Acute low-glucose exposure induces mitochondrial fragmentation in endothelial cells via Drp1 and is associated with impaired endothelial function in human arterioles. Targeting of Drp1 prevents fragmentation, improves vasofunction, and may provide a therapeutic target for improving cardiovascular complications among diabetics.
Keywords: endothelial dysfunction, mitochondria, nitric oxide, glucose, vasodilation
Abstract
Intensive glycemic regulation has resulted in an increased incidence of hypoglycemia. Hypoglycemic burden correlates with adverse cardiovascular complications and contributes acutely and chronically to endothelial dysfunction. Prior data indicate that mitochondrial dysfunction contributes to hypoglycemia-induced endothelial dysfunction, but the mechanisms behind this linkage remain unknown. We attempt to determine whether clinically relevant low-glucose (LG) exposures acutely induce endothelial dysfunction through activation of the mitochondrial fission process. Characterization of mitochondrial morphology was carried out in cultured endothelial cells by using confocal microscopy. Isolated human arterioles were used to explore the effect LG-induced mitochondrial fission has on the formation of detrimental reactive oxygen species (ROS), bioavailability of nitric oxide (NO), and endothelial-dependent vascular relaxation. Fluorescence microscopy was employed to visualize changes in mitochondrial ROS and NO levels and videomicroscopy applied to measure vasodilation response. Pharmacological disruption of the profission protein Drp1 with Mdivi-1 during LG exposure reduced mitochondrial fragmentation among vascular endothelial cells (LG: 0.469; LG+Mdivi-1: 0.276; P = 0.003), prevented formation of vascular ROS (LG: 2.036; LG+Mdivi-1: 1.774; P = 0.005), increased the presence of NO (LG: 1.352; LG+Mdivi-1: 1.502; P = 0.048), and improved vascular dilation response to acetylcholine (LG: 31.6%; LG+Mdivi-1; 78.5% at maximum dose; P < 0.001). Additionally, decreased expression of Drp1 via siRNA knockdown during LG conditions also improved vascular relaxation. Exposure to LG imparts endothelial dysfunction coupled with altered mitochondrial phenotypes among isolated human arterioles. Disruption of Drp1 and subsequent mitochondrial fragmentation events prevents impaired vascular dilation, restores mitochondrial phenotype, and implicates mitochondrial fission as a primary mediator of LG-induced endothelial dysfunction.
NEW & NOTEWORTHY Acute low-glucose exposure induces mitochondrial fragmentation in endothelial cells via Drp1 and is associated with impaired endothelial function in human arterioles. Targeting of Drp1 prevents fragmentation, improves vasofunction, and may provide a therapeutic target for improving cardiovascular complications among diabetics.
Listen to this article’s corresponding podcast @ http://ajpheart.podbean.com/e/mitochondrial-dynamics-impact-endothelial-function/.
clinical hypoglycemia (blood glucose < 70 mg/dl; 3.9 mmol/l) is associated with adverse cardiovascular outcomes among Type 2 diabetic patients receiving intensive glucose control therapy (15, 16, 24, 43). Large randomized trials suggest a relationship between increased cardiac mortality and incidence of hypoglycemia (20, 48). Hypoglycemia rapidly leads to biological responses that adversely affect the vasculature and increase cardiovascular risk (1).
Exciting recent data show that exposure to a clinically relevant hypoglycemic condition, experimentally defined as a state of low-glucose (LG) concentration, results in vascular endothelial dysfunction, manifested by both vascular inflammation and impairment of nitric oxide (NO)-mediated, endothelium-dependent dilation (2, 7, 33, 52, 59). However, the mechanisms by which LG exposure impairs human endothelial function remain incompletely elucidated. Data from our laboratory and others strongly suggest that abnormal mitochondrial function, alterations in mitochondrial membrane potential, and increased oxidative stress may all have been mechanistically linked with vascular dysfunction associated with both diabetes mellitus and LG exposure (4, 5, 36, 55). These critical changes in mitochondrial function may be the result of a pathological imbalance of the mitochondrial fission and fusion processes (22, 27, 28, 38, 57, 62).
Importantly, a loss of mitochondrial networks within endothelial cells has been observed in both murine and human subjects afflicted with diabetes, indicating a pathophysiological connection between mitochondrial fission and diabetes (38, 50). Mitochondrial fission is regulated through a small GTPase, dynamin-related protein 1 (Drp1), a necessary component of the fission/fusion machinery (10, 47, 51, 54). Based on our prior work demonstrating LG-induced endothelial dysfunction and the prior foundational data linking mitochondrial fragmentation to diabetes, we hypothesize that acute LG exposure (2.2 mmol/l) would impair NO-dependent endothelium-dependent vasodilation by inducing Drp1 activity, phenotypically manifested as increased mitochondrial fission and subsequent mitochondrial dysfunction (increased mitochondrial inner membrane polarization and mitochondrial superoxide production).
MATERIALS AND METHODS
Materials.
Human umbilical vein endothelial cells (HUVECs) were obtained from the Hybridoma Core at the Blood Research Institute (Milwaukee, WI). Culture reagents including M199, fetal bovine serum, penicillin-streptomycin-glutamine solution (×100), Dulbecco’s PBS (DPBS), and Hanks’ balanced salt solution were purchased from Invitrogen (Carlsbad, CA). Mdivi-1 was purchased from Sigma-Aldrich (product no. M0199, St. Louis, MO). MitoSOX Red and CellLight Mitochondria-Red Fluorescent Protein (Mito-RFP) were purchased from Invitrogen (product nos. M36008 and C10601). Tetramethylrhodamine methyl ester (TMRM) and 4,5-diaminofluorescein diacetate (DAF2-DA) were purchased from Invitrogen (product no. T-668) and Cayman Chemical (product no. 85165, Ann Arbor, MI), respectively. Three RNAi constructs (sequences: AGAGUGUAACUGAUUCAAUCCGUGA, AGGAUAUUGAGCUUCAAAUCAGAGA, and CCCUUAAACUGAGUCAAGAUCUGAA) for Drp1 were obtained from Origene Technologies (product no. SR306774, Rockville, MD) and Lipofectamine RNAiMAX was acquired from Invitrogen. The following antibodies were used: Drp1 and Phospho-Drp1 (Cell Signaling Technology, Danvers, MA), β-actin (Sigma-Aldrich), anti-mouse and anti-rabbit IgG-HRP-conjugated antibodies (Santa Cruz Biotechnology, Dallas, TX).
Human subjects.
Unless otherwise noted, isolated human microvessels were obtained from healthy human volunteers lacking evidence of major medical illness, diabetes, or metabolic syndrome through either subcutaneous gluteal adipose biopsy or from subcutaneous fat discarded at the time of abdominal surgery. Overall, tissue samples were used from 33 subjects composed of 10 men and 23 women aged 31–79 yr. All procedures and subject consent were performed in accordance with the policies of the Medical College of Wisconsin Human Research Protection Program and were approved by the Institutional Review Board.
Cell culture.
HUVECs were plated on culture dishes coated with 0.1% gelatin. Growth medium was M199 supplemented with 20% FBS (vol/vol), 100 μg/ml heparin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.292 mg/ml glutamine. Final glucose concentration was adjusted to 5 mmol/l. Cells were either grown to confluency or allowed to adhere for 24 h before experimental protocols. For LG treatment media, M199 was diluted with DPBS to achieve 2.2 mmol/l glucose and supplemented as described above. Normal glucose (NG) treatment was prepared by supplanting the LG media with glucose to achieve 5 mmol/l. Determination of the target LG concentration was performed previously (55). Cells were maintained and passaged in the normal growth medium until the time of treatment when the growth medium was exchanged for NG or LG treatment medium as described. Cells were used from passage 3 to passage 6.
Mitochondrial fission.
HUVECs were plated onto a Nunc Laboratory-Tek II 4-chamber glass-bottom culture slide (155382, Thermo Fisher Scientific, Waltham, MA) suitable for confocal microscopy. The cells were incubated with a mitochondrial-specific fluorescent probe, Mito-RFP, for 16–24 h. Postincubation, cells were exposed to media with either low (2.2 mmol/l) or normal (5.5 mmol/l) levels of glucose for 2 h. At the end of treatment, cells were fixed with 4% paraformaldehyde. Fluorescent images of mitochondria were captured with a Leica TCS SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a ×63 oil immersion lens with an excitation wavelength of 543 nm and an emission wavelength range of 575–700 nm. Image size was set to 1,024 × 1,024 pixels with a scan speed of 100 Hz with line averaging of four. Resultant images were analyzed by the following method adapted from Rehman et al. (45) and referenced in numerous publications (28, 40, 49). Ten to fifteen cells were randomly and individually imaged and then analyzed with ImageJ (National Institutes of Health, Bethesda, MD) by using a unique processing macro designed to count the number of fluorescent pixels that represent mitochondria and determine the number of fragments present within the image via “particle analysis.” Images were assessed to determine the number of discrete mitochondrial objects within a cell (numerator) and normalized to the total mitochondrial signal within the cell (denominator) to generate a fragmentation ratio for each cell analyzed. The number of fragments divided by the number of mitochondrial pixels yields a fragmentation ratio. Cells with a higher ratio display greater amounts of mitochondrial fragmentation.
Bioenergetics.
The mitochondrial metabolic profile of HUVECs during LG exposure was assessed with a Seahorse XF96 Analyzer (Seahorse Bioscience, North Billerica, MA) and an experimental protocol modified from Dranka et al. (13). The day before analysis, HUVECs were seeded onto a 96-well flux plate (product no. 102416-100, Seahorse Bioscience) at a density of 15,000 cells/well with normal culture media. The following day, the culture media was removed and replaced with either NG or LG treatment media and incubated for 2 h. After incubation, the treatment media was removed and replaced with analysis media containing no buffering capacity (bicarbonate) and no pH dependent indicators, while retaining the same glucose concentrations as treatment. The plate was subjected to a mitochondrial stress test protocol as outline by Seahorse Bioscience and performed by the Bioenergetics Core within the Department of Biophysics at the Medical College of Wisconsin (Milwaukee, WI). The protocol consisted of obtaining baseline measurements of the oxygen consumption rate for ~40 min, at which time the cells were treated with oligomycin (1 mg/ml) and observed for ~30 min. Next was the addition of FCCP (1 μmol/l) and another ~30 min of observation. The last step was the addition of antimycin A (10 μmol/l), followed by recording of the oxygen consumption rate for a final 30 min. The data were then normalized to protein content analyzed via a Bradford assay.
Western blot analysis.
HUVECs pretreated with NG and LG for time periods up to 2 h were lysed in MOPS lysis buffer as described (55). Protein concentration was measured with DC protein assay reagent (Bio-Rad, Hercules, CA). Sample lysates containing 25–30 μg of protein were separated on a 10% Tris·HCL Criterion Gel (Bio-Rad) and transferred onto a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% of nonfat dry milk in PBS for 1 h. After blocking, the membrane was washed with PBS containing 0.1% Tween 20 (PBST), probed with either anti-phospho-Drp1 (Ser637), anti-phospho-Drp1(Ser616) (1:1,000) (product no. 4867S and product no. 3455S, Cell Signaling Technology), or β-actin (1:40,000) (Sigma-Aldrich), and incubated overnight at 4°C. To detect the expression of total Drp1, the membrane was stripped and reprobed with anti-Drp1 (1:1,000) (product no. 8570S, Cell Signaling Technology). Membranes were subsequently washed with PBST and incubated with goat anti-rabbit IgG-HRP-conjugated secondary antibody (Santa Cruz Biotechnology) and goat anti-mouse-HRP conjugate secondary antibody (Bio-Rad). Blots were developed with ECL Plus Western blotting detection system (GE Healthcare Biosciences, Pittsburgh, PA). Densitometry measures were performed with ImageJ 1.48.
Microvessel preparation and assessment of endothelial function.
Human small resistance arterioles from gluteal fat pad biopsies or surgical discards were prepared as previously described (12, 18, 36, 55). Arterioles were preconstricted with endothelin-1 to ~30–50% of maximum diameter under resting condition. Endothelium-dependent vasodilation was determined by adding acetylcholine (ACh) into organ bath to reach concentrations of 10−10 to 10−5 mol/l. Vessel diameter is measured after each addition of ACh by video microscopy using digital calipers (Boeckeler Instruments, Tucson, AZ). For studies on the impact of LG and vasomotor activity, vessels were exposed to LG condition for 2 h before vasomotor measurements. Control vessels exposed to NG conditions were similarly incubated in NG conditions for 2 h before testing. Exposure to intraluminal Mdivi-1 (10 μmol/l) and/or NG-nitro-l-arginine methyl ester (l-NAME) (100 μmol/l) was performed for 30 min before incubation in either NG or LG conditions. Concentrations of Mdivi-1 and l-NAME were determined based on empirical tests (unpublished results) and literature review. At the end of each series of vessel study, smooth muscle reactivity was determined by adding papaverine (0.2 mmol/l).
NO expression.
NO measurements were made with a fluorescent NO marker, DAF-2 DA (Cayman Chemical) by the method previously described (37). Untreated vessels served as controls and were examined in parallel with the treated vessels to remove any fluorescence confounding. The images were analyzed with the same gain and intensity settings to ensure comparability between samples and controls. Results are expressed as the mean fluorescence intensity of a defined vessel region and measured with MetaMorph 6.1 (Universal Imaging, West Chester, PA).
Mitochondrial superoxide production.
After 2 h of incubation in either NG or LG buffer, vessels were exposed to 10 μmol/l 1MitoSox Red and were incubated at 37°C for 30 min DPBS. Samples were subsequently washed twice with DPBS and mounted on a microscope slide with fluorescent mounting media (product no. S302380-2, Dako, Carpinteria, CA) and mounted on coverslips. MitoSox signal (Ex: 510 nm; Em: 580 nm) was measured by fluorescence microscopy (Eclipse TE 200, Nikon, Japan) and analyzed with MetaMorph 6.1 (Universal Imaging). Measurements of mitochondrial superoxide production were also made in the presence of Mdivi-1 (10 μmol/l) or carbonyl cyanide m-chlorophenyl hydrazine (CCCP, 1 μmol/l) to determine the effect fission inhibition or mitochondrial membrane depolarization had on superoxide level. Concentration of CCCP was determined empirically (unpublished results) and has been shown to be effective at modest membrane depolarization (55). Results are expressed as the mean fluorescence intensity of a defined vessel region and measured with MetaMorph 6.1 (Universal Imaging).
Mitochondrial membrane potential.
TMRM (Invitrogen) is a cell-permeable fluorescent dye that is readily sequestered by active mitochondria and has been previously used to measure mitochondrial membrane potential (36). After 2 h of incubation in either NG or LG buffer, vessels were incubated with TMRM (1 µmol/l) at 37°C for 30 min in DPBS. The vessels were washed twice and then mounted on a microscope slide with fluorescent mounting medium (Dako) and mounted on coverslips. TMRM signal (Ex: 548 nm; Em: 573 nm) was measured with a fluorescence microscope (Eclipse TE 200; Nikon). Results were expressed as relative fluorescence intensity of the defined vessel region. Results are expressed as the mean fluorescence intensity of a defined vessel region and measured with MetaMorph 6.1 (Universal Imaging).
Knockdown of Drp1 expression.
Lipofectamine RNAi Max (Invitrogen) was used to introduce Drp1 RNAi constructs into HUVECs. Constructs were acquired from Origene along with a negative scrambled control. The Lipofectamine vehicle was added to RNAi constructs per manufacturer’s protocol, and the mixture was diluted in culture media and incubated with endothelial cells for 4–6 h prior replacement with normal culture media and subsequent incubation for 24 h before treatment and analysis. For human arterioles, the Lipofectamine/RNAi mixture was passed through the lumen of the vessel and allowed to remain within the lumen for 4–6 h before washout at a low flow rate (shear stress < 5 dyn/cm2). Vessels were then incubated under culture conditions for 24 h before vasoactivity testing under either NG or LG conditions (2-h exposure before testing). Microvessels were maintained during the transfection and incubation period with a culture myograph tissue chamber system from Danish Myograph Technology (DMT-USA, Ann Arbor, MI).
Statistical analysis.
Differences between two groups were evaluated by unpaired Student’s t-test. Comparisons of NO production, mitochondrial superoxide production, mitochondrial membrane potential, and mitochondrial fission were made by one-way ANOVA for overall differences with post hoc testing to assess differences between groups. To maintain normality, data from MitoSOX and DAF fluorescence and mitochondrial networks were log transformed before analysis. Analysis of functional vessel data was performed by two-way ANOVA with post hoc testing to determine differences between groups and dose-response. P values of ≤0.05 were considered significant. Data are presented as means ± SE unless otherwise stated. All analyses were performed with SigmaPlot ver. 12.5 (Systat Software, San Jose, CA).
RESULTS
Impact of LG on mitochondrial network fragmentation.
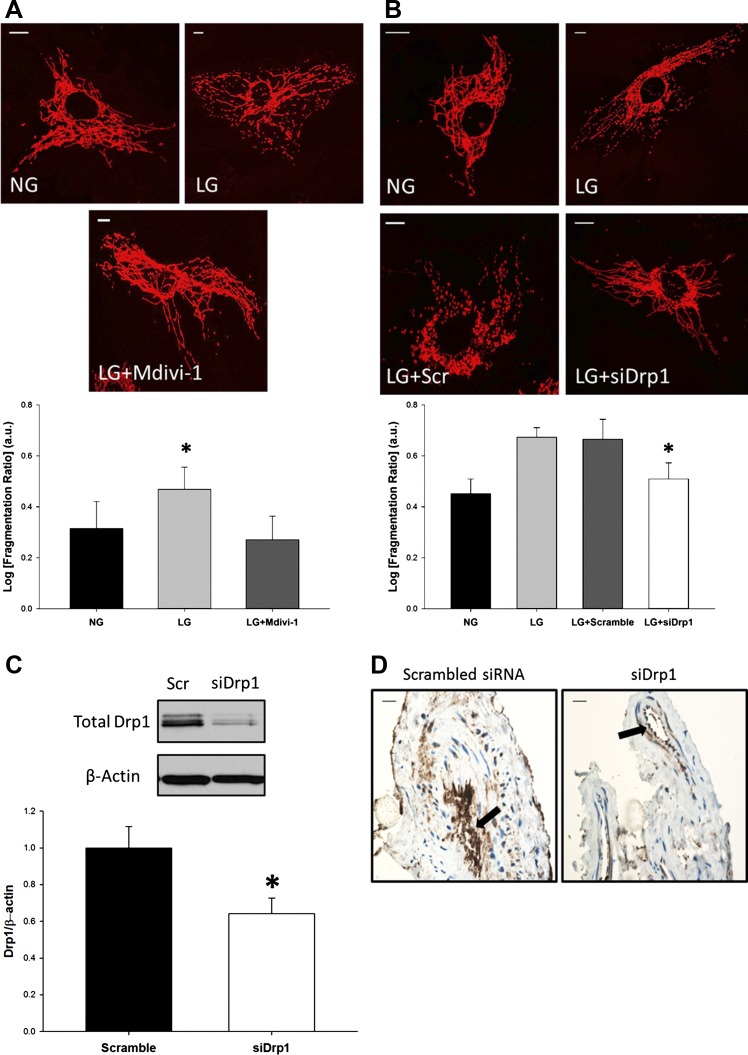
To test whether alterations in glucose levels impacted mitochondrial morphology, we first exposed HUVECs to LG and assessed the degree of mitochondrial fragmentation. Fragmentation is expressed as a ratio of discrete mitochondrial objects in relation to the total amount of mitochondria present in a cell: the higher the fragmentation ratio, the more fragmented the mitochondrial network. After acute LG exposure, the fragmentation ratio of the LG group was markedly greater than the normal glucose control (NG: 0.314; LG: 0.469; P = 0.011) (Fig. 1A). These data suggest that acute LG exposure (2 h) increases mitochondrial fragmentation.
Fig. 1.
Treatment with low glucose (LG) elicits a mitochondrial fission response. A: LG induced mitochondrial fragmentation in endothelial cells and inhibition of dynamin-related protein 1 (Drp1) activity with Mdivi-1 (10 μM) attenuated the response (P = 0.003 overall, *P < 0.02 for LG vs. NG and LG + Mdivi-1, N = 6). Scale bars represent 10 μm. B: similarly, when expression of Drp1 was decreased via siRNA knockdown, mitochondrial fragmentation was decreased in LG-exposed cells (P < 0.001 overall, *P = 0.025 for LG + Scramble vs. LG + siDrp1 and NG, N = 6). Scale bars represent 10 μm. C: Human umbilical vein endothelial cells (HUVECs) exposed to siRNA constructs against Drp1 displayed ~50% decrease in protein expression when analyzed by Western blot (*P < 0.05, N = 6). D: proof of concept in translating cellular knockdown of Drp1 expression to our ex vivo preparation of human tissues. Vessels were exposed to siDrp1 constructs per protocol and then analyzed for Drp1 expression via immunohistochemistry. The endothelium of samples exposed to siDrp1 displayed a decrease in Drp1 expression when compared with scrambled control (arrows). Scale bars represent 10 μm.
The experiment was repeated in the presence of Mdivi-1, a pharmacological inhibitor of Drp1 activity, to determine the role of Drp1 in this process. Incubation with Mdivi-1 reduced the level of fragmentation seen during LG exposure (LG: 0.469; LG+Mdivi-1: 0.276; P = 0.003) (Fig. 1A). Knockdown of Drp1 expression Drp1-targeted siRNA also diminished LG-induced fragmentation (LG+Scramble: 0.665; LG+siDrp1: 0.509; P = 0.025) (Fig. 1, B and C). Transfected cells exposed to NG maintained decreased fragmentation ratios compared with their LG counterparts, indicating that LG is the main driving force behind increased fragmentation (data not shown). Thus both pharmacological and molecular inhibition of Drp1 prevents LG-induced mitochondrial fragmentation, suggesting LG promotes mitochondrial fission in a Drp1-dependent manner.
Effect of LG on endothelium-dependent vasodilation of human arterioles.
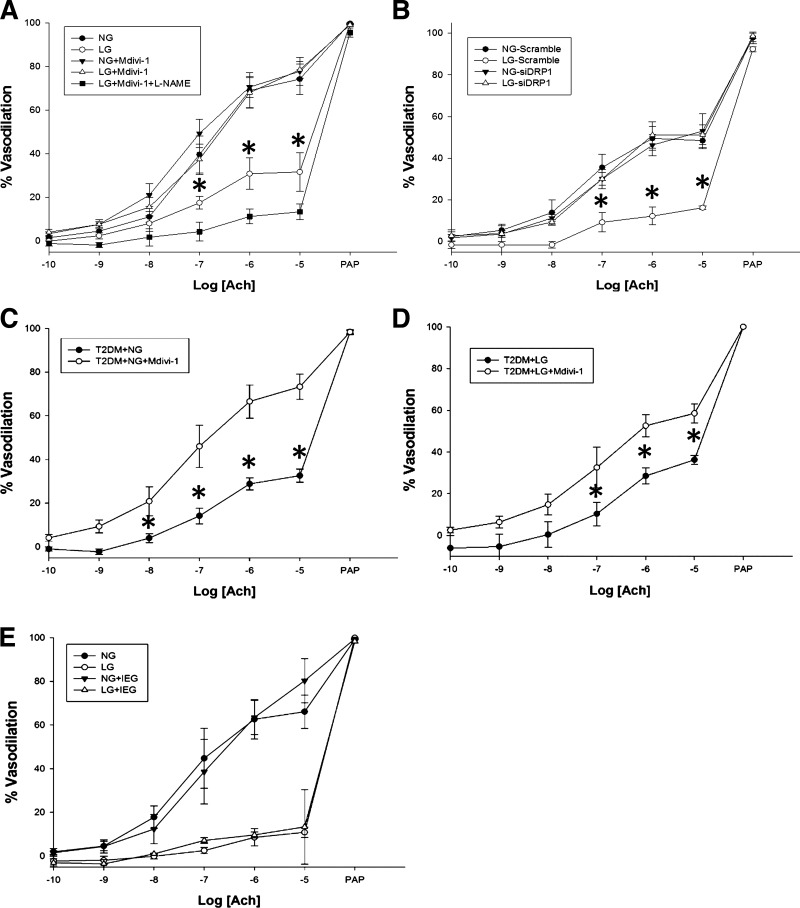
We had previously shown arterioles incubated in LG conditions displayed impaired endothelium-dependent vasodilation (55). We now examined the involvement of Drp1 with endothelium-dependent dilation of human microvasculature (Fig. 2A). The LG group had significantly impaired vasodilatory response to ACh when compared with NG (P < 0.001 overall) in human arterioles as measured by videomicroscopy. Addition of Mdivi-1 reversed the impact of LG (P < 0.02 at indicated doses) with no effect on NG endothelium-dependent vasodilation. Addition of l-NAME abrogated the vasodilation response (P < 0.01), indicating that the improved response to Mdivi-1 is related to the production of NO from eNOS.
Fig. 2.
LG-induced endothelial dysfunction is attenuated through mitochondrial fission inhibition. A: percent vasodilation in response to acetylcholine (ACh) was measured in vessels incubated in either NG or LG buffer for 2 h either with or without addition of Mdivi-1 (10 μmol/l) for the final 30 min of incubation. Overall, LG exposure displayed significant impairment of endothelial function compared with the other groups (P < 0.001 overall, *P < 0.02 at the indicated doses for LG vs. NG, NG+Mdivi-1, and LG+Mdivi-1, N = 5 subjects). Additionally, l-NAME appears to completely abrogate the ameliorative effect of Mdivi-1 on vasodilation under LG conditions (N = 4 subjects, P = 0.01 vs. all other conditions) B: impact of molecular knockdown of Drp1 expression on endothelial function. LG exposure caused impaired vasodilatory response and was improved under knockdown conditions (P < 0.001 overall, *P ≤ 0.002 at indicated ACh doses for LG+Scrambled siRNA vs. all other exposures, N = 3 subjects). C: inhibition of Drp1 activity improves vasofunction in arterioles isolated from clinically diagnosed T2DM subjects displaying glycosylated hemoglobin (HbA1c) levels ranging from 6.5 to 9.3% (mean and SD; 7.3 ± 1.2) and blood glucose levels between 115 and 162 mg/dl (mean and SD; 135 ± 19) (P < 0.005 overall, *P < 0.05 at indicated dose, N = 5 subjects). D: effect of Mdivi-1 on vasofunction of T2DM arterioles (HbA1c: 7.1 ± 1.3; blood glucose: 134 ± 23) were repeated with exposure to LG (P = 0.032 overall, *P < 0.05 at indicated dose, N = 4 subjects). E: microvessels incubated in either NG (N = 3) or LG (N = 4) buffer were exposed to physiologically relevant concentrations of insulin (I, 0.25 nmol/l), epinephrine (E, 5.5 nmol/l), and glucagon (G, 23 nmol/l) to simulate compensatory signaling of the endocrine system to a LG stress. Hormonal effects were negligible where P = 0.937 and P = 0.745 overall for NG and LG groups, respectively. ACh, acetylcholine; PAP, papaverine.
The experiment was repeated using molecular suppression of Drp1 expression with Drp1-targeted siRNA (Fig. 1D). Similar to Mdivi-1, decreased Drp1 expression improves endothelium-dependent vasodilation under LG conditions (Fig. 2B; P < 0.001 overall). Taken together, these data indicate that LG-induced endothelial dysfunction is mediated through Drp1 activity and expression.
An examination of the vascular response in the presence of Mdivi-1 was performed in resistance arterioles obtained from subjects clinically diagnosed with Type 2 diabetes mellitus (T2DM) (Fig. 2, C and D). NO-dependent vasodilation was assessed in arterioles incubated in either NG buffer ± Mdivi-1 or LG buffer ± Mdivi-1. Vascular function was significantly improved among the Mdivi-1 administered group in both studies (P < 0.005 overall for NG and P < 0.05 overall for LG), suggesting altered mitochondrial dynamics during T2DM contributes to endothelial dysfunction.
During systemic hypoglycemic exposure, patients experience a counterregulatory response that includes increased circulating glucagon and epinephrine levels which could affect endothelial function. We tested this in a small set of vessels exposed to LG concomitantly with epinephrine, glucagon, and insulin at levels that mimic those seen in a clinical insulin overdose and did not find the addition of these factors affected endothelium-dependent vasodilation (Fig. 2E).
Effect of Drp1 activity on NO bioavailability measured by DAF fluorescence.
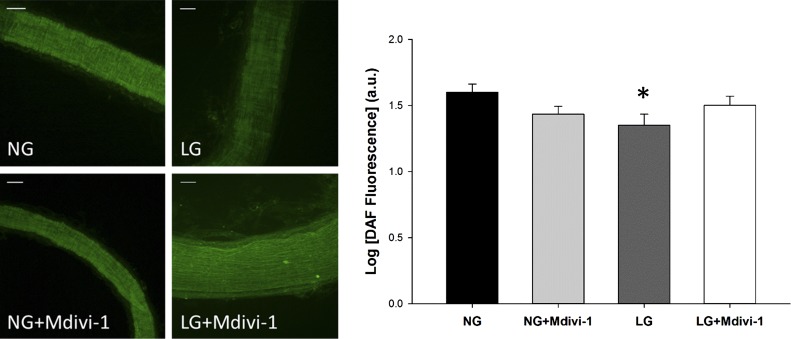
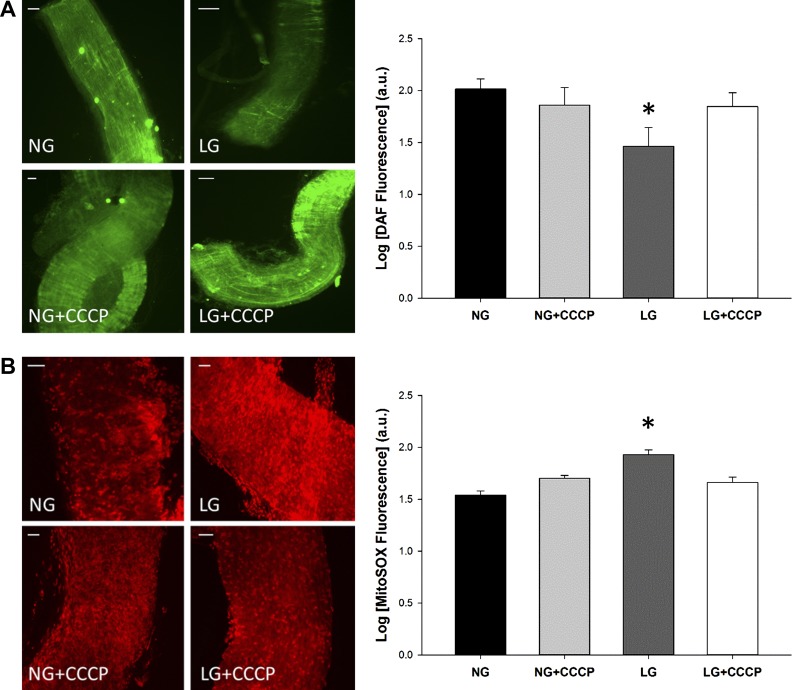
We next wanted to directly investigate the relationship between mitochondrial fragmentation and NO levels. To evaluate NO levels in the human microvessels, the fluorescent NO probe DAF2-DA was used to semiquantify NO bioavailability. After exposure to LG conditions, vessels were incubated with DAF2-DA and imaged (Fig. 3). Intensity of the DAF2-DA signal positively correlates with NO presence, and LG-exposed vessels had reduced DAF fluorescence intensity relative to NG exposed vessels, indicating that LG-treated vessels experience decreased NO bioavailability.
Fig. 3.
Increased NO bioavailability in human arterioles with pharmacologic inhibition of fission. Human arterioles were incubated in either NG or LG buffer with or without fission inhibitor, Mdivi-1 (10 µmol/l). NO was visualized with the fluorescent NO sensitive fluorescent probe DAF2-DA. LG treatment reduced NO presence in isolated microvessels, and blockade of the fission process with Mdivi-1 increased NO levels in vessels exposed to LG (P < 0.001 overall, *P < 0.05 for LG vs. NG and LG+Mdivi-1, N = 4). Scale bars represent 50 μm.
In the presence of Mdivi-1, NO levels measured by DAF2-DA fluorescence increased in vessels exposed to LG in comparison with vessels not exposed to Mdivi-1 (P < 0.04). These data suggest that LG exposure decreases NO bioavailability in human arterioles and inhibition of Drp1 activity is effective at reestablishing NO levels.
Impact of inhibition of Drp1 on mitochondrial membrane potential and superoxide production.
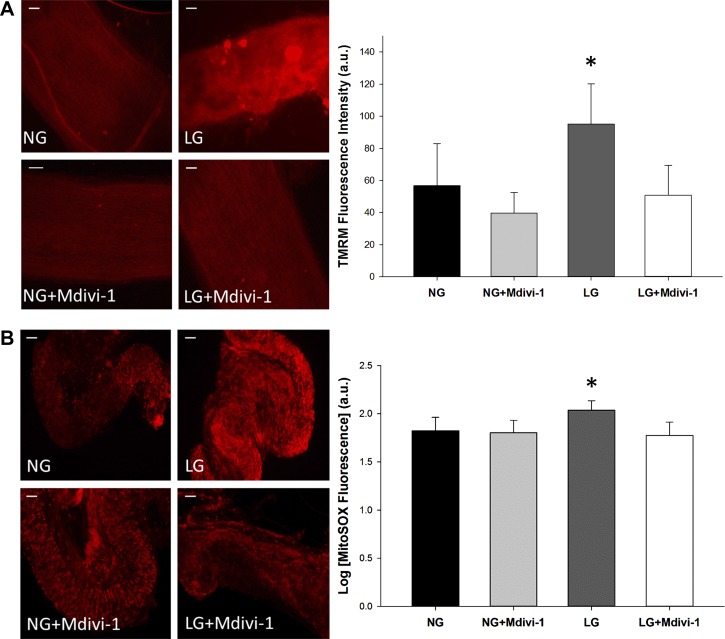
Our prior work demonstrated that exposure to LG resulted in hyperpolarization of the mitochondrial membrane potential (MMP) within endothelial cells (55). We next wanted to examine the relationship between LG-induced mitochondrial membrane polarization and Drp1 in human arterioles (Fig. 4A). Treatment with LG increased TMRM fluorescence relative to NG exposure (P < 0.02). When repeated in the presence of Mdivi-1, TMRM intensity decreased, indicating a reversal of the hyperpolarizing effect of LG (P < 0.01). Mdivi-1 decreased the MMP to that of the control, suggesting inhibition of Drp1 can restore homeostatic mitochondrial membrane polarization. We conclude mitochondrial fission is implicated in mitochondrial membrane hyperpolarization under LG conditions.
Fig. 4.
Fission inhibition depolarizes mitochondrial membrane potential and reduces mitochondrial superoxide in human arterioles. A: human arterioles were incubated in either NG or LG buffer with or without Mdivi-1 (10 μmol/l) to block the fission response (P = 0.008 overall, N = 4). Treatment with LG alone displayed increased membrane hyperpolarization compared with NG alone (*P < 0.02). When LG-treated vessels were coincubated with Mdivi-1, hyperpolarization was reversed (*P < 0.01 vs. LG alone). B: isolated vessels were exposed to either NG or LG buffer with/or without pretreatment with Mdivi-1 (10 μmol/l). MitoSOX was used to detect for the presence of superoxide; greater fluorescence intensity equals more superoxide present. Vessels treated with LG displayed increased superoxide production (P = 0.003 overall, *P < 0.02 for LG vs. NG, N = 5). Pharmacologic inhibition of mitochondrial fission via administration of Mdivi-1 prevented the increase in superoxide production seen in vessels exposed to LG alone (*P = 0.005 for LG vs. LG+Mdivi-1). Scale bars represent 50 μm.
We have previously demonstrated that LG increased mitochondrial superoxide levels in HUVECs and use of a mitochondrial-targeted antioxidant, mito-TEMPOL, was successful in restoring nitric oxide levels during LG exposure (55). Given the correlation between mitochondrial superoxide and LG, we investigated the impact Drp1 inhibition had on superoxide levels. Human arterioles were subjected to LG conditions followed by exposure to MitoSOX, a fluorescent mitochondrial superoxide probe, and signal intensity monitored (Fig. 4B). Treatment of vessels with LG increased the superoxide signal compared with control (P < 0.02). The addition of Mdivi-1 to LG-treated vessels reduced superoxide by almost 40% when compared with vessels exposed to LG alone and recapitulated levels similar to those seen under NG conditions (P < 0.01). These data demonstrate that disruption of Drp1 activity during LG conditions prevents mitochondrial superoxide production within arterioles.
Impact of partial mitochondrial membrane depolarization on NO bioavailability and mitochondrial superoxide production under LG conditions.
We observed that suppression of Drp1 activity during LG exposure partially depolarized the mitochondrial membrane, and additionally, increased NO bioavailability and decreased mitochondrial superoxide levels. In an attempt to determine the role of MMP in this sequence of events, the effects of targeted MMP partial depolarization on NO bioavailability and superoxide levels in human arterioles subjected to LG were investigated. To accomplish this, vessels were incubated in LG buffer with CCCP (100 nmol/l), a membrane depolarization agent, while NO was monitored as described above (Fig. 5A). Vessels treated with CCCP had a twofold increase in the amount of DAF2-DA fluorescence compared with vessels incubated in LG alone (P < 0.03) with no impact on DAF2-DA fluorescence under NG conditions. This suggests that MMP hyperpolarization during LG exposure reduces NO levels and depolarization can rescue NO bioavailability.
Fig. 5.
Mitochondrial membrane depolarization improves bioavailability of NO and reduces mitochondrial superoxide in isolated arterioles. A: vessels were incubated with DAF-2DA with or without exposure to carbonyl cyanide m-chlorophenyl hydrazine (CCCP) (100 nmol/l), a membrane depolarizing agent. LG-treated vessels displayed a significant decrease in NO level compared with other treatment groups (P = 0.003 overall, *P < 0.03 for LG vs. all other exposures, N = 5), while membrane depolarization with CCCP increased NO levels approximating that of the NG+CCCP group. B: superoxide was detected with MitoSOX in the presence of CCCP (100 mol/l). LG-treated vessels displayed a significant increase in superoxide compared with other treatment groups based on increased fluorescence, while membrane depolarization with CCCP decreased MitoSox fluorescence (P < 0.001 overall, *P < 0.01 for LG vs. all other exposures, N = 4). Scale bars represent 50 μm.
Observation of mitochondrial superoxide under the same conditions yielded similar results (Fig. 5B). Superoxide production measured with MitoSOX increased within the LG group when compared with the NG group (P < 0.001). In the presence of CCCP, superoxide levels decreased and showed no significant difference when compared with the NG+CCCP group. These results indicate that increased MMP polarization results in increased superoxide production in human arterioles exposed to LG. These experiments taken together support the link between depolarization of the MMP and improved NO bioavailability and reduced mitochondrial superoxide production. In a similar fashion, perturbation of Drp1 activity had the same effects: depolarization of the MMP, increased NO, and decreased superoxide.
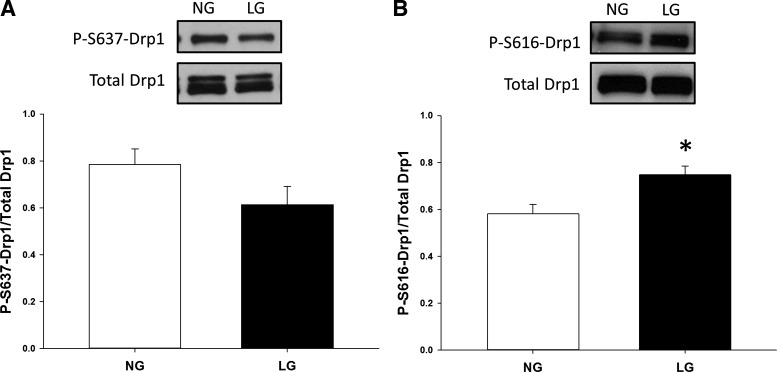
Drp1 activation under LG conditions.
We also wanted to investigate whether increased mitochondrial fragmentation is due to activation of Drp1 during LG conditions. Drp1 activation occurs in part through posttranslational modifications regarding the phosphorylation state of two distinct serine residues, Ser616 and Ser637 (9, 11, 54). Phosphorylation at Ser616 is thought to increase Drp1 activity, while, conversely, dephosphorylation at Ser637 also increases activity. To examine the effect LG has on the phosphorylation state of Drp1, endothelial cells were incubated in either NG or LG conditions for 2 h, and Western blot analysis was employed to assess phosphorylation level. LG-induced activation of Drp1 is favored by increased phosphorylation of Ser616 (P = 0.007) during LG exposure. Inactivating dephosphorylation at Ser637 under LG conditions trended toward significance, but did not reach it (P = 0.10) (Fig. 6). These data indicate that LG conditions may confer activating changes to Drp1 and drive more network fragmentation through increased Drp1 activity.
Fig. 6.
Posttranslational regulation of Drp1 via LG exposure. A: phosphorylation of Drp1 at Ser 637 acts as an inhibitor of Drp1 activity. Expression of P-S637-Drp1 was analyzed in HUVECs treated with LG via Western blot. Overall, the data appear to support that LG exposure decreases phosphorylation at Ser 637 and may allow for increased Drp1 activity and subsequent fission. B: conversely, phosphorylation of Drp1 at Ser 616 acts as an activating event and LG exposure appears to increase phosphorylation at this site (*P < 0.01, N = 10).
Bioenergetic implications of LG exposure.
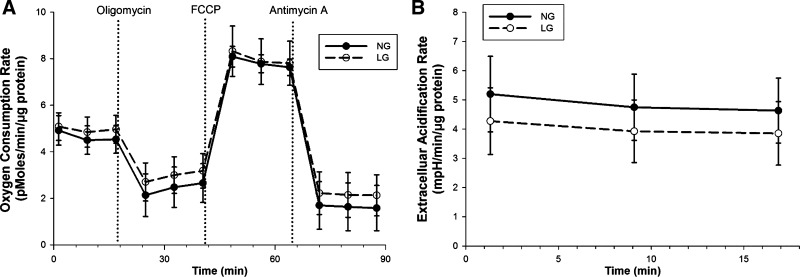
Glucose supplies a important portion of the energy requirements of endothelial cells, and alterations in substrate may alter the energy balance between glycolysis and oxidative phosphorylation. It is possible the changes in mitochondrial dynamics observed are the result of the mitochondria compensating for lost glycolytic energy. To examine this idea, utilization of oxygen by the electron transport chain was measured in HUVECs with a Seahorse XF96 Analyzer (Fig. 7). Treatment of endothelial cells with LG did little to alter the oxygen consumption rate in comparison with NG. This would indicate that the mitochondrial changes observed are not linked to metabolic alterations regarding mitochondrial function.
Fig. 7.
LG does not alter mitochondrial bioenergetics. A: endothelial cells were exposed to either NG or LG media and the rate of oxygen consumption was analyzed with a Seahorse XF96 Analyzer. B: glycolytic activity was also recorded concurrently during the basal data collection period. No significant differences were observed between the two groups. Data represent results from four independent experiments (P = not significant at all data points).
DISCUSSION
We proposed that clinically relevant LG exposure disturbs normal mitochondrial dynamics leading to excessive mitochondrial fission via Drp1 activation, and these changes would subsequently be responsible for LG-induced endothelial dysfunction. This study provided mechanistic insight into the effect of LG on endothelial cells and established three unique findings. First, endothelial cells subjected to a clinically relevant LG concentration exhibited an increase in mitochondrial fission that appears to be mediated through the primary fission protein Drp1. Second, human arterioles exposed to LG display the hallmarks of endothelial dysfunction with decreased NO bioavailability, increased ROS production, and impaired vasodilatory response. Last, when mitochondrial fission is suppressed either pharmacologically or molecularly, the impairment in endothelium-dependent vasodilatory response of vessels exposed to LG is reversed, showing a similar response to normal controls.
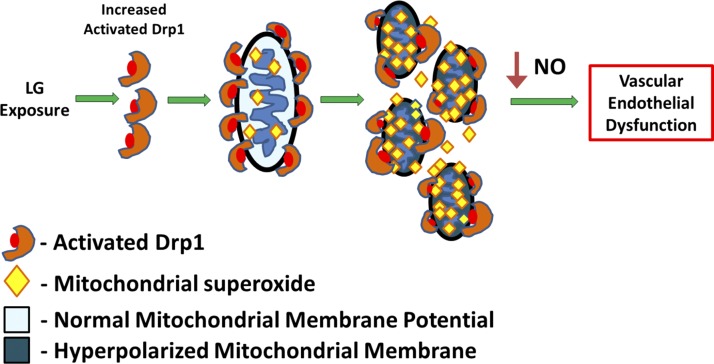
Importantly, many of our studies were performed in intact human arterioles, supporting the clinical relevance of our findings. Our data not only expand the knowledge regarding mechanisms for inducing endothelial dysfunction, but also suggest a potential therapeutic opportunity for reducing the impact of vascular disease in T2DM patients who experience hypoglycemia through inhibition of Drp1. The data indicate that a chain of events that start with LG exposure and ends with impaired endothelium-dependent vasodilation and support that a reduction in Drp1 activity could improve vascular function (Fig. 8).
Fig. 8.
Impairment of endothelial function through LG exposure. Acute LG conditions appear to increase mitochondrial fragmentation by creating a pro-fission state via activation of Drp1. This in turn causes hyperpolarization of the mitochondrial membrane potential, an increase in superoxide production, and subsequent decrease in NO bioavailability. Taken together, these modifications yield an impairment of human microvessels to vasodilate in an endothelium-dependent manner. Blockade of LG-induced mitochondrial fragmentation via Drp1 attenuates the adverse effects and restores vasodilation, indicating the potential of therapeutic targets to improve endothelial function.
The data support a situation in which the exposure of human arterioles to LG conditions results in a reduction in detectable NO that appears dependent on alterations in the mitochondrial fragmentation balance within endothelial cells. We believe the imbalance yields hyperpolarized vascular mitochondria, as demonstrated, and is coupled with increased generation of mitochondrial superoxide. Restoration of the fragmentation balance through inhibition of Drp1 activity ameliorates these responses and improves NO expression in intact arterioles, potentially via decreased scavenging by superoxide. This is further supported by the vascular function data where pharmacologic or molecular perturbation of Drp1 improved endothelial-dependent vasodilation in response to ACh.
Hypoglycemia is strongly associated with cardiovascular mortality (19, 39, 60, 61). LG exposure appears to attenuate the intended benefits from intensive glucose control in part through the induction and aggravation of endothelial dysfunction (8). Repeated exposure to LG has been shown to impair macrovascular endothelial function in patients with diabetes at least in part through a loss of NO bioavailability (23). Acute exposure to systemic hypoglycemia impairs endothelium-dependent vasodilation and increases systemic inflammatory markers along with cell adhesion molecule expression for an extended period in both T2DM and non-T2DM subjects (8, 23, 32). Our prior data strongly suggested mitochondrial changes may play a key role in LG-induced endothelial dysfunction in humans with T2DM by way of increased mitochondrial superoxide (36). The current data build on those findings by suggesting a Drp1-dependent mechanism for the loss of NO bioavailability and impaired endothelium-dependent vasodilation, which was reversed when arterioles from T2DM subjects were treated with Mdivi-1. Interestingly, vessels sourced from T2DM subjects displayed similar levels of vascular impairment under either NG or LG conditions. Acute exposures to abnormal glucose concentrations impair endothelial function and increase mitochondrial fission, supporting a role for glucose in the development of endothelial dysfunction during diabetes (14, 35, 46, 55). While the mechanism of vascular endothelial dysfunction in diabetes involves multiple cellular processes, insights into the epigenetic changes during diabetes resulting in vascular disease provide a link between repeated transient abnormal glucose exposures and chronic adverse changes in the vascular phenotype that may be regulated to abnormal mitochondrial function (17, 30). These epigenetic changes manifest as a type of “molecular memory” that dictates an impaired phenotype even though the stimulus has been removed. This may explain why the NG-treated group of vessels from T2DM subjects was as impaired as the LG-exposed group of these vessels, while both were improved in response to Mdivi-1.
The mechanism by which LG signals for Drp1 activation remains to be elucidated, but evidence suggest that it could be governed by alterations of O-linked glycosylation of Drp1, which also have a strong interplay with protein phosphorylation, and this potential signaling pathway merits further investigation (21, 25, 26). Interestingly, our data also suggest that LG does not alter mitochondrial function as it pertains to energy production, implying the changes observed are not in response to an energy deficit and the increased production of ROS is not linked to increased electron transport chain flux as proposed by Brownlee and colleagues (4, 5) during instances of hyperglycemia. The underlying pathophysiologic cause of LG-induced ROS production remains an interesting area for further exploration.
While the overall regulation of mitochondrial dynamics is still being explored, under normal homeostatic conditions, factors encouraging fission, including mitochondrial fission protein 1, dynamin-2, and Drp-1, and those promoting fusion, including mitofusin-1 and mitofusin-2 are balanced. However, external conditions can disrupt this balance. There are numerous biological implications due to an imbalance in the fission/fusion process. Increased mitochondrial fission has been implicated to be a detrimental phenotype resulting in altered mitochondrial morphology, increased oxidative stress, and cellular apoptosis (3, 27, 41, 42, 58, 62, 63). Relevant to our work, Shenouda et al. (50) demonstrated that expression of the fission proteins Drp1 and Fis1 is increased in endothelial cells harvested from diabetic patients, as well as marked mitochondrial fragmentation within these cells. Mitochondrial fragmentation coupled with elevated superoxide levels is observed in endothelial cells from hearts of diabetic mice (38). Mitochondrial fission has also been implicated in ischemia-reperfusion injury studies where Drp1 is activated during ischemia-reperfusion events and pharmacologic inhibition of Drp1 improved mitochondrial morphology and prevented cell death (42, 49). These studies indicate an association between mitochondrial fragmentation and vascular dysfunction, which may be alleviated by targeting the fission process.
Paradoxically, other works indicate that genetic knockout of Drp1 results in lethal cardiomyopathies in mice (31, 34, 53). Additionally, ablation of Drp1 or loss of function mutation caused impairment of normal autophagic processes necessary to maintain normal mitochondrial function and ultimately lead to mitochondrial dysfunction presented as impaired respiratory function coupled with a decline in ATP production in either mouse embryonic fibroblasts or cardiac myocytes (6, 29, 34). Altered mitochondrial morphology was also observed as impaired fission or elongation of mitochondrial networks (6, 29, 34). We believe these data support the importance of a proper balance of mitochondrial fusion and fission forces to maintain normal cellular function. Our data support this concept by suggesting that reducing excessive mitochondrial fission under LG conditions is beneficial. However, we suspect complete abrogation of Drp1 activity would be detrimental by driving excessive mitochondrial elongation and subsequent dysfunction in other cell types. This concept is corroborated by our experience that significantly Mdivi-1 concentrations two to three orders of magnitude higher than those used in this study have been shown to be toxic to cells in prior work (44, 56). Our work expands upon the knowledge of negative phenotypes associated with fission/fusion imbalance by demonstrating a clear impact of increased mitochondrial fission through Drp1 activation leading to impaired NO bioavailability and defective endothelium-dependent vasodilation in human vessels.
Our study has some limitations. Use of MitoSOX to detect mitochondrial ROS fluorescently has come under scrutiny regarding its ability to detect superoxide specifically, but it is accepted as a marker of mitochondrial oxidants. Its use is supported by our previous work, showing a change in MitoSOX intensity in response to various mitochondrial antioxidants, such as mito-TEMPOL (36, 55). Employment of DAF2-DA to detect NO, while having specificity, suffers from interference from background sources and limited photostability, but improved signal intensity vs. alternate probes. We chose fluorescent detection of NO in arterioles over activity assays and protein regulation because of the short life of NO and the small amount produced from relatively few endothelial cells in our small vessel samples precluding other measurement methods. Also, while we had success in imaging mitochondrial networks in cultured endothelial cells, it has proven difficult to directly observe these organelles in the endothelium within our human arteriole samples. This is due to a lack of fidelity in introducing the mitochondrial probe into the limited number of cells present in a tissue sample and an inability to target endothelial cells specifically in exclusion of the vascular smooth muscle cells surrounding the vessel lumen. Current methodologies do not offer the feasibility to directly image the endothelial cells of isolated arterioles. However, the same pharmacologic and molecular perturbation of Drp1 resulting in decreased mitochondrial fission among HUVECs also improved vasodilation in human arterioles, indicating the vascular response is linked to mitochondrial fission. Balanced against these limitations are the originality of our findings and the novel mechanistic insight they provide concerning regulation of human endothelial function.
Our study describes a novel mechanism by which LG induces human endothelial dysfunction. This allows us to better cast LG as a potential mechanistic contributor to the adverse cardiovascular events associated with clinical hypoglycemia and intensive glycemic control. The central findings demonstrate that brief exposure to hypoglycemia levels of LG induced mitochondrial fission among endothelial cells, leading to decreased NO bioavailability, mitochondrial membrane hyperpolarization, increased ROS production, and impaired endothelium-dependent vasodilatory responses in isolated human arterioles. Subsequent interventions to inhibit or reverse the LG-induced fission response resulted in a great improvement in all measures, seeming to prevent the negative phenotypes associated with endothelial dysfunction. The use of intact human arterioles allows us to impart strong human translational relevance to the data generated in the study and, therefore, hopefully brings us closer to understanding the mechanisms behind clinical endothelial dysfunction that seem to precede adverse cardiovascular events in patients with diabetes and hypoglycemic exposures. Insight into the causal links between clinical hypoglycemia and cardiovascular complications among patients with T2DM creates an opportunity to develop targeted pharmacological intervention designed to alleviate the adverse cardiovascular manifestations caused by diabetes.
GRANTS
M. Widlansky received funding from National Heart, Lung, and Blood Institute Grants K23 HL-089326, P01 HL-081587, and R01 HL-125409; the Doris Duke Foundation; and Merck Sharp & Dohme Corp. J. Wang was supported by National Heart, Lung, and Blood Institute GrantT32 HL-007792. M. Tanner was supported by a predoctoral fellowship from the Midwest Affiliate of the American Heart Association (14PRE18710040).
DISCLOSURES
M.E.W. has a significant research contribution from Merck Sharp and Dohme Corp and Everist Health. All other authors have no disclosures to report.
AUTHOR CONTRIBUTIONS
M.J.T, J.W., R.Y. performed experiments. T.B.S., M.M., and M.S. performed tissue biopsies and provided samples from human subjects. A.C. and A.B. coordinated subject recruitment and enrollment. M.J.T. drafted the manuscript, analyzed the data, prepared figures, and interpreted the results. M.J.T. and M.E.W edited and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Blake Hill (Department of Biochemistry, Medical College of Wisconsin) for critical review of this manuscript and expertise in mitochondrial fission proteins.
REFERENCES
- 1.Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 58: 360–366, 2009. doi: 10.2337/db08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedenis R, Price AH, Robertson CM, Morling JR, Frier BM, Strachan MWJ, Price JF. Association between severe hypoglycemia, adverse macrovascular events, and inflammation in the Edinburgh Type 2 Diabetes Study. Diabetes Care 37: 3301–3308, 2014. doi: 10.2337/dc14-0908. [DOI] [PubMed] [Google Scholar]
- 3.Brady NR, Hamacher-Brady A, Gottlieb RA. Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochim Biophys Acta 1757: 667–678, 2006. doi: 10.1016/j.bbabio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Cahill TJ, Leo V, Kelly M, Stockenhuber A, Kennedy NW, Bao L, Cereghetti G, Harper AR, Czibik G, Lao C, Bellahcene M, Steeples V, Ghaffari S, Yavari A, Mayer A, Poulton J, Ferguson DJP, Scorrano L, Hettiarachchi NT, Peers C, Boyle J, Hill RB, Simmons A, Watkins H, Dear TN, Ashrafian H. Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure. J Biol Chem 290: 25907–25919, 2015. doi: 10.1074/jbc.M115.665695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Bucciarelli L, Rondinelli M, Genovese S. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care 36: 4104–4108, 2013. doi: 10.2337/dc13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R, Bonfigli AR, Esposito K, Giugliano D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 61: 2993–2997, 2012. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 10.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci 1201: 34–39, 2010. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens 25: 528–534, 2012. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med 51: 1621–1635, 2011. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108: 1341–1348, 2001. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 16.Duh E, Feinglos M. Hypoglycemia-induced angina pectoris in a patient with diabetes mellitus. Ann Intern Med 121: 945–946, 1994. doi: 10.7326/0003-4819-121-12-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 17.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417, 2008. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol 32: 467–473, 2012. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hébert PC, Heyland DK, Robinson BG; NICE-SUGAR Study Investigators . Hypoglycemia and risk of death in critically ill patients. N Engl J Med 367: 1108–1118, 2012. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 20.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 34, Suppl 2: S132–S137, 2011. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR III, Hoshijima M, Dillmann W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem 287: 30024–30034, 2012. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med 52: 348–356, 2012. doi: 10.1016/j.freeradbiomed.2011.10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giménez M, Gilabert R, Monteagudo J, Alonso A, Casamitjana R, Paré C, Conget I. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care 34: 198–203, 2011. doi: 10.2337/dc10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha WC, Oh SJ, Kim JH, Lee JM, Chang SA, Sohn TS, Son HS. Severe hypoglycemia is a serious complication and becoming an economic burden in diabetes. Diabetes Metab J 36: 280–284, 2012. doi: 10.4093/dmj.2012.36.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 26.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80: 825–858, 2011. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hom J, Yoon Y, Nadtochiy S, Brookes P, Sheu SS. Calcium-mediated translocation of fission protein DLP1 to mitochondria and augmentation of reactive oxygen species (ROS) levels in heart. Biophys J 96: 245a, 2009. doi: 10.1016/j.bpj.2008.12.1205. [DOI] [Google Scholar]
- 28.Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, Trivedi K, Weir EK, Archer SL. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res 112: 802–815, 2013. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116: 264–278, 2015. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 30.Intine RV, Sarras MP Jr. Metabolic memory and chronic diabetes complications: potential role for epigenetic mechanisms. Curr Diab Rep 12: 551–559, 2012. doi: 10.1007/s11892-012-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, Mizushima N, Mihara K, Ishihara N. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol 35: 211–223, 2015. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 34: 1164–1170, 2011. doi: 10.2337/dc10-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joy NG, Tate DB, Younk LM, Davis SN. Effects of acute and antecedent hypoglycemia on endothelial function and markers of atherothrombotic balance in healthy humans. Diabetes 64: 2571–2580, 2015. doi: 10.2337/db14-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J 33: 2798–2813, 2014. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemeny SF, Figueroa DS, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One 8: e66176, 2013. doi: 10.1371/journal.pone.0066176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kizhakekuttu TJ, Wang J, Dharmashankar K, Ying R, Gutterman DD, Vita JA, Widlansky ME. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol 32: 2531–2539, 2012. doi: 10.1161/ATVBAHA.112.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol 290: R57–R65, 2006. doi: 10.1152/ajpregu.00424.2005. [DOI] [PubMed] [Google Scholar]
- 38.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53: 1783–1794, 2010. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenström A; DIGAMI 2 Investigators . Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 26: 650–661, 2005. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 40.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang Y-H, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong S-B, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res 88: 16–29, 2010. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong S-B, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 43.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 44.Qian W, Wang J, Roginskaya V, McDermott LA, Edwards RP, Stolz DB, Llambi F, Green DR, Van Houten B. Novel combination of mitochondrial division inhibitor 1 (Mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells. Oncotarget 5: 4180–4194, 2014. doi: 10.18632/oncotarget.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J 26: 2175–2186, 2012. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salt IP, Morrow VA, Brandie FM, Connell JMC, Petrie JR. High glucose inhibits insulin-stimulated nitric oxide production without reducing endothelial nitric-oxide synthase Ser1177 phosphorylation in human aortic endothelial cells. J Biol Chem 278: 18791–18797, 2003. doi: 10.1074/jbc.M210618200. [DOI] [PubMed] [Google Scholar]
- 47.Santel A, Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 60: 448–455, 2008. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 48.Sardar P, Udell JA, Chatterjee S, Bansilal S, Mukherjee D, Farkouh ME. Effect of intensive versus standard blood glucose control in patients with type 2 diabetes mellitus in different regions of the world: systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 4: e001577, 2015. doi: 10.1161/JAHA.114.001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28: 316–326, 2014. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess M-A, Levit A, Kim B, Hartman M-L, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124: 444–453, 2011. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirnova E, Griparic L, Shurland D-L, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommerfield AJ, Wilkinson IB, Webb DJ, Frier BM. Vessel wall stiffness in Type 1 diabetes and the central hemodynamic effects of acute hypoglycemia. Am J Physiol Endocrinol Metab 293: E1274–E1279, 2007. doi: 10.1152/ajpendo.00114.2007. [DOI] [PubMed] [Google Scholar]
- 53.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW II. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21: 273–285, 2015. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32: 712–720, 2012. doi: 10.1161/ATVBAHA.111.227389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Li J, Santana-Santos L, Shuda M, Sobol RW, Van Houten B, Qian W. A novel strategy for targeted killing of tumor cells: induction of multipolar acentrosomal mitotic spindles with a quinazolinone derivative mdivi-1. Mol Oncol 9: 488–502, 2015. doi: 10.1016/j.molonc.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 156: 15–25, 2010. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol 298: H633–H642, 2010. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 33: 1591–1597, 2010. doi: 10.2337/dc10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yakubovich N, Gerstein HC. Serious cardiovascular outcomes in diabetes: the role of hypoglycemia. Circulation 123: 342–348, 2011. doi: 10.1161/CIRCULATIONAHA.110.948489. [DOI] [PubMed] [Google Scholar]
- 61.Younk LM, Davis SN. Hypoglycemia and vascular disease. Clin Chem 57: 258–260, 2011. doi: 10.1373/clinchem.2010.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653–2658, 2006. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu T, Sheu S-S, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341–351, 2008. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]