylation of eukaryotic initiation factor (eIF)4E-binding protein-1 (4E-BP1) and ribosomal protein S6 kinase-1 (S6K1), with a commensurate rapid increase in protein synthesis (10, 37, 38). Conversely, inhibition of mTOR activity decreases adipose tissue mass (7). As sepsis-induced changes in mTOR activity are tissue dependent, being increased in the liver and decreased in the skeletal muscle and heart (19), it is not possible to extrapolate as to the effect of sepsis on mTOR activity in WAT. Cellular protein and mass are also regulated by a number of degradative pathways, including the ubiquitin-proteasome pathway (UPP), autophagy, and apoptosis, which have also not been characterized in WAT in response to sepsis.
Therefore, to address these knowledge gaps, we investigated the signal transduction pathways related to protein synthesis, autophagy, and apoptosis as well as inflammation and the endoplasmic reticulum (ER) stress response as potential underlying mechanisms mediating the loss of WAT during the acute septic period (24-h post-CLP) and what we have determined to be the failure to adequate replenish WAT during recovery from sepsis (10 days post-CLP).
MATERIALS AND METHODS
Animal care.
Adult male C57BL/6 mice (10 wk of age, 26.9 ± 1.9 g) were purchased from Charles River Laboratories (Wilmington, MA). Mice were acclimated at the Pennsylvania State College of Medicine animal facility for 1 wk before study and provided standard rodent chow (Teklad Globak 2019, Harlan Teklad, Boston, MA) and water ad libitum. Mice were housed individually in shoebox cages with corncob bedding and maintained in a controlled environment (23°C) with a 12:12-h light-dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of Pennsylvania State University College of Medicine (protocol no. 46946) and adhered to National Institutes of Health (NIH) guidelines.
Experimental design.
Polymicrobial peritonitis was induced using CLP, and separate groups of mice were studied in the acute phase of sepsis (i.e., 24 h post-CLP) (54) or during the recovery phase (i.e., 10 days post-CLP) (60). Briefly, mice were anesthetized with isoflurane (3–4% induction with 2–3% maintenance, Vedco, St. Joseph, MO) in 100% O2. The abdomen was shaved and cleaned with betadine, and a 1-cm midline laparotomy was performed. The cecum was ligated 0.8 cm from the distal end using 4-0 silk (Covidien, Minneapolis, MN) and punctured twice with a 25-gauge needle. A small amount of cecal material was extruded from the puncture sites to ensure patency, and the cecum was returned to the abdominal cavity. The abdominal wall was sutured closed using 5-0 silk, and the skin was closed with metal wound clips. Mice were resuscitated subcutaneously with 1 ml warm sterile saline (0.9%) that contained 0.05 mg/kg buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) for postoperative analgesia. Sham control mice underwent the same surgical procedure except that the cecum was only identified without further manipulation. Mice were individually housed and were permitted free access to water. However, because septic mice consume little or no food during the first night after CLP, all mice (both control and septic) were fasted to ensure the same basal nutritional state. Mice were observed for 24 h after laparotomy with or without CLP, and this group is referred to as the “acute sepsis” group.
A separate cohort of mice was used to examine changes during the recovery phase of sepsis. The initial CLP procedure was as described above. In this second study, septic mice had free access to food and water for the entire experiment. Daily body weights and food consumption were measured each morning. Starting on day 1, 24 h post-CLP, antibiotic (0.5 mg meropenem, Fresenius Kabi, Lake Zurich, IL) and buprenorphine were injected subcutaneously (total volume of 1 ml sterile saline) twice daily for the next 5 days (days 1–5). Antibiotic treatment was instituted to better mimic the clinical situation; however, antibiotics do perturb the intestinal microbiome and thereby may alter immune and metabolic function, including storage of lipid (17). Forty-eight hours post-CLP, mice were anesthetized using isoflurane as described above, and the original incision was reopened. At this time, the ischemic portion of the cecum distal to the original suture ligation was resected, and the abscess around the cecum was removed. The peritoneal cavity was washed with 5 ml warmed saline or until the debris, and the abscess was cleared. The abdominal incision was again closed in two layers. Mice were kept on a warming pad during the procedure and until they regained consciousness. Resuscitation was performed with 1 ml warmed saline injected subcutaneously. Mice were observed through day 10, and this group is referred to as the “sepsis-recovery” group. To assure that any metabolic changes observed resulted from sepsis and not differences in food intake, each morning mice in the sham control group were pair fed the average quantity of food consumed by the sepsis-recovery mice the previous day. There were no deaths in the control group (n = 10) during the 10-day experimental protocol. For the septic mice (n = 30), there were no deaths within the first 24 h or after day 5; overall 10-day mortality was 43%. Other general characteristics of this sepsis-recovery model have been previously reported (12).
Analytic methods.
For the sepsis-recovery study, longitudinal changes in body composition were determined using noninvasive NMR (1H NMR) spectroscopy (Minispec LF90, Bruker, Woodlands, TX) before the start of the study and at days 5 and 10 post-CLP.
In vivo protein synthesis was determined by the incorporation of puromycin into tissue protein, as previously described (12, 13). Thirty minutes before euthanasia and tissue excision, mice received an intraperitoneal injection of puromycin (0.04 µmol/g body wt dissolved in sterile saline). Puromycin incorporation into protein was determined by Western blot analysis (Kerafast, Boston, MA). Mice were then anesthetized with isoflurane inhalation as described above.
The entire epididymal (e)WAT depot was excised bilaterally, freeze clamped to the temperature of liquid nitrogen, and weighed. Tissues were subsequently stored at −70°C until analyzed. Because of the amount of tissue needed to assure completion of the analyses described below, no tissue was processed for histological examination. While this experimental design limits the interpretation of some end points (as noted below), it has the advantage of permitting systematic multiparameter analysis of mRNA and protein content and enzymatic activity from the same tissue. Furthermore, while it is recognized that various WAT depots have fundamentally different metabolic and hormonal characteristics (47), only a single depot was sampled because of the large number of end points to be determined. eWAT is an intra-abdominal WAT depot and is generally considered to be more representative of visceral than subcutaneous adipose tissue (47). It was selected over other depots because of its mass, greater inflammatory response than subcutaneous WAT, and negative consequences on cardiometabolic health (30).
Western blot analysis was performed as previously described (53). Briefly, a portion of the tissue was homogenized in ice-cold homogenizing buffer [20 HEPES (pH 7.4), 2 EGTA, 0.2 EDTA, 100 KCl, 50 β-glycerophosphate, 50 NaF, 0.5 sodium orthovanadate, 1 benzamidine, 0.1 PMSF, and 1 DTT]. An additional centrifugation step at 10,000 g for 10 min was performed, and the liquid phase under the top layer was used in subsequent analyses. The protein concentration of each tissue homogenate was quantified (Bio-Rad Protein Assay, Hercules, CA), and SDS-PAGE was performed using equal amounts of total protein per sample. Each polyvinylidene difluoride membrane was stained with Ponceau S (Aqua Solutions, Deer Park, TX) to verify equal protein loading. Blots were then blocked in 5% nonfat dry milk and incubated overnight with primary antibody at 4°C. Antibodies included (Cell Signaling, Beverly, MA, unless otherwise noted) were 4E-BP1 (Bethyl Laboratories, Montgomery, TX), 4E-BP1 (Thr65), S6K1 (Santa Cruz Biotechnology, Santa Cruz, CA), S6K1 (Thr389), ribosomal protein S6, ribosomal protein S6 (Ser240/244), eukaryotic elongation factor (eEF)2, eEF2 (Thr56), Akt, Akt (Thr308 and Thr473), ERK, ERK1/2 (Thr202/Tyr204), 90-kDa ribosomal S6K (RSK), RSK1/2 (Ser380), eEF2 kinase (eEF2K), eEF2K (Ser366), AMP-activated kinase-α (AMPK), AMPK (Thr172), tuberous sclerosis complex 2 (TSC2), TSC2 (Thr1462 and Ser1387), Unc-51-like autophagy activating kinase 1 (ULK1), ULK1 (Ser757), p62, light chain (LC)3B, autophagy-related protein (Atg)12, Atg7, adipose tissue triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), HSL (Ser660), CCAAT enhancer-binding protein homologous protein (CHOP), peroxisome proliferator-activated receptor (PPAR)-γ, CCAAT/enhancer-binding protein (C/EBP)α, cleaved caspase-3, cleaved nuclear poly(ADP-ribose) polymerase (PARP), eIF2α (Thr56), activating transcription factor 4 (ATF4), JNK (Thr183/Tyr185), NLR pyrin domain containing 3 (NLRP3), adaptor protein containing a COOH-terminal caspase-recruitment domain (ACS; also known as TMS1), and caspase-1 (BioVision, Milpitas, CA). Blots were then washed with an appropriate secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG) and developed with enhanced chemiluminescence reagents (Pierce Chemical, Rockford, IL) according to the manufacturer’s instructions. Blots were imaged using the FluorChem (ProteinSimple, San Jose, CA), and densities in the linear range were quantified using ImageJ (NIH, Bethesda, MD). Western blots for each protein or phospho-protein were run, and data were quantified from adipose tissue obtained from all sham control (n = 10) and septic (n = 17) mice. However, for clarity and presentation purposes, representative Western blots are presented for only the sepsis-recovery study.
A portion of eWAT was homogenized and reconstituted in cold assay buffer (Proteasome Activity Fluorometric Assay Kit, BioVision), and the assay was performed exactly as previously described (33). The protein content was assayed in a sample aliquot (Bio-Rad). Each sample was measured in the presence and absence of the provided proteasome inhibitor to account for nonproteasomal degradation of the substrate and then subtracted from each measurement. Proteasome activity (in nmol·min−1·mg protein−1) was calculated by the change in the fluorescence signal, where 1 unit of activity equals 1.0 nmol of the fluorophore 7-amino-4-methylcoumarin per minute.
Total RNA was extracted using Tri-reagent (Molecular Research Center, Cincinnati, OH) and RNeasy mini kit (Qiagen, Valencia, CA) following the manufacturers’ protocols. On-column DNase I treatment was used to remove residual DNA contamination. RNA was eluted from the column with RNase-free water, and an aliquot was used for quantitation (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA). RNA quality was analyzed on a 1% agarose gel. Total RNA (1 µg) was reversed transcribed using superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed using 25 ng cDNA in a StepOnePlus system using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) using primers as described previously by our laboratory (13). The comparative quantitation method 2-ΔΔCt (where Ct is threshold cycle) was used in presenting gene expression of target genes in reference to the endogenous control. Samples for the determination of adipose tissue mRNA content in mice from both acute and recovery sepsis were run simultaneously.
Blood was collected from the abdominal aorta into heparinized syringes before euthanasia of the animals and centrifuged, and plasma cytokine concentrations were measured using a mouse-specific V-PLEX Proinflammatory assay platform (MSD, Rockville, MD); samples from mice in both the acute sepsis and sepsis-recovery studies were run at the same time for direct comparisons among all four groups.
Data and statistical analysis.
Because this study focused on the changes that occurred during sepsis-recovery, representative Western blots are presented for only this experimental group. However, bar graphs are presented for acute (24 h post-CLP) sepsis-induced changes in key signaling end points. As samples from the acute sepsis and sepsis-recovery studies were run independently (i.e., not on the same gel), the magnitude of the changes determined by Western blot analysis between these two time points are not directly comparable.
Data are presented as means ± SE with the number of mice in each group presented in the figures. Unless otherwise noted, statistical analysis of the data was performed using a two-sided t-test to compare groups (GraphPad Prism version 6.0, La Jolla, CA), and group differences were considered significant when P < 0.05. As tissue cytokine mRNA content and plasma cytokine concentrations from both the acute sepsis and sepsis-recovery studies were run simultaneously, data were analyzed on commercial statistical software (SigmaPlot, Systat, San Jose, CA) using a two-way ANOVA (sepsis × time) with a Student-Newman-Keuls post hoc test.
RESULTS
Body composition.
CLP decreased body weight by 24 h, with the peak decrement in body weight occurring between 2 and 4 days post-CLP (Fig. 1A). Thereafter, body weight gradually increased back toward basal starting values. A decrease in body weight was also seen in sham control mice because their food intake was restricted to match that of septic mice. However, the sepsis-induced decrement in body weight between days 2 and 8 (except day 5) was greater than that seen in the pair-fed control group. Whole body fat mass determined by 1H NMR revealed a comparable decrease in fat mass in both control and septic mice at day 5 (Fig. 1B). However, by day 10, whole body fat mass had returned to basal presurgery levels in control mice, but fat mass in septic mice was 35% lower than time-matched pair-fed control values. At the 10-day time point, eWAT mass normalized for body weight was reduced 48% compared with control values (Fig. 1C). This decrease was independent of food consumption as all control mice were pair fed. That is, between days 5 and 10 post-CLP, sepsis-recovery mice consumed an average of 4.2 ± 0.2 g food/day, and this amount did not differ from that consumed by these same mice before CLP (4.1 ± 0.3 g food/day) or by pair-fed nonseptic control mice during this time period (4.1 ± 0.3 g food/day).
Fig. 1.
Sepsis-induced changes in body composition. A: decrement in body weight where the starting body weight did not differ between control (27.6 ± 2.0 g) and septic (26.9 ± 1.7 g) mice. B and C: bar graphs for whole body fat mass (B) and epididymal fat mass (C) normalized for body weight in control and septic mice. CLP, cecal ligation and puncture. Values are means ± SE; n = 30 mice initially for sepsis-recovery and 10 mice for pair-fed controls. *P < 0.05 comparing groups on the same day; +P < 0.05 compared with day 0 values in the same treatment group.
Protein synthesis.
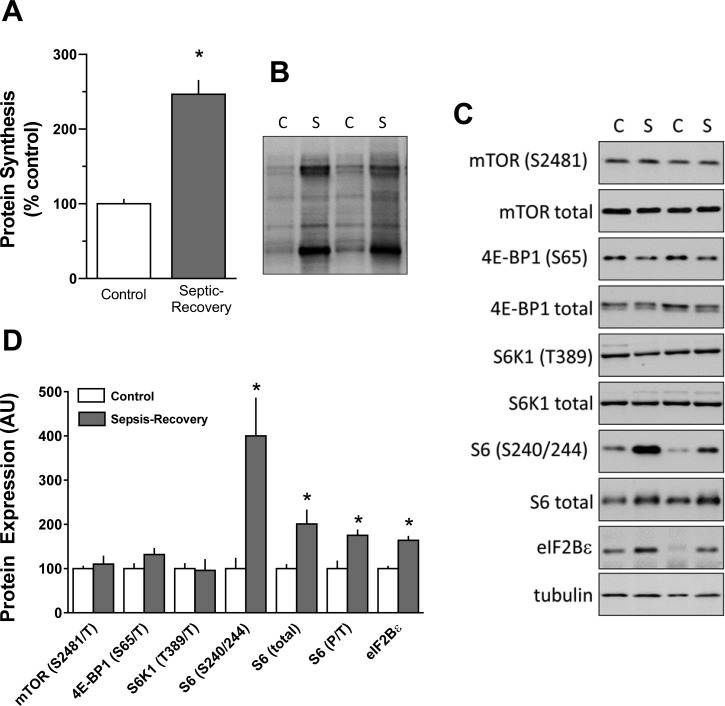
The relative rate of global in vivo protein synthesis was increased more than twofold in eWAT from mice during sepsis-recovery compared with control values (Fig. 2, A and B). To determine the mechanism for this increase, we assessed mTOR complex (mTORC)1 activity as a possible mechanism for this observed increase by determining the phosphorylation of mTOR itself as well as the phosphorylation of its known endogenous downstream substrates, S6K1 and 4E-BP1 (15). Contrary to expectations, both total and Ser2481-phosphorylated (autophosphorylation site) mTOR (Fig. 2, C and D) did not differ between control and sepsis-recovery mice. Likewise, total or phosphorylated 4E-BP1 (Ser65) and S6K1 (Thr389) (Fig. 2, C and D) were not altered during sepsis-recovery.
Fig. 2.
Protein synthesis and mammalian target of rapamycin (mTOR) proteins regulating mRNA translation in epididymal white adipose tissue (eWAT) from sepsis-recovery and pair-fed control mice. A: global protein synthesis was determined in eWAT by measuring puromycin incorporation into protein. Data are expressed as the percentage of pair-fed control values. B: representative puromycin Western blot demonstrating differences in incorporation between control (C) and sepsis-recovery (S) mice. C: representative Western blot of total and/or phosphorylated mTOR substrates between control and sepsis-recovery mice. A representative blot for loading control (tubulin) is also presented. D: values in the bar graph represent means ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. 4E-BP1, eukaryotic initiation factor (eIF)4E-binding protein-1; S6K1, S6 kinase-1; AU, arbitrary units. *P < 0.05 compared with pair-fed control values.
Although S6K1 phosphorylation was unaltered, Ser240/244 phosphorylation of ribosomal protein S6 was increased nearly fourfold during recovery from sepsis (Fig. 2, C and D). As this increase was greater than that seen for the increase in total S6, there was an approximately twofold elevation in the ratio of phosphorylated to total S6 (Fig. 2D). In contrast, there was no increase in S6 phosphorylation of Ser235/236 or its upstream kinase ERK1/2 (Thr202/Tyr204) or RSK1/2 (Ser380) (data not shown). Finally, eIF4B phosphorylation (another known protein substrate for S6K1) in eWAT did not differ between control and sepsis-recovery mice (data not shown).
eIF2B is a guanine nucleotide exchange factor for eIF2, and increases in eIF2B can drive global protein synthesis (58). Total eIF2Bε, the catalytic subunit of this multimeric protein, was increased in eWAT during recovery from sepsis (Fig. 2, C and D).
Upstream of mTORC1.
Akt phosphorylation can be differentially regulated, thereby affecting its kinase activity (35). A discordant phosphorylation pattern for Akt was detected in eWAT from sepsis-recovery mice (Fig. 3). That is, Ser473-phosphorylated Akt was decreased, whereas Thr308-phosphorylated Akt was increased, independent of a change in total Akt content. Downstream of Akt lies TSC2, which regulates mTORC1 (24). While the extent of Thr1462-phosphorylated (Akt-dependent site) TSC2 did not differ between groups, TSC2 phosphorylation at Ser1387 (AMPK-dependent site) was increased during recovery. In accord with this latter observation, AMPK activity appeared to be increased, as evidenced by the increased Thr172 phosphorylation of AMPK. Finally, the protein content for REDD1, a negative regulator of mTORC1, did not differ between control and sepsis-recovery mice (Fig. 3).
Fig. 3.

Regulators of signal transduction upstream of mTOR in eWAT from sepsis-recovery and pair-fed control mice. A: representative Western blots for selected proteins proximal to mTOR complex 1 (mTORC1) between control and sepsis-recovery mice. B: bar graph shows the quantitation of total and/or phosphorylated proteins normalized to the total amount of the respective protein or tubulin. For bar graphs, values represent means ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. TSC, tuberous sclerosis complex; AMPK, AMK-activated kinase. *P < 0.05 compared with pair-fed controls for the same protein.
Protein breakdown.
Increased activation of AMPK can stimulate the UPP (31). In vitro-determined chemotrypsin-like proteasome activity was significantly (P < 0.05) increased in eWAT from sepsis-recovery (2.44 ± 0.35 nmol·h−1·mg protein−1) compared with control (1.51 ± 0.23 nmol·h−1·mg protein−1) mice.
In other conditions, the aforementioned increase in AMPK activity coupled with unchanging mTORC1 activity is associated with enhanced autophagy (27). Hence, we assessed the phosphorylation state of ULK1, a major regulator of this degradative pathway. Phosphorylation of ULK1 at Ser555 (AMPK-dependent site) was increased, whereas Ser757 phosphorylation of ULK1 (mTOR-dependent site) was decreased in eWAT of mice recovering from sepsis (Fig. 4). Consistent with a stimulation of autophagy, beclin, Atg5/12 complex, and LC3B-II were all increased, whereas p62 content was decreased (Fig. 4).
Fig. 4.
Alterations in autophagy-related proteins in eWAT from sepsis-recovery and pair-fed control mice. A: representative Western blots for selected proteins regulating autophagy between control and sepsis-recovery mice. B: values in the bar graph represent means ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. Quantitation of total and/or phosphorylated proteins is normalized to the total amount of the respective protein or tubulin. ULK1, Unc-51-like autophagy activating kinase-1; Atg, autophagy-related protein; LC, light chain. *P < 0.05 compared with pair-fed controls for the same protein.
Sepsis can also induced ER stress, potentially leading to apoptosis and a decrease in cell number (22). During recovery from sepsis, there was an increase in both the amount of total and phosphorylated eIF2α as well as an increase in the transcription factor ATF4 compared with control values (Fig. 5). Cleaved caspase-3 and cleaved PARP were also increased nearly threefold during sepsis-recovery. In contrast, the amount of CHOP protein as well as total and phosphorylated JNK did not differ between sepsis-recovery and control mice.
Fig. 5.
Alterations in proteins related to cellular stress and apoptosis in eWAT from sepsis-recovery and pair-fed control mice. A: representative Western blots for selected stress-related proteins between control and sepsis-recovery mice. B: values in the bar graph represent mean ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. Quantitation of total and/or phosphorylated proteins is normalized to the total amount of the respective protein or tubulin. ATF, activating transcription factor; CHOP, CCAAT enhancer-binding protein homologous protein; PARP. poly(ADP-ribose) polymerase. *P < 0.05 compared with pair-fed controls for the same protein.
Inflammasome.
Persistent low-level inflammation in eWAT can potentially stimulate pyroptosis via inflammasome activation (46). Western blot analysis of key elements of the NLRP3 inflammasome, including NLRP3, TMS1 (ACS), and cleaved caspase-1, were all increased in eWAT during recovery from sepsis (Fig. 6).
Fig. 6.
Alterations in the total amount proteins related to inflammasome activation in eWAT from sepsis-recovery and pair-fed control mice. A: representative Western blots for selected proteins regulating inflammasome activity between control and sepsis-recovery mice. B: values in the bar graph represent means ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. Quantitation of the protein of interest is normalized to tubulin or actin. NLRP3, NLR pyrin domain containing 3. *P < 0.05 compared with pair-fed controls for the same protein.
Lipid metabolism.
Lipid accumulation in adipocytes represents a balance between lipolysis and lipogenesis. ATGL, a major lipase responsible for lipolysis in adipose tissue (42), was decreased during sepsis-recovery compared with time-matched pair-fed control values (Fig. 7). In contrast, there was no difference in either the total or Ser660-phosphorylated HSL in eWAT between control and sepsis-recovery mice. The relative amount of PPAR-γ and C/EBPα protein, two transcription factors regulating lipogenesis, were both decreased in during sepsis-recovery (Fig. 7). Finally, uncoupling protein (UCP)-1 was increased more than twofold in eWAT from mice recovering from sepsis.
Fig. 7.
Alterations in the total amount or phosphorylation state of proteins related to lipid metabolism in eWAT from sepsis-recovery and pair-fed control mice. A: representative Western blots for selected proteins regulating lipid metabolism between control and sepsis-recovery mice. For peroxisome proliferator-activated receptor (PPAR)-γ, the antibody recognizes both PPAR-γ1 and PPAR-γ2. B: values in the bar graph represent means ± SE for Western blot data from all sepsis-recovery (n = 17) and pair-fed control (n = 10) mice. Quantitation of total and/or phosphorylated proteins is normalized to the total amount of the respective protein or tubulin. ATGL, adipose tissue triglyceride lipase; HSL, hormone-sensitive lipase; UCP, uncoupling protein; C/EBP, CCAAT/enhancer-binding protein. *P < 0.05 compared with pair-fed controls for the same protein.
Protein and lipid metabolism in eWAT during the acute phase of sepsis.
A separate cohort of mice was included from which eWAT was obtained 24 h post-CLP to identify altered signaling networks associated with the onset of metabolic dysfunction of adipose tissue. The data from this “acute sepsis” study are shown in Fig. 8. It is important to note that we cannot directly compare the magnitude of the changes in septic mice in the two studies because samples from the two time points (i.e., 24 h and 10 days) were not run on the same gel. Representative blots are not presented for the acute sepsis study to simplify presentation of the data but are similar in quality to those illustrated for the sepsis-recovery group (data not shown).
Fig. 8.
Alterations in the total amount or phosphorylation state of proteins related to proteostasis and lipid metabolism in eWAT during the acute phase of sepsis. Bar graphs represent the quantitation for proteins that did not differ (A) or were significant altered (B) between time-matched control and septic mice at 24 h post-CLP. For bar graphs, values are means ± SE; n = 8 and 10, respectively. *P < 0.05 compared with control value for the same protein which was arbitrarily set at 100 AU for each protein.
At this early time point, there were no differences between control and septic values for global protein synthesis, 4E-BP1 and S6K1 phosphorylation, or total eIF2Bε protein content (Fig. 8A). Markers of autophagy (Ser555-phosphorylated ULK1, Atg5/12, beclin, and LC3B-II) also did not differ between control and acute sepsis mice. There was also no acute sepsis-induced change in Ser660-phosphorylated HSL.
In contrast, acute sepsis appeared to increase ER stress (eIF2α, ATF4, and phosphorylated JNK), apoptosis (caspase-3 and PARP cleavage), proteasome activity, inflammasome activity (NLRP3 and cleaved caspase-1), and lipolysis (ATGL) (Fig. 8B). Finally, acute sepsis decreased PPAR-γ and C/EBPα in eWAT compared with time-matched pair-fed control values (Fig. 8B).
Adipose tissue and plasma cytokines.
Figure 9 shows data for proinflammatory cytokine mRNA content in eWAT and plasma cytokine concentrations. Because samples from both time points were analyzed simultaneously, a direct comparison of the data for the acute sepsis and sepsis-recovery groups is possible for these end points. Acute sepsis increased mRNA expression for TNF-α, IL-1β, and IL-6 in eWAT as well as circulating plasma concentrations. For TNF-α, both the mRNA content and plasma concentration remained elevated during sepsis-recovery, but levels were decreased 66% and 97%, respectively, compared with 24-h post-CLP values. For IL-β, the sepsis-induced increase in mRNA content in eWAT was comparable during the acute and sepsis-recovery phases; however, the plasma IL-1β concentration did not differ between control and septic mice during recovery. For IL-6, the mRNA content of eWAT had returned to control values during sepsis-recovery. Although the plasma IL-6 concentration remained significantly elevated during sepsis-recovery, compared with time-matched control values, this value represented a >99.5% reduction from the sepsis-induced increase in circulating IL-6 protein seen during the acute phase of sepsis.
Fig. 9.
TNF-α, IL-1β, and IL-6 mRNA content in eWAT and protein concentrations in plasma from mice after acute sepsis or sepsis-recovery. Samples from mice with acute sepsis were obtained 24 h post-CLP in the fasted condition, whereas samples from mice during recovery from sepsis were collected at day 10 in the freely fed condition where food consumption did not differ between groups. For mRNA content (left), samples were quantitated by quantitative RT-PCR and normalized to L32, which did not differ with treatment; the plasma cytokine concentrations were determined by multiplex array (right). All values are means ± SE; n = 8 and 10 (control and septic, respectively) mice for acute sepsis; n = 17 and 10 (control and sepsis, respectively) mice for sepsis-recovery. Because the assays for the samples from both the acute sepsis and sepsis-recovery studies were run simultaneously, two-way ANOVA was performed for data analysis, with a subsequent Student-Newman-Keuls test for significance. a−dFor each parameter, means with different letter are significantly different (P < 0.05); means with the same letter did not differ statistically.
DISCUSSION
The present study elucidated the molecular signature in eWAT that ensues rapidly within the first 24 h after CLP as well as the signaling changes that manifest during the recovery phase preventing the normal repletion of adipose tissue. Our data indicate that eWAT responds acutely to infection with an increase in inflammasome and UPP activity as well as enhanced apoptosis. Moreover, despite the removal of the infectious nidus and the general recovery process, eWAT remains a site of continued inflammation with activation of apoptosis, UPP, and autophagy. In addition, UCP-1 is increased during recovery, suggesting a “browning” of this white adipose tissue depot. These data have been used to model the signaling network in eWAT by which sepsis produces lipoatrophy and slows adipose tissue accretion during recovery (Fig. 10).
Fig. 10.
Schematic signal transduction network representing the observed changes in protein and lipid metabolism in eWAT during sepsis-recovery. Abbreviations are identified within the text. Bold arrows indicate the activation of selected pathway components; red stars indicate an increase phosphorylation and the presumed activation of the respective substrates; light colored circles indicate phosphorylation sites not altered during sepsis-recovery; dark blue stars indicate a decrease in phosphorylation. Note that the illustration does not depict the intracellular localization of proteins and metabolic pathways and does not identify the cell type within adipose tissue where the sepsis-induced changes occur.
We originally posited that sepsis would acutely decrease mTOR-dependent protein synthesis in eWAT, similar to that seen in muscle (32), with the synthetic rate returning back to basal levels during recovery. However, eWAT protein synthesis was unaltered at the 24-h time point and was twofold greater during the recovery phase. In contrast to sepsis-induced changes in protein synthesis in other tissues, the increased protein synthesis in eWAT during sepsis-recovery was independent of mTORC1 (e.g., no hyperphosphorylation of S6K1 and 4E-BP1) and most likely mediated by the concomitant increase in eIF2Bε, which is consistent with its ability to stimulate translation initiation (58). As increased protein synthesis is associated with adipocyte hypertrophy (26), this response may support the restoration of adipocyte structure and function. Alternatively, an elevated protein synthetic rate may also be deleterious as it might also lead to the accumulation of nonstoichiometric amounts of either normal or damaged proteins stimulating the ER stress response and proteasomal degradation (discussed below).
The UPP is critical for the removal of intracellular proteins and is activated in other tissues during sepsis (14). UPP activity in eWAT was increased as early as 24 h post-CLP and remained elevated during recovery. Such an increase would be important for removal of damaged cellular proteins. Alternatively, because UPP activity is highest during the early stages of preadipocyte differentiation (48), our data may suggest an increased differentiation of adipose-derived stem cells.
The unfolded protein response is a cellular adaptation activated by ER stress (50). Activation of PRKR-like ER kinase (PERK) promotes the phosphorylation of eIF2α, thereby increasing ATF4 and the translation of selected mRNAs. At both the 24-h and 10-day time points, the amount of eIF2α (total and phosphorylated) and ATF4 was increased, suggesting a sustained elevation in ER stress. An increase in the PERK pathway has also been reported in adipocytes cultured with endotoxin (2) as well as in WAT in rats 24-h post-CLP (9) and from burn patients (8). Misfolded proteins can be shuttled to the cytosol and degraded by the proteasome, and this process is referred to as ER-associated degradation (6); such a process may explain the coordinated upregulation of the unfolded protein response and proteasome activity. This stress response may be induced in part by the increased demand for protein synthesis, the increased release of fatty acids, and/or the continued elevation in one or more of the various proinflammatory cytokines.
The decrease in p62 protein as well as the increases in LC3B-II, Atg5/12 complex, and beclin during the recovery phase of sepsis are consistent with increased autophagy. However, in contrast to the changes detected for the UPP and apoptosis (see below), increased autophagy was restricted to the recovery phase and was not detected acutely at the 24-h time point after CLP. Protein recycling via autophagy is tightly regulated by the phosphorylation of ULK1, which is governed by the interplay between mTORC1 and AMPK (27). Activation of the latter kinase during sepsis-recovery was evidenced by the enhanced Thr172 phosphorylation of AMPK and the parallel increase in Ser555 phosphorylation of ULK1 (AMPK dependent). Conversely, autophagy can be inhibited by mTORC1activation, which phosphorylates Ser757 on ULK1 (27). Hence, the reduction in Ser757-phosphorylated ULK1 is consistent with an mTORC1-independent induction of autophagy. The molecular mechanisms linking autophagy with ER stress are varied, but increased Atg gene expression can be driven by activation of the PERK/eIF2α/ATF4 pathway (21).
Apoptosis is a normal physiological process regulating adipose tissue mass (22). The observed increase in cleaved caspase-3, a common apoptotic pathway effector, and the concomitant increase in PARP cleavage are strongly suggestive of activation of the apoptosis cascade. However, future studies should endeavor to confirm apoptotic cell death using validated morphological and cytochemical techniques. The apoptotic phenotype was associated with increases in eIF2α and ATF4 but independent of JNK activation (phosphorylation) or an increase in CHOP. Increased inflammasome activity was present during both acute sepsis and recovery, as evidenced by the increase in NLRP3, TMS1, and cleaved caspase-1. A comparable response has been previously reported in humans after burn injury (52). Although adipocytes represent ≈90% of total cellular volume in whole adipose tissue (30), we cannot definitively conclude whether inflammasome activation detected in whole WAT was specific to adipocytes or was localized to stromal and/or infiltrating inflammatory cells (52). Hence, it is possible that the death of infiltrating immune cells my contribute at least in part to increased apoptosis, as seen in other organs (23). The early and sustained increases in apoptosis, UPP, unfolded protein response, and inflammasome activity were temporally associated with increases in TNF-α and IL-1β mRNA content in eWAT. It was particularly striking that the magnitude of the sepsis-induced increase in IL-1β in eWAT was comparable during both the acute and recovery phases of sepsis. This may be a manifestation of the early and sustained increased inflammasome activity in adipocytes, the stromal fraction, and/or infiltrating macrophages that generate IL-1β (30, 46, 52). In contrast, while plasma concentrations of IL-6 and TNF-α remained statistically elevated during the recovery period, levels were reduced by >95% compared with those detected during the acute phase, and the physiological importance of this low residual inflammation remains to be assessed. Additional studies are required to delineate which of these cytokines are responsible for activation of these degradative pathways.
Sepsis enhances adipose tissue lipolysis, and lipids are the preferred oxidative fuel for whole body energy balance during this condition (49). However, lipotoxicity can result when fatty acid release from adipose tissue exceeds the metabolic capacity of peripheral tissues, leading to lipid accumulation, cellular dysfunction, and potential cell death. We are not aware of previously published data on ATGL and HSL in WAT in response to sepsis per se, although total HSL has been reported to by decreased acutely by endotoxin (20). ATGL was increased 24 h post-CLP and decreased during sepsis-recovery. As ATGL is the predominant triglyceride lipase in fat, having an essential role in regulating basal and stimulated lipolysis (55), these data are consistent with the increased adipose tissue lipolysis and fatty acid efflux seen during the early phase of sepsis in humans (18). Two lipogenic protein transcription factors (PPAR-γ and C/EBPα) in WAT were also decreased acutely after CLP, and this decrease was sustained during the recovery phase. Likewise, C/EBPα and PPAR-γ have been reported to be acutely decreased in a JNK-dependent manner when adipose tissue was incubated ex vivo with endotoxin (59). Although mTORC1 plays a key role in adipogenesis, as evidenced by the ability of the mTOR inhibitor rapamycin to block adipogenesis and the expression of PPAR-γ and C/EBPα (28), such a mechanism does not appear operational in the current experimental context.
Upregulation of UCP-1 is indicative of a switch in the WAT phenotype in a process termed “browning” (51). Such an increase in UCP-1 in subcutaneous fat has been observed after burn injury (43) and cancer cachexia (44) but not previously in sepsis. While we have provided molecular evidence supporting browning, future studies will need to provide morphological evidence as well as to directly determine thermogenesis. Moreover, because of the well-established ability of UCP-1 to uncouple mitochondrial ATP synthesis, such a transition in WAT may in part be responsible for the elevated rate of whole body O2 consumption in septic (4) and burn patients (43) and may be responsible for the elevated rate of thermogenesis (3). Although the mechanism for the sepsis-induced browning was not elucidated, systemic inflammation and IL-6 mediate this transition in cancer cachexia and are in part responsible for the increase in lipid mobilization (44). As plasma IL-6 levels are increased acutely after CLP and remain elevated after 10 days, this mechanism may also explain in part the browning of WAT during sepsis. In the current model, the sepsis-induced increase in ER stress precedes WAT browning and is consistent with data suggesting that increased ER stress governs the transcriptional induction of UCP-1 in brown adipocytes (5).
Perspectives and Significance
Adipose tissue exerts dynamic metabolic control at the whole body and tissue levels by its release of fatty acids and an expanding array of hormones and inflammatory cytokines. Dysregulation of this tissue, especially visceral or intraabdominal fat, has been implicated in the pathogenesis of disease-related complications (e.g., hepatic steatosis and insulin resistance) that worsen outcomes in critical ill patients (41). Currently, there are few data pertaining to the metabolic dysfunction of WAT during the acute and recovery phases of sepsis, and our present results reveal the temporally dependent interconnected multilevel network governing proteostasis in eWAT. The acute response was characterized by an increase in ER stress and an extensive proinflammatory response, changes associated with increased proteasome activity and apoptosis in eWAT. Despite diminution of the local and systemic cytokine response, inflammasome activity remained elevated during recovery and was associated with a sustained elevation in proteasome activity and apoptosis as well as autophagy. Our data highlight the dynamic regulation of adipose tissue phenotype that leads to the original atrophy of WAT during early sepsis and the failure to replenish fat stores during convalescence. Further studies are needed to define the mechanisms for these temporally dependent metabolic changes and to assess whether they are causally related to damaging metabolic and functional changes in distant peripheral tissues, such as the liver and skeletal muscle. We speculate that by so doing novel therapeutic targets may be identified that have the potential to lessen the initial adipose tissue atrophy and thereby the detrimental effects of lipid overload in nonadipose tissues.
GRANTS
This work was supported by National Institutes of Health Grant GM-38032 (to C. H. Lang) and by National Institutes of Health postdoctoral fellowship award F32-GM-112401 (to K. T. Crowell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.T.C., D.I.S., and C.H.L. conceived and designed research; K.T.C. and C.H.L. performed experiments; K.T.C. and C.H.L. analyzed data; K.T.C., D.I.S., and C.H.L. interpreted results of experiments; K.T.C. and C.H.L. prepared figures; K.T.C., D.I.S., and C.H.L. drafted manuscript; K.T.C., D.I.S., and C.H.L. edited and revised manuscript; K.T.C., D.I.S., and C.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Maithili Navaratnarajah and Anne Pruznak for the excellent technical assistance.
REFERENCES
- 1.Abdullahi A, Jeschke MG. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab 27: 542–552, 2016. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhusaini S, McGee K, Schisano B, Harte A, McTernan P, Kumar S, Tripathi G. Lipopolysaccharide, high glucose and saturated fatty acids induce endoplasmic reticulum stress in cultured primary human adipocytes: Salicylate alleviates this stress. Biochem Biophys Res Commun 397: 472–478, 2010. doi: 10.1016/j.bbrc.2010.05.138. [DOI] [PubMed] [Google Scholar]
- 3.Arnold J, Little RA, Rothwell NJ. Energy balance and brown adipose tissue thermogenesis during chronic endotoxemia in rats. J Appl Physiol 66: 1970–1975, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Arnold J, Shipley KA, Scott NA, Little RA, Irving MH. Thermic effect of parenteral nutrition in septic and nonseptic individuals. Am J Clin Nutr 50: 853–860, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Asada R, Kanemoto S, Matsuhisa K, Hino K, Cui M, Cui X, Kaneko M, Imaizumi K. IRE1α-XBP1 is a novel branch in the transcriptional regulation of Ucp1 in brown adipocytes. Sci Rep 5: 16580, 2015. doi: 10.1038/srep16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avci D, Lemberg MK. Clipping or extracting: two ways to membrane protein degradation. Trends Cell Biol 25: 611–622, 2015. doi: 10.1016/j.tcb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard PG, Festuccia WT, Houde VP, St-Pierre P, Brûlé S, Turcotte V, Côté M, Bellmann K, Marette A, Deshaies Y. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J Lipid Res 53: 1117–1125, 2012. doi: 10.1194/jlr.M021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovic E, Kraus N, Patsouris D, Diao L, Wang V, Abdullahi A, Jeschke MG. Endoplasmic reticulum stress in adipose tissue augments lipolysis. J Cell Mol Med 19: 82–91, 2015. doi: 10.1111/jcmm.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calisto KL, Camacho AC, Mittestainer FC, Carvalho BM, Guadagnini D, Carvalheira JB, Saad MJ. Diacerhein attenuates the inflammatory response and improves survival in a model of severe sepsis. Crit Care 16: R158, 2012. doi: 10.1186/cc11478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 59: 775–781, 2010. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung YM, Joham A, Marks S, Teede H. The obesity paradox: an endocrine perspective. Intern Med J. In press. doi: 10.1111/imj.13257. [DOI] [PubMed] [Google Scholar]
- 12.Crowell KT, Soybel DI, Lang CH. Restorative mechanisms regulating protein balance in skeletal muscle during recovery from sepsis. Shock. In press. doi: 10.1097/SHK.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowell KT, Steiner JL, Coleman CS, Lang CH. Decreased whole-body fat mass produced by chronic alcohol consumption is associated with activation of S6K1-mediated protein synthesis and increased autophagy in epididymal white adipose tissue. Alcohol Clin Exp Res 40: 1832–1845, 2016. doi: 10.1111/acer.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlmann B. Role of proteasomes in disease. BMC Biochem 8, Suppl 1: S3, 2007. doi: 10.1186/1471-2091-8-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13: 252–259, 2007. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333: 233–238, 2011. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 17.Defazio J, Fleming ID, Shakhsheer B, Zaborina O, Alverdy JC. The opposing forces of the intestinal microbiome and the emerging pathobiome. Surg Clin North Am 94: 1151–1161, 2014. doi: 10.1016/j.suc.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forse RA, Leibel R, Askanazi J, Hirsch J, Kinney JM. Adrenergic control of adipocyte lipolysis in trauma and sepsis. Ann Surg 206: 744–751, 1987. doi: 10.1097/00000658-198712000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011. doi: 10.1152/physiol.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Gao S, Liu Z, Zhao R, Yang X. Alpha-lipoic acid alleviates acute inflammation and promotes lipid mobilization during the inflammatory response in white adipose tissue of mice. Lipids 51: 1145–1152, 2016. doi: 10.1007/s11745-016-4185-2. [DOI] [PubMed] [Google Scholar]
- 21.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 22.Herold C, Rennekampff HO, Engeli S. Apoptotic pathways in adipose tissue. Apoptosis 18: 911–916, 2013. doi: 10.1007/s10495-013-0848-0. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822, 2006. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 24.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke MG, Finnerty CC, Emdad F, Rivero HG, Kraft R, Williams FN, Gamelli RL, Gibran NS, Klein MB, Arnoldo BD, Tompkins RG, Herndon DN. Mild obesity is protective after severe burn injury. Ann Surg 258: 1119–1129, 2013. doi: 10.1097/SLA.0b013e3182984d19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao Q, Pruznak AM, Huber D, Vary TC, Lang CH. Castration differentially alters basal and leucine-stimulated tissue protein synthesis in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 297: E1222–E1232, 2009. doi: 10.1152/ajpendo.00473.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JE, Chen J. regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 53: 2748–2756, 2004. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- 29.Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 15: 277–287, 2014. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 30.Koenen TB, Stienstra R, van Tits LJ, Joosten LA, van Velzen JF, Hijmans A, Pol JA, van der Vliet JA, Netea MG, Tack CJ, Stalenhoef AF, de Graaf J. The inflammasome and caspase-1 activation: a new mechanism underlying increased inflammatory activity in human visceral adipose tissue. Endocrinology 152: 3769–3778, 2011. doi: 10.1210/en.2010-1480. [DOI] [PubMed] [Google Scholar]
- 31.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292: E1555–E1567, 2007. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 33.Lang CH, Korzick DH. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol 306: R23–R33, 2014. doi: 10.1152/ajpregu.00414.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol 19: R1046–R1052, 2009. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res 2: 19–42, 2010. [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr 131: 861S–865S, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab 283: E824–E835, 2002. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- 38.Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab 283: E503–E513, 2002. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- 39.Marques M, Perre S, Aertgeerts A, Derde S, Güiza F, Casaer MP, Hermans G, Van den Berghe G, Langouche L. Critical illness induces nutrient-independent adipogenesis and accumulation of alternatively activated tissue macrophages. Crit Care 17: R193, 2013. doi: 10.1186/cc12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittwede PN, Clemmer JS, Bergin PF, Xiang L. Obesity and critical illness: insights from animal models. Shock 45: 349–358, 2016. doi: 10.1097/SHK.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso G, Cassader M, Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov 15: 249–274, 2016. doi: 10.1038/nrd.2015.3. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen TS, Jessen N, Jørgensen JO, Møller N, Lund S. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol 52: R199–R222, 2014. doi: 10.1530/JME-13-0277. [DOI] [PubMed] [Google Scholar]
- 43.Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Reports 13: 1538–1544, 2015. doi: 10.1016/j.celrep.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20: 433–447, 2014. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Plank LD, Connolly AB, Hill GL. Sequential changes in the metabolic response in severely septic patients during the first 23 days after the onset of peritonitis. Ann Surg 228: 146–158, 1998. doi: 10.1097/00000658-199808000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Próchnicki T, Mangan MS, Latz E. Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Res 5: 1469, 2016. doi: 10.12688/f1000research.8614.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sackmann-Sala L, Berryman DE, Lubbers ER, Zhang H, Vesel CB, Troike KM, Gosney ES, List EO, Kopchick JJ. Age-related and depot-specific changes in white adipose tissue of growth hormone receptor-null mice. J Gerontol A Biol Sci Med Sci 69: 34–43, 2014. doi: 10.1093/gerona/glt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakamoto K, Sato Y, Sei M, Ewis AA, Nakahori Y. Proteasome activity correlates with male BMI and contributes to the differentiation of adipocyte in hADSC. Endocrine 37: 274–279, 2010. doi: 10.1007/s12020-009-9298-4. [DOI] [PubMed] [Google Scholar]
- 49.Samra JS. Sir David Cuthbertson Medal Lecture. Regulation of lipid metabolism in adipose tissue. Proc Nutr Soc 59: 441–446, 2000. doi: 10.1017/S0029665100000604. [DOI] [PubMed] [Google Scholar]
- 50.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833: 3460–3470, 2013. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seale P, Lazar MA. Brown fat in humans: turning up the heat on obesity. Diabetes 58: 1482–1484, 2009. doi: 10.2337/db09-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanojcic M, Chen P, Harrison RA, Wang V, Antonyshyn J, Zúñiga-Pflücker JC, Jeschke MG. Leukocyte infiltration and activation of the NLRP3 inflammasome in white adipose tissue following thermal injury. Crit Care Med 42: 1357–1364, 2014. doi: 10.1097/CCM.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steiner JL, Crowell KT, Kimball SR, Lang CH. Disruption of REDD1 gene ameliorates sepsis-induced decrease in mTORC1 signaling but has divergent effects on proteolytic signaling in skeletal muscle. Am J Physiol Endocrinol Metab 309: E981–E994, 2015. doi: 10.1152/ajpendo.00264.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steiner JL, Lang CH. Sepsis attenuates the anabolic response to skeletal muscle contraction. Shock 43: 344–351, 2015. doi: 10.1097/SHK.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsoli M, Swarbrick MM, Robertson GR. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin Cell Dev Biol 54: 68–81, 2016. doi: 10.1016/j.semcdb.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 56.Utzolino S, Ditzel CM, Baier PK, Hopt UT, Kaffarnik MF. The obesity paradox in surgical intensive care patients with peritonitis. J Crit Care 29: e881–e885, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Wacharasint P, Boyd JH, Russell JA, Walley KR. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care 17: R122, 2013. doi: 10.1186/cc12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wortham NC, Proud CG. eIF2B: recent structural and functional insights into a key regulator of translation. Biochem Soc Trans 43: 1234–1240, 2015. doi: 10.1042/BST20150164. [DOI] [PubMed] [Google Scholar]
- 59.Xiang X, An W, Jiang C, Zhao J, Wang X, Sun G, Li Y, Zhang W. Lipopolysaccharide inhibits the expression of resistin in adipocytes. J Mol Endocrinol 51: 287–299, 2013. doi: 10.1530/JME-13-0117. [DOI] [PubMed] [Google Scholar]
- 60.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun 74: 5227–5235, 2006. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS One 11: e0163677, 2016. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]