Cardiac function is evaluated, for the first time, in progeny following maternal nanomaterial inhalation. The findings indicate that exposure to nano-sized titanium dioxide (nano-TiO2) during gestation negatively impacts cardiac function and mitochondrial respiration and bioenergetics. We conclude that maternal nano-TiO2 inhalation contributes to adverse cardiovascular health effects, lasting into adulthood.
Keywords: nanomaterials, cardiac dysfunction, pregnancy, mitochondria
Abstract
Nanomaterial production is expanding as new industrial and consumer applications are introduced. Nevertheless, the impacts of exposure to these compounds are not fully realized. The present study was designed to determine whether gestational nano-sized titanium dioxide exposure impacts cardiac and metabolic function of developing progeny. Pregnant Sprague-Dawley rats were exposed to nano-aerosols (~10 mg/m3, 130- to 150-nm count median aerodynamic diameter) for 7–8 nonconsecutive days, beginning at gestational day 5–6. Physiological and bioenergetic effects on heart function and cardiomyocytes across three time points, fetal (gestational day 20), neonatal (4–10 days), and young adult (6–12 wk), were evaluated. Functional analysis utilizing echocardiography, speckle-tracking based strain, and cardiomyocyte contractility, coupled with mitochondrial energetics, revealed effects of nano-exposure. Maternal exposed progeny demonstrated a decrease in E- and A-wave velocities, with a 15% higher E-to-A ratio than controls. Myocytes isolated from exposed animals exhibited ~30% decrease in total contractility, departure velocity, and area of contraction. Bioenergetic analysis revealed a significant increase in proton leak across all ages, accompanied by decreases in metabolic function, including basal respiration, maximal respiration, and spare capacity. Finally, electron transport chain complex I and IV activities were negatively impacted in the exposed group, which may be linked to a metabolic shift. Molecular data suggest that an increase in fatty acid metabolism, uncoupling, and cellular stress proteins may be associated with functional deficits of the heart. In conclusion, gestational nano-exposure significantly impairs the functional capabilities of the heart through cardiomyocyte impairment, which is associated with mitochondrial dysfunction.
NEW & NOTEWORTHY Cardiac function is evaluated, for the first time, in progeny following maternal nanomaterial inhalation. The findings indicate that exposure to nano-sized titanium dioxide (nano-TiO2) during gestation negatively impacts cardiac function and mitochondrial respiration and bioenergetics. We conclude that maternal nano-TiO2 inhalation contributes to adverse cardiovascular health effects, lasting into adulthood.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/gestational-nanomaterial-exposure-and-cardiac-dysfunction/.
growing production, distribution, and use of engineered nanomaterials (ENMs) necessitates more thorough evaluations of the physiological effects that exposure to these ENMs induces. One of the most widely used ENM in the world is titanium dioxide (nano-TiO2): in 2006, 40,000 metric tons of nano-TiO2 were estimated to be produced domestically, with 2015 projections reaching as high as 260,000 metric tons (43). Specifically, it is well accepted that inhaled ENMs can interact in one of three ways: an acute inflammatory response, direct translocation, and through the autonomous nervous system in the lungs (19); the link between pulmonary exposure and extrapulmonary effects provides a picturesque example of the complications that may arise when trying to decipher a prominent mechanism for nano-TiO2 toxicity.
In the heart, it has been shown that, through pulmonary exposure (15, 36, 57), intravenous administration (30), and orally (16), ENMs can affect cardiac function, although this is not without disagreement (8, 20). Nano-TiO2 accumulation in cardiac tissue has been demonstrated to increase edema and apoptosis during embryonic development (37, 64). In adult exposures, nano-TiO2 causes a decrease in bioenergetics, increase in inflammatory signaling, and DNA damage (15, 16, 67). While measures of direct interaction between an organism and nano-TiO2 have revealed much about cardiac dysregulation, very little has been shown regarding the mechanisms affecting cardiac tissue of gestational-exposed progeny (22). Recent studies have demonstrated a role for diesel exhaust particulate matter air pollution in causing negative cardiac implications in progeny from maternal exposures (18, 58, 59), but no study has focused specifically on the longitudinal effects of a single, characterized ENM on cardiac function.
Although many extrapulmonary effects are well recognized, in vivo cardiac functional impacts are less commonly identified as toxicological end points, making investigation into the effects essential for expanding our knowledge. Studies have examined the effects of directly placing TiO2 nanoparticles on cardiomyocytes in vitro. These studies have concluded that high enough concentrations of nano-TiO2 (>10 µg/ml) can directly affect the functionality of cardiomyocytes, including measures of shortening, relengthening, and amplitude of contraction (26, 46); decreased functionality remained consistent across varying frequencies of contractions. Previous work has examined the cardiac bioenergetics from young adult Sprague-Dawley rats exposed during gestation to nano-TiO2; this study demonstrated that state 3 and 4 respiration was significantly altered (54), providing the rationale for defining the specific impacts on cardiomyocyte bioenergetics more closely.
Recent literature has examined how ENM exposure can affect the in utero environment. Maternal nano-TiO2 inhalation disrupts uterine microvascular function and creates a hostile gestational environment for development (51); furthermore, intravenous injection of carbon nanotubes has been shown to translocate to the placenta, resulting in fetal deformities and lower progeny yields (7, 23, 42). With known physiological impacts to the developing fetus following ENM exposure, the need for an initial understanding of the cardiac consequences resulting from changes within the in utero environment is crucial. Ultimately, this understanding could have profound effects, including the following: ENM exposure standards for pregnant women, defining kinetics of nanoparticles across the placental barrier, and outlining mechanisms affecting cardiac/mitochondrial development in exposed progeny. To determine how nano-TiO2 could affect progeny, we subjected pregnant Sprague-Dawley rats to pulmonary exposure of nano-TiO2 throughout gestation. These exposures allowed for measurement across three critical developmental points: fetal, neonatal, and young adult. To date, no study has examined cardiomyocyte function following gestational exposure to ENMs. We hypothesized that maternal nano-TiO2 exposure would negatively impact cardiac function and cardiomyocyte bioenergetics compared with air-exposed progeny. Our results provide evidence that gestational exposure to nano-TiO2 significantly affects the heart and cardiomyocyte function of progeny. Furthermore, changes in mitochondrial respiratory capacity, coupled with decreasing mitochondrial complex activity, provide a potential mechanism for the observed functional deficits.
METHODS
Animal Model
The experiments in this study conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (8th Ed.) and were approved by the West Virginia University Animal Care and Use Committee. Sprague-Dawley rats (250–275 g female; 300–325 g male) were purchased from Hilltop Laboratories (Scottsdale, PA). Rats were housed at the West Virginia University Health Sciences Center with food and water provided ad libitum. Rats were acclimated for at least 72 h before mating, as previously described (53). At gestational day ~6 (5.85, mean of exposure times), pregnant rats were exposed to nano-TiO2, as described below.
Engineered Nanomaterial
Nano-TiO2 P25 powder, containing anatase (80%) and rutile (20%) TiO2, was purchased from Evonik (Aeroxide TiO2, Parsippany, NJ). Primary particle size was 21 nm, with a surface area of 48.08 m2/g (35, 44, 45). Aerosolization by drying, sieving, and storing are described previously for nano-TiO2 (27, 35).
Inhalation Exposure
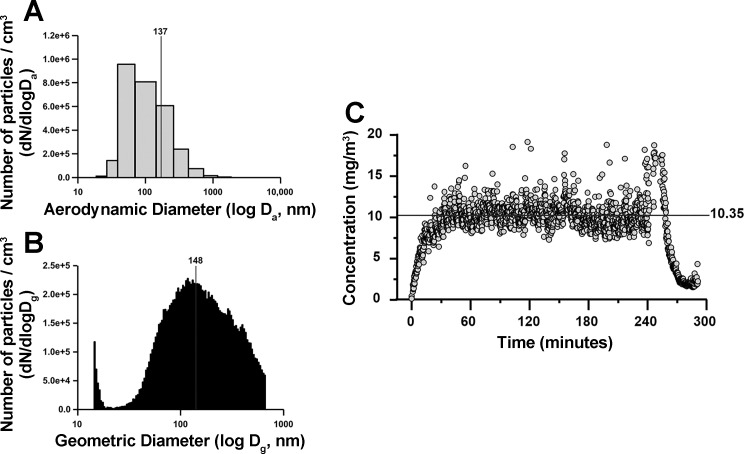
The design and use of the nanoparticle exposure system has been previously described (65). Briefly, aerosol size was measured through both electrical low-pressure impactor (136.47 ± 1.44 nm) (Fig. 1A) and scanning particle mobility sizer (134.80 ± 1.24 nm) (Fig. 1B). Aerosol concentration of engineered TiO2 was determined to be 10.35 ± 0.13 mg/m3 (Fig. 1C). Average aerosol concentration was measured over a total of approximately eight exposures (7.79 ± 0.26 days) to each of the 13 animals in the gestational nano-TiO2 exposure group. The first exposure to nano-TiO2 was given on gestational day ~6, with the last exposure given 24 h before birth. Exposures consisted of 5 h/day treatments, with a calculated lung daily deposition of 43.8 ± 1.2 μg. Control animals were exposed to filtered air only. Calculation of total exposure volume has been previously described (29, 35, 41, 52). To calculate the dose required to match the appropriate lung deposition paradigm for Sprague-Dawley rats, the formula D = F × V × C × T was used, where F is the deposition fraction (10%), V is the minute ventilation based on body weight, C equals the mass concentration (mg/m3) and T equals the exposure duration (minutes).
Fig. 1.
Characterization of nano-TiO2 used in aerosol exposure paradigm. Total daily deposition = 43.8 ± 1.2 μg. A: electrical low-pressure impactor size distribution (aerodynamic diameter) of nano-TiO2 particles for n = 89 individual measurements (136.465 ± 1.436 nm). B: scanning mobility particle sizer size distribution (mobility diameter) of nano-TiO2 particles for n = 89 individual measurements (134.802 ± 1.241 nm). C: aerosol concentration of engineered nano-TiO2 (10.352 ± 0.134 mg/m3) during exposures.
Echocardiography–B-mode, M-mode, and Doppler Imaging
Nano-TiO2-exposed and control filtered-air young adult (6–12 wk) Sprague-Dawley rats were used in analysis and, subsequently, for cardiomyocyte isolation. Anesthetization was performed similarly to that shown in James et al. (25); by slowly reducing the total isoflurane given, measurements were taken as the animal awakens, reducing the anesthetic-induced effect on cardiac function. B-mode, M-mode echocardiograph, as well as Doppler imaging procedures, have been described previously in mice (48), but with some adaptations, including the use of a lower frequency transducer (13–24 MHz instead of 32–55 MHz) and 3% isoflurane induction with sustained isoflurane at 1.5% or higher. Ultrasound images were taken using the Vevo 2100 Imaging System (Visual Sonics, Toronto, Canada). Briefly, ultrasound slightly left of the sternum, at the midpapillary layer, was used for long-axis B-mode imaging. Rotation 90°, perpendicular to the animal, allowed for short-axis B-mode images. B-mode procurement provided detailed information, such as area and ejection volume of the left ventricle (LV). Gating midway between the short-axis B-mode images allowed for acquisition of M-mode parameters. In M-mode, measures to determine the LV thickness (interventricular septal, inner, and posterior wall) were conducted on adjacent end-systolic and end-diastolic peaks in relation to LV trace analysis. Doppler echocardiography measured mitral valve function, E- and A-wave velocity, deceleration time, E-wave-to-A-wave ratio (E/A ratio), mitral valve area, etc. All ultrasound procedures were carried out by one imaging specialist, taken at a frame rate of 109–284 frames/s. M-mode and Doppler echocardiography were examined using averaged values from at least three replicate analyses from each animal. All measures were completed by one analyst.
Stress Strain-Speckle Tracking
B-mode images, in both the short and long axis, were used for speckle-tracking-based strain analyses. Peak strain and strain rates were measured across radial, longitudinal, and circumferential dimensions. Speckle-tracking-based strain analysis has been described previously in mice (48), with caveats for rat analyses mentioned above. Briefly, during each cardiac cycle, strain was measured as a function of total deformation length divided by the original length of a segment (3, 10, 38). Displacement length, velocity, strain, and strain rate were measured in the endocardium over a minimum of three cycles. As previously described, short-axis images in the LV were split into anterior free, lateral, posterior, inferior free, posterior septum, and anterior septum, while long-axis was split into anterior base, anterior mid, anterior apex, posterior apex, posterior mid, and posterior base, as previously described (48).
Cardiomyocyte Isolation
Fetal/neonatal.
For the fetal time point, pregnant dams were euthanized, and pups were removed at gestational day 20 from the exposed and control rat uterus. For the neonatal time point, rats were killed at 4–10 days postbirth. Animals at both time points were kept on ice during dissection. During cardiomyocyte isolation, pregnant rats were anesthetized using 5% isoflurane, with maintenance isoflurane levels sustained at 2.5%. Hearts were removed through a midsagittal cut in the thoracic cavity. Hearts were collected in groups of four to eight per litter, and atrial and ventricular appendages trimmed. Digestive solution included 2 mg pancreatin (Sigma Adrich, St. Louis, MO) and 2 mg of collagen type II (Worthington Biochemical, Lakewood, NJ), mixed with 2 ml of physiological 1× ADS buffer (0.1 M NaCl, 1.2 mM NaH2PO4, 0.8 mM MgSO4, 5.4 mM KCl, and 5 mM glucose at pH 7.4) per heart digested. Digestive solution was used to initially wash the hearts (15 min and discarded) and to begin separating single cells from the tissue (3–4 washes at 22 min each, which were saved). After each saved digestion, the supernatant was removed from the tissue debris and centrifuged at 1,000 rpm for 7 min. The supernatant was discarded, and 2 ml of newborn calf serum was added to the cell suspension and transferred to a 37°C incubator.
In a 15-ml conical tube, a Percoll gradient was made for separation of cardiomyocytes from other cells of the heart. The Percoll gradient (density = 1.130 g/ml) was made of two layers: clear (top) and red (bottom) Percoll solutions. Four milliliters of top Percoll (density = 1.059 g/ml, 90 ml Percoll stock + 110 ml 1× ADS buffer) were first added, followed by pipetting bottom Percoll (density = 1.082 g/ml, 130 ml Percoll stock + 70 ml 1× ADS buffer with phenyl red) below the first solution. After all cell collection steps, cells were centrifuged at 1,000 rpm and resuspended in 2 ml of 1× ADS buffer, per five hearts, which was added to the top of the Percoll gradient. Cells on top of the Percoll gradient were centrifuged at 3,000 rpm for 30 min, with 9-min acceleration and deceleration time. Cells were then extracted from the middle layer, washed with newborn calf serum, and placed in plating media (with fetal bovine serum). Two hours after placing in plating media, cells were exchanged to maintenance media (no fetal bovine serum) and media was changed every 2 days. Cells were counted using a hemocytometer.
Adult.
Young adult rats were anesthetized using 5% isoflurane. The isolation procedure was adapted from Xu and Colecraft (61). Briefly, hearts were removed, rinsed in physiological salt solution, and suspended on a Langendorff perfusion system, with retrograde flow through the aorta. Enzymatic digestion with 20 mg collagen type II (Worthington Biochemical, Lakewood, NJ), 10 mg neutral protease (Worthington Biochemical, Lakewood, NJ), 20 mg carnitine (Sigma Aldrich), 25 mg 2,3-butanedione monoxime (Sigma Aldrich), 31 mg taurine (Sigma Aldrich), 20 mg glutamine (Sigma Aldrich), and 1.25 µl of 1 M CaCl2 in 50 ml of buffer A. After digestion (total calcium concentration added 0.6 mM), gravimetric separation of cells was performed with continual calcium loading during the three wash steps. Cells were counted using hemocytometer.
Cardiomyocyte Contractility
A video-based edge detection system (Ionoptix, Westwood, MA) was used to assess contractility of cardiomyocytes. Phase-contrast microscopy coupled with Ionoptix software was used to track cardiomyocyte dimensions. Cells were placed in contractility buffer (50.55 mg 2,3-butanedione monoxime, 8.82 mg CaCl2, 119.5 mg HEPES, 10.16 mg MgCl2, 394.47 mg NaCl, 90.075 mg glucose, 14.91 mg KCl, and 2.276 mg NaH2PO4) and then diluted 10:1 with contractility buffer from the original cell suspension. Field stimulation at 0.5 Hz at 10 V was used for all measures. Dynamic measures taken included departure velocity, peak height, peak height percentage to baseline, return velocity, 10 and 90% time to shortening, 10 and 90% time to relengthening, area of departure, area of return, and retention of baseline. Cells included in the study matched the following specific criteria: 1) chosen based on the best morphology compared with others within the field (striations, no blebbing); and 2) cells sustain consistent contractions for at least 60 s. For reference, Ye et al. (63) provides more detailed parameters of the contractile apparatus.
Cardiomyocyte Mitochondrial Bioenergetics
Isolated cardiomyocytes were plated on XF96 V3 cell culture microplates, using the Seahorse XF96 for analysis (Agilent Technologies, Santa Clara, CA). Measurements were taken 24–48 h after plating cardiomyocytes. Concentrations of fetal and neonatal cells ranged from 35,000 to 85,000 per well. Adult cardiomyocytes, due to slight variation in cell viability and time before analysis for each sample, were not normalized based on cell number, but rather on basal respiration between groups. Replicates were generally performed in sets of five to six. Yoshida et al. (66) provides a comprehensive overview of the Seahorse applications. Briefly, oxygen consumption rate, extracellular acidification rate, and proton production rate were measured using oligomycin, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone, antimycin A, and rotenone. Measures of mitochondrial respiration included ATP production, proton leak, basal respiration, maximal respiration, and spare capacity.
Electron Transport Chain Complex Activities
Electron transport chain (ETC) complexes I, III, IV (11, 12) and V (ATP synthase) activities were measured as previously described (9), with minor modifications. Briefly, cardiomyocytes from young adult animals were treated with RIPA buffer (Life Technologies, Grand Island, NY). A Bradford assay was carried out on each sample to provide a basis for normalization to protein content. Activity was measured in complex I (reduction of decyclubiquinone), complex III (reduction of cytochrome c), complex IV (oxidation of reduced cytochrome c), and complex V (pyruvate kinase and phosphoenolpyruvate and ATP production).
ETC Complex Protein Expression
Blue native polyacrylamide gel electrophoresis (BN-PAGE) was performed as previously described (56), with standardization to protein content through the Bradford method (4). Briefly, young adult cardiomyocytes were digested with RIPA buffer (Life Technologies, Grand Island, NY) and 40 µg of protein were added to 1% digitonin on ice. Coomassie G-250 was added, and samples were run on 4–16% NativePAGE gels. Gels were placed in GelCode Blue Stain Reagent (Thermo Fisher, Rockford, IL) to fix bands for representation, followed by washes until the desired background staining was removed. Densitometry was measured using Image J Software (NIH, Bethesda, MD). Complexes were identified using a 480-kDa ladder with criteria for identifying the molecular weight of each complex matching Wittig et al. (60).
Quantitative PCR
RNA was isolated from fetal progeny hearts using the Vantage Total RNA Purification Kit (Origene), following the manufacturer’s instructions. RNA was then converted to cDNA for quantitative PCR (qPCR) analysis using the cDNA Synthesis Kit for micro-RNA (product no.: HP100042, Origene), following the manufacturer’s instructions. cDNA was used to assess three markers: peroxisome proliferator-activated receptor-γ (PPAR-γ) (product no.: Rn00440945_m1, Thermo Fisher), carnitine palmitoyltransferase I (CPT1A) (product no.: Rn00580702_m1, Thermo Fisher), and uncoupling protein 2 (UCP2) (product no.: Rn01754856_m1, Thermo Fisher). Samples were standardized to GAPDH (product no.: Rn01775763_g1, Thermo Fisher). Experiments were conducted on the Applied Biosystems 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA), in a total reaction volume of 20 µl with TaqMan 2X Universal PCR Master Mix (Applied Biosystems).
Western Blot Analyses
Twelve-well, 4–12% gradient gels were used in SDS-PAGE, as previously described (3, 4, 6, 11, 12, 28, 29), with modifications. Briefly, cardiomyocytes from the young adult group were used in all protein quantification, with the Bradford method being employed to standardize protein concentration using bovine serum albumin (4). Primary antibodies used in the study included the following: anti-adenine nucleotide translocase (product no.: sc-9300, Santa Cruz Biotechnology, Dallas, TX), anti-apoptotic protease activating factor 1 (APAF-1) (product no.: ab2000, Abcam, Cambridge, MA), anti-B-cell lymphoma 2 (Bcl-2)-like protein 4 (BAX) (product no.: ab32503, Abcam), anti-BCL-2 (product no.: sc-7382, Santa Cruz Biotechnology), anti-histone 3 lysine 4 trimethylation (H3K4me3) (product no.: G.532.8, Thermo Fisher), anti-histone 3 lysine 27 trimethylation (H3K27me3) (product no.: G.299.10, Thermo Fisher), anti-GAPDH (product no.: ab8245, Abcam), anti- histone deacetylase 6 (product no.: ab61058, Abcam), anti-optic atrophy 1 (product no.: ab42364, Abcam), anti-pyruvate dehydrogenase (PDH) (product no. 2784; Cell Signaling Technology, Danvers, MA), anti-PDH E1-α-subunit (phospho S293) (product no.: ab177461, Abcam), anti-PPAR-α (product no.: ab2779, Abcam), anti-UCP3 (product no.: ab3477, Abcam), anti-voltage-dependent anion channel (product no. 4866; Cell Signaling Technology). Secondary antibodies used included the following: goat anti-mouse IgG horseradish peroxidase (HRP) conjugate (product no. 31430; Pierce Biotechnology, Rockford, IL), goat anti-rabbit IgG HRP conjugate (product no. 10004301; Cayman Chemical, Ann Arbor, MI), and rabbit anti-goat IgG HRP conjugate (product no. 31402; Pierce Biotechnology). Standardization of protein levels for each blot were performed with both Ponceau S solution (Sigma Adrich) and the anti-GAPDH antibody. Pierce enhanced chemiluminescence Western blotting substrate (Pierce Biotechnology) was used to detect signal per manufacturer’s instructions. G:Box Bioimaging system (Syngene, Frederick, MD) was used to detect signals, and data were captured using GeneSnap/GeneTools software (Syngene). Densitometry was analyzed using Image J Software (NIH, Bethesda, MD). All values were expressed as optical density with arbitrary units.
Statistics
A two-tailed Student’s t-test was used to determine statistical significance between control and gestational nano-TiO2-exposed groups. Differences between control and exposed groups were considered statistically trending (P = 0.075–0.055, denoted by †), or statistically different (P ≤ 0.05, denoted by *, and P ≤ 0.01, denoted by **), unless explicitly stated otherwise. All data are presented as means ± SE.
RESULTS
Echocardiography and Speckle-Tracking-Based Strain Analysis
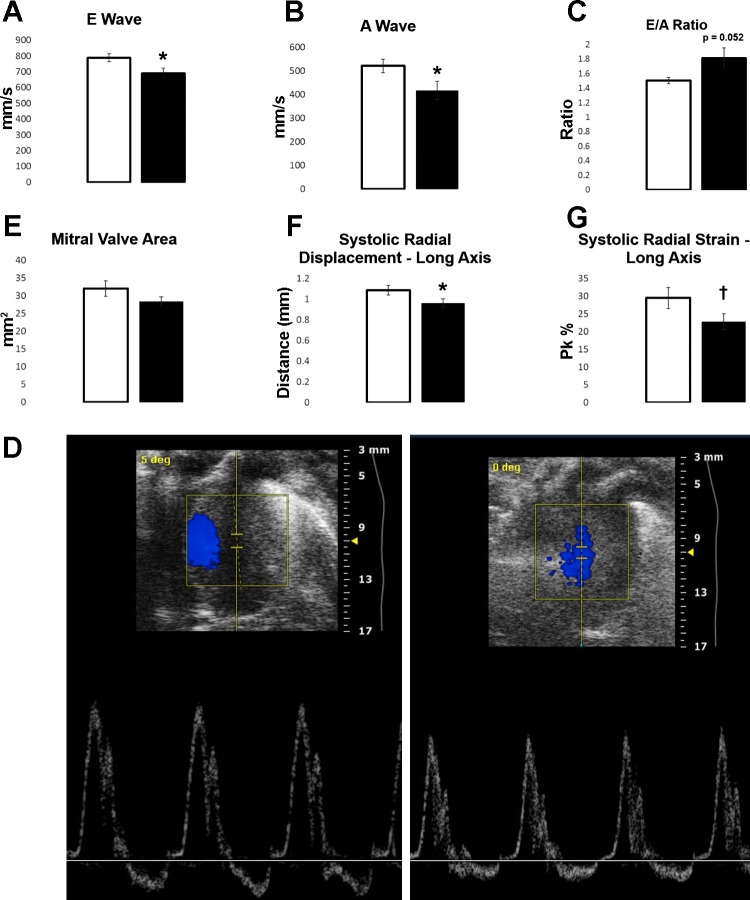
Young adult animals (6–12 wk) were assessed using a variety of ultrasound techniques to evaluate in vivo cardiac function. M-mode, measuring end-systolic and end-diastolic diameters and volumes, and B-mode, measuring the short- and long-axis diameter of the LV, are provided in Tables 1 and 2, respectively. M-mode was found to have significant changes in the total mass of the LV between groups (P = 0.033) and a trending difference in stroke volume (P = 0.072). LV thickness was measured across end diastole and end systole for changes in interventricular septal, inner, and posterior wall thickness. Interventricular end-systole septal thickness showed a significant decrease (P = 0.015) in the experimental group (Table 3). B-mode showed no changes between groups. Doppler echocardiography revealed differences in LV filling between the control and gestational nano-TiO2-exposed group (Fig. 2); this included differences in the velocities of both the E wave (P = 0.025) (Fig. 2A) and the A wave (P = 0.040) (Fig. 2B), with the experimental group showing a significant decrease in both velocities.
Table 1.
B-Mode echocardiography for control vs. gestational TiO2-exposed animals at the young adult (6-12 wk) stage
| Calculation | Units | Control Average ± SE | Exposed Average ± SE |
|---|---|---|---|
| Area;d (LV trace) | mm2 | 60.39 ± 3.87 | 56.39 ± 2.49 |
| Area;s (LV trace) | mm2 | 25.64 ± 2.69 | 25.59 ± 1.97 |
| CO (LV trace) | ml/min | 196.01 ± 18.54 | 177.18 ± 12.10 |
| EF (LV trace) | % | 77.29 ± 2.69 | 75.70 ± 2.27 |
| FS (LV trace) | % | 20.64 ± 2.07 | 16.26 ± 1.64 |
| SV (LV trace) | µl | 200.21 ± 18.21 | 179.19 ± 12.07 |
| V;d (LV trace) | µl | 262.54 ± 24.54 | 238.86 ± 16.45 |
| V;s (LV trace) | µl | 62.33 ± 9.68 | 59.67 ± 7.022 |
Values are averages ± SE for each measure. Area;d, diastolic area; Area;s, systolic area; CO, cardiac output; EF, ejection fraction; FS, fractional shortening; SV, stroke volume; V;d, volume at diastole; V;s, volume at systole.
Table 2.
M-mode echocardiography for control vs. gestational TiO2-exposed animals at the young adult (6-12 wk) stage
| Calculation | Units | Control Average ± SE | Exposed Average ± SE |
|---|---|---|---|
| CO (LV trace) | ml/min | 70.86 ± 1.79 | 65.14 ± 3.05 |
| Diameter;d (LV trace) | mm | 6.72 ± 0.12 | 6.38 ± 0.16 |
| Diameter;s (LV trace) | mm | 2.90 ± 0.14 | 2.83 ± 0.11 |
| EF | % | 79.41 ± 1.48 | 80.07 ± 1.05 |
| EF (LV trace) | % | 85.56 ± 1.23 | 84.96 ± 0.72 |
| FS | % | 49.54 ± 1.54 | 49.84 ± 1.09 |
| FS (LV trace) | % | 57.33 ± 1.63 | 56.18 ± 0.99 |
| LV Mass (LV trace) | mg | 841.82 ± 51.34* | 696.89 ± 40.93* |
| LV Mass Corr (LV trace) | mg | 673.45 ± 41.07* | 557.51 ± 32.75* |
| LV Vol;d | µl | 214.37 ± 10.19 | 195.27 ± 11.32 |
| LV Vol;s | µl | 45.78 ± 5.05 | 38.90 ± 3.11 |
| SV (LV trace) | µl | 200.09 ± 6.95† | 178.31 ± 9.19† |
| V;d (LV trace) | µl | 236.10 ± 9.63 | 211.59 ± 11.45 |
| V;s (LV trace) | µl | 36.01 ± 4.02 | 33.28 ± 2.73 |
Values are averages ± SE for each measure. CO, cardiac output, Diameter;d, diameter at diastole, Diameter;s, diameter at systole; EF, ejection fraction; FS, fractional shortening; LV Mass, left ventricular mass; LV Vol;d, left ventricular volume at diastole; LV Vol;s, left ventricular volume at systole; SV, stroke volume; V;d, volume at diastole; V;s, volume at systole.
P = 0.075–0.055.
P ≤ 0.05.
Table 3.
Left ventricle thickness, using M-mode echocardiography for control vs. gestational TiO2-exposed animals at the young adult (6-12 wk) stage
| Measurement | Units | Control Average ± SE | Exposed Average ± SE |
|---|---|---|---|
| IVS;d | mm | 1.92 ± 0.06 | 1.77 ± 0.06 |
| IVS;s | mm | 3.21 ± 0.06* | 2.91 ± 0.07* |
| LVID;d | mm | 6.45 ± 0.13 | 6.14 ± 0.17 |
| LVID;s | mm | 3.28 ± 0.15 | 3.07 ± 0.10 |
| LVPW;d | mm | 1.88 ± 0.10 | 1.77 ± 0.07 |
| LVPW;s | mm | 2.87 ± 0.09 | 2.69 ± 0.08 |
Values are averages ± SE for each measure. IVS;d, interventricular end-diastolic septal thickness; IVS;s, interventricular end-systolic septal thickness; LVID;d, left ventricular end-diastolic internal thickness; LVID;s, left ventricular end-systolic thickness; LVPW;d, left ventricular end-diastolic posterior wall thickness; LVPW;s, left ventricular end-systolic posterior wall thickness.
P ≤ 0.05.
Fig. 2.
Echocardiography and speckle-tracking analysis, for control (open bars, n = 17) vs. gestational TiO2-exposed (solid bars, n = 20) animals at the young adult (6-12 wk) stage. A: velocity of E wave (P = 0.025). B: velocity of A wave (P = 0.04). C: E/A ratio (P = 0.052). D: representative image of Doppler echocardiography for control (left) and exposed (right) animals. Images were chosen to represent the average E/A ratio for control (1.506 ± 0.043) and exposed (1.813 ± 0.140) animals during ultrasound. Other parameters of echocardiography were found to be insignificantly different. E: mitral valve area (P = 0.16). Stress-strain analysis of systolic long-axis radial displacement (P = 0.05; F) and radial strain (P = 0.065; G) is shown. Values are means ± SE. †P = 0.075–0.055. *P ≤ 0.05.
Also, even though both the E- and A-wave velocities were altered, the E/A ratio between groups was found to be higher in the experimental group (P = 0.052). Control animals exhibited a normal E/A ratio (1.505), while the experimental group exhibited a higher ratio (1.813) (Fig. 2C). This is represented by Fig. 2D, which depicts a control (left) and gestational-exposed (right) animal with E/A ratios similar to that of the average for each group. Other parameters were also measured, including mitral valve area (Fig. 2E), and were not found to be significant. For the stress-strain analysis, only two parameters appeared to be altered during contraction of the LV: the long-axis systolic displacement and strain (Fig. 2, F and G) showed a significant (P = 0.049) and trending (P = 0.065) difference, respectively. These results demonstrate that maternal exposure can produce adverse effects in cardiac systolic and diastolic function, while also interfering with cardiac development.
Cardiomyocyte Contractility
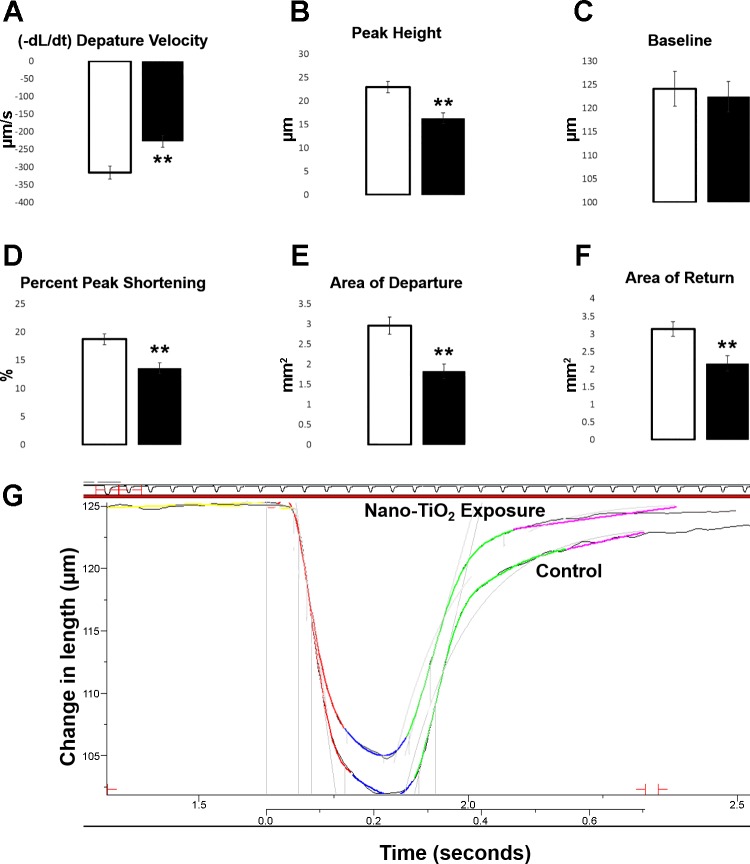
To further delineate the cardiac outcomes of progeny predisposed to gestational nano-TiO2, cardiomyocyte contractile function was assessed. Cells were isolated from young adult rats in the control and experimental groups. Cardiomyocyte contractile function was decreased in the gestational-exposed compared with the control (Fig. 3). An assessment of decreasing function was demonstrated through changes in departure velocity, peak height, baseline percentage to peak height, and area under the contractile curve. Departure velocity was significantly decreased in the young adult group (P = 0.0008) after gestational exposure (Fig. 3A). In addition, the peak height of contraction was significantly decreased (P = 0.0001) (Fig. 3B), with no differences observed in the resting baseline (P = 0.73) (Fig. 3C). The exposed group also exhibited a decreased baseline percentage to peak height (P = 0.0003) (Fig. 3D). Lastly, total area of the contraction was shown to be significantly reduced in the exposed group compared with the control, both in area of departure (P = 0.0001) and area of return (P = 0.002) (Fig. 3, E and F). Changes in return velocity, time to 90% peak, and time to 90% baseline were not significant. Two initial transients, one from both the control and experimental contractile data, are overlaid for a visual representation of differences in the contractile curves (Fig. 3G); the departure velocity and peak height measurements are similar to the averages for each group. All measures were performed on the first contraction after stimulation, referred to further as the first transient. Second transient, or the second contractile peak, measures displayed a similar trend (data not shown). At least three cardiomyocytes were used per control or exposed litter. Attenuated responsiveness during stimulation in the maternal exposure model establish modifications to cardiomyocytes function, which remain into adulthood.
Fig. 3.
All first transient contractions (measured as the first contraction after stimulation) at 10 V and 0.5 Hz, control (open bars, n = 39 cardiomyocytes from n = 8 animals) vs. gestational TiO2-exposed (solid bars, n = 42 cardiomyocytes from n = 9 animals) cardiomyocytes at the young adult (6-12 wk) stage. A: departure velocity (P = 0.0008). B: peak height (P = 0.0001). C: baseline (P = 0.73). D: baseline percentage to peak height (P = 0.0003). E: area of departure (P = 0.0001). F: area of return (P = 0.0018). G: illustration of contractile properties from the control and exposed cardiomyocytes. Specific contractions were chosen to match the average departure velocity and peak height found in the controls (−315.446 ± 18.611 µm/s, 22.920 ± 1.174 µm) and the exposed (−227.054 ± 17.268 µm/s, 16.275 ± 1.158 µm) groups. Values are means ± SE. **P ≤ 0.01.
Mitochondrial Bioenergetics
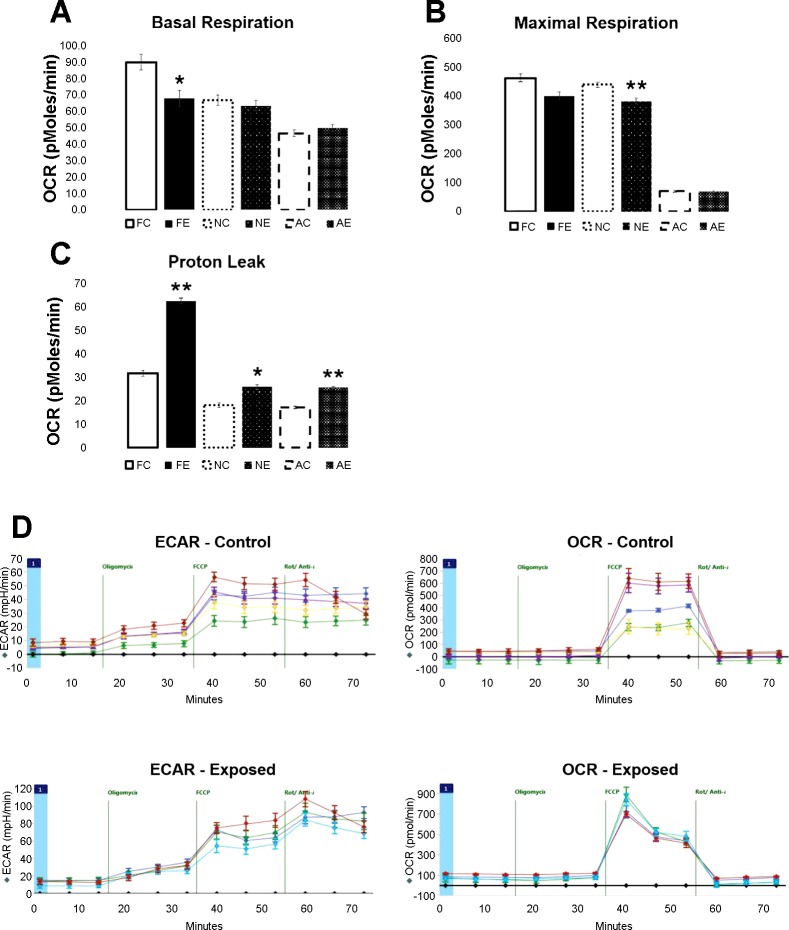
To determine whether changes in contractility resulted from changes in cellular energetics that drive contractile function, the Seahorse XF96 was implemented to determine the effects of gestational nano-TiO2 exposure on the bioenergetics of the growing progeny. Data from the Seahorse XF96 was compiled at three time points: fetal (gestational day 20), neonatal (4–10 days postbirth), and young adult (6–12 wk). The results indicated mitochondrial respiration dysfunction, with varying degrees of impairment across developmental stages (Fig. 4). Fetal cardiomyocytes, seeded at a 65,000 cell density, showed a decrease in basal respiration (P = 0.0001) (Fig. 4A) and an increase in proton leak (P = 0.003) in the experimental group. When cardiomyocytes were extracted from the neonatal hearts and seeded at a 55,000 cell density, effects on basal respiration diminished, but significant decreases in maximal respiration (P = 0.0031) (Fig. 4B) and spare capacity (P = 0.003) were observed within the nano-TiO2-exposed groups. Also, proton leak continued to show a significant increase in the exposed group (P = 0.044). Because of slight variations in the recovery percentage of viable adult cardiomyocytes and limited cell viability in culture, normalization of the young adult group was processed differently than the fetal and neonatal groups.
Fig. 4.
Seahorse XFe96 measurement for oxygen consumption rate (OCR) at pmol/min, for controls (open bars) vs. gestational TiO2-exposed (solid bars) cardiomyocytes at the fetal (gestational day 20), neonatal (4-10 days), and young adult (6-12 wk) stage. For all measures, an average of 65,000 cells were used for fetal measurements, 55,000 cells for neonatal, and the young adult group was normalized to basal respiration between the control and exposed groups. A: basal respiration rates, with a change observed between the fetal groups (P = 0.013). B: proton leak across all three groups, changing in the fetal (P = 0.0003), neonatal (P = 0.045), and young adult (P= 0.0072) groups. C: maximal respiration across all three groups, with changes in the neonatal group (P = 0.003). D: neonatal cardiomyocyte OCR and extracellular acidification rate (ECAR) in the control (top) and experimental animals (bottom). Also, an illustration is shown of how values from the Seahorse analysis (basal respiration, maximal respiration, spare capacity, proton leak, etc.) are obtained using inhibitor agents, such as oligomycin, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), antimycin A, and rotenone. Values are means ± SE. Fetal n = 5 control and n = 3 exposed, neonatal n = 4 control and n = 5 exposed, and young adult n = 5 control and n = 5 exposed animals were used for analysis. FC, fetal control; FE, fetal exposed; NC, neonatal control; NE, neonatal exposed; AC, adult control; AE, adult exposed. *P ≤ 0.05. **P ≤ 0.01.
Due to changes in basal respiration diminishing in the neonatal group, this parameter was used to normalize the young adult age group. Basal respiration normalization resulted in a significant increase in the proton leak in the experimental group (P = 0.0072) (Fig. 4C). Normalization to both maximal respiration and spare capacity also showed a significant increase in proton leak in the experimental group (data not shown). A depiction of the oxygen consumption rate, extracellular acidification rate, and proposed bioenergetics capacity for neonatal cardiomyocytes in the control and experimental groups are depicted in Fig. 4D to provide an example of the overall procedure. This experiment validates the sustained, longitudinal effects of gestational exposure to ENMs, highlighting changes occurring in the bioenergetics of the cardiomyocytes.
ETC Complex I, III, IV, and V Activities and Expression
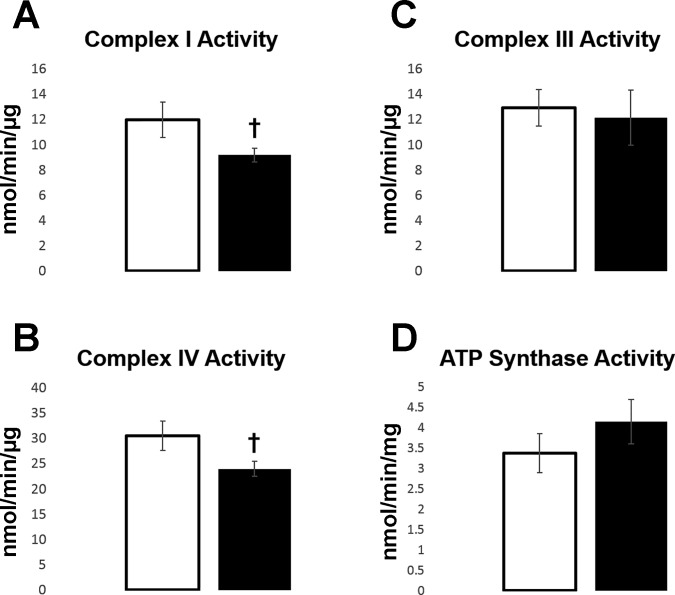
To further target the molecular effects of gestational nano-TiO2 exposure on bioenergetics, mitochondrial complex activities was measured on young adult cardiomyocytes. Trends were shown in both complex I (P = 0.067) (Fig. 5A) and complex IV (P = 0.056) (Fig. 5B), with as much as a 20% decrease in activity in the gestational-exposed group compared with the controls. Complex III and complex V (ATP synthase) showed no significant differences in activity between groups (Fig. 5, C and D). Although trending differences, complex activity changes indicate potential lasting effects to the quantity, or function, of the mitochondrial ETC constituents. To determine whether a reduction in complex activity was due to a decrease in the expression level of the complexes, BN-PAGE was performed. No significant changes were found in any of the major ETC complex expressions (Table 4).
Fig. 5.
Electron transport chain complex activity, control (open bars, n = 7) vs. gestational TiO2-exposed (solid bars, n = 8) cardiomyocytes at the young adult (6-12 wk) stage. A: complex I activity (P = 0.067). B: complex III activity. C: complex IV activity (P = 0.056). D: ATP synthase activity. Activity was measured through the mass of cardiomyocyte cell lysates, not isolated mitochondria. Values are means ± SE. †P = 0.075–0.055.
Table 4.
Blue native polyacrylamide gel electrophoresis for control vs. gestational TiO2-exposed animals at the young adult (6-12 wk) stage
| Electron Transport Chain | Optical Density | Control Average ± SE | Exposed Average ± SE |
|---|---|---|---|
| Complex I | AU | 8.69 ± 1.66 | 8.61 ± 2.26 |
| Complex II | AU | 7.02 ± 1.07 | 6.58 ± 1.98 |
| Complex III | AU | 9.80 ± 1.10 | 10.2 ± 2.45 |
| Complex IV | AU | 10.6 ± 0.27 | 9.83 ± 1.82 |
| Complex V | AU | 8.59 ± 0.76 | 8.66 ± 2.00 |
Values are averages ± SE for each measure; n = 3 control and n = 4 gestational TiO2-exposed animals. Complex I (P = 0.94), complex II (P = 0.74), complex III (P = 0.82), complex IV (P = 0.5), and complex V (P = 0.96) were evaluated and found to be statistically insignificant.
Metabolism and Cell Stress
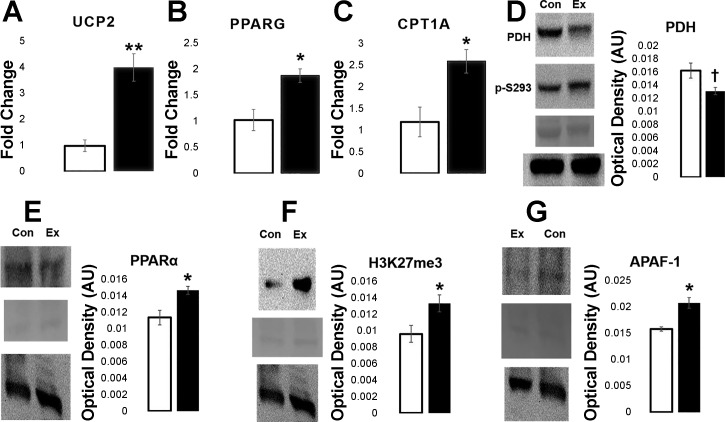
To understand some of the molecular changes resulting from gestational nano-TiO2 exposure to progeny, qPCR and Western blot analysis were used to probe changing mRNA and protein levels, respectively. At the fetal time point, mRNA of proteins contributing to proton leak and fatty acid metabolism was examined. For proton leak, qPCR of UCP2 revealed increased (P = 0.006) mRNA levels in the experimental group compared with the control (Fig. 6A), with qPCR of fatty acid metabolism constituents PPAR-γ (P = 0.039) and CPT1A (P = 0.023) exhibiting a similar increase (Fig. 6, B and C). Because molecular markers involving proton leak and fatty acid metabolism were changed in progeny at the fetal level of development, the next step was to evaluate protein expression in the young adult cardiomyocytes.
Fig. 6.
qPCR and Western blot data from fetal and young adult progeny. Fetal heart tissue was analyzed using qPCR for mRNA levels, while young adult cardiomyocytes were evaluated using Western blotting for protein content. Control (n = 5) vs. exposed (n = 5) mRNA fold change levels for uncoupling protein 2 (UCP2; A), peroxisome proliferator-activated receptor-γ (PPAR-γ; B), and carnitine palmitoyltransferase I (CPT1A; C) are shown. mRNA levels were normalized to GAPDH. Control (n = 4) vs. exposed (n = 4) optical density for pyruvate dehydrogenase (PDH) and pyruvate dehydrogenase E1 α-subunit (phospho S293) (PDH p-S29; D), peroxisome proliferator-activated receptor-α (PPAR-α; E), histone 3 lysine 27 trimethylation (H3K27me3; F), and APAF-1 (G) are shown. Blots are organized as follows: sample (top), Ponceau S staining (middle), and GAPDH (bottom). One representative image from both the control (con) and gestational exposed (ex) groups were chosen to display. Values are means ± SE. †P = 0.075–0.055. *P ≤ 0.05. **P ≤ 0.01.
Subsequent experiments to measure changes in metabolism involved assessing PDH, which was found to be trending toward reduced expression (P = 0.061) in the experimental group (Fig. 6D), with activity of PDH, evaluated through phosphorylation at serine 293 (p-S293), found to be statistically insignificant. When comparing the fluorescence ratio of PDH p-S293 to PDH, the amount of p-S293 per PDH expression was higher in the experimental group, but not significantly (P = 0.082). PPAR-α (Fig. 6E) was found to be significantly increased (P = 0.017). As an epigenetic evaluation of the proteome, H3K4me3 and H3K7me3 were used to assess global histone activation/deactivation patterns in the cells, respectively. H3K4me3 expression remained similar between groups (data not shown), while H3K27me3 (Fig. 6F) showed a significant, increased expression (P = 0.045) in the experimental animals. Signals for cell stress were also evaluated. BAX, BCL-2, and optic atrophy 1 were found to be similarly expressed between groups (data not shown), whereas APAF-1 (Fig. 6G) was found to be significantly increased (P = 0.037) in the experimental group. Lastly, proton leak was further evaluated through voltage-dependent anion channel, adenine nucleotide translocase, and UCP3, showing no significant alterations between groups (data not shown). Changes in the epigenetic profile, metabolism, and mitochondrial stress of the experimental progeny demonstrate the implications of initial, as well as prolonged, effects of gestational nano-TiO2 exposure.
DISCUSSION
Nanotechnology holds the potential to significantly improve the lives of people around the world through a wide range of applications, including tumor abolition (2) or delivery of medication (31), but the risks associated with nanomaterial use must first be properly identified. The most widely manufactured nanomaterial in the world is TiO2, with up to 80% of its uses being involved in cosmetics and other personal care products (40). The ratio of nano-TiO2-to-TiO2 production continues to increase, with some predictions speculating total conversion of the market to nano-TiO2 by 2025 (43). With the growing interest in nano-TiO2 applications in the consumer market, nanotoxicological work is needed to fully define the extent to which this ENM contributes to human health and safety. Nano-TiO2 has demonstrated the potential to cause adverse health outcomes after exposure; what remains poorly understood is how nano-TiO2 can interact through indirect, gestational exposure on growing progeny. More specifically, it is unclear as to how nano-TiO2 impacts cardiac development and function.
The results of our study provide evidence that gestational nano-TiO2 exposure can significantly affect the cardiac function of progeny, leading to a decreased functional capacity in both the whole heart and the cardiomyocyte. A mechanism for this dysfunction was illustrated through changes to cardiomyocyte bioenergetics after gestational exposure, which was then validated more specifically through mitochondrial complex activity, suggesting a role of nano-TiO2 in decreasing cardiac function by the disruption of mitochondrial oxidative phosphorylation and respiration rates. The dose of nano-TiO2 administered was calculated as a conservative estimate of ENM occupational exposure projected as 2.3–8.5 yr of human exposure in a manufacturing setting. This calculation represents the maximum estimated dose deposited in the lungs. Because variables such as age, mass, clearance, and health states are not part of the calculation; we consider this dose very relevant to occupational settings. The exposures were also carried out over multiple, nonconsecutive days to more appropriately simulate human exposures. Low nanomaterial lung deposition, extended time courses for dosing, and the use of an inhalation facility all support study observations that have a much higher potential of being attributable to true, physiological outcomes in humans and possessing repeatability across studies.
In vivo measurements, including M-mode, B-mode, Doppler echocardiography, and speckle-tracking-based strain analysis were taken to assess changes in contraction mechanics of the LV and the flow of blood through the mitral valve. In systole, radial strain and displacement of the LV walls were decreased in the experimental group, demonstrating initial signs of dysfunction in LV contraction mechanics. Decreasing radial strain and displacement during contraction of the LV is coupled with more overt measures of LV systolic function presented in the study, including a decreased interventricular end-systolic septal thickness and decreased total mass of the LV in the experimental group [with total mass falling into the lower percentile of normative values for Sprague-Dawley rats (39)]. From this data, we conclude that gestational nano-TiO2 exposure weakens the contractile properties of the heart, potentially leading to increased risk factors of heart disease in older age. This is also supported by the fact that a decreased stroke volume is also seen to be trending in the experimental group. By decreasing the size of the LV, the thickness of its contractile walls, and measures of systolic radial strain and displacement, the function of the heart becomes more susceptible to perturbation and may indicate early predictive signs of heart complications.
In diastole, a decrease in E- and A-wave velocity and an increase in total E/A ratio were observed in the experimental group. Studies have suggested that E/A ratios > 1.5 can indicate diastolic dysfunction (1, 21), potentially leading to restrictive filling. Furthermore, the total decrease in both the E- and A-wave velocities seems to indicate that diastole is not only dysregulated through atrial pumping abnormalities (restrictive filling) but potentially through asymmetric, long-axis filling (33). Changes in systolic and diastolic parameters demonstrate that the heart of gestational nano-TiO2-exposed progeny develops a maladaptive phenotype. These findings raised questions as to the link between the observed cardiac dysfunction and the potential contribution of the cellular contractile unit, the cardiomyocyte.
In cardiomyocytes, changes in the departure velocity, baseline percentage to peak height, and both the area of departure and return under the curve were detected (Fig. 3F), although no changes were observed in other common parameters of cardiomyocyte contractility (time to peak 90% and time to baseline 90%). The changes found with cardiomyocyte contractility in the experimental group further support the hypothesis that gestational nano-TiO2 exposure weakens the contractile properties of the heart. A reduced baseline percentage to peak height and length of contraction in cardiomyocytes could be responsible for the attenuation in systolic radial strain and displacement, with reduced contractility also supporting the presentation of restrictive, diastolic filling. The decreased area of contraction in the experimental group (ultimately leading to a diminished cardiac output) could also be linked to the decreasing stroke volume.
Changes in cardiomyocyte contractility have been observed in the presence of nano-TiO2, but these experiments have been through direct incubation with the nanoparticle. Exceeding 10 µg/ml nano-TiO2 concentrations, changes in the departure velocity, return velocity, and peak shortening were observed in the cardiomyocytes (26, 46). Differences between the two approaches (direct application and gestational inhalation exposure) are that return velocity parameters remained unchanged in the present study. The speed of the return velocity is indicative of the effectiveness of calcium shuttling in the cytoplasm (5). Primarily, calcium return to the endoplasmic reticulum from the cytoplasm is accomplished through sarco(endo)plasmic reticulum Ca2+-ATPase pumps and Na+/Ca2+ transporters. Because return velocity remained constant across both groups, this provides an initial indication that cardiomyocyte dysfunction may not be the result of calcium handling, but rather a mechanism of bioenergetics and mitochondrial function.
Examining fetal/neonatal cardiomyocytes for changes in bioenergetics provided information on the longitudinal effects that gestational nano-TiO2 exposure may produce on the development of cardiomyocytes, illuminating time points during development that may be more fragile to perturbation. During fetal exposure to nano-TiO2, substrate unavailability/increased cytoplasmic oxidation could provide the reasoning for decreased basal respiration of cells and ultimately compromised bioenergetics, resulting from either direct or indirect effects of nano-TiO2 exposure. As observed, this decrease in basal respiration was also coupled with increased proton leak. Proton leak is a factor controlled by both environment (mitochondrial membrane potential and pH) as well as protein constituents of the mitochondrion (14, 47). What is significant about these findings is that, following normal conditions, increases in basal respiration should coincide with increases in proton leak. But, as indicated in the results, a significant decrease in basal respiration was followed by an increase in proton leak.
Examining the data from the neonatal cardiomyocytes, it becomes evident that basal respiration is restored, depicting that at some point between the fetal and neonatal time point, basal respiration levels in the exposed group recovers, matching those found in the controls. This may be an indication that disturbances of basal respiration in the fetal cardiomyocytes were due to environmental conditions [likely through limitations of substrate availability (5, 55) promoted by nano-TiO2 interactions with the mother or placenta]. Restoration of substrate supplies, no longer limited by nano-TiO2 interactions, restored basal respiration to that of controls in the neonatal group, although the neonatal group exhibited a decreased maximal respiration and spare respiratory capacity, with a similar pattern of increased proton leak in the experimental group. We conclude that changes in maximal respiration and spare capacity may reflect persistent effects of nano-TiO2 exposure, limiting mitochondrial health and function as the animal matures.
Normalization to basal respiration for the young adult group was performed for two reasons: 1) no significant change in basal respiration were observed in the neonatal group; and 2) cell number for adult cardiomyocytes will vary, depending on preparation and time before recording oxygen consumption. After normalizing to basal respiration, as well as maximal respiration and spare capacity, proton leak was seen to be increased in the exposed group over the control. With proton leak increasing over all three time points, unbalancing of the proton motive force, proton shuttling protein dysfunction/underexpression, and/or increased total reactive oxygen species could all be mechanisms contributing to the process.
To more specifically pinpoint the mechanisms behind changes in the respiration rates of cardiomyocytes from progeny exposed to nano-TiO2, ETC complex activities were examined. Complex I, III, IV, and V activities were measured in the young adult group, to determine whether changes in mitochondrial ETC complex activities provide support for the longitudinal, detrimental effects of gestational nano-TiO2 on energy production. Our data revealed no changes in complex III and complex V (ATP synthase), but strong trends for a decrease in complex I and complex IV, as indicated by an average of 20% decrease in activity for both. Because overall complex expression is not changing, a decrease in ETC complex activities, whether through transcriptional, translational, or posttranslational mechanisms, demonstrates that mitochondrial function, disturbed at both the fetal and neonatal time points, may still have an impact later in life.
Molecular changes at both the mRNA and protein level suggest that alterations in the metabolic profile of the heart become disrupted after gestational nano-TiO2 exposure, with variations also seen in the epigenetic profile and mitochondrial stability. Research suggests that increasing levels of fatty acid oxidation in the heart, with decreasing glucose metabolism, can be indicative of the initial stages of insulin insensitivity, hyperglycemia, and/or heart complications (6, 17, 28). In this study, PPAR-α, PPAR-γ, and CPT1A are seen to increase in the experimental group, while PDH, although trending, is reduced; these molecular changes, coupled with the observed diastolic dysfunction, are supportive of the hypothesis that increasing utilization of fatty acids could be coupled to a decrease in diastolic function resulting from an insult (32). Increases in PPAR-α protein expression may also be linked to proton leak and reactive oxygen species (ROS) production.
PPAR-α acts as a transcriptional regulator of UCPs by activating expression of the proteins (17, 62). This study found increasing mRNA levels of UCP2 in gestational nano-TiO2-exposed progeny, together with increased proton leak. Because increasing fatty acid metabolism leads to higher levels of incomplete β-oxidation, changes in ATP utilization, and changing initial substrates for the ETC, ROS production is likely to be increased (34, 68). With an increase in ROS, a greater proton leak could help to ameliorate damaging effects. The molecular data also reveal increasing APAF-1 expression in the experimental group, potentially pointing to decreased cell viability due to changing metabolic profiles. Because BAX and BCL-2 levels were similar between groups (data not shown), APAF-1 may be activated independently of the canonical apoptotic pathway, which has been shown to be possible in certain tissue types (24).
Limitations/Future Directions
This study focused specifically on the changing bioenergetics of gestational nano-TiO2-exposed cardiac tissue and cardiomyocytes, but future experimentation could examine alterations in calcium handling. Data presented in this paper, through molecular markers of metabolism and complex activity, suggest that bioenergetics plays a role in contractile dysfunction and reduced diastolic function, although measures of complex activity are likely underpowered due to variability in progeny birth and cohort sizes. Fluctuations in calcium availability or shuttling may also contribute to some of the phenotype, but would need further exploration. The study was limited by not directly assessing mitochondrial membrane potential and measures of ROS. This study demonstrated that UCP2 could be contributing to proton leak and should indicate a lower mitochondrial membrane potential, but, without direct measures of mitochondrial membrane potential, it is unclear if molecular changes resulted in functional changes, which we acknowledge as a limitation. With changing levels of proton leak, examining ROS production is also important in understanding if proton leak is a compensatory mechanism for preserving function, or a result of mitochondrial disease and dysregulation.
Molecularly, changes in constituents involved in fatty acid metabolism suggest an increase of β-oxidation in the gestational nano-TiO2-exposed group. To validate these conclusions, analysis of mitochondrial respiration in the presence of fatty acids, instead of glucose metabolites, would have been insightful. Lastly, a more in-depth understanding of epigenetic changes could provide greater insight into how function is regulated in gestational nano-TiO2-exposed progeny. This study showed that transcriptional activation marks on histones (H3K4me3) were not changed between groups (data not shown), but global transcriptional repression on histones (H3K27me3) was upregulated in the experimental group, suggesting decreased global gene expression. Further evaluating epigenetic consequences through whole genome bisulfite sequencing or chromatin immunoprecipitation sequencing could greatly enhance our understanding of nano-TiO2 effects on growing progeny.
Conclusions
In summary, gestational nano-TiO2 exposure impacts cardiac function of the progeny. Manifestations of cardiac impairment in the maturing adult can be seen functionally through systolic and diastolic abnormalities and cardiomyocyte contractile attenuation. Mechanisms driving these deficiencies were found to include changes in electron transport of the mitochondria, including decreasing basal and maximal respiration, decreases in complex I and IV activities, and increased proton leak. Our study demonstrates that exposure to nano-TiO2 indirectly, either through translocation across the placental barrier, change of the in utero environment, or through other mechanisms of interaction with the nanomaterial, induces significant cardiac deficiencies in development and adulthood.
This study has begun to define the molecular and physiological health outcomes related to progeny development after maternal ENM exposures, building on previous work that has examined uterine and developing progeny microvascular function (51, 54). These studies may provide valuable information regarding the effects of ENMs during gestational exposure, but insight into how the progeny heart is adversely affected has yet to be fully elucidated, although it is likely that inflammatory signaling in the placenta could be linked to improper development of the progeny (13, 49), without even the need for direct translocation of the nanomaterial (50). The purpose of this paper is to better characterize the effects resulting from changes that can alter the uterine environment, providing insight into the possible mediators involved in nanomaterial exposures. The physiological changes observed in this paper through nano-TiO2 also begin to raise questions regarding other engineered metal oxides which may share similar physiochemical characteristics and pathologies. Although nanomaterials continue to be a vital part of our industrial society, the effects of prenatal nanomaterial exposure are important to consider as their use continues to increase.
GRANTS
This work was supported by National Science Foundation Integrative Graduate Education and Research Traineeship: Research and Education in Nanotoxicology at West Virginia University Fellowship (1144676), awarded to Q. A. Hathaway; the National Institutes of Health (NIH) from the National Heart, Lung and Blood Institute (R56 HL-128485), awarded to J. M. Hollander; the NIH from the National Institute of Environmental Safety and Health (R01 ES-015022), awarded to T. R. Nurkiewicz; an American Heart Association Predoctoral Fellowship (13PRE16850066), awarded to C. E. Nichols; the NIH from the National Institute of Environmental Safety and Health (K99 ES024783), awarded to P. A. Stapleton. Small-animal imaging and image analysis were performed in the West Virginia University Animal Models & Imaging Facility, which has been supported by the West Virginia University Cancer Institute and NIH Grants P20 RR-016440, P30 GM-103488 and S10 RR-026378. Seahorse data acquisition was supported by the West Virginia Stroke Centers for Biomedical Research Excellence (P20 GM-109098).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Q.A.H., C.E.N., D.L.S., P.A.S., S.L.M., J.C.S., S.L.R., M.V.P., A.B.A., C.R.M., J.Y., and S.M.S. performed experiments; Q.A.H., C.E.N., D.L.S., S.L.R., and J.Y. analyzed data; Q.A.H., C.E.N., D.L.S., T.R.N., and J.M.H. interpreted results of experiments; Q.A.H. and J.Y. prepared figures; Q.A.H. drafted manuscript; Q.A.H., C.E.N., D.L.S., P.A.S., J.C.S., A.B.A., T.R.N., and J.M.H. edited and revised manuscript; Q.A.H., C.E.N., D.L.S., P.A.S., S.L.M., J.C.S., S.L.R., M.V.P., A.B.A., C.R.M., J.Y., S.M.S., T.R.N., and J.M.H. approved final version of manuscript.
REFERENCES
- 1.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation 105: 1928–1933, 2002. doi: 10.1161/01.CIR.0000015076.37047.D9. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66: 2–25, 2014. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: clinical applications. Heart 96: 2032–2040, 2010. doi: 10.1136/hrt.2010.199885. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349, 2005. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 7.Campagnolo L, Massimiani M, Palmieri G, Bernardini R, Sacchetti C, Bergamaschi A, Vecchione L, Magrini A, Bottini M, Pietroiusti A. Biodistribution and toxicity of pegylated single wall carbon nanotubes in pregnant mice. Part Fibre Toxicol 10: 21, 2013. doi: 10.1186/1743-8977-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol 29: 330–337, 2009. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 9.Croston TL, Shepherd DL, Thapa D, Nichols CE, Lewis SE, Dabkowski ER, Jagannathan R, Baseler WA, Hollander JM. Evaluation of the cardiolipin biosynthetic pathway and its interactions in the diabetic heart. Life Sci 93: 313–322, 2013. doi: 10.1016/j.lfs.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 1: 154–170, 2000. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 11.Dabkowski ER, Williamson CL, Bukowski VC, Chapman RS, Leonard SS, Peer CJ, Callery PS, Hollander JM. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol 296: H359–H369, 2009. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med 31: 181–190, 2001. doi: 10.1016/S0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 13.de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, Furukawa LN, de Castro I, Saldiva PH, Heimann JC. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett 232: 475–480, 2015. doi: 10.1016/j.toxlet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 26: 192–205, 2011. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 15.Duan Y, Liu H, Zhao J, Liu C, Li Z, Yan J, Ma L, Liu J, Xie Y, Ruan J, Hong F. The effects of nano-anatase TiO(2) on the activation of lactate dehydrogenase from rat heart. Biol Trace Elem Res 130: 162–171, 2009. doi: 10.1007/s12011-009-8326-9. [DOI] [PubMed] [Google Scholar]
- 16.Faddah LM, Abdel Baky NA, Al-Rasheed NM, Al-Rasheed NM. Biochemical responses of nanosize titanium dioxide in the heart of rats following administration of idepenone and quercetin. Afr J Pharm Pharmacol 7: 2639–2651, 2013. doi: 10.5897/AJPP2013.3426. [DOI] [Google Scholar]
- 17.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109: 121–130, 2002. doi: 10.1172/JCI0214080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 307: H1353–H1360, 2014. doi: 10.1152/ajpheart.00526.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwinn MR, Vallyathan V. Nanoparticles: health effects--pros and cons. Environ Health Perspect 114: 1818–1825, 2006. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han W, Wang Y, Zheng Y. In vivo biocompatibility studies of nano TiO2 materials. Adv Mat Res 79–82: 389–392, 2009. doi: 10.4028/www.scientific.net/AMR.79-82.389. [DOI] [Google Scholar]
- 21.Haney S, Sur D, Xu Z. Diastolic heart failure: a review and primary care perspective. J Am Board Fam Pract 18: 189–198, 2005. doi: 10.3122/jabfm.18.3.189. [DOI] [PubMed] [Google Scholar]
- 22.Hougaard KS, Campagnolo L, Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D, Valentino S, Park MV, de Jong WH, Wolterink G, Piersma AH, Ross BL, Hutchison GR, Hansen JS, Vogel U, Jackson P, Slama R, Pietroiusti A, Cassee FR. A perspective on the developmental toxicity of inhaled nanoparticles. Reprod Toxicol 56: 118–140, 2015. doi: 10.1016/j.reprotox.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Zhang F, Sun X, Choi KY, Niu G, Zhang G, Guo J, Lee S, Chen X. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials 35: 856–865, 2014. doi: 10.1016/j.biomaterials.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imao T, Nagata S. Apaf-1- and caspase-8-independent apoptosis. Cell Death Differ 20: 343–352, 2013. doi: 10.1038/cdd.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James JF, Hewett TE, Robbins J. Cardiac physiology in transgenic mice. Circ Res 82: 407–415, 1998. doi: 10.1161/01.RES.82.4.407. [DOI] [PubMed] [Google Scholar]
- 26.Jawad H, Boccaccini AR, Ali NN, Harding SE. Assessment of cellular toxicity of TiO2 nanoparticles for cardiac tissue engineering applications. Nanotoxicology 5: 372–380, 2011. doi: 10.3109/17435390.2010.516844. [DOI] [PubMed] [Google Scholar]
- 27.Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V, Nurkiewicz TR. Nanoparticle inhalation alters systemic arteriolar vasoreactivity through sympathetic and cyclooxygenase-mediated pathways. Nanotoxicology 6: 724–735, 2012. doi: 10.3109/17435390.2011.606926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbé SM, Grenier-Larouche T, Noll C, Phoenix S, Guérin B, Turcotte EE, Carpentier AC. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes 61: 2701–2710, 2012. doi: 10.2337/db11-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leavens TL, Parkinson CU, James RA, House D, Elswick B, Dorman DC. Respiration in Sprague-Dawley rats during pregnancy. Inhal Toxicol 18: 305–312, 2006. doi: 10.1080/08958370500444361. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Ma L, Zhao J, Liu J, Yan J, Ruan J, Hong F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129: 170–180, 2009. [Erratum. Biol Trace Elem Res 160 (Jul): 152, 2014.] 10.1007/s12011-008-8285-6. [DOI] [PubMed] [Google Scholar]
- 31.Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, Feng SS. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev 65: 880–890, 2013. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Mori J, Basu R, McLean BA, Das SK, Zhang L, Patel VB, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail 5: 493–503, 2012. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 33.Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart 91: 681–695, 2005. doi: 10.1136/hrt.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 32: 874–883, 2007. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 35.Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol 5: 1, 2008. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65: 1531–1543, 2002. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 37.Park H, Yeo M. Comparison of gene expression changes induced by exposure to Ag, Cu-TiO2, and TiO2 nanoparticles in zebrafish embryos. Mol Cell Toxicol 9: 129–139, 2013. doi: 10.1007/s13273-013-0017-0. [DOI] [Google Scholar]
- 38.Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging 24: 479–491, 2008. doi: 10.1007/s10554-007-9286-9. [DOI] [PubMed] [Google Scholar]
- 39.Pawlush DG, Moore RL, Musch TI, Davidson WR Jr. Echocardiographic evaluation of size, function, and mass of normal and hypertrophied rat ventricles. J Appl Physiol (1985) 74: 2598–2605, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Piccinno F, Gottschalk F, Seeger S, Nowack B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14: 1109, 2012. doi: 10.1007/s11051-012-1109-9. [DOI] [Google Scholar]
- 41.Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 7: 1179–1194, 2012. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi W, Bi J, Zhang X, Wang J, Wang J, Liu P, Li Z, Wu W. Damaging effects of multi-walled carbon nanotubes on pregnant mice with different pregnancy times. Sci Rep 4: 4352, 2014. doi: 10.1038/srep04352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol 43: 4227–4233, 2009. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- 44.Sager TM, Castranova V. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part Fibre Toxicol 6: 15, 2009. doi: 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol 5: 17, 2008. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savi M, Rossi S, Bocchi L, Gennaccaro L, Cacciani F, Perotti A, Amidani D, Alinovi R, Goldoni M, Aliatis I, Lottici PP, Bersani D, Campanini M, Pinelli S, Petyx M, Frati C, Gervasi A, Urbanek K, Quaini F, Buschini A, Stilli D, Rivetti C, Macchi E, Mutti A, Miragoli M, Zaniboni M. Titanium dioxide nanoparticles promote arrhythmias via a direct interaction with rat cardiac tissue. Part Fibre Toxicol 11: 63, 2014. doi: 10.1186/s12989-014-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah U, Bien H, Entcheva E. Microtopographical effects of natural scaffolding on cardiomyocyte function and arrhythmogenesis. Acta Biomater 6: 3029–3034, 2010. doi: 10.1016/j.actbio.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepherd DL, Nichols CE, Croston TL, McLaughlin SL, Petrone AB, Lewis SE, Thapa D, Long DM, Dick GM, Hollander JM. Early detection of cardiac dysfunction in the type 1 diabetic heart using speckle-tracking based strain imaging. J Mol Cell Cardiol 90: 74–83, 2016. doi: 10.1016/j.yjmcc.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirasuna K, Usui F, Karasawa T, Kimura H, Kawashima A, Mizukami H, Ohkuchi A, Nishimura S, Sagara J, Noda T, Ozawa K, Taniguchi S, Takahashi M. Nanosilica-induced placental inflammation and pregnancy complications: different roles of the inflammasome components NLRP3 and ASC. Nanotoxicology 9: 554–567, 2015. doi: 10.3109/17435390.2014.956156. [DOI] [PubMed] [Google Scholar]
- 50.Sood A, Salih S, Roh D, Lacharme-Lora L, Parry M, Hardiman B, Keehan R, Grummer R, Winterhager E, Gokhale PJ, Andrews PW, Abbott C, Forbes K, Westwood M, Aplin JD, Ingham E, Papageorgiou I, Berry M, Liu J, Dick AD, Garland RJ, Williams N, Singh R, Simon AK, Lewis M, Ham J, Roger L, Baird DM, Crompton LA, Caldwell MA, Swalwell H, Birch-Machin M, Lopez-Castejon G, Randall A, Lin H, Suleiman MS, Evans WH, Newson R, Case CP. Signalling of DNA damage and cytokines across cell barriers exposed to nanoparticles depends on barrier thickness. Nat Nanotechnol 6: 824–833, 2011. doi: 10.1038/nnano.2011.188. [DOI] [PubMed] [Google Scholar]
- 51.Stapleton PA, McBride CR, Yi J, Nurkiewicz TR. Uterine microvascular sensitivity to nanomaterial inhalation: an in vivo assessment. Toxicol Appl Pharmacol 288: 420–428, 2015. doi: 10.1016/j.taap.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stapleton PA, Minarchick VC, Cumpston AM, McKinney W, Chen BT, Sager TM, Frazer DG, Mercer RR, Scabilloni J, Andrew ME, Castranova V, Nurkiewicz TR. Impairment of coronary arteriolar endothelium-dependent dilation after multi-walled carbon nanotube inhalation: a time-course study. Int J Mol Sci 13: 13781–13803, 2012. doi: 10.3390/ijms131113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stapleton PA, Minarchick VC, Yi J, Engels K, McBride CR, Nurkiewicz TR. Maternal engineered nanomaterial exposure and fetal microvascular function: does the Barker hypothesis apply? Am J Obstet Gynecol 209: e221–e211, 2013. doi: 10.1016/j.ajog.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stapleton PA, Nichols CE, Yi J, McBride CR, Minarchick VC, Shepherd DL, Hollander JM, Nurkiewicz TR. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology 9: 941–951, 2015. doi: 10.3109/17435390.2014.984251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda K, Suzuki K, Ishihara A, Kubo-Irie M, Fujimoto R, Tabata M, Sugamata M.. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J Health Sci 55: 95–102, 2009. doi: 10.1248/jhs.55.95. [DOI] [Google Scholar]
- 56.Thapa D, Nichols CE, Lewis SE, Shepherd DL, Jagannathan R, Croston TL, Tveter KJ, Holden AA, Baseler WA, Hollander JM. Transgenic overexpression of mitofilin attenuates diabetes mellitus-associated cardiac and mitochondria dysfunction. J Mol Cell Cardiol 79: 212–223, 2015. doi: 10.1016/j.yjmcc.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JX, Fan YB, Gao Y, Hu QH, Wang TC. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 30: 4590–4600, 2009. doi: 10.1016/j.biomaterials.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Weldy CS, Liu Y, Chang YC, Medvedev IO, Fox JR, Larson TV, Chien WM, Chin MT. In utero and early life exposure to diesel exhaust air pollution increases adult susceptibility to heart failure in mice. Part Fibre Toxicol 10: 59, 2013. doi: 10.1186/1743-8977-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weldy CS, Liu Y, Liggitt HD, Chin MT. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS One 9: e88582, 2014. doi: 10.1371/journal.pone.0088582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittig I, Braun HP, Schägger H. Blue native PAGE. Nat Protoc 1: 418–428, 2006. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 61.Xu X, Colecraft HM. Primary culture of adult rat heart myocytes. J Vis Exp 28: 1308, 2009. doi: 10.3791/1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med (Berl) 85: 697–706, 2007. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- 63.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53: 1336–1343, 2004. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 64.Yeo M, Kim H. Gene expression in zebrafish embryos following exposure to TiO2 nanoparticles. Mol Cell Toxicol 6: 97–104, 2010. doi: 10.1007/s13273-010-0013-6. [DOI] [Google Scholar]
- 65.Yi J, Chen BT, Schwegler-Berry D, Frazer D, Castranova V, McBride C, Knuckles TL, Stapleton PA, Minarchick VC, Nurkiewicz TR. Whole-body nanoparticle aerosol inhalation exposures. J Vis Exp 75: e50263, 2013. doi: 10.3791/50263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida S, Tsutsumi S, Muhlebach G, Sourbier C, Lee MJ, Lee S, Vartholomaiou E, Tatokoro M, Beebe K, Miyajima N, Mohney RP, Chen Y, Hasumi H, Xu W, Fukushima H, Nakamura K, Koga F, Kihara K, Trepel J, Picard D, Neckers L. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc Natl Acad Sci USA 110: E1604–E1612, 2013. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu X, Hong F, Zhang YQ. Cardiac inflammation involving in PKCε or ERK1/2-activated NF-κB signalling pathway in mice following exposure to titanium dioxide nanoparticles. J Hazard Mater 313: 68–77, 2016. doi: 10.1016/j.jhazmat.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta 1801: 1–22, 2010. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]