Abstract
In order to evaluate alternative tests and strategies to simplify pediatric human immunodeficiency virus (HIV) screening at the district hospital level, a cross-sectional exploratory study was organized in the Democratic Republic of the Congo. Venous and capillary phlebotomies were performed on 941 Congolese children, aged 1 month to 12 years (153 children under 18 months and 788 children more than 18 months old). The HIV prevalence rate was 4.7%. An algorithm for children more than 18 months old, using serial rapid tests (Determine, InstantScreen, and Uni-Gold) performed on capillary blood stored in EDTA tubes, had a sensitivity of 100.0% (95% confidence interval [CI], 88.9 to 100.0%) and a specificity of 100.0% (95% CI, 99.5 to 100.0%). The results of this study suggest that the ultrasensitive p24 antigen assay may be performed on capillary plasma stored on filter paper (sensitivity and specificity, 100.0%; n = 87) instead of venous plasma (sensitivity, 92.3%; specificity, 100.0%; n = 150). The use of glucolets (instruments used to perform capillary phlebotomies), instead of syringes and needles, may reduce procedural pain and the risk of needle stick injuries at a comparable cost. Compared to the reference, HIV could have been correctly excluded based on one rapid test for at least 90% of these children. The results of this study point towards underutilized opportunities to simplify phlebotomy and pediatric HIV screening.
A serostatus-based approach and early human immunodeficiency virus (HIV) diagnosis are crucial for optimal pediatric HIV care, but so far few African children have been tested (5, 8, 9, 13, 14). Due to the presence of passive HIV antibodies from the mother and prolonged exposure through breastfeeding, HIV diagnosis in infants requires more expensive tests, such as PCR. These are rarely available or affordable at the district hospital level, which makes HIV counseling for children even more challenging (1, 18). In children, phlebotomy is often more difficult and is accompanied by greater risks in cases of needle stick injuries (28, 39). A recent survey among African doctors reported a high degree of fatalism with respect to the treatment of HIV-infected children (12). Since the majority of these children rely on district hospitals for their health care, simplification of HIV screening at that level could substantially facilitate programs for the rollout of pediatric antiretroviral therapy (16).
While previous research in Africa showed that rapid tests have performance equivalent to that of serial enzyme immunoassays (EIA), children were usually not included in these study populations (17, 41). A validation of the accuracy of rapid tests in African children could facilitate decentralization of pediatric HIV screening (20, 33).
Previous studies have demonstrated that the ultrasensitive or heat-denatured signal-amplified p24 antigen (Up24 Ag) assay may have a sensitivity and specificity comparable to those of DNA-PCR (10). Most of these studies were done among patients with sub-type B infections in research settings. Because of the high genetic heterogeneity of HIV type 1 (HIV-1) in the Democratic Republic of the Congo (DRC), assessment of test performance on venous plasma from Congolese children could facilitate further operationalization of the test (21, 22). It has not yet been documented whether the Up24 Ag assay could also be performed on capillary plasma stored on filter paper. Experience with DNA PCR on dried blood spots demonstrated that this could greatly simplify phlebotomy and transport of samples (3, 27).
Glucolets, i.e., instruments used to perform capillary phlebotomies (Glucolet 2 combo; Bayer), have allowed simplification of diabetes monitoring. Their use could also simplify pediatric phlebotomy and reduce procedural pain and the risk of needle stick injuries (3, 33).
This study aims to investigate opportunities for simplification of pediatric HIV screening at the district hospital level in Africa. The first goal is to assess the accuracy of an alternative screening strategy for children more than 18 months old, based on serial rapid tests performed on capillary blood stored in EDTA tubes. A second goal of this study is to assess the accuracy of the diagnostic Up24 Ag assay performed on venous plasma and capillary plasma from children less than 18 months old that has been stored on filter paper in case of non-subtype B infection. Finally, this study aims to evaluate the use of glucolets to simplify phlebotomy.
MATERIALS AND METHODS
A cross-sectional study was organized in the DRC to evaluate the performance of alternative tests (Up24 Ag assay and rapid tests) and alternative strategies (test performance with capillary blood and plasma, storage of blood in EDTA tubes, storage of plasma on filter paper, and use of glucolets).
Selection of sites and study population.
Seven sites were selected in Lubumbashi and Kinshasa, DRC. The selection criteria for sites included availability of pre-and posttest counseling and a plan for medical follow-up for the HIV-infected children. The site settings were diverse, including a center for ambulatory HIV care, an association for HIV-infected mothers, an organization responsible for HIV orphans in the community, orphanages, and a center for reintegration of street children.
The sites were responsible for the selection of children. Selection criteria for children included the following. There were a signed informed consent and documented legal guardianship in case parental consent could not be obtained. The age was between 1 month and 12 years. (One month was chosen as the cutoff because of the lower sensitivity of DNA PCR for children less than 1 month old [25, 30, 46]. Twelve years was an arbitrary cutoff.) Antiretroviral therapy was not yet started, and there was a previously unmet need for HIV testing, because of medical reasons or a request of the legal guardian. The project tried as much as possible to attend to the unmet need without creating additional demand for HIV testing. All selected children were accepted for testing, except when the number exceeded the capacity of the phlebotomy team. In such case, the staff of the site was asked to reduce the number. The research team was blind to the serostatus of all children. No attempts were made to apply a clinical case definition or to obtain medical information.
Phlebotomy.
Phlebotomy was organized at the sites to respect familiarity and to mimic real-life conditions and patient load. Venous and capillary phlebotomies were performed on each child, to allow comparison with the reference. Multifly (Sarstedt, Essen, Belgium) and S-monovette 4-ml EDTA tubes (Sarstedt) were used for the venous phlebotomy. Glucolet 2 Combo (Bayer, Brussels, Belgium) and microtainer EDTA tubes (Becton Dickinson, Erembodegem, Belgium) were used for the capillary phlebotomy. Unistick 2 Super (Owen Mumford, Klinipath, Duiven, The Netherlands) was also available.
Child friendliness was high on the agenda. When the child was less cooperative, priority was given to venous phlebotomy in order to secure diagnosis. Consequently, there was not always sufficient sample for the full analysis. Local artists helped with psychological preparation of the children. The children's hands were soaked in warm soapy water to facilitate finger pricks. Biscuits and a toy or a set of stationery were given to all participants, and transportation fees were refunded.
Local phlebotomists were trained and informed about free postexposure prophylaxis. The six phlebotomists from Lubumbashi were less experienced in pediatric phlebotomy than the six phlebotomists from Kinshasa. This allowed evaluation of the effects of training. All phlebotomies were supervised by the principal investigator.
Laboratory work.
Diagnosis was based on the reference only and communicated via the local laboratory staff. All samples were coded. Only the principal investigator knew the code. Local lab technicians were trained by a lab technician from the Institute of Tropical Medicine (ITM) or the director of the National AIDS Reference Laboratory (ARL) of the DRC, who supervised nearly all laboratory work done in the DRC. The temperature was continuously monitored and remained within the required range. All tests were performed within 4 months.
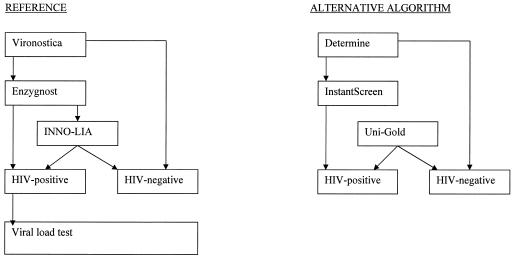
The reference for children more than 18 months old was the result of an algorithm using Vironostica HIV Uni-Form II Plus O (bioMérieux bv, Boxtel, The Netherlands), Enzygnost Anti-HIV 1/2 Plus (Dade Behring Marburg GmbH, Marburg, Germany), and INNO-LIA HIV (Innogenetics S.A., Ghent, Belgium) on the same sample of venous plasma. An HIV-negative status was based on the first test or two of the three tests being negative. An HIV-positive status was based on two positive tests. This was according to the proposed adapted World Health Organization-United Nations AIDS HIV testing strategy III (33) (Fig. 1). A viral load test (Cobas AmpliPrep/Amplicor HIV-1 [V1.5]; Roche, Branchburg, N.J.) was performed on each HIV-positive patient. Since the viral load was always above 50 copies/ml, this test confirmed all diagnoses. The proposed alternative screening strategy followed the same principle to determine the HIV status but used serial rapid tests on the same sample of capillary blood stored in EDTA tubes (6, 45) (Fig. 1). The Determine HIV-1/2 (Abbott Laboratories, Tokyo, Japan), InstantScreen Rapid HIV-1/2 (Gaifar GmbH, Potsdam, Germany), and Uni-Gold HIV (Trinity Biotech PLC, Bray, Ireland) tests were chosen because of characteristics making them more suitable for the African context (Table 1).
FIG. 1.
Reference versus alternative algorithm for children over 18 months of age.
TABLE 1.
Characteristics of the chosen rapid tests
| Parameter | Test characteristic
|
||
|---|---|---|---|
| Test | Determine HIV-1/2 | Instant Screen Rapid HIV-1/2 | Uni-Gold HIV |
| Company | Abbott Laboratories | Gaifar GmbH | Trinity Biotech PLC |
| Temperature range (°C) | 2-30 | −20-45 | 2-27 |
| Shelf life of kit (mo) | 18 | 18 | 20 |
| Volume of blood needed (μl) | 50 | 20 | 60 |
| Time to perform assay for one specimen (min) | 17 | 3 | 11 |
| Ease of performing test | Very easy | Very easy | Very easy |
| Volume of kit (length-width-height [cm]) | Small (27-16-1) | Large (30-19-9) | Large (23-14-10.5) |
| Price in developing countriesa | $0.9 | $2-4 | $2-4 |
| Principle of test | Immunochromatography | Immunofiltration | Immunochromatography |
| Recombinant proteins and synthetic peptides useda | gp41 and gp36 | gp120, gp41, and gp36 | gp120, gp41, and gp34 |
Information obtained through communication with the respective companies.
The reference for children less than 18 months old was the DNA PCR performed on dried blood spots stored on filter paper (Schleicher & Schuell, Ghent, Belgium) following an in-house method. A viral load test was performed on each PCR-positive patient. Since the viral load was always above 50 copies/ml, this test confirmed all diagnoses. The alternative test for infants was the Up24 Ag assay. This was performed on frozen venous and capillary plasma, as well as on the respective samples stored on filter paper (Schleicher & Schuell), according to the procedure described by Schüpbach et al. (35). For part of this group of infants, the Determine test was performed to assess its power to identify HIV-affected children.
Laboratory work was done locally when possible. Rapid tests and EIA were performed on fresh samples by the local (Lubumbashi or Kinshasa) laboratory staff. The staff of the National ARL of the DRC (Kinshasa) also performed the viral load tests for children over 18 months of age. All other tests were performed by the staff of the ARL of the ITM (Belgium), which has assisted the Congolese National ARL with quality control since 1999. Viral load tests and the INNO-LIA test were performed on frozen plasma. Samples for the viral load test in children less than 18 months old were diluted 1:10 in negative human serum (Table 2).
TABLE 2.
Summary of the performed testsa
| Patient group | Reference strategy (no. of assays; lab site[s]) | Alternative strategy | Additional tests |
|---|---|---|---|
| Children under 18 months | DNA PCRC-FP (153; A) | Up24 Ag assayV-F (150; A) | Up24 Ag assayC-F (125; A) |
| Up24 Ag assayC-FP (87; A) | Quantitative Up24 Ag assayV-F for false-negative diagnostic Up24 Ag assay (1; A) | ||
| Viral load testV-F (13; A) | Up24 Ag assayV-FP for HIV-positive samples only (12; A) | ||
| Determinec (9; L); Determine (107; K/L) | |||
| Tests for quality control (A) | |||
| Children over 18 months | VironosticaV (788; K/L) | DetermineC (788; K/L) | InstantScreenC for samples with blood clots (46; K/L) |
| EnzygnostV (43; K/L) | InstantScreenC (45; K/L) | ||
| INNO-LIAV-F (8; A) | Uni-GoldC (8; K/L) | DetermineV instead of INNO-LIA (8; K/L) | |
| Viral load testV-F (31; K) | Tests for quality control (K/A) |
Abbreviations: K, Kinshasa, DRC; L, Lubumbashi, DRC; A, Antwerp, Belgium. Superscripts: v, venous plasma; c, capillary plasma; f, frozen; fp, stored on filter paper (if not fresh samples).
Procedures for the ultrasensitive p24 antigen assays.
The Alliance HIV-1 p24 enzyme-linked immunosorbent assay kit, the ELAST enzyme-linked immunosorbent assay amplification system, and a confirmatory reagent (Perkin-Elmer Life Science, Boston, Mass.) were used to perform the Up24 assays. To each 100-μl plasma sample, 50 μl of an in-house viral lysis buffer and 450 μl of 0.5% Triton X-100 were added. The diluted plasma was heated for 5 min at 100°C. Two hundred fifty microliters of each sample was inserted into a well and tested in duplicate. Two negative controls, tested in duplicate, were included in each run. The plate was incubated on a shaker for 2 h at room temperature. After washing with diluted phosphate-buffered saline (PBS), 100 μl of biotinylated detector antibody was added. The samples were incubated at 37°C for 1 h and washed. One hundred microliters of streptavidin-horseradish peroxidase (1:50 dilution) was added, and the plate was incubated for 15 min at 37°C. After washing, 100 μl of biotinyl tyramide working solution was added. The mixture was incubated for 15 min at room temperature in the dark. After washing, 100 μl of streptavidin-horseradish peroxidase solution diluted 1:2,000 was added, and the plate was incubated for 15 min in the dark. After a final washing step, 100 μl of ortho-phenylen-diamin was added, and the plate was incubated for 30 min at room temperature in the dark. The reaction was stopped, and the absorbance was read with a Bio-Rad 550 microplate reader at 490 nm with the reference at 630 nm. A sample was considered initially reactive if the optical density (OD) was equal to or greater than the sum of the means of the four negative controls plus five times the standard deviation of these means. An initially reactive sample was further tested in a neutralization assay to eliminate false-positive results. For this assay, a 1:6 dilution of the samples, a positive and a negative control, were made. After heat denaturation, 10 μl of control reagent was added to 300 μl of the diluted plasma, and 10 μl of confirmatory reagent was added to another 300 μl of the same diluted plasma sample. After 30 min of incubation at 37°C, the procedure described above was followed. Again two negative controls were incorporated in each run and tested in duplicate. If the OD of the sample with the control reagent was equal to or greater than the cutoff, the percent neutralization was calculated with the following formula: 100 × [(OD of sample with control reagent − OD of sample with neutralizing reagent)/(OD of sample with control reagent − OD of negative control with control reagent)]. If the calculated percent neutralization for a sample was higher than 50%, the sample was considered HIV Ag positive.
For the elution of dried plasma spots, an in-house method was used: the entire spot on the filter paper (on which 100 μl of plasma had been spotted) was used for the Up24 assay. Six hundred thirty microliters of 0.5% Triton X-100 and 70 μl of viral lysis buffer were added. The mixture was incubated at room temperature for 3 h on a shaker and centrifuged (2.000 × g), and the procedure described above was followed for the supernatant.
Procedure for DNA PCR on dried blood spots.
An in-house method was used for extraction of DNA from dried blood spots. Three disks of 7-mm diameter were cut from the filter paper and washed with 1 ml of 0.5% saponin-PBS. The mixture was incubated overnight at 4°C and centrifuged, and the supernatant was discarded. One milliliter of PBS was added, and the tube was incubated at 4°C for 2 h. Subsequently, the mixture was centrifuged; the supernatant was discarded, and 100 μl of 10% Chelex-100 was added. The tube was incubated at 95°C for 10 min while being mixed continuously. The mixture was centrifuged for 6 min at 16,100 × g. The supernatant was transferred to another tube and centrifuged for another 6 min, which produced a supernatant that was stored at −20°C and used for the PCR.
Fragments of the HIV-1 pol and env genes were amplified by a nested PCR. For this reaction, 10 μl of the extracted supernatant was used in a total PCR volume of 50 μl. The amplified DNA was separated by rapid agarose gel electrophoresis and stained by ethidium bromide, and visualization was done by transillumination. A fragment of the β2 microglobulin gene was amplified in a single PCR assay to ensure DNA quantity and quality after extraction (11). The detection limit for the PCR on dried blood spots was set at 250 copies per 10 μl of eluate by the use of an 8E5 cell line and HIV-negative blood spotted on filter paper. Each run incorporated a positive extraction control of approximately 250 copies per 10 μl and a negative extraction control.
In the case of repeatedly discordant results between the pol and env primer sets, a third nested PCR with a long terminal repeat primer set (HLTR67, AGCCTCAATAAAGCTTGCCTTG outer, sense; HLTR336, CTGACGCTCTCGCACCCAT outer, antisense; HLTR126, TCTGGTAACTAGAGAGATCCCTCA inner, sense; HLTR228, GAGTCCTGCGTCGAGAGA inner, antisense) was performed under the same conditions as the pol and env PCR (unpublished data).
A sample was considered HIV positive if a positive result was observed for at least two of the primer sets. A sample was considered indeterminate if a positive result was obtained for only one primer set and HIV negative if the results for both initial primer sets (pol and env) were negative (40).
Statistical analysis.
Data analysis was done with Intercooled Stata version 7.0 (StataCorp, College Station, Tex.). Because preliminary data were scarce and it was expected to be difficult to enroll the required number of patients for each age group, it was decided to start with an exploratory study (2, 29). For each age group, the proposed strategy was compared to the reference, and two-sided confidence intervals (CI) for sensitivity and specificity were calculated with the Wilson score method as described by Newcombe (26).
Ethics.
Permission for the project was granted by the ethics committee of the Institute of Tropical Medicine in Antwerp, Belgium, the ethics committee of the University of Kinshasa, and the Social Welfare Office, DRC.
RESULTS
Between 8 September and 11 October, 2003, phlebotomy was performed on 941 Congolese children between 1 month and 12 years of age (153 children were under 18 months, and 788 children were over 18 months). Two sites in Kinshasa (28.2% of all children) and five sites in Lubumbashi (71.8% of all children) participated in the study. A total of 50.7% were boys, and 49.3% were girls. The HIV prevalence was 4.7% for the total group (3.7% for the Lubumbashi group and 7.2% for the Kinshasa group). Table 3 contains a more detailed description of the study population.
TABLE 3.
Characteristics of the study populationa
| Parameter | Characteristic for:
|
||
|---|---|---|---|
| Total population | Children below 18 months | Children above 18 months | |
| No. of patients (% of total group) | 941 | 153 (16.2) | 788 (83.8) |
| % of subjects (no. of sites from which children were selected) from: | |||
| Lubumbashi | 71.8 (7) | 63.4 (4) | 73.5 (5) |
| Kinshasa | 28.2 (7) | 36.6 (2) | 26.5 (2) |
| Mean age (±SD) | 9.7 (±4.9) mo | 6.7 (±3.3) yr | |
| Male/female (%) | 50.7/49.3 | 60.1/39.9 | 48.9/51.1 |
| No. of HIV-infected children (% within subgroup) | 44 (4.7) | 13 (8.5) | 31 (3.9) |
| Mean age of HIV-infected children (±SD) | 11.8 (±4.8) mo | 7 (±3.1) yr | |
| Mean viral load (no. of copies/ml) | 985,262 | 2,602,146 | 307,213 |
| Viral range load (log no. of copies/ml) | 3.28-7.19 | 4.84-7.19 | 3.28-6.21 |
SD, standard deviation.
The proposed algorithm for children over 18 months, based on serial rapid tests performed on capillary blood stored in EDTA tubes, had a sensitivity of 100.0% (95% CI, 88.9 to 100.0%) and a specificity of 100.0% (95% CI, 99.5 to 100.0%) (Table 4). In case there was some doubt about the reading of the Determine test line (1.3%), the result was considered indeterminate, and an InstantScreen test was performed. All these InstantScreen results were also negative. Separate analysis of the Determine results for children over 18 months revealed no false-negative and two false-positive results out of 788 cases (excluding indeterminate cases). Among the 116 samples from children under 18 months, there were no false-negative and only three false-positive Determine results out of 116 cases. Small dots, caused by blood clots, were observed on 6.4% of all Determine test strips for children over 18 months. High percentages (more than 10%) of these results were observed for the first 300 samples, but the percentages quickly decreased to less than 4% for other subsets of 100 consecutive samples. Although small dots were observed, all these tests could be categorized as clearly negative (the control line was present). For some of them (n = 46), InstantScreen was performed, and all these tests confirmed the negative Determine results. The dots were observed only in samples from the less experienced phlebotomists, suggesting effects of training. For one InstantScreen test (0.8%), flowthrough did not occur.
TABLE 4.
Evaluation of the proposed rapid-test algorithma
| Proposed rapid test algorithm | No. of tests with indicated result by EIA-based strategy
|
|
|---|---|---|
| HIV positive | HIV negative | |
| HIV positive | 31 | 0 |
| HIV negative | 0 | 757 |
Sensitivity, 100.0% (95% CI, 88.9 to 100.0%); specificity, 100.0% (95% CI, 99.5 to 100.0%).
The sensitivity of the Up24 Ag assay was 92.3% (95% CI, 66.7 to 98.6%), and the specificity was 100.0% (95% CI, 97.3 to 100.0%) when performed on frozen venous plasma samples (n = 150). When the Up24 Ag assay was performed on capillary plasma stored on filter paper, the sensitivity and specificity were both 100.0% (95% CI, 56.5 to 100.0 and 95.5 to 100.0%, respectively; n = 87). As only 5 of the 13 HIV-infected infants had a drop of capillary plasma stored on filter paper, the Up24 Ag assay was also performed on venous plasma stored on filter paper (n = 12). All 12 cases were detected (Table 5).
TABLE 5.
Evaluation of the Up24 Ag assay performed on venous plasma and capillary plasma stored on filter paper
| Test and result | No. of DNA PCR tests with indicated result
|
|
|---|---|---|
| HIV positive | HIV negative | |
| Up24 Ag assay with venous plasmaa | ||
| HIV positive | 12 | 0 |
| HIV negative | 1 | 137 |
| Up24 Ag assay with capillary plasma stored on filter paperb | ||
| HIV positive | 5 | 0 |
| HIV negative | 0 | 82 |
For the assay with venous plasma, the sensitivity was 92.3% (95% CI, 66.7 to 98.6%), and the specificity was 100.0% (95% CI, 97.3 to 100.0%).
For the assay with capillary plasma stored in filter paper, the sensitivity was 100.0% (95% CI, 56.5 to 100.0%), and the specificity was 100.0% (95% CI, 95.5 to 100.0%).
No needle stick injuries were observed during the entire project. Glucolets were perceived as a valuable and user-friendly means to perform capillary phlebotomy on children.
DISCUSSION
The data obtained by this exploratory study suggest that there are several opportunities to simplify phlebotomy and pediatric HIV screening at the level of an African district hospital.
Proposed rapid-test algorithm.
The proposed algorithm based on serial rapid tests performed on capillary blood stored in EDTA tubes had a sensitivity of 100.0% (95% CI, 88.9 to 100.0%) and a specificity of 100.0% (95% CI, 99.5 to 100.0%), which is in agreement with test performance using venous blood from adult Africans (17, 41). The use of rapid tests instead of EIA for children may allow same-day receipt of test results at a lower cost and facilitate further decentralization of pediatric HIV screening (33, 44). The use of the Determine test instead of the INNO-LIA test as a third test of the EIA-based strategy could also allow further simplification and reduction of costs (33).
Ultrasensitive p24 antigen assay.
When performed on frozen venous plasma samples (n = 150), the sensitivity of the Up24 Ag assay was 92.3% (95% CI, 66.7 to 98.6%), and the specificity was 100.0% (95% CI, 97.3 to 100.0%). One of the 13 HIV-positive samples was missed by the diagnostic Up24 Ag assay but had a p24 antigen level of 1.2 pg/ml. The viral load was 5.58 log copies/ml. This raises the question of whether the cutoff value for a positive diagnostic Up24 Ag assay needs to be lowered or a certain grey area needs to be taken into consideration. Previous studies demonstrated a high and rather stable specificity (99 to 100%) for test performance with venous plasma (10, 36, 38). Only Bonard et al. found a suboptimal specificity of 94.7% when samples with low p24 antigen levels were included (4). Pediatric studies of treatment-naïve children found sensitivities between 96 and 100% (19, 24, 35, 36, 38). The results of this study confirm previous research and are a step forward towards operationalization of the test for non-subtype B infants in field settings (32, 34).
When performed on capillary plasma samples stored on filter paper (n = 87), the sensitivity and specificity of the Up24 assay were both 100.0% (95% CI, 56.5 to 100.0 and 95.5 to 100.0%, respectively). These findings suggest that the test may be performed on capillary plasma stored on filter paper instead of venous plasma and that samples for DNA PCR may be stored on the same filter paper. This could facilitate future research and transport of samples where power cuts and delays in transport are common.
Phlebotomy.
No needle stick injuries were observed during this study. But not only health care workers but also children playing on hospital compounds and other people exposed to medical waste material are at high risk (28, 39, 46). The use of glucolets would allow a reduction of biohazard material. This study documented a minimum capacity of about 300 finger pricks per instrument. According to Bayer, a capacity of 7,000 finger pricks per instrument could be easily achieved, making this instrument very cost effective.
Exclusion of HIV infection.
For the children over 18 months of age, despite the increased risk for false-positive tests due to the low HIV prevalence rate, there were only 0.2% false-positive Determine results observed (6). There were no false-negative Determine results. Compared to the reference (breastfeeding interference not being taken into consideration), HIV could have been correctly excluded in 94% of these children. Basing a negative HIV diagnosis on a single negative rapid test (provided that breastfeeding has been stopped for more than 6 months) is an acceptable and more affordable strategy but is presently underutilized, even for groups with low HIV prevalence and asymptomatic individuals (33, 44).
For children under 2 years of age, conclusions based on a single antibody test result could cause misclassification due to transient negative test results or persistent passive antibodies in the second year of life (7, 37). No false-negative and 2.6% false-positive Determine results were observed in this group. Compared to the DNA PCR result, a negative Determine test could have correctly excluded HIV infection in 90% of these children.
While people may feel overwhelmed by the complexity of diagnosing HIV in children, too little attention has been paid to the opportunities and advantages of HIV exclusion. In this study population, 77% of the children were older than 2 years and likely to have been weaned for more than 6 months. HIV suspicion could have been eliminated by one simple test that costs less than a dollar. Acceptability and feasibility to guide care and counseling by offering rapid tests to all infants should be further investigated. A first rapid test at 6 weeks of age could identify HIV-affected children needing special follow-up, including Pneumocystis carinii pneumonia prophylaxis. In asymptomatic children more than 6 months old, two consecutive negative serological tests within a minimum interval of 1 month may be used to exclude HIV (provided that breastfeeding interference is taken into consideration) (42).
Strengths and weaknesses of the study design.
Imperfect “gold standard” bias could be a factor if the exact age of the child were not known. A child could then be classified incorrectly as being older than 18 months (receiving an EIA as a reference instead of DNA PCR). Of the study population, 2.4% were between 18 and 24 months old. None were HIV infected. One baby was incorrectly classified as an older child but appeared to be HIV uninfected. This did not affect the final conclusions of the study. Furthermore, the most conservative cutoff for DNA PCR (18 months) was chosen, and viral load results were able to confirm all HIV diagnoses (23, 31, 37) (Table 3). Specimen handling errors would be more difficult to exclude but would only influence the final conclusions if an HIV-positive sample were exchanged for an HIV-negative one or vice versa. The discordance between the results of venous and capillary samples and the results of different tests would have raised suspicion. Reporting bias is unlikely, since tests were performed by different lab technicians in different laboratories and all were blinded to the code. Bias due to the exclusion of hospitalized, terminally ill children can be ruled out, as the very high viral loads were also observed among the younger HIV-infected children of this study population.
The low HIV prevalence rate in this study population (4.7%) is in accordance with previous surveillance studies but is a serious limitation to obtaining accurate information about the sensitivity of the alternative tests and strategies and thus calls for a replication of the study in a group with high HIV prevalence (15, 43). However, evaluation of the community effect of this project revealed that the high number of HIV exclusions facilitated communication about HIV testing in children and the step towards obtaining consent for testing of other children.
CONCLUSION
More can be done to simplify phlebotomy and HIV diagnosis in children than is currently being undertaken. An algorithm based on serial rapid tests seems to give performance equivalent to that of a serial EIA-based strategy at a lower cost. The results of the present study confirm that the Up24 Ag assay may be an acceptable alternative to DNA PCR and suggest that the test may be performed on capillary plasma stored on filter paper instead of venous plasma. This may facilitate future research and use in field settings. Glucolets can be used for capillary phlebotomy at comparable cost and could reduce procedural pain and the risk of needle stick injuries. While the cost and complexity of HIV diagnosis in children may be overwhelming, for many children, exclusion of HIV can be simple and cheap and deserves more attention.
Acknowledgments
This project was funded by the Belgian Directorate General for Development Cooperation, the Institute of Tropical Medicine of Antwerp, Belgium, and the Deutsche Gesellschaft für Zusammenarbeit (GTZ).
Abbott sent Determine tests and chase buffers free of charge to the DRC, Fometro provided part of the viral load tests for free, Perkin-Elmer and Klinipath gave price reductions for the test kits, M. Meersman reduced the price of the medical kit, L. Verelst provided toys, and P. Jeannin provided vaccines and medication for postexposure prophylaxis. We thank the staff of the Programme National de Lutte contre le Sida, especially J. Kokolomami, L. Lepira, and L. Kabila; Social Welfare; the Medical Inspector for the Province Katanga; the national and regional Ordre des Medecins, especially T. Tombe, and the ethics committee from the School of Public Health of the University of Kinshasa, especially M. Mukungo, for their enthusiasm and help; GTZ for financial and logistical support, especially D. Van Daele, V. Makwelebi, J. Colson, G. Caris, and K. De Clercq; the staff from the ARL in Kinshasa and Lubumbashi and the phlebotomists for their dedicated work; N. Mama and J. Muwonga for helping to adapt the protocol; O. Ngongo; the Missionnaires ICM sisters; the staff from all sites and the volunteers who helped to streamline the project; the artists; the counseling teams and people responsible for the medical follow-up of these children; Rev. Meert and Rev. Ginneberge for sharing from their long-term experience with regard to the life world of these children; H. Klein, S. Light, and J. Dennis for editing; and M. Bulterys for his thoughtful comments. Our deepest appreciation is for the Congolese children and their caregivers, without whom this study would not have been possible. We thank also the staff from the ITM, especially S. Ribas, P. Ondoa, J. Vielfont, L. Casier, L. Kestens, and last but not least, the director, R. Gryseels, who has been the driving force behind this project.
REFERENCES
- 1.Akpede, G. O., R. S. Lawal, and S. O. Momoh. 2002. Perception of voluntary screening for paediatric HIV and response to post-test counselling by Nigerian parents. AIDS Care 14:683-697. [DOI] [PubMed] [Google Scholar]
- 2.Arkin, C. F., and M. S. Wachtel. 1990. How many patients are necessary to assess test performance? JAMA 263:275-278. [PubMed] [Google Scholar]
- 3.Beck, I. A., K. D. Drennan, A. J. Melvin, K. M. Mohan, A. M. Herz, J. Alarcón, J. Piscoya, C. Velázquez, and L. M. Frenkel. 2001. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J. Clin. Microbiol. 39:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonard, D., F. Rouet, T. A. Toni, A. Minga, C. Huet, D. K. Ekouévi, F. Dabis, R. Salamon, C. Rouzioux, and the ANRS 1220 PRIMO-CI Study Group. 2003. Field evaluation of an improved assay using a heat-dissociated p24 antigen for adults mainly infected with HIV-1 CRF02_AG strains in Côte d'Ivoire, West Africa. J. Acquir. Immune Defic. Syndr. 34:267-273. [DOI] [PubMed] [Google Scholar]
- 5.Burns, D. N., and L. M. Mofenson. 1999. Paediatric HIV-1 infection. Lancet 354:1-13. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2001. Revised guidelines for HIV counseling, testing, and referral. Morb. Mort. Wkly. Rep. Recomm. Rep. 50(RR-19):1-57. [PubMed] [Google Scholar]
- 7.Chantry, C. J., E. R. Cooper, S. I. Pelton, C. Zorilla, G. V. Hillyer, and C. Diaz. 1995. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr. Infect. Dis. J. 14:382-387. [DOI] [PubMed] [Google Scholar]
- 8.Dabis, F., and E. R. Ekpini. 2002. HIV-1/AIDS and maternal and child health in Africa. Lancet 359:2097-2104. [DOI] [PubMed] [Google Scholar]
- 9.De Cock, K. M., E. Marum, and D. Mbori-Ngacha. 2003. A serostatus-based approach to HIV/AIDS prevention and care in Africa. Lancet 362:1847-1849. [DOI] [PubMed] [Google Scholar]
- 10.Forum for Collaborative HIV Research. 2002. Transfer of HIV diagnostic and monitoring technologies into resource-poor settings. [Online.] Forum for Collaborative HIV Research, Washington, D.C. http://www.hivforum.org. Accessed 20 May 2004.
- 11.Fransen, K., P. Zhong, H. De Beenhouwer, G. Carpels, M. Peeters, J. Louwagie, W. Janssens, P. Piot, and G. van der Groen. 1994. Design and evaluation of new, highly sensitive and specific primers for polymerase chain reaction detection of HIV-1 infected primary lymphocytes. Mol. Cell. Probes 8:317-322. [DOI] [PubMed] [Google Scholar]
- 12.Fransman, D., M. McCulloch, D. Lavies, and G. Hussey. 2000. Doctors' attitudes to the care of children with HIV in South Africa. AIDS Care 12:89-96. [DOI] [PubMed] [Google Scholar]
- 13.Graham, S. M. 2002. Prophylaxis against Pneumocystis carinii pneumonia for HIV-exposed infants in Africa. Lancet 360:1966-1968. [DOI] [PubMed] [Google Scholar]
- 14.Harries, A. D. 2002. Management of HIV in resource-poor countries, with a focus on sub-Saharan Africa. Lepr. Rev. 73:268-275. [PubMed] [Google Scholar]
- 15.Joint United Nations Programme on HIV/AIDS. 2002. Report on the global HIV/AIDS epidemic. July report. UNAIDS, Geneva, Switzerland.
- 16.Jong-wook, L. 2003. Global health improvement and W.H.O.: shaping the future. Lancet 362:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koblavi-Dème, S., C. Maurice, D. Yavo, T. S. Sibailly, K. N′guessan, Y. Kamelan-Tano, S. Z. Wiktor, T. H. Roels, T. Chorba, and J. N. Nkengasong. 2001. Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast. J. Clin. Microbiol. 39:1808-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lester, P., M. Chesney, M. Cooke, R. Weiss, P. Whalley, B. Perez, D. Glidden, A. Petru, A. Dorenbaum, and D. Wara. 2002. When the time comes to talk about HIV: factors associated with diagnostic disclosure and emotional distress in HIV-infected children. J. Acquir. Immune Defic. Syndr. 31:309-317. [DOI] [PubMed] [Google Scholar]
- 19.Lyamuya, E., U. Bredberg-Rådén, A. Massawe, E. Urassa, G. Kawo, G. Msemo, T. Kazimoto, A. Östborn, K. Karlsson, F. Mhalu, and G. Biberfeld. 1996. Performance of a modified HIV-1 p24 antigen assay for early diagnosis of HIV-1 infection in infants and prediction of mother-to-infant transmission of HIV-1 in Dar es Salaam, Tanzania. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:421-426. [DOI] [PubMed] [Google Scholar]
- 20.Malonza, I. M., B. A. Richardson, J. K. Kreiss, J. J. Bwayo, and G. C. J. Stewart. 2003. The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial. AIDS 17:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokili, J. L., M. Rogers, J. K. Carr, P. Simmonds, J. M. Bopopi, B. T. Foley, B. T. Korber, D. L. Birx, and F. E. McCutchan. 2002. Identification of a novel clade of human immunodeficiency virus type 1 in Democratic Republic of Congo. AIDS Res. Hum. Retrovir. 18:817-823. [DOI] [PubMed] [Google Scholar]
- 22.Montavon, C., L. Vergne, A. Bourgeois, E. Mpoudi-Ngole, G. Malonga-Mouellet, C. Butel, C. Toure-Kane, E. Delaporte, and M. Peeters. 2002. Identification of a new circulating recombinant form of HIV type 1, CRF11-cpx, involving subtypes A, G, J, and CRF01-AE, in Central Africa. AIDS Res. Hum. Retrovir. 18:231-236. [DOI] [PubMed] [Google Scholar]
- 23.Moodley, D., R. A. Bobat, A. Coutsoudis, and H. M. Coovadia. 1995. Predicting perinatal human immunodeficiency virus infection by antibody patterns. Pediatr. Infect. Dis. J. 14:850-852. [DOI] [PubMed] [Google Scholar]
- 24.Nadal, D., J. Böni, C. Kind, O. E. Varnier, F. Steiner, Z. Tomasik, and J. Schüpbach. 1999. Prospective evaluation of amplification-boosted ELISA for heat-denatured p24 antigen for diagnosis and monitoring of pediatric human immunodeficiency virus type 1 infection. J. Infect. Dis. 180:1089-1095. [DOI] [PubMed] [Google Scholar]
- 25.Nesheim, S., P. Palumbo, K. Sullivan, F. Lee, P. Vink, E. Abrams, and M. Bulterys. 2003. Quantitative RNA testing for diagnosis of HIV-infected infants. J. Acquir. Immune Defic. Syndr. 32:192-195. [DOI] [PubMed] [Google Scholar]
- 26.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857-872. [DOI] [PubMed] [Google Scholar]
- 27.Nyambi, P. N., K. Fransen, H. De Beenhouwer, E. N. Chomba, M. Temmerman, J. O. Ndinya-Achola, P. Piot, and G. van der Groen. 1994. Detection of human immunodeficiency virus type 1 (HIV-1) in heel prick blood on filter paper from children born to HIV-1-seropositive mothers. J. Clin. Microbiol. 32:2858-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obimbo, E., D. Mbori-Ngacha, P. Otieno, A. Isavwa, D. Wamalwa, C. Farquhar, R. Bosire, B. Lohman, B. Richardson, and G. John-Stewart. 2003. Pattern and correlates of viral load in Kenyan HIV-1 infected children. Antivir. Ther. 8(Suppl. 1):S191-S192. [Google Scholar]
- 29.Obuchowski, N. 1998. Sample size calculations in studies of test accuracy. Stat. Methods Med. Res. 7:371-392. [DOI] [PubMed] [Google Scholar]
- 30.Owens, D. K., M. Holodniy, T. W. McDonald, J. Scott, and S. Sonnad. 1996. A meta-analytic evaluation of the polymerase chain reaction for the diagnosis of HIV infection in infants. JAMA 275:1342-1348. [PubMed] [Google Scholar]
- 31.Palasanthiran, P., P. Robertson, J. B. Ziegler, and G. G. Graham. 1994. Decay of transplacental human immunodeficiency virus type 1 antibodies in neonates and infants. J. Infect. Dis. 170:1593-1596. [DOI] [PubMed] [Google Scholar]
- 32.Pascual, A., A. Cachafeiro, M. L. Funk, and S. A. Fiscus. 2002. Comparison of an assay using signal amplification of the heat-dissociated p24 antigen with the Roche Monitor human immunodeficiency virus RNA assay. J. Clin. Microbiol. 40:2472-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Respess, R. A., M. A. Rayfield, and T. J. Dondero. 2001. Laboratory testing and rapid HIV assays: applications for HIV surveillance in hard-to-reach populations. AIDS 15:S49-S59. [DOI] [PubMed] [Google Scholar]
- 34.Ribas, S. G., P. Ondoa, J. Schüpbach, G. van der Groen, and K. Fransen. 2003. Performance of a quantitative human immunodeficiency virus type 1 p24 antigen assay on various HIV-1 subtypes for the follow-up of human immunodeficiency type 1 seropositive individuals. J. Virol. Methods 113:29-34. [DOI] [PubMed] [Google Scholar]
- 35.Schüpbach, J., J. Böni, Z. Tomasik, J. Jendis, R. Seger, C. Kind, and the Swiss Neonatal HIV Study Group. 1994. Sensitive detection and early prognostic significance of p24 antigen in heat-denatured plasma of human immunodeficiency virus type 1-infected infants. J. Infect. Dis. 170:318-324. [DOI] [PubMed] [Google Scholar]
- 36.Sherman, G. G., G. Stevens, and W. S. Stevens. 2004. Affordable diagnosis of human immunodeficiency virus infection in infants by p24 antigen detection. Pediatr. Infect. Dis. J. 23:173-175. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, B. J., and W. A. Andiman. 1994. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention. Pediatrics 93:840-842. [PubMed] [Google Scholar]
- 38.Sutthent, R., N. Gaudart, K. Chokpaibulkit, N. Tanliang, C. Kanoksinsombath, and P. Chaisilwatana. 2003. p24 antigen detection assay modified with a booster step for diagnosis and monitoring of human immunodeficiency virus type 1 infection. J. Clin. Microbiol. 41:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taha, T. E., S. M. Graham, N. I. Kumwenda, R. L. Broadhead, D. R. Hoover, D. Markakis, L. van der Hoeven, G. N. Liomba, J. D. Chiphangwi, and P. G. Miotti. 2000. Morbidity among human immunodeficiency virus-1-infected and -uninfected African children. Pediatrics 106:1-8. [DOI] [PubMed] [Google Scholar]
- 40.Vandamme, A.-M., K. Fransen, L. Debaisieux, D. Marissens, S. Sprecher, D. Vaira, A. T. Vandenbroucke, C. Verhofstede, and the Belgian AIDS Reference Laboratories. 1995. Standardisation of primers and an algorithm for HIV-1 diagnostic PCR evaluated in patients harbouring strains of diverse geographical origin. J. Virol. Methods 51:305-316. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson, D., N. Wilkinson, C. Lombard, D. Martin, A. Smith, K. Floyd, and R. Ballard. 1997. On-site HIV testing in resource-poor settings: is one rapid test enough? AIDS 11:377-381. [DOI] [PubMed] [Google Scholar]
- 42.Working Group on Antiretroviral Therapy, the National Pediatric and Family HIV Resource Center, the Health Resources and Services Administration, and the National Institutes of Health. 2003. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Online.] U.S. Department of Health and Human Resources, Washington, D.C. http://www.AIDSinfo.nih.gov. Accessed 20 May 2004.
- 43.World Health Organization. 2002. Democratic Republic of the Congo, p. 123-126. In HIV/AIDS epidemiological surveillance update for the W.H.O. African region 2002. [Online.] www.who.int/hiv/pub/epidemiology/en/country_profiles_en.pdf.
- 44.World Health Organization and Joint United Nations Programme on HIV/AIDS. 1999. Operational characteristics of commercially available assays to determine antibodies to HIV-1 and/or HIV-2 in human sera. Report 11. World Health Organization, Geneva, Switzerland.
- 45.World Health Organization and Joint United Nations Programme on HIV/AIDS. 2002. HIV simple/rapid assays: operational characteristics (phase 1). Report 12. World Health Organization, Geneva, Switzerland.
- 46.Young, N. L., N. Shaffer, T. Chaowanachan, T. Chotpitayasunondh, N. Vanparapar, P. A. Mock, N. Waranawat, K. Chokephaibulkit, R. Chuachoowong, P. Wasinrapee, T. D. Mastro, R. J. Simonds, and the Bangkok Collaborative Perinatal HIV Transmission Study Group. 2000. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assays sensitive to non-B subtypes. J. Acquir. Immune Defic. Syndr. 24:401-407. [DOI] [PubMed] [Google Scholar]