Sulfate clearance is decreased in chronic heart failure patients compared with healthy individuals. Among patients, sulfate clearance is positively associated with favorable disease outcome, i.e., a decreased rehospitalization rate and increased patient survival. Hence, decreased sulfate clearance may be involved in the pathophysiology of heart failure.
Keywords: chronic heart failure, sulfate, renal handling, hydrogen sulfide, disease outcome
Abstract
New leads to advance our understanding of heart failure (HF) pathophysiology are urgently needed. Previous studies have linked urinary sulfate excretion to a favorable cardiovascular risk profile. Sulfate is not only the end product of hydrogen sulfide metabolism but is also directly involved in various (patho)physiological processes, provoking scientific interest in its renal handling. This study investigates sulfate clearance in chronic HF (CHF) patients and healthy individuals and considers its relationship with disease outcome. Parameters related to renal sulfate handling were determined in and compared between 96 previously characterized CHF patients and sex-matched healthy individuals. Among patients, sulfate clearance was analyzed for associations with clinical and outcome parameters. In CHF patients, plasma sulfate concentrations are significantly higher, whereas 24-h urinary excretion, fractional excretion, and clearance of sulfate are significantly lower, compared with healthy individuals. Among patients, sulfate clearance is independently associated with diuretics use, creatinine clearance and 24-h urinary sodium excretion. Sulfate clearance is associated with favorable disease outcome [hazard ratio per SD increase 0.38 (95% confidence interval 0.23–0.63), P < 0.001]. Although significance was lost after adjustment for creatinine clearance, the decrease of sulfate clearance in patients is independent of this parameter, indicating that sulfate clearance is not merely a reflection of renal function. This exploratory study reveals aberrant sulfate clearance as a potential contributor to CHF pathophysiology, with reduced levels in patients and a positive association with favorable disease outcome. Further research is needed to unravel the nature of its involvement and to determine its potential as a biomarker and target for therapy.
NEW & NOTEWORTHY Sulfate clearance is decreased in chronic heart failure patients compared with healthy individuals. Among patients, sulfate clearance is positively associated with favorable disease outcome, i.e., a decreased rehospitalization rate and increased patient survival. Hence, decreased sulfate clearance may be involved in the pathophysiology of heart failure.
despite therapeutic advances, heart failure (HF) remains a leading cause of morbidity and mortality, in particular among the elderly (33). In fact, as the world is aging, the burden of HF is anticipated to increase in coming years (20). Accordingly, there is a pressing need for new leads to further our understanding of the pathophysiology of HF.
In this context, renal handling of sulfate is of interest. Indeed, a study of renal transplant recipients has shown that urinary sulfate excretion is positively associated with a favorable cardiovascular risk profile (31). This profile includes a lower serum level of NH2-terminal pro-B-type natriuretic peptide (NT-proBNP), which is an established marker of HF progression (13).
Sulfate is the fourth most abundant anion in human plasma and essential to all cells (14). It is derived from the diet, both directly and through oxidation of the sulfur-containing amino acids (SAAs) cysteine and methionine (14, 23). The latter links sulfate to the production of hydrogen sulfide (H2S), which is a reactive metabolite formed in the so-called transsulfuration pathway. H2S has been recognized as a cardiovascular signaling molecule with various protective properties (22). Furthermore, by way of sulfate conjugation, or sulfation, sulfate itself is involved in the biotransformation and detoxification of many endogenous and exogenous substances. Sulfated proteoglycans, for example, are indispensible for the structural integrity of cells and tissues (5, 14). Despite its physiological importance, sulfate has also been connected to pathology through sulfation of toxic intermediates derived form dietary compounds, which in turn have been implicated in carcinogenesis, as well as heart failure events (2, 9, 34). Even though sulfation is generally considered to increase hydrophilicity and thereby to promote excretion, the way in which sulfate conjugation relates to the toxicity of these compounds remains to be elucidated (4).
Sulfate homeostasis is maintained by the kidneys, which freely filter sulfate and then reabsorb it in the proximal tubule. Under normal conditions up to 90% of the filtered sulfate is reabsorbed (15, 19, 27). This active reabsorption is capacity limited, but also dynamic as the expression of the sodium/sulfate cotransporter NaS1 is up- or downregulated in response to various stimuli. Known positive stimuli include pregnancy, thyroid hormone, growth hormone, and vitamin D, whereas negative stimuli include the diet sulfate content and medication, such as nonsteroidal anti-inflammatory drugs (15). Furthermore, in children with chronic kidney disease even net secretion of sulfate has been described, underlining the dynamic nature of renal sulfate handling (18).
The present study aimed at exploring changes in sulfate clearance in CHF patients compared with healthy individuals and assessing its relation to disease outcome.
MATERIALS AND METHODS
Study subjects.
This study is a post hoc analysis of an open-label, blinded end point, randomized prospective trial (VitD-CHF trial) (24). In the period of March 2010 to November 2011, 101 stable CHF patients presenting at the outpatient clinic of the University Medical Center Groningen, in Groningen, The Netherlands were included in this trial. These patients were ≥18 yr of age, had a left ventricular ejection fraction (LVEF) <45%, and were treated with optimal HF medication [i.e., angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs), β-blockers, and mineralocorticoid-receptor antagonists (MRAs) when indicated]. Study participants have previously been described in more detail (17, 24). Patients were randomized to receive either a daily dose of 2,000 IU of vitamin D3 (vitD) or no extra medication for 6 wk. For the current analysis baseline samples were used, which were taken before the start of vitD treatment. Due to unavailability of plasma or urine samples, 5 patients had to be excluded, leaving 96 eligible for analysis. For comparison a group of 96 sex-matched healthy subjects was included, consisting of individuals who were approved for living kidney donation in our center. None had a history of cardiovascular events. The study was conducted in accordance with the Declaration of Helsinki and the local Institutional Review Board approved the study protocol. All CHF patients and healthy subjects included in this analysis provided written informed consent.
Baseline characteristics.
Data on participants’ disease state, medical history and medication were extracted from patient records. Systolic and diastolic blood pressure and heart rate were measured according to protocol. The body mass index (BMI) was calculated by dividing body weight by height squared. Participants were instructed to collect 24-h urine the day before visiting the outpatient clinic. On the day of the visit, after an overnight fast, serum and plasma samples were obtained and routine laboratory measurements, including NH2-terminal pro-B-type natriuretic peptide (NT-proBNP), albumin, total protein, creatinine, urinary albumin, creatinine and sodium, HbA1c, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), calcium, and parathyroid hormone (PTH), were performed. Aliquots of blood and urine samples were stored at −80°C for future analysis. Measurements of plasma renin concentration (PRC), plasma renin activity (PRA), and aldosterone have been described before (24). The estimated glomerular filtration rate (eGFR) was determined using the four-variable Modification of Diet in Renal Disease (MDRD) formula. Creatinine clearance was calculated as follows: [urine concentration (mM) × [volume (ml) of 24-h urine/urine collection time (min)]/plasma concentration (mM).
Sulfate clearance.
Plasma and urinary sulfate concentrations were measured by means of a validated ion-exchange chromatography assay (type 861; Metrohm, Herisau, Switzerland). The intra- and interassay coefficients of variation were 3.4 and 6.1% for plasma and 2.0% and 4.3% for urine analysis, respectively. Fractional excretion of sulfate was calculated using the following formula: [urinary sulfate (mM) × plasma creatinine (mM)]/[plasma sulfate (mM) × urinary creatinine (mM)] × 100. Sulfate clearance was calculated as follows: [urine concentration (mM) × [volume (ml) of 24-h urine/urine collection time (min)]/plasma concentration (mM).
Outcome parameter.
The outcome parameter of this study is composed of HF-related rehospitalization and all-cause mortality. The mean follow-up period was 5.1 ± 0.5 yr. No patients were lost to follow-up.
Statistical analysis.
Healthy subjects were matched to CHF patients based on sex using point-and-click case-control matching in SPSS (version 22; IBM, Armonk, NY).
Statistical analysis was performed with STATA software (version 14.0; Stata, College Station, TX). Graphs were drawn in GraphPad Prism (version 5.0; GraphPad Software, La Jolla, CA).
The distribution of all variables was examined using histograms and probability plots. Normally distributed continuous data are presented as means ± SD. Skewed data are presented as median [interquartile range (IQR)] and were normalized by logarithmic transformation for analysis. Nominal data are presented as n (%).
The mean ages of CHF patients and healthy subjects were compared by means of the Student’s t-test. Linear regression analysis was applied to assess the association between sulfate clearance and age among healthy individuals. Differences in plasma and urinary sulfate concentrations, creatinine clearance, fractional excretion of sulfate, and sulfate clearance between CHF patients and healthy subjects were also studied using linear regression analysis, which allowed us to correct for age and, when testing the difference in sulfate clearance, for creatinine clearance.
Univariable and multivariable linear regression analyses were performed on data from CHF patients to identify variables that are independently associated with sulfate clearance. Next, these variables were included in a Cox proportional hazard model. Associations are shown with sulfate clearance as a continuous variable. Hazard ratios are shown per SD increase for normally distributed data and per doubling for logarithmically transformed skewed data.
All reported P values are two tailed. Values of P < 0.05 were considered statistically significant.
RESULTS
Patient characteristics.
Baseline characteristics of the 96 stable CHF patients are presented in Table 1. The mean age of the study subjects was 63 ± 10.1 yr and 89 (93%) were male. The median duration of HF was 61 (29–106) mo. Most patients were categorized in New York Heart Association (NYHA) class II (n = 85, 89%) and their mean LVEF was 34.8 ± 8.3%. All patients were treated with medication according to the current European Society of Cardiology guidelines, including ACEi/ARBs (n = 96, 100%), β-blockers (n = 93, 97%), MRAs (n = 28, 29%), and diuretics (mainly furosemide, n = 49, 49%).
Table 1.
Baseline characteristics CHF patients
| Stable CHF Patients | |
|---|---|
| Characteristics, n | 96 |
| Age, yr | 63.4 ± 10.1 |
| Male, n (%) | 89 (93) |
| Current smoker, n (%) | 22 (23) |
| BMI, kg/m2 | 28 ± 4.4 |
| Systolic blood pressure, mmHg | 116 ± 16.9 |
| Diastolic blood pressure, mmHg | 70.9 ± 10.3 |
| Heart rate, beats/min | 67.7 ± 9.3 |
| HF history | |
| Duration HF,* mo | 61 (29–106) |
| Ischemic etiology, n (%) | 68 (71) |
| NYHA class II/III, n (%) | 85/11 (89/11) |
| LVEF, % | 34.8 ± 8.3 |
| Medication | |
| ACEi/ARB, n (%) | 96 (100) |
| β-Blocker, n (%) | 93 (97) |
| MRA, n (%) | 28 (29) |
| Diuretic, n (%) | 47 (49) |
| Laboratory measurements | |
| NT-proBNP,* ng/l | 381 (200–904) |
| Serum albumin, g/l | 44.4 ± 2.4 |
| Total serum protein, g/l | 72.2 ± 3.9 |
| eGFR, ml/min/1.73m2 | 80.6 ± 16.3 |
| Creatinine clearance, ml/min | 97.6 ± 31.3 |
| 24-h Urinary albumin, mg/24 h | 41.7 ± 207 |
| 24-h Urinary sodium, mmol/24 h | 166 ± 75.7 |
| HbA1C, % | 6.1 ± 0.6 |
| Cholesterol, mmol/l | 4.5 ± 1.1 |
| HDL, mmol/l | 1.2 ± 0.4 |
| LDL, mmol/l | 2.7 ± 0.9 |
| Calcium, mmol/l | 2.3 ± 0.1 |
| PTH, pmol/l | 7.6 ± 4.0 |
| PRC,* ng/l | 58.7 (17.1–195) |
| PRA,* ng/ml/h | 5.1 (1.4–20.6) |
| Aldosterone,* pmol/l | 0.3 ± 0.4 |
| VitD supplementation† | 48 (50) |
| 1,25(OH)2D, pmol/l | 143.9 ± 44.91 |
Normally distributed continuous data are presented as means ± SD. CHF, chronic heart failure; BMI, body mass index; HF, heart failure; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid-receptor antagonists; NT-proBNP, NH2-terminal pro-B-type natriuretic peptide; HDL, high density lipoprotein; LDL, low density lipoprotein; PTH, parathyroid hormone; PRC, plasma renin concentration; PRA, plasma renin activity; VitD, vitamin D3 (cholecalciferol); IQR, interquartile range.
Skewed data are presented as median (IQR).
2,000 IU of VitD daily for 6 wk.
Renal sulfate handling in CHF patients and healthy individuals.
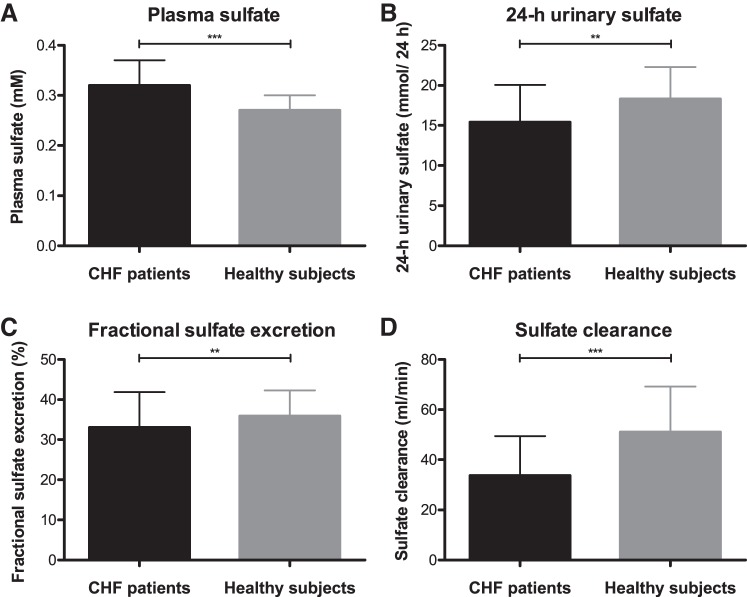
Table 2 shows the plasma sulfate concentration, 24-h urinary excretion of sulfate, creatinine clearance, fractional excretion of sulfate and sulfate clearance in CHF patients and healthy subjects. Because of the association between sulfate clearance and age in CHF patients (Table 3, coefficient: −0.545, P < 0.001), as well as in healthy individuals (coefficient: −0.378, P < 0.001) and patients being significantly older compared with healthy subjects (63.4 ± 10.1 vs. 51.9 ± 10.7 yr, P < 0.001), a correction for age was applied when comparing these groups. Linear regression analysis demonstrated that 24-h urinary sulfate excretion (15.5 ± 6.2 vs. 19.4 ± 6.8, P = 0.007), fractional excretion of sulfate [33.1 (25.5–41.9) vs. 35.9 (31.6–42.3), P = 0.005], and sulfate clearance [33.7 ± 15.7 vs. 51.0 ± 18.2, P < 0.001) are significantly lower in patients, whereas their plasma sulfate concentration (0.34 (0.29–0.37) vs. 0.27 (0.24–0.30), P < 0.001] is significantly higher. These differences are illustrated in Fig. 1. Even though creatinine clearance (97.6 ± 31.3 vs. 132.6 ± 34.0, P < 0.001) is also lower in patients compared with healthy individuals, the difference in sulfate clearance between these groups remained significant after additional adjustment for this parameter (P = 0.005).
Table 2.
Parameters related to renal sulfate handling in CHF patients and healthy individuals
| Parameters | CHF Patients (n = 96) | Healthy Subjects (n = 96) | P Value† |
|---|---|---|---|
| Plasma sulfate,* mM | 0.34 (0.29–0.37) | 0.27 (0.24–0.30) | <0.001‡ |
| 24-h Urinary sulfate, mmol/24 h | 15.5 ± 6.2 | 19.4 ± 6.8 | 0.007‡ |
| Creatinine clearance, ml/min | 97.6 ± 31.3 | 133 ± 34.0 | <0.001‡ |
| Fractional sulfate excretion,* % | 33.1 (25.5–41.9) | 35.9 (31.6–42.3) | 0.005‡ |
| Sulfate clearance, ml/min | 33.7 ± 15.7 | 51.0 ± 18.2 | 0.001‡ |
Normally distributed continuous data are presented as mean ± SD. CHF, chronic heart failure; IQR, interquartile range.
Skewed data are presented as median (IQR) and were normalized by logarithmic transformation for analysis.
Based on linear regression analysis, corrected for age. ‡Significant P value.
Table 3.
Univariable and multivariable linear regression analyses of sulfate clearance and clinical parameters in CHF
| Univariable Regression for Sulfate Clearance |
Multivariable Regression for Sulfate Clearance |
|||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Characteristics | ||||
| Age | −0.545 | <0.001‡ | ||
| Male | −11.354 | 0.066 | ||
| Current smoker | −0.975 | 0.710 | ||
| BMI | 0.141 | 0.703 | ||
| Systolic blood pressure | 0.035 | 0.713 | ||
| Diastolic blood pressure | 0.275 | 0.079 | ||
| Heart rate | 0.188 | 0.281 | ||
| Heart failure history | ||||
| Duration HF* | −0.793 | 0.649 | ||
| Ischemic etiology | −2.542 | 0.475 | ||
| NYHA class II/III | −14.112 | 0.005‡ | ||
| LVEF | −0.040 | 0.838 | ||
| Treatment | ||||
| β-Blocker | 10.838 | 0.243 | ||
| MRA | −5.285 | 0.136 | ||
| Diuretic | −9.389 | 0.003‡ | −4.322 | 0.048‡ |
| Laboratory measurements | ||||
| NT-proBNP* | −6.689 | <0.001‡ | ||
| Serum albumin | 1.877 | 0.004‡ | ||
| Total serum protein | −0.651 | 0.117 | ||
| Creatinine clearance | 0.369 | <0.001‡ | 0.331 | <0.001‡ |
| 24-h Urinary albumin | 0.046 | 0.098 | ||
| 24-h Urinary sodium | 0.177 | <0.001‡ | 0.077 | 0.023‡ |
| HbA1C | 2.422 | 0.356 | ||
| Cholesterol | 1.547 | 0.315 | ||
| HDL | −7.268 | 0.088 | ||
| LDL | 1.930 | 0.271 | ||
| Calcium | −21.940 | 0.257 | ||
| PTH | −0.446 | 0.270 | ||
| PRC* | −1.640 | 0.086 | ||
| PRA* | −1.623 | 0.060 | ||
| Aldosterone* | −4.281 | 0.032‡ | ||
| 1,25(OH)2D | 0.046 | 0.207 | ||
CHF, chronic heart failure; BMI; body mass index; HF, heart failure; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid-receptor antagonists; NT-proBNP; NH2-terminal pro-B-type natriuretic peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PTH, parathyroid hormone; PRC, plasma renin concentration; PRA, plasma renin activity.
Skewed data, normalized by logarithmic transformation. ‡Significant P value.
Fig. 1.
Parameters related to renal sulfate handling in chronic heart failure (CHF) patients and healthy individuals. In CHF patients, plasma sulfate concentration (A) is significantly higher, whereas 24-h urinary sulfate excretion, fractional excretion of sulfate, and sulfate clearance (B–D) are significantly lower compared with healthy individuals. Normally distributed data are presented as means ± SD (D). Skewed data are presented as median (interquartile range; A–C). P values are based on linear regression analysis, corrected for age: **P < 0.01, ***P < 0.001.
Factors associated with sulfate clearance in CHF patients.
Univariable and multivariable linear regression analyses showed that in CHF patients use of diuretics, creatinine clearance, and 24-h urinary sodium are independently associated with sulfate clearance (Table 3).
Renal sulfate handling and outcome in CHF patients.
During follow-up for 5.1 ± 0.5 yr, 12 patients (13%) were rehospitalized and 21 patients (22%) died. The composite outcome was recorded 29 times. Sulfate clearance was significantly higher in patients with favorable disease outcome, compared with those who were rehospitalized or died (37.7 ± 15.4 vs. 24.4 ± 12.2, P < 0.001). Rehospitalization and/or death occurred in eight patients (17%) with above-average sulfate clearance compared with 21 patients (44%) with below-average levels (log-rank test, P = 0.004). The corresponding Kaplan-Meier plot is shown as Fig. 2.
Fig. 2.
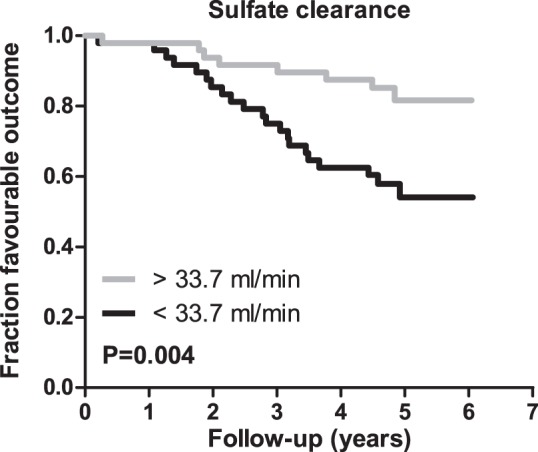
Kaplan-Meier analysis of the association of sulfate clearance above and below the mean with outcome in chronic heart failure (CHF). Kaplan-Meier plot with log-rank test for outcome (a composite of HF-related rehospitalization and all-cause mortality). Sulfate clearance above the mean is significantly associated with favorable outcome in stable CHF patients (P = 0.004).
As presented in Table 4, crude Cox regression analyses showed that 24-h urinary sulfate excretion {hazard ratio (HR) per SD increase 0.58 [95% confidence interval (CI) 0.39–0.89], P = 0.012} and sulfate clearance [HR per SD increase 0.38 (95% CI 0.23–0.63), P < 0.001] are positively associated with favorable disease outcome, i.e., a decreased rehospitalization rate and increased patient survival. Accordingly, the plasma sulfate concentration was found to be negatively associated with disease outcome [HR per doubling 4.45 (95% CI 1.94–10.2), P < 0.001].
Table 4.
Cox proportional hazards models of the association of the plasma sulfate concentration, 24-h urinary excretion of sulfate, fractional excretion of sulfate, and sulfate clearance with disease outcome in CHF
| Model | HR (95% CI) | P Value |
|---|---|---|
| 1: Plasma sulfate concentration* | 4.45 (1.94–10.2) | <0.001‡ |
| 2: 24-h Urinary sulfate excretion | 0.58 (0.39–0.89) | 0.012‡ |
| 3: Fractional excretion of sulfate* | 0.55 (0.26–1.19) | 0.130 |
| 4: Sulfate clearance | 0.38 (0.23–0.63) | <0.001‡ |
For normally distributed data the HR is presented per SD increase. CHF, chronic heart failure; HR, hazard ratio; CI, confidence interval.
For logarithmically transformed skewed data the HR is presented per doubling. ‡Significant P value.
While adjustment for use of diuretics and 24-h urinary sodium excretion only marginally affected the association of sulfate clearance with favorable disease outcome, adjustment for creatinine clearance caused significance to be lost [Table 5, model 6, HR per SD increase 0.59 (0.30–1.18), P = 0.140].
Table 5.
Cox proportional hazards model of the association of sulfate clearance with disease outcome in CHF, adjusted for associated clinical parameter separately and in conjunction
| Sulfate Clearance |
||
|---|---|---|
| Model | HR* (95% CI) | P value |
| 1: Crude | 0.38 (0.23–0.63) | <0.001‡ |
| 2: Adjusted for diuretics use | 0.42 (0.25–0.71) | 0.001‡ |
| 3: Adjusted for diuretics use and creatinine clearance | 0.71 (0.35–1.44) | 0.344 |
| 4: Adjusted for diuretics use and 24-h urinary sodium | 0.43 (0.25–0.75) | 0.003‡ |
| 5: Adjusted for diuretics use, creatinine clearance and 24-h urinary sodium | 0.72 (0.35–1.45) | 0.354 |
| 6: Adjusted for creatinine clearance | 0.59 (0.30–1.18) | 0.140 |
| 7: Adjusted for creatinine clearance and 24-h urinary sodium | 0.60 (0.30–1.19) | 0.149 |
| 8: Adjusted for 24-h urinary sodium | 0.39 (0.23–0.67) | 0.001‡ |
HR per SD increase. CHF, chronic heart failure; HR, hazard ratio; CI, confidence interval. ‡Significant P value.
Table 6 shows that the association of sulfate clearance with favorable disease outcome is independent of established prognostic factors in HF, age, eGFR, and NT-proBNP [model 4, HR 0.55 (95% CI 0.31–0.98), P = 0.042].
Table 6.
Cox proportional hazards model of the association of sulfate clearance and established prognostic factors with disease outcome in CHF
| Sulfate Clearance |
||
|---|---|---|
| Model | HR* (95% CI) | P value |
| 1: Crude | 0.38 (0.23–0.63) | <0.001‡ |
| 2: Adjusted for age | 0.46 (0.27–0.79) | 0.005‡ |
| 3: Adjusted for age and eGFR | 0.48 (0.28–0.83) | 0.009‡ |
| 4: Adjusted for age, eGFR and NT-proBNP | 0.55 (0.31–0.98) | 0.042‡ |
CHF, chronic heart failure; HR, hazard ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; NT-proBNP, NH2-terminal pro-B-type natriuretic peptide.
HR per SD increase. ‡Significant P value.
DISCUSSION
This exploratory study indicates that aberrant sulfate clearance is a potential contributor to the pathophysiology of CHF. Specifically, our data show that, compared with healthy individuals, CHF patients exhibit decreased sulfate clearance. Furthermore, among patients we have found sulfate clearance to be positively associated with favorable disease outcome, i.e., a decreased rehospitalization rate and increased patient survival. After adjustment for creatinine clearance this association was no longer significant. Sequentially, one might assume that the association of sulfate clearance with disease outcome is explained by its relationship with creatinine clearance, i.e., renal function. In this respect, it is important to note that creatinine clearance itself is very strongly associated with disease outcome in HF and thus leaves limited room for other variables to improve the proportional hazards model. Combined with limited statistical power, this likely explains the nonsignificant P value for the association of sulfate clearance with disease outcome when creatinine clearance is corrected for. Accordingly, in a larger cohort the association may well have remained significant. This assumption is substantiated by the fact that the decrease of sulfate clearance in patients compared with healthy individuals is independent of creatinine clearance, indicating that sulfate clearance is not merely a reflection of renal function and potentially provides new insight into the pathophysiology of HF.
While creatinine clearance caused significance to be lost, the association between sulfate clearance and disease outcome did retain its significance after adjustment for established prognostic factors in HF, age, eGFR, and NT-proBNP. This not only incites interest in the renal handling of sulfate but also underscores the need for cautiousness when eGFR is used to represent renal function. The latter applies to disease cohorts in particular, as in patients plasma creatinine levels may deviate, for example, due to lower muscle mass or increased catabolism.
The potential relevance of renal sulfate handling to CHF pathology lies in the connection of sulfate to H2S metabolism and the physiological importance of sulfation on the one hand and the apparent need for regulation of sulfate homeostasis and the implication of sulfated toxic intermediates in HF on the other.
To the best of our knowledge, we are the first to assess sulfate clearance in CHF patients. In general, very few studies have related renal handling of sulfate to outcome of disease. As mentioned above, a study of renal transplant recipients has reported urinary sulfate excretion to be associated with a favorable cardiovascular risk profile (31). Furthermore, in patients with type 1 and type 2 diabetes a high urinary sulfate concentration has been found to be associated with a reduced risk of renal disease progression (1, 32).
It is unclear whether urinary sulfate excretion primarily reflects intake and production of sulfate or rather the rate of sulfate clearance by the kidneys. Our study unfortunately lacks information on dietary intake. However, in renal transplant recipients and patients with diabetes mellitus type 1 nephropathy the association of sulfate excretion with all-cause mortality or renal disease progression was shown to be independent of dietary protein intake (1, 31). Perhaps sulfate production through oxidation of SAAs is a stronger determinant, especially because of the intermediate enzymatic production of H2S as part of this process that could explain the benefit of high urinary sulfate excretion (11). In relation to this, degradation of nonenzymatically produced H2S, through reduction of bound sulfur and by sulfate reducing bacteria in the gut represents another source (10, 26). H2S is a gaseous signaling molecule involved in numerous (patho)physiological processes, featuring vasodilatory, angiogenic, anti-apoptotic, anti-inflammatory, and antioxidant properties (21, 25, 30). Accordingly, various preclinical studies have convincingly shown H2S to be protective in HF (22). Unfortunately, reliable methods to capture with confidence the entire pool of H2S in biological samples are lacking, which impedes determination of the exact relationship between sulfate and H2S production. The only way to accomplish this experimentally would involve the use of radio- or stable-isotope tracer methodology, which to the best of our knowledge has not been carried out to quantify H2S metabolism to sulfate in humans. Besides, sulfate itself is an essential anion. The physiological importance of sulfation, pointed out above, is emphasized by the necessary increase of circulating sulfate concentrations in pregnancy (6). As the fetus is unable to generate sulfate, it relies completely on the maternal sulfate supply. Hyposulfatemia and consequent inadequate sulfation capacity have been linked to fetal growth abnormalities and even intrauterine death (6). The maternal increase of the serum sulfate concentration is mediated through upregulation of sulfate reabsorption in the kidneys, demonstrating the importance of renal handling to the regulation of sulfate homeostasis (3). The implication of sulfated toxic intermediates, such as indoxyl sulfate and p-cresyl sulfate, in HF led us to suppose that in fact sulfate clearance would be of interest in this context. As sulfation is generally considered to increase hydrophilicity and thereby to promote excretion of its targets, decreased sulfate clearance in HF may reflect a need for sulfate-mediated detoxification (4). However, the way in which sulfate conjugation relates to the toxicity of these specific compounds has not been shown. Furthermore, the benefit of increased sulfate clearance is substantiated by the finding that decreased sulfate reabsorption by knockout of the sodium/sulfate cotransporter NaS1 in mice resulted in an increase of their lifespan by ~25% (16). This was shown to be associated with significant upregulation of anti-aging genes, including Sirt1, Hdac3, and Cat, which other studies have linked to HF. Indeed, Sirt1 has been reported to be downregulated in cardiomyocytes of patients with advanced HF (12). Also, aberrant expression of the enzymes encoded by Hdac3 and Cat has been shown to affect myocardial lipid metabolism and antioxidant defense, respectively (7, 28, 29).
NaS1 has a major role in regulating the reabsorption of sulfate in the proximal tubule (15). As it facilitates sodium/sulfate cotransport, the association we have found between 24-h urinary sodium excretion and sulfate clearance is unsurprising. While one could argue that in HF sodium excretion is beneficial, as opposed to sodium retention, this could only in part explain the association of sulfate clearance with favorable disease outcome. Hence, as is the case for creatinine clearance, sulfate clearance is not just a reflection of sodium excretion either and presents an independent benefit in CHF patients.
To our knowledge, a relationship between use of diuretics and renal handling of sulfate has not been described. While the need to use a diuretic may simply reflect severity of disease, the association may also be related to the tubular sodium/sulfate cotransport. In this regard, sulfate clearance, through sodium excretion, may positively affect volume status, thereby decreasing the need to use a diuretic. Conversely, decreased sodium reabsorption by specific transporters, induced by a diuretic, perhaps results in a compensatory increase of sodium/sulfate cotransport, decreasing sulfate clearance.
Whereas a study in vitamin D-deficient rats has shown supplementation to upregulate NaS1 expression and, consequently, sulfate reabsorption, in our study vitamin D and sulfate clearance were not found to be associated. In contrast to the rats, CHF patients had relatively high vitamin D levels at baseline, suggesting that vitamin D is relevant to sulfate reabsorption only in the case of a deficiency (8).
Besides NaS1, two anion exchangers, Sat1 and CFEX, are also known to be involved in tubular sulfate reabsorption (15). Changes in the expression of these transporters have been linked to disease states, including chronic renal failure and hypothyroidism (14). The extent to which aberrant sulfate clearance in HF can be attributed to altered expression of sulfate transporters in the kidney remains to be elucidated. However, as sulfate reabsorption is capacity limited, without upregulation of tubular transport, one would expect the filtered load to start exceeding the reabsorption capacity with increasing plasma sulfate levels, resulting in increased fractional excretion (15). Interestingly, we have found fractional excretion to be lower in patients compared with healthy individuals in spite of higher plasma sulfate concentrations, suggesting that tubular reabsorption is upregulated in CHF.
Regardless of the involvement of specific transporters in the renal handling of sulfate, the exact mechanism through which a higher sulfate clearance leads to an improved outcome in HF is yet unclear. Hence, further research is warranted to substantiate the role of sulfate clearance in HF pathology.
In addition to the lack of dietary information, our study has other limitations. First of all, statistical power is limited by the small size of the CHF cohort and number of times the composite outcome was recorded. Furthermore, it is a single center study of Caucasian subjects, confining the validity of extrapolating our results to other ethnicities. Also, our study population mainly consists of males, precluding analysis of gender-related differences. Finally, because of its cross-sectional design, possible causality of the relationship between sulfate clearance and disease outcome could not be examined. Strengths of this study include the homogenous and extensive characterization of the CHF patients and the relatively long follow-up period of on average 5.1 yr.
In conclusion, sulfate clearance is reduced in CHF patients compared with healthy subjects and positively associated with favorable disease outcome among patients. Aberrant sulfate clearance may thus serve as a new lead to advance our understanding of the pathophysiology of HF. Further research is needed to unravel the nature of its involvement and to determine its potential as a biomarker and target for therapy.
GRANTS
This work was supported by grants from the Dutch Kidney Foundation (IP13–114 to H. van Goor), the Innovational Research Incentives Scheme Program of The Netherlands Organization of Scientific Research (VIDI Grant 917.13.350), The Netherlands Foundation for Cardiovascular Excellence (both to R. A. de Boer), The Netherlands Heart Foundation (Dekker Grant 2015 T034 to W. C. Meijers), and the Groningen University Institute for Drug Exploration (to A. M. Koning).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.M. and A. Post performed experiments; A.M.K. and W.C.M. analyzed data; A.M.K. prepared figures; A.M.K., W.C.M., I.M., A. Post, M.F., A. Pasch, H.G.D.L., R.A.d.B., S.J.L.B., and H.v.G edited and revised manuscript; H.v.G approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marinda Dekker and Marian Bulthuis for technical assistance.
REFERENCES
- 1.Andrésdóttir G, Bakker SJ, Hansen HP, Parving HH, Rossing P. Urinary sulphate excretion and progression of diabetic nephropathy in Type 1 diabetes. Diabet Med 30: 563–566, 2013. doi: 10.1111/dme.12131. [DOI] [PubMed] [Google Scholar]
- 2.Cao XS, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, Lv WL, Xiang FF, Tan X, Ding XQ. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol 10: 111–119, 2015. doi: 10.2215/CJN.04730514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole DE, Baldwin LS, Stirk LJ. Increased renal reabsorption of inorganic sulfate in third-trimester high-risk pregnancies. Obstet Gynecol 66: 485–490, 1985. [PubMed] [Google Scholar]
- 4.Coughtrie MW, Bamforth KJ, Sharp S, Jones AL, Borthwick EB, Barker EV, Roberts RC, Hume R, Burchell A. Sulfation of endogenous compounds and xenobiotics–interactions and function in health and disease. Chem Biol Interact 92: 247–256, 1994. doi: 10.1016/0009-2797(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 5.Dawson PA. Role of sulphate in development. Reproduction 146: R81–R89, 2013. doi: 10.1530/REP-13-0056. [DOI] [PubMed] [Google Scholar]
- 6.Dawson PA, Elliott A, Bowling FG. Sulphate in pregnancy. Nutrients 7: 1594–1606, 2015. doi: 10.3390/nu7031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich S, Bieligk U, Beulich K, Hasenfuss G, Prestle J. Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation 101: 33–39, 2000. doi: 10.1161/01.CIR.101.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes I, Hampson G, Cahours X, Morin P, Coureau C, Couette S, Prie D, Biber J, Murer H, Friedlander G, Silve C. Abnormal sulfate metabolism in vitamin D-deficient rats. J Clin Invest 100: 2196–2203, 1997. doi: 10.1172/JCI119756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatt H, Engelke CE, Pabel U, Teubner W, Jones AL, Coughtrie MW, Andrae U, Falany CN, Meinl W. Sulfotransferases: genetics and role in toxicology. Toxicol Lett 112-113: 341–348, 2000. doi: 10.1016/S0378-4274(99)00214-3. [DOI] [PubMed] [Google Scholar]
- 10.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 11.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC, Hsu CP. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci 21: 57, 2014. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JGF, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 10: 824–839, 2008. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Markovich D. Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 81: 1499–1533, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Markovich D, Aronson PS. Specificity and regulation of renal sulfate transporters. Annu Rev Physiol 69: 361–375, 2007. doi: 10.1146/annurev.physiol.69.040705.141319. [DOI] [PubMed] [Google Scholar]
- 16.Markovich D, Ku MC, Muslim D. Increased lifespan in hyposulfatemic NaS1 null mice. Exp Gerontol 46: 833–835, 2011. doi: 10.1016/j.exger.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Meijers WC, van der Velde AR, Ruifrok WP, Schroten NF, Dokter MM, Damman K, Assa S, Franssen CF, Gansevoort RT, van Gilst WH, Silljé HH, de Boer RA. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J Am Heart Assoc 3: e000962–e000962, 2014. doi: 10.1161/JAHA.114.000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalk D, Manz F, Muller-Wiefel DE, Scharer K. Renal handling of inorganic sulfate in children with chronic kidney disorders. Miner Electrolyte Metab 8: 255–260, 1982. [PubMed] [Google Scholar]
- 19.Morris ME, Murer H. Molecular mechanisms in renal and intestinal sulfate (re)absorption. J Membr Biol 181: 1–9, 2001. doi: 10.1007/s0023200100028. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 21.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA 106: 21972–21977, 2009. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 114: 730–737, 2014. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabry ZI, Shadarevian SB, Cowan JW, Campbell JA. Relationship of dietary intake of sulphur amino-acids to urinary excretion of inorganic sulphate in man. Nature 206: 931–933, 1965. doi: 10.1038/206931b0. [DOI] [PubMed] [Google Scholar]
- 24.Schroten NF, Ruifrok WPT, Kleijn L, Dokter MM, Silljé HH, Lambers Heerspink HJ, Bakker SJ, Kema IP, van Gilst WH, van Veldhuisen DJ, Hillege HL, de Boer RA. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am Heart J 166: 357–364.e2, 2013. doi: 10.1016/j.ahj.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silve C. Tubular handling and regulation of sulphate. Nephrol Dial Transplant 15, Suppl 6: 34–35, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8: 132–140, 2002. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun Z, Singh N, Mullican SE, Everett LJ, Li L, Yuan L, Liu X, Epstein JA, Lazar MA. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. J Biol Chem 286: 33301–33309, 2011. doi: 10.1074/jbc.M111.277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo C. Medicinal chemistry and therapeutic applications of the gasotransmitters NO, CO, and H2S and their prodrugs. In: Burger’s Medicinal Chemistry and Drug Discovery. Hoboken, NJ: John Wiley & Sons, 2010, p. 265–368. doi: 10.1002/0471266949.bmc178. [DOI] [Google Scholar]
- 31.van den Berg E, Pasch A, Westendorp WH, Navis G, Brink EJ, Gans RO, van Goor H, Bakker SJ. Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 25: 1303–1312, 2014. doi: 10.1681/ASN.2013050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Born JC, Frenay AR, Bakker SJ, Pasch A, Hillebrands JL, Lambers Heerspink HJ, van Goor H. High urinary sulfate concentration is associated with reduced risk of renal disease progression in type 2 diabetes. Nitric Oxide 55-56: 18–24, 2016. doi: 10.1016/j.niox.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 33.van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 18: 242–252, 2016. doi: 10.1002/ejhf.483. [DOI] [PubMed] [Google Scholar]
- 34.Wang CH, Cheng ML, Liu MH, Shiao MS, Hsu KH, Huang YY, Lin CC, Lin JF. Increased p-cresyl sulfate level is independently associated with poor outcomes in patients with heart failure. Heart Vessels 31: 1100–1108, 2016. doi: 10.1007/s00380-015-0702-0. [DOI] [PubMed] [Google Scholar]