Abstract
This study investigated the role of γ-aminobutyric acid subtype B (GABAB) receptors in tibial and pudendal neuromodulation of bladder overactivity induced by intravesical administration of dilute (0.5%) acetic acid (AA) in α-chloralose-anesthetized cats. To inhibit bladder overactivity, tibial or pudendal nerve stimulation (TNS or PNS) was applied at 5 Hz and two or four times threshold (T) intensity for inducing toe or anal sphincter twitch. TNS at 2T or 4T intensity significantly (P < 0.05) increased the bladder capacity to 173.8 ± 16.2 or 198.5 ± 24.1%, respectively, of control capacity. Meanwhile, PNS at 2T or 4T intensity significantly (P < 0.05) increased the bladder capacity to 217 ± 18.8 and 221.3 ± 22.3% of control capacity, respectively. CGP52432 (a GABAB receptor antagonist) at intravenous dosages of 0.1–1 mg/kg completely removed the TNS inhibition in female cats but had no effect in male cats. CGP52432 administered intravenously also had no effect on control bladder capacity or the pudendal inhibition of bladder overactivity. These results reveal a sex difference in the role of GABAB receptors in tibial neuromodulation of bladder overactivity in cats and that GABAB receptors are not involved in either pudendal neuromodulation or irritation-induced bladder overactivity.
Keywords: GABA, bladder, neuromodulation, cat
overactive bladder (OAB) is clinical diagnosis characterized by urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence. It is a prevalent disorder affecting as many as 33–43% of women and 15–27% of men worldwide and has a significant economic and clinical impact (1, 5, 12, 14). First line management of OAB is pharmacological usually with anticholinergics or more recently with β3-adrenergic agonists. Pharmacological management has traditionally been plagued by poor efficacy and/or substantial side effects so alternative therapies are needed (2, 4). Sacral neuromodulation has been used clinically as second line therapy for more than a decade and more recently tibial and pudendal neuromodulation have also been shown to be effective in the management of OAB (24–26, 34, 35). Despite the clinical success, substantial questions about the mechanisms of action for bladder neuromodulation still remain (6).
We hypothesize that stimulation of tibial or pudendal nerves delays the initiation of reflex micturition by sending action potentials in sensory nerves to the central nervous system and causing the release of inhibitory neurotransmitters. Our previous studies in cats have shown that pudendal and tibial neuromodulation utilize different neurotransmitters. GABAA receptors are important in pudendal neuromodulation (36), while opioid receptors are not involved (19, 27). In contrast, opioid receptors play an important role in tibial neuromodulation (30). In this study, we examined the role of GABAB receptors in pudendal and tibial neuromodulation of bladder overactivity in cats.
GABA, an inhibitory neurotransmitter, has been implicated in the neural control of micturition in animals (2). It has been shown that baclofen (a GABAB receptor agonist) induces both spinal and supraspinal inhibition of micturition in rats (11). A further study extended this observation and showed GABAB agonists attenuated bladder overactivity in rats and suggested that this mechanism could potentially be useful therapeutically for treating bladder disorders caused by C-fiber afferent activation (23). Based on these previous studies, we hypothesize that tibial or pudendal neuromodulation may activate the inhibitory GABAB mechanism to inhibit bladder overactivity. In this study using anesthetized cats, dilute (0.5%) acetic acid (AA) was infused to irritate the bladder, activate the nociceptive bladder C-fiber afferents and induce bladder overactivity. Stimulation of tibial or pudendal nerves was used to inhibit the bladder overactivity. CGP25432 (a GABAB receptor antagonist) was administered intravenously to determine if it blocked the inhibition and if GABAB receptors are involved in tibial or pudendal neuromodulation.
METHODS
The protocol and animal use in this study were approved by Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedures.
A total of 20 cats (10 males and 10 females, 2.6–4.3 kg; Liberty Research, Waverly, NY) were used in this study. The animals were initially anesthetized with isoflurane (2–5% in O2) during surgery. The left cephalic vein was catheterized for intravenous administration of drugs and fluids. A tracheostomy was performed and an endotracheal tube was inserted to maintain the airway open. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the ear. A laparotomy was performed and the ureters were isolated, tied, and cut for external drainage. Via this laparotomy incision, a double lumen catheter was inserted into the bladder through a small incision in the proximal urethra ~15 mm from the bladder neck and secured by a ligature around the urethra close to the incision. One lumen of the catheter was connected to a pump to slowly (1–2 ml/min) infuse saline or 0.5% AA. This AA concentration was shown previously to be effective in irritating the bladder and reducing bladder capacity in cats (15). The other lumen was attached to a pressure transducer for recording bladder pressure. The abdominal fascia and skin were closed by sutures. The right pudendal nerve was isolated in the sciatic notch in 10 cats (5 female and 5 male) for pudendal nerve stimulation (PNS), and the left tibial nerve was isolated on the medial side of the hindlimb above the ankle in another 10 cats (5 female and 5 male) for tibial nerve stimulation (TNS). Tripolar cuff electrodes (NC223pt; MicroProbe, Gaithersburg, MD) were placed around the pudendal and tibial nerves and connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA). Anesthesia was then switched to α-chloralose (initial 65 mg/kg followed by supplemental dosing as needed) during data collection. Although it is known that α-chloralose can change the micturition reflex (17, 28), our previous studies (7, 16, 19, 21, 27, 30, 31, 36, 37) have shown that the micturition reflex is stable for many hours after the cats are anesthetized with this agent. The temperature of the animal was maintained at 36–38°C using a heating pad during the experiments.
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width), which have been shown to be effective in inhibiting the bladder reflex (16, 30), were delivered to the tibial or pudendal nerve via the cuff electrode. The threshold intensities (T) for inducing toe or anal sphincter twitch were determined by gradually increasing the stimulation intensity. Based on our previous studies (16, 31) stimulation intensities of 2T and 4T for both TNS and PNS were used in this study to inhibit the bladder activity.
At the beginning of each experiment, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that was defined as the bladder volume threshold to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). The multiple CMGs ensured the reproducibility of the saline control bladder capacity. Then, 0.5% AA was infused into the bladder to irritate the bladder and induce bladder overactivity that was indicated by a significantly reduced bladder capacity. A smaller bladder capacity produces a higher voiding frequency which is one of the symptoms of OAB. Once the control bladder capacity stabilized during repeated AA CMGs, the animals were divided into two groups (TNS and PNS groups).
In the TNS group of 10 cats (5 male and 5 female), four AA CMGs were performed: 1) control CMG without stimulation; 2) CMG during 2T TNS; 3) CMG during 4T TNS; and 4) control CMG again to evaluate a poststimulation effect. Then, cumulative doses (0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg) of CGP52432 (a GABAB receptor antagonist; Tocris, Bristol, UK) were administered intravenously. Ten minutes after each dose of drug, the four CMGs (control, 2T TNS, 4T TNS, and control) were repeated. The same drug testing protocol was also used in the PNS group of another 10 cats (5 male and 5 female). A waiting period of 2–3 min was used between CMGs for the bladder reflex to recover.
Data analysis.
The bladder capacity was measured for each CMG and normalized to the capacity measurement of the initial AA control CMG. Repeated measurements (2–3 CMGs) of AA control bladder capacity in the same animal were averaged. The normalized data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by Bonferroni multiple comparisons following ANOVA. Two-way ANOVA was performed with CGP52432 as one factor (6 levels of dosage: 0–1 mg/kg) and CMG condition as another factor (3 levels: control, 2T, and 4T) to detect the significant difference between bladder capacities at each dosage of the drug (see Figs. 2–4 and 6–8). One-way ANOVA was performed for each CMG condition (control, 2T or 4T) to detect significant difference of bladder capacities among six different doses of CGP52432 (see Fig. 2). Before drug treatment, one-way ANOVA was also performed to detect any significant difference of bladder capacities measured under different CMG conditions (4 levels: control, 2T, 4T, and postcontrol) (see Figs. 1 and 5), while two-way ANOVA was performed to determine any difference between genders with CMG condition as one factor (4 levels: control, 2T, 4T, and postcontrol) and gender as another factor (2 levels: male and female). The significant reduction of bladder capacity by AA irritation was detected by paired t-test (P < 0.05).
Fig. 2.
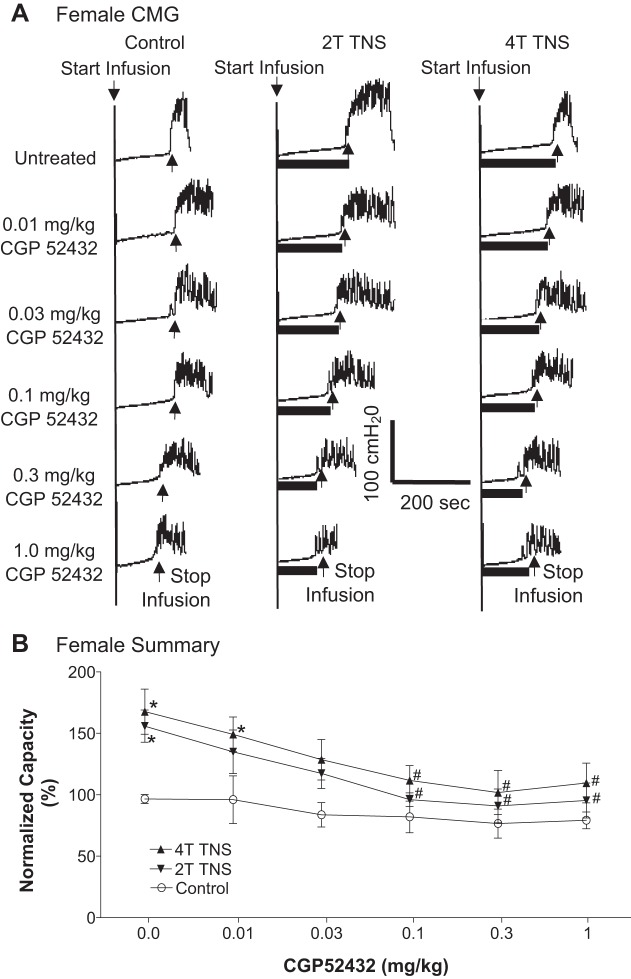
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS) in female cats. A: repeated CMGs at different cumulative doses of CGP52432 were performed during acetic acid (AA) infusion with or without TNS. Black bars under the pressure trace indicate TNS duration. TNS: 5 Hz, 0.2 ms, T = 3 V. Infusion rate = 2 ml/min. B: normalized bladder capacity measured under different conditions (n = 5 cats). *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA). #Significantly (P < 0.05, Bonferroni comparisons) different from the bladder capacity measured during TNS before CGP52432 treatment (one-way ANOVA).
Fig. 4.
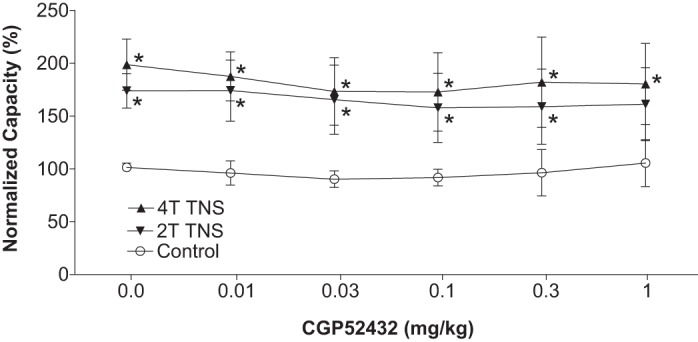
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS) in cats of different genders (n = 5 female + 5 male). Normalized bladder capacity measured under different conditions. *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA).
Fig. 6.
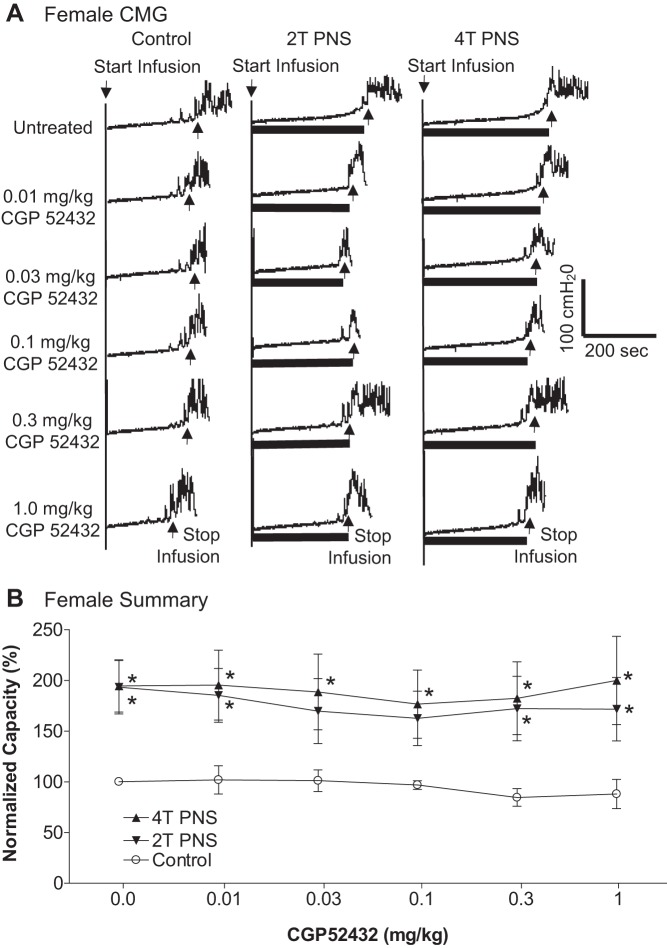
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by pudendal nerve stimulation (PNS) in female cats. A: repeated CMGs at different cumulative doses of CGP52432 were performed during acetic acid (AA) infusion with or without PNS. Black bars under the pressure trace indicate PNS duration. PNS: 5 Hz, 0.2 ms, T = 0.42 V. Infusion rate = 2 ml/min. B: normalized bladder capacity measured under different conditions (n = 5 cats). *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA).
Fig. 8.
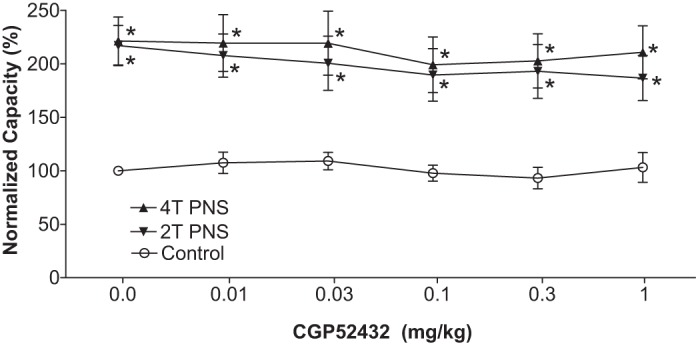
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by pudendal nerve stimulation (PNS) in cats of different genders (n = 5 female + 5 male). Normalized bladder capacity measured under different conditions. *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA).
Fig. 1.
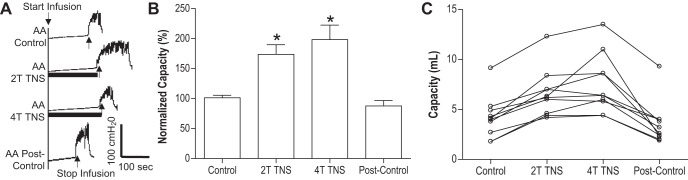
Tibial nerve stimulation (TNS) inhibited the bladder overactivity induced by acetic acid (AA) irritation. A: representative cystometrogram (CMG) tracings with/without TNS during AA infusion into the bladder. Black bars under the pressure trace indicate TNS duration. TNS: 5 Hz, 0.2 ms, T = 3 V. Infusion rate = 2 ml/min. B: normalized bladder capacities measured during CMGs with/without TNS (n = 10 cats). The capacity was normalized to the AA control CMG. *Significantly (P < 0.05, Bonferroni comparisons) different from the control (one-way ANOVA). C: nonnnormalized bladder capacities for every animal.
Fig. 5.
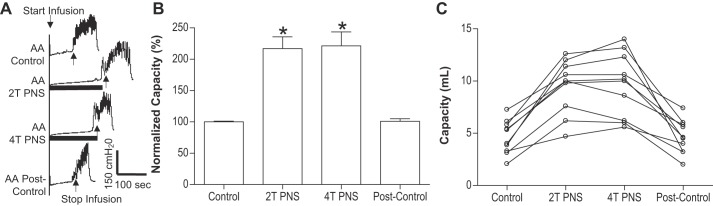
Pudendal nerve stimulation (PNS) inhibited the bladder overactivity induced by acetic acid (AA) irritation. A: representative CMG tracings with/without PNS during AA infusion into the bladder. Black bars under the pressure trace indicate PNS duration. PNS: 5 Hz, 0.2 ms, T = 0.75 V. Infusion rate = 2 ml/min. B: normalized bladder capacities measured during CMGs with/without PNS (n = 10 cats). The capacity was normalized to the AA control CMG. *Significantly (P < 0.05, Bonferroni comparisons) different from the control (one-way ANOVA). C: nonnormalized bladder capacities for every animal.
RESULTS
Effect of CGP52432 on TNS inhibition of bladder overactivity.
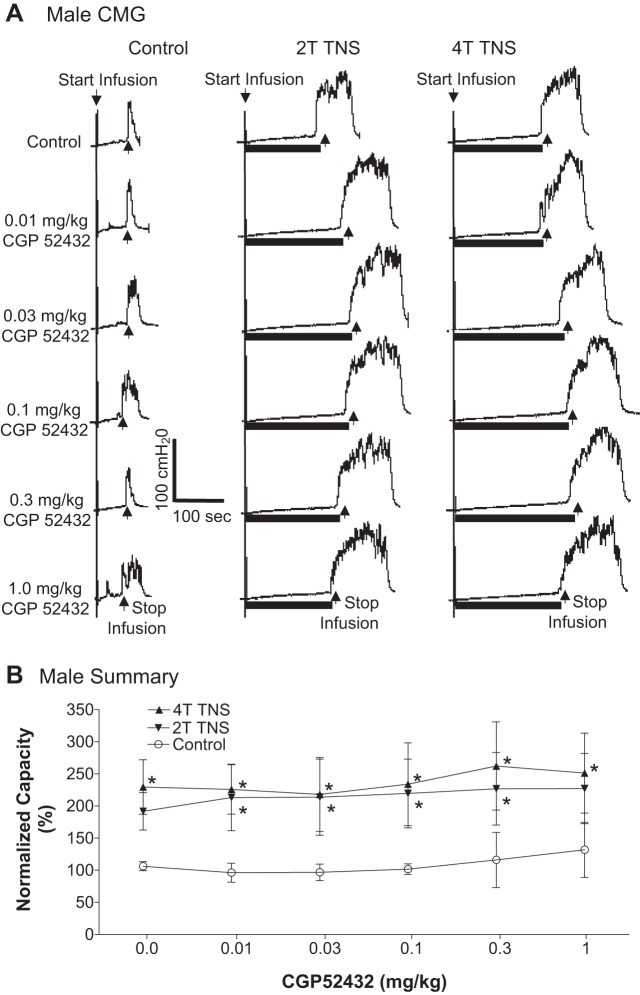
In the TNS group, intravesical infusion of dilute (0.5%) AA irritated the bladder, induced bladder overactivity, and significantly (P < 0.0001) reduced bladder capacity to 4.1 ± 0.7 ml from 9.6 ± 1.1 ml measured during saline CMGs. TNS inhibited bladder overactivity induced by AA irritation and significantly (P < 0.05) increased bladder capacity to 173.8 ± 16.2% (6.6 ± 0.7 ml) or 198.5 ± 24.1% (7.5 ± 0.9 ml) of AA control capacity, respectively, at 2T or 4T stimulation intensities (Fig. 1). The amplitude and duration of bladder contractions were also significantly increased after TNS (see CMG traces in every figure). However, these increases are likely due to the larger bladder volumes during neuromodulation rather than a direct effect of TNS. Poststimulation AA control capacity was not different from prestimulation AA control demonstrating that there was no poststimulation effect (Fig. 1). When the data in Fig. 1 were separated into male and female groups, no difference could be detected between the two genders (P > 0.05, Bonferroni multiple comparisons following two-way ANOVA). However, CGP52432 at doses of 0.1 – 1.0 mg/kg iv completely (P < 0.05) removed the inhibition induced by 2T and 4T TNS in female cats (n = 5, Fig. 2) but had no effect in male cats (n = 5, Fig. 3). When all cats were analyzed regardless of gender as a single group, the reduction in inhibition was not significant (n = 10, Fig. 4).
Fig. 3.
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS) in male cats. A: repeated CMGs at different cumulative doses of CGP52432 were performed during acetic acid (AA) infusion with or without TNS. Black bars under the pressure trace indicate TNS duration. TNS: 5 Hz, 0.2 ms, T = 0.6 V. Infusion rate = 1 ml/min. B: normalized bladder capacity measured under different conditions (n = 5 cats). *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA).
Effect of CGP52432 on PNS inhibition of bladder overactivity.
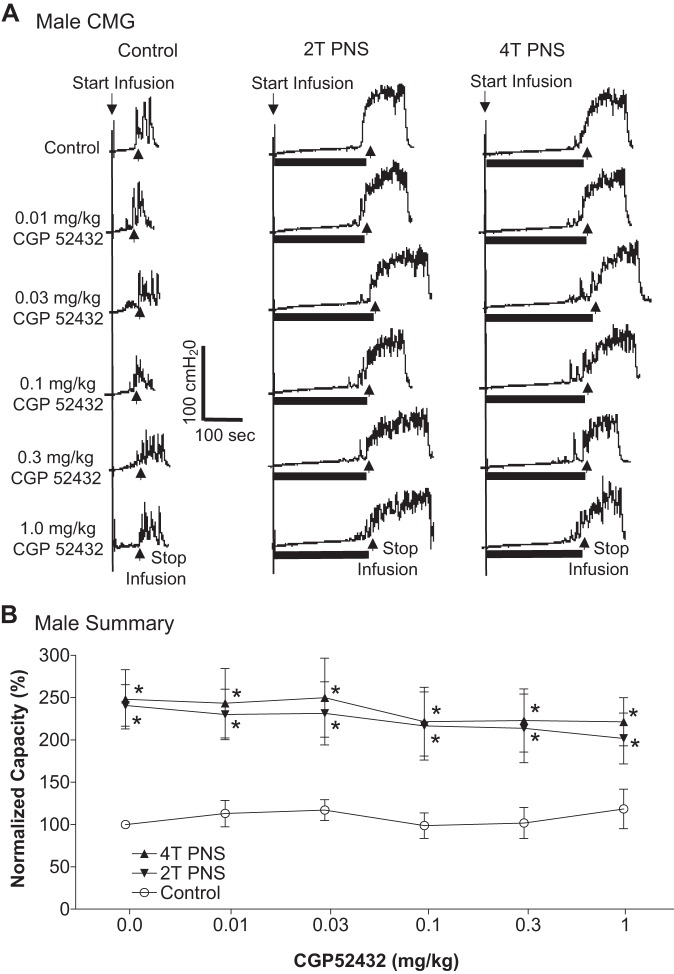
In the PNS group, intravesical infusion of dilute (0.5%) AA irritated the bladder, induced bladder overactivity, and significantly (P = 0.0001) reduced bladder capacity to 4.6 ± 0.5 ml from 10.9 ± 1.0 ml measured during saline CMGs. Similar to TNS, PNS also inhibited bladder overactivity induced by AA irritation and significantly (P < 0.05) increased bladder capacity to 217 ± 18.8% (9.5 ± 0.8 ml) or 221.3 ± 22.3% (9.7 ± 1.0 ml) of AA control capacity, respectively, at 2T or 4T stimulation intensities (Fig. 5). As noted above the amplitude and duration of bladder contractions were also significantly increased after PNS (see CMG traces in every figure); however, these increases are likely due to the larger bladder volumes during neuromodulation. Poststimulation AA control capacity was not different from prestimulation AA control demonstrating that there was no poststimulation effect (Fig. 5). When the data in Fig. 5 were separated into male and female groups, no difference could be detected between the two genders (P > 0.05, Bonferroni multiple comparisons following two-way ANOVA). However, in contrast to the results in the TNS group, CGP52432 had no effect on PNS inhibition of bladder overactivity in either female cats (n = 5, Fig. 6) or male cats (n = 5, Fig. 7). The drug also had no effect when the data were analyzed irrespective of gender (n = 10, Fig. 8).
Fig. 7.
Effect of CGP52432 (intravenous) on the inhibition of bladder overactivity induced by pudendal nerve stimulation (PNS) in male cats. A: repeated CMGs at different cumulative doses of CGP52432 were performed during acetic acid (AA) infusion with or without PNS. Black bars under the pressure trace indicate PNS duration. PNS: 5 Hz, 0.2 ms, T = 1.5 V. Infusion rate = 2 ml/min. B: normalized bladder capacity measured under different conditions (n = 5 cats). *Significantly (P < 0.05, Bonferroni comparisons) different from control at different dosages of CGP52432 (two-way ANOVA).
DISCUSSION
This study in anesthetized cats shows that GABAB receptors play an important role in tibial neuromodulation of irritation-induced bladder overactivity, while they have no role in pudendal neuromodulation. However, GABAB receptors are only involved in tibial neuromodulation in female cats but not in male cats. This result indicates for the first time that the mechanisms of action for bladder neuromodulation can be gender specific.
Previous studies have also shown that GABAB receptors can have very different roles in male or female animals. After neonatal brain damage induced by hypoxia-ischemia, adult male and female mice respond to the blockade of GABAB receptors differently in regard to motor function, spatial learning, and memory (9, 10). It has also been reported in mice that anxiety-like behavior is only increased in the female adult offspring of mothers treated with a GABAB receptor antagonist, while hyperactivity is only observed in the male adult offspring (29). These differences are very likely due in part to different effects of male and female steroid hormones on 1) expression of proteins involved in the synthesis and transport of GABA (33); 2) expression of the GABAB receptor subunits (18); or 3) coupling of the GABAB receptors to downstream signaling mechanisms such as inwardly rectifying K+ channels (20). The prominent gender difference in the contribution of GABAB receptors to tibial neuromodulation may be related to one or more of these mechanisms.
Because previous studies in male and female cats revealed that tibial neuromodulation of bladder overactivity involves the activation of a supraspinal μ-opioid receptor mechanism (30, 37), it would be important to know how this mechanism interacts with the GABAB receptor mechanism identified in the present experiments in female cats. The interaction might be complex because administration of either an opioid receptor antagonist (30, 37) or a GABAB receptor antagonist (Fig. 2) completely eliminates tibial neuromodulation, suggesting that both mechanisms are essential for eliciting inhibition. Thus the opioid and GABAB receptor-mediated inhibitions could occur at separate sites arranged sequentially in the inhibitory reflex circuit or occur by a synergistic interaction between the two inhibitory mechanisms at one site where both mechanisms have to be activated to elicit a detectable inhibition.
Interactions between opioid and GABAB receptor inhibitory mechanisms have also been identified in studies of noxious stimulation-induced antinociception (NSIA) in rats (32). NSIA elicited by activation of C-fiber nociceptors following hindpaw intraplantar injection of capsaicin is suppressed by GABAB and μ-opioid receptor antagonists. Spinal administration of the GABAB agonist (baclofen) mimicked the NSIA in that it could be blocked by a μ-opioid receptor antagonist administered either in the spinal cord or at a supraspinal site in the nucleus accumbens, suggesting that tonic activation of μ-opioid receptors facilitates GABAB receptor-mediated antinociception or that the baclofen-induced antinociception is elicited by μ-opioid receptors located downstream of GABAB receptor activation. The pharmacological data obtained in the NSIA model are strikingly similar to those in the AA bladder irritation model, which also involves activation of C-fiber nociceptors (13). A further similarity is the role of supraspinal μ-opioid receptors in both tibial neuromodulation of bladder overactivity (30, 37) and in NSIA-induced antinociception (32).
Because of the importance of the opioid receptor-GABAB receptor interaction in tibial neuromodulation and the minimal role of opioid receptor mechanisms in pudendal neuromodulation (27), it was not surprising that the latter was resistant to blockade of GABAB receptors by CGP52432. However, it is noteworthy that activation of GABAA receptors in the spinal cord plays an important role in pudendal neuromodulation of bladder overactivity (36). Thus electrical stimulation of pudendal nerve afferents must release GABA at synapses in the spinal cord but GABAB receptors must not be expressed at these synapses or not activated by the transmitter. The latter possibility is supported by electrophysiological studies in rats that showed that sacral parasympathetic preganglionic neurons express both GABAA and GABAB receptors (3). Both receptors can be activated by agonists, but electrical stimulation of local inhibitory interneurons activates only the GABAA receptors. Thus it is possible that pudendal neuromodulation excites this direct GABAergic inhibitory input to the preganglionic neurons but selectively activates only GABAA receptor-mediated inhibition.
The control of bladder capacity is not changed by CGP52432 treatment (Figs. 2–4 and 6–8), indicating that GABAB receptors do not play a physiological role in the bladder overactivity induced by AA irritation in cats. However, previous studies in rats have shown that GABAB receptors play an important role in both normal and overactive bladder reflexes (6). Experiments in rats have shown that baclofen (a GABAB receptor agonist) can inhibit normal bladder reflex activity induced by saline distention when it is administered intravenously, intrathecally and intracerebroventricularly (11, 23). Meanwhile, CGP62349 (a GABAB receptor antagonist) excited the bladder during saline distention, indicating a tonic inhibitory GABAergic mechanism (23). In addition, bladder overactivity induced by intravesical oxyhemoglobin could be attenuated or abolished by baclofen administered intravenously or intrathecally (23). Furthermore, antagonizing GABAB receptors reduced detrusor overactivity in chronic spinal cord injured rats (22). These results indicate that in cats and rats the GABAB receptors play a very different role in bladder overactivity. Whether GABAB receptors play a role in normal bladder activity induced by saline distention still needs to be determined in cats.
An important issue is the relevance of the effects of neuromodulation in an AA-induced overactive bladder model to the mechanisms of action of neuromodulation when treating patients with OAB. Currently the pathophysiology of OAB is unknown. Therefore, it is impossible to develop an animal model of OAB. However, it is reasonable to propose that OAB symptoms such as urgency and frequency are induced by bladder afferent firing in response to bladder distension. Furthermore we know that the bladder is innervated by two types of afferent nerves 1) nonnociceptive afferent Aδ fibers and 2) nociceptive afferent C fibers (8). Saline distension of bladder activates the nonnociceptive afferent Aδ fibers that trigger a supraspinal micturition reflex while the nociceptive afferent C fibers are silent (13). Bladder irritation can activate the nociceptive afferent C fibers (13) that trigger a spinal reflex and cause bladder overactivity (8). Therefore, AA was used in this study to activate afferent C fibers in the bladder and induce bladder overactivity. Additional studies are needed to determine whether GABAB receptors are involved in tibial or pudendal neuromodulation of normal bladder activity induced by saline distention. Understanding neuromodulation of bladder reflexes mediated by either Aδ or C fibers is certainly important for understanding the possible mechanisms of action underlying neuromodulation of OAB, because OAB could be caused by overactivity in either Aδ- or C-fiber afferent-mediated reflex pathways.
In regard to clinical relevance of our data, it is also worth noting that TNS and PNS were tested at both 2T and 4T intensities in this study. Since stimulation intensity greater than 6–8T likely activates small nociceptive afferent C fibers, we choose 2T and 4T intensities to activate the majority of nonnociceptive large afferent fibers at two different levels and avoid activating the nociceptive C fibers at the same time. This experimental design could maximize the opportunity to identify different neurotransmitter mechanisms involved in stimulation of different groups of nonnociceptive large afferent nerve fibers. However, in clinical application, the 30-min TNS is applied at 1T or slightly below 1T intensity to achieve a poststimulation inhibitory effect that can last for weeks. In contrast, our current study only investigated the acute TNS inhibition during CMGs. A chronic animal study of bladder inhibition induced by TNS at 1T intensity is warranted to further reveal the possible mechanisms of action.
Perspectives and Significance
This study in cats reveals a sex difference of GABAB receptors in tibial neuromodulation of bladder overactivity. It also shows that GABAB receptors are not involved in overactive bladder reflex and pudendal neuromodulation of the reflex. These results are important for understanding the neurotransmitter mechanisms of bladder neuromodulation and for discovering new molecular targets to further improve neuromodulation therapies for OAB patients.
GRANTS
This study is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-102427, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; T.W.F., X.J., U.B., V.L., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein A; Standardisation Sub-Committee of the International Continence Society . The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61: 37–49, 2003. doi: 10.1016/S0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63: 2595–2611, 2003. doi: 10.2165/00003495-200363230-00003. [DOI] [PubMed] [Google Scholar]
- 3.Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J Neurophysiol 72: 2903–2910, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54: 543–562, 2008. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101: 1388–1395, 2008. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 6.Elkilini MS, Abuzqaya A, Hassouna MM. Mechanism of action of sacral neuromodulation. Int Urogynecol J Pelvic Floor Dysfunct 21, Suppl 2: S439–S446, 2010. doi: 10.1007/s00192-010-1273-3. [DOI] [PubMed] [Google Scholar]
- 7.Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol 309: F242–F250, 2015. doi: 10.1152/ajprenal.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillani QA, Akbar A, Ali M, Iqbal F. Gender-specific effects of CGP 55845, GABAB receptor antagonist, on neuromuscular coordination, learning and memory formation in albino mouse following neonatal hypoxia-ischemia insult. Neurol Sci 36: 961–969, 2015. doi: 10.1007/s10072-015-2205-2. [DOI] [PubMed] [Google Scholar]
- 10.Gillani Q, Ali M, Iqbal F. CGP 35348, GABA B receptor antagonist, has a potential to improve neuromuscular coordination and spatial learning in albino mouse following neonatal brain damage. BioMed Res Int: 295215, 2014. doi: 10.1155/2014/295215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliani S, Lecci A, Santicioli P, Del Bianco E, Maggi CA. Effect of the GABAB antagonist, phaclofen, on baclofen-induced inhibition of micturition reflex in urethane-anesthetized rats. Neuroscience 48: 217–223, 1992. doi: 10.1016/0306-4522(92)90350-B. [DOI] [PubMed] [Google Scholar]
- 12.Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodynamics, Female Pelvic Medicine . Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 193: 1572–1580, 2015. doi: 10.1016/j.juro.2015.01.087. [DOI] [PubMed] [Google Scholar]
- 13.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50: 1306–1314, 2006. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Kullmann FA, Wells GI, Langdale CL, Zheng J, Thor KB. Stability of the acetic acid-induced bladder irritation model in alpha chloralose-anesthetized female cats. PLoS One 8: e73771, 2013. doi: 10.1371/journal.pone.0073771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees P. Pharmacology and toxicology of alpha chloralose: a review. Vet Rec 91: 330–333, 1972. doi: 10.1136/vr.91.14.330. [DOI] [PubMed] [Google Scholar]
- 18.Lux-Lantos VA, Bianchi MS, Catalano PN, Libertun C. GABA(B) receptors in neuroendocrine regulation. Cell Mol Neurobiol 28: 803–817, 2008. doi: 10.1007/s10571-008-9263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013. doi: 10.1016/j.juro.2012.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malyala A, Kelly MJ, Rønnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids 70: 397–406, 2005. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Matsuta Y, Mally AD, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Contribution of opioid and metabotropic glutamate receptor mechanisms to inhibition of bladder overactivity by tibial nerve stimulation. Am J Physiol Regul Integr Comp Physiol 305: R126–R133, 2013. doi: 10.1152/ajpregu.00572.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol 179: 1178–1183, 2008. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pehrson R, Lehmann A, Andersson KE. Effects of gamma-aminobutyrate B receptor modulation on normal micturition and oxyhemoglobin induced detrusor overactivity in female rats. J Urol 168: 2700–2705, 2002. doi: 10.1016/S0022-5347(05)64247-4. [DOI] [PubMed] [Google Scholar]
- 24.Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 26.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29: 1267–1271, 2010. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 27.Rogers MJ, Xiao Z, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C. Propranolol, but not naloxone, enhances spinal reflex bladder activity and reduces pudendal inhibition in cats. Am J Physiol Regul Integr Comp Physiol 308: R42–R49, 2015. doi: 10.1152/ajpregu.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudy DC, Downie JW, McAndrew JD. Alpha-chloralose alters autonomic reflex function of the lower urinary tract. Am J Physiol Regul Integr Comp Physiol 261: R1560–R1567, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Stratton MS, Staros M, Budefeld T, Searcy BT, Nash C, Eitel C, Carbone D, Handa RJ, Majdic G, Tobet SA. Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety- and depression-like behaviors sex specifically in mice. PLoS One 9: e106015, 2014. doi: 10.1371/journal.pone.0106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged poststimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385–F392, 2011. doi: 10.1152/ajprenal.00526.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tambeli CH, Levine JD, Gear RW. Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord. Pain 143: 228–232, 2009. doi: 10.1016/j.pain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umorin M, Stinson C, Bellinger LL, Kramer PR. Genes in the GABA pathway increase in the lateral thalamus of Sprague-Dawley rats during the proestrus/estrus phase. J Cell Physiol 231: 1057–1064, 2016. doi: 10.1002/jcp.25198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JP, Debruyne FM, Kiemeney LA, Bemelmans BL. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn 22: 227–232, 2003. doi: 10.1002/nau.10111. [DOI] [PubMed] [Google Scholar]
- 35.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Z, Reese J, Schwen Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 306: F781–F789, 2014. doi: 10.1152/ajprenal.00679.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Slater RC, Ferroni MC, Kadow BT, Lyon TD, Shen B, Xiao Z, Wang J, Kang A, Roppolo JR, de Groat WC, Tai C. Role of µ, κ, and δ opioid receptors in tibial inhibition of bladder overactivity in cats. J Pharmacol Exp Ther 355: 228–234, 2015. doi: 10.1124/jpet.115.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]