Abstract
Dendritic cells (DCs) are antigen-presenting cells with the ability to induce primary immune responses necessary in innate immunity and adaptive immunity. Osteopontin (OPN) is a secreted acidic phosphoprotein containing an arginine-glycine-aspartate sequence and has been suggested to play an important role in early cellular immune responses. The interaction between DCs and OPN has not been clarified. We hypothesized that there is an important interaction between DCs and OPN, which is an indispensable extracellular matrix component in early cellular immune responses. Human monocyte-derived DCs synthesized OPN especially during the differentiation from monocytes to immature DCs. By blocking of OPN with anti-OPN antibody, cultured DCs became smaller and expressed lower levels of costimulatory molecules and major histocompatibility complex class II antigens than untreated DCs. Furthermore, DCs treated with anti-OPN antibody easily underwent apoptosis. These results suggest that human DCs can produce OPN and that OPN may play a role in the differentiation, maturation, and survival of DCs by autocrine and/or paracrine pathways.
Dendritic cells (DCs) play critical roles in innate immunity and adaptive immunity (4). Immature DCs reside in peripheral tissues, where they serve as sentinels for foreign antigens and microbial pathogens. Upon activation, immature DCs undergo maturation and migrate to the lymph nodes. During maturation, DCs acquire an enhanced capacity to form and accumulate peptides, major histocompatibility complex (MHC) class II molecules, costimulatory molecules (such as CD40, CD80, and CD86), and antigens of unknown functions (such as CD83 and DC-LAMP) (10). Mature DCs can prime naïve T cells and initiate primary T-cell-mediated immune responses (4). In addition, there is increasing evidence that DCs in situ induce antigen-specific unresponsiveness or tolerance in central lymphoid organs and in peripheral tissues (4, 31). Thus, DCs play a crucial role during the initiation and regulation of immune responses. Recently, we and others reported that DCs are essential for granuloma formation against bacterial antigens in animal models (12, 33, 36).
Osteopontin (OPN), also known as early T-lymphocyte activation-1 (Eta-1), is a phosphoprotein that contains arginine-glycine-aspartate (RGD). Although OPN is classified as an extracellular matrix (ECM) protein, OPN has only recently been shown to be an important component of early cellular immune responses (18). OPN has various functions in chemotaxis for immune cells, tumor metastasis, neovascularization, and host defense, including control of nitric oxide production, control of infection, and control of cell adhesion (3, 5, 9, 21, 25). These mechanisms are regulated by posttranslational modifications, such as cleavage by thrombin, addition of a glucose chain, and phosphorylation. Various immunological disorders are associated with high levels of OPN expression (8, 15). Analyses of OPN-deficient mice revealed that OPN plays an important immunological role in granuloma formation (23), acid-fast bacillus disease (21), and carcinoma metastasis (5). The role of OPN in inflammation suggests that ECM-related proteins may function as pleiotropic cytokines to regulate immune responses. Activated macrophages, lymphocytes, and natural killer (NK) cells produce OPN in response to various stimuli (23). However, there are no reports of the effects of OPN on DCs, with the exception of a single report of the migratory effect of OPN on cutaneous Langerhans cells and DCs in a mouse allergic cutaneous hypersensitivity model (34). The direct effect of OPN on the development and activation of DCs has not been clarified. Thus, we sought to characterize the functional interaction between OPN and DCs by examining the effects of OPN on differentiation, maturation, and function of human monocyte-derived immature and mature DCs. We report here that human monocyte-derived dendritic cell (Mo-DC) can produce OPN that enhances differentiation, maturation, and survival of DCs by autocrine and/or paracrine pathways.
MATERIALS AND METHODS
Reagents.
Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) was kindly provided by Kirin Brewery (Tokyo, Japan). Recombinant human interleukin-4 (IL-4) was purchased from R&D Systems (Minneapolis, Minn.). Anti-OPN monoclonal antibody (MAb) (mouse immunoglobulin G1 [IgG1]) was from IBL (Gunma, Japan). Phycoerythrin (PE)-conjugated anti-human HLA-DR antibody, Fc receptor, and fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 were from Sigma (St. Louis, Mo.). Anti-human CD86 antibody was purchased from BD PharMingen (San Diego, Calif.), and anti-human CD83 and isotype control IgG were from Immunotech (Marseille, France). Lipopolysaccharide (LPS) (Escherichia coli) (catalog no. L4391), β-d-glucan (barley) (catalog no. G6513), and lipoteichoic acid (LTA) (Staphylococcus aureus) (catalog no. L2515) were from Sigma.
Generation of DCs and macrophages from purified human CD14+ monocytes.
Mo-DCs were obtained as previously described (29) but with a minor modification. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples from healthy volunteers by standard density gradient centrifugation with Lymphoprep (Axis-Shield, Oslo, Norway). PBMCs at the interface were pelleted and washed twice with phosphate-buffered saline (PBS). CD14+ monocytes were isolated from mononuclear fractions through positive selection with microbeads coated with anti-CD14 antibody and Midi-Macs separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity was checked by flow cytometry with anti-CD14 MAb and was >95%.
For Mo-DC generation, purified CD14+ monocytes were cultured in complete medium (CM), which consisted of RPMI 1640 medium supplemented with NaHCO3, l-glutamine (Nipro, Osaka, Japan), 10% fetal calf serum (FCS), 10 mg of streptomycin per ml, 10,000 U of penicillin G per ml, 55 mM 2-mercaptoethanol, and HEPES, at a concentration of 5 × 105 or 2.5 × 105 cells/ml in 24-well flat-bottom microplates (Becton Dickinson, Franklin Lakes, N.J.). GM-CSF (800 U/ml) and IL-4 (500 U/ml) were added to the CM to generate Mo-DCs. Cells were incubated at 37°C in a 5% CO2 atmosphere. On day 5, the cultured cells progressed to immature DCs as confirmed by fluorescence-activated cell sorting (FACS) analysis of surface markers and by morphology. To generate mature DCs, immature DCs were harvested on day 5, washed with PBS, and seeded (5 × 105 cells/ml/well) in fresh CM supplemented with GM-CSF (800 U/ml) and IL-4 (500 U/ml), and stimulated with LPS (1 μg/ml), β-d-glucan (5 μg/ml), LTA (20 μg/ml), or CD40L (1 μg/ml) for 48 h.
Monocyte-derived macrophages (Mo-Mφs) were generated as described previously (16). CD14+ monocytes were cultured (37°C, 5% CO2) in CM supplemented with M-CSF (104 U/ml) for 5 days. During culture, monocytes underwent morphological changes characteristic of macrophages differentiated from monocytes, such as an increasing size and adherence. Purity was >95% as verified by FACS analysis with an anti-CD14 MAb and an anti-human Fc receptor MAb and by morphology and enhanced phagocytosis of latex particles.
Cytology.
DCs generated in vitro were cytocentrifuged for 5 min at 500 × g (Cytospin 3; Shandon, Astmoor, United Kingdom) and stained with Diff-Quick (Kokusai Shiyaku, Kobe, Japan). Mature DCs were larger, double the size of monocytes in diameter with long cytoplasmic projections (dendrites), eccentric multilobulate lateral nuclei, and abundant cytoplasm. Immature DCs had small cytoplasmic projections or no projections at all.
Flow cytometry.
Cells were washed twice with PBS supplemented with 2% FCS and resuspended in PBS supplemented with 2% FCS. Cells were incubated with Abs at saturating concentrations for 30 min at 4°C and then washed with PBS two more times. Cells were stained with the following Abs: FITC-conjugated anti-CD14 antibody (Sigma), PE-conjugated anti-HLA-DR antibody (Sigma), anti-CD83 antibody (Immunotech), and anti-CD86 antibody (PharMingen). Rabbit FITC-conjugated anti-mouse immunoglobulin (DakoCytomation, Kyoto, Japan) was used as a secondary antibody. Cell surface antigen expression was evaluated by single- or double-immunofluorescence staining, and analysis was performed with a FACScan analyzer and CellQuest software (Becton Dickinson).
Measurement of OPN.
The concentration of OPN in PBMC culture supernatants was measured with a human OPN enzyme immunoassay (EIA) kit (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) according to the manufacturer's instructions.
Detection of apoptosis.
Apoptosis was detected by staining with an annexin V-FITC kit (Immunotech) according to the manufacturer's protocol. Cells were harvested, washed twice with PBS, and labeled with annexin V-FITC and propidium iodide (PI) for 10 min on ice. Annexin V and PI staining was examined with a FACScan analyzer and CellQuest software (Becton Dickinson).
Statistical analysis.
Student's paired t test was used to determine the significance of different mean values, and a P value of <0.05 was taken to indicate statistical significance.
RESULTS
Production of OPN by human monocytes and immature DCs.
A previous study revealed that OPN is produced by activated macrophages and T cells and that expression of OPN is induced by GM-CSF signaling in hematopoietic cells (17). We hypothesized that Mo-DCs produce OPN and that there is some functional interaction between OPN and GM-CSF, a cytokine necessary for generation of DCs. We analyzed changes in OPN production by Mo-DCs during maturation from monocytes to DCs. Immature Mo-DCs were generated by culturing CD14+ monocytes (5 × 105 cells/ml/well) in CM supplemented with GM-CSF (800 U/ml) and IL-4 (500 U/ml) for 5 days. Monocytes cultured in CM without GM-CSF and IL-4 for 5 days were used as a control. Control cells showed no morphological changes and still expressed CD14 antigen after culture (data not shown). In contrast, immature Mo-DCs lost CD14 surface antigen and were twice as large as monocytes in diameter, as previously described (30).
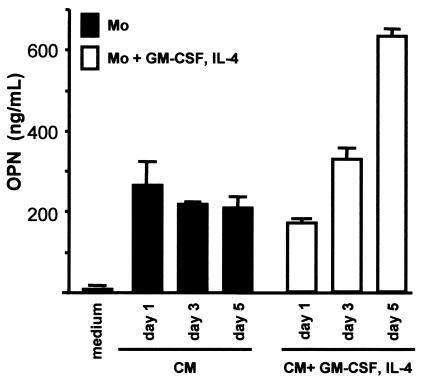
As shown in Fig. 1, monocytes and cells maturing to immature DCs produced OPN. OPN was detected at levels as high as 200 ng/ml in culture supernatants of monocytes cultured in the absence of GM-CSF and IL-4. However, a meaningful increase in OPN production was not observed during the culture period. In contrast, for immature DCs incubated with GM-CSF and IL-4, a significant increase in OPN production during the culture period was observed. The supernatants on day 5 contained more than 600 ng of OPN per ml, which is triple that of the monocytes. This suggests that human monocytes can produce OPN and that OPN production is enhanced by maturation to immature DCs.
FIG. 1.
OPN production by human monocytes and immature DCs. Human CD14+ monocytes (Mo) were incubated for 5 days in the presence and absence of GM-CSF (800 U/ml) and IL-4 (500 U/ml). Twenty-four, 72, and 120 h after incubation, OPN in the culture supernatant was quantified with an EIA kit. OPN production by monocyte-derived maturing DCs increased during the culture period, whereas production by monocytes did not change. Data are shown as the means ± standard errors of the means (error bars) from three independent experiments.
OPN production by immature DCs decreased during their maturation.
Immature Mo-DCs obtained by 5-day culture of CD14+ monocytes with GM-CSF (800 U/ml) and IL-4 (500 U/ml) were collected, washed with PBS, and cultured for another 48 h with or without stimulants, such as LPS (1 μg/ml), LTA (20 μg/ml), and β-d-glucan (5 μg/ml). Pathogen-associated molecular patterns (PAMPs), such as LPS and LTA, stimulate maturation of DCs. This leads to enhanced antigen processing, increased MHC class II expression, and induction of costimulatory molecules, such as CD80 and CD86.
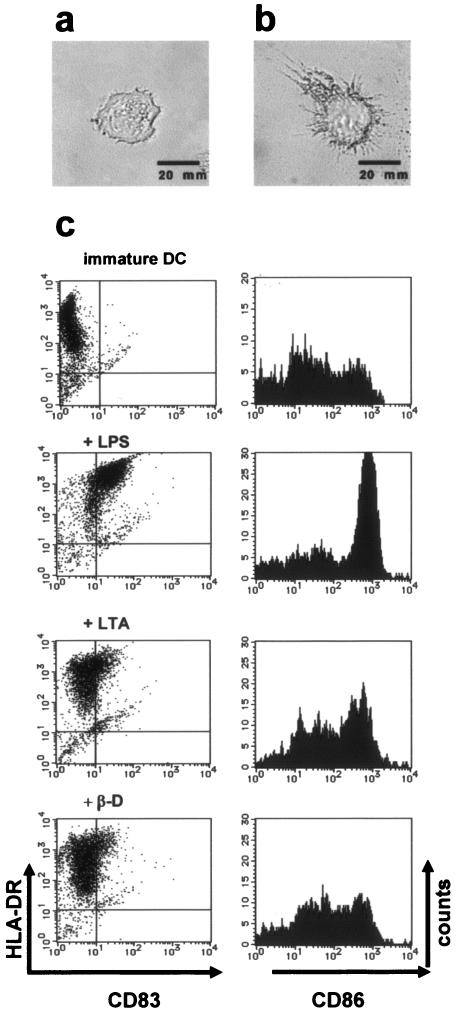
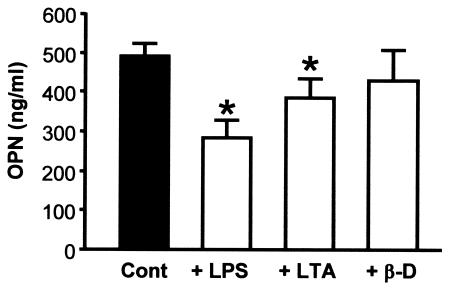
As shown in Fig. 2c, LPS (1 μg/ml) and LTA (20 μg/ml) enhanced surface expression of CD83, CD86, and HLA-DR, although β-d-glucan (5 μg/ml) did not up-regulate expression of costimulatory molecules and HLA-DR as strongly as LPS did. OPN levels in culture supernatants were measured by EIA. Human Mo-DCs that matured in response to stimulation by LPS (1 μg/ml) or LTA (20 μg/ml) produced significantly less OPN than immature DCs did (P < 0.05) (Fig. 3). Analysis of surface markers of immature DCs stimulated with β-d-glucan (5 μg/ml) revealed that the maturation signal in our study system was insufficient to obtain fully mature DCs (Fig. 2c), and the significant decrease in OPN production that occurs with DC maturation was not observed (Fig. 3).
FIG. 2.
Changes in morphology and immunophenotype of immature and mature monocyte-derived DCs. (a) Immature DCs. After magnetic cell sorting, CD14+ monocytes that were incubated in CM with GM-CSF (800 U/ml) and IL-4 (500 U/ml) for 5 days acquired the morphological characteristics of immature DCs (magnification, ×200). (b) Mature DCs. Immature DCs were stimulated with LPS (1 μg/ml) for 48 h to generate mature DCs. LPS induced maturation of DCs with characteristic dendrites (magnification, ×200). (c) Flow cytometric analysis of CD86, HLA-DR, and CD83 on immature DCs and mature DCs treated with PAMPs. Immature DCs were stimulated with various PAMPs and harvested, and expression of CD83, CD86, and HLA-DR antigens was examined by flow cytometry. DCs stimulated with LPS (1 μg/ml) showed increased expression of CD83, CD86, and HLA-DR. In comparison, LTA (20 μg/ml) and β-d-glucan (β-D) (5 μg/ml) also triggered DC maturation, although they did not lead to full maturation. The results shown are from a single experiment using cells from a single donor and are representative of three independent experiments that gave similar results.
FIG. 3.
OPN production by immature DCs and mature DCs treated with PAMPs. Immature DCs were stimulated with LPS (1 μg/ml), LTA (20 μg/ml), or β-d-glucan (β-d) (5 μg/ml) for 48 h to generate mature DCs or left alone as a control (Cont). Maturation of DCs was confirmed by morphology and expression of surface markers (Fig. 2c). OPN production by immature and mature DCs was measured with an EIA kit. Immature human DCs synthesized OPN, and production decreased during maturation. Production of OPN by LPS-treated mature DCs decreased to half that of immature DCs. LTA-stimulated mature DCs also produced less OPN than immature DCs. β-d-glucan-stimulated DCs showed a tendency to produce less OPN, but the difference was not statistically significant. The results shown are means ± standard errors of the means (error bars) from a single experiment using cells from a single donor and are representative of three experiments that gave similar results. Values that were significantly different (P < 0.05) from the control value (asterisk) are indicated.
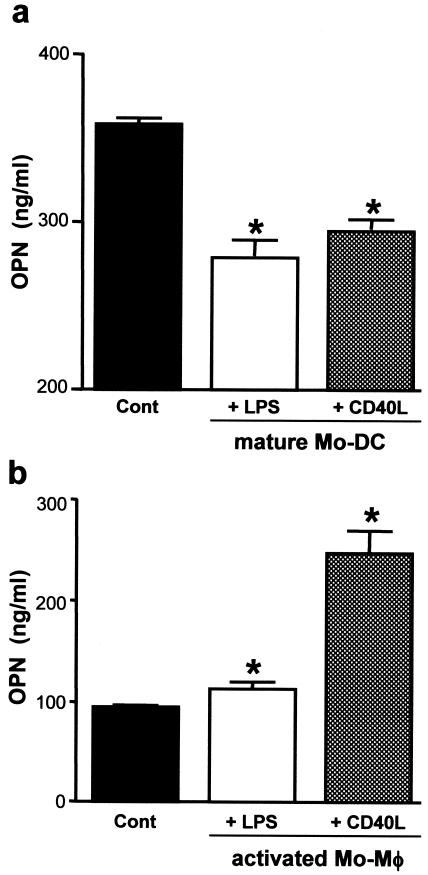
Maturation of DCs was confirmed by analysis of surface expression of CD83 and HLA-DR and by morphology. These results suggest that OPN production decreases when DCs mature fully. To confirm this, we used 1 μg of CD40L per ml as a nonpathogenic stimulant to obtain fully mature DCs. Immature DCs were incubated for 48 h in CM with 1 μg of CD40L per ml. OPN was produced in CD40L-treated mature DCs; however, the mature DCs produced significantly less OPN than the nonstimulated immature DCs (Fig. 4a). These results suggest that OPN production decreases when DCs mature fully in response to various stimulants.
FIG. 4.
Comparison of OPN production by mature DCs and activated macrophages. (a) OPN production by LPS- or CD40L-stimulated mature DCs. Immature DCs (5 × 106 cells/ml/well) were incubated with LPS (1 μg/ml) or CD40L (1 μg/ml) to obtain mature monocyte-derived DC (Mo-DC). LPS- or CD40L-treated DCs acquired the morphology characteristic of mature DCs and showed increased expression of HLA-DR, CD86, and CD83 (data not shown). Both LPS- and CD40L-induced mature DCs produced OPN. However, production of OPN by LPS- and CD40L-induced mature DCs was lower than that induced by control (Cont) immature DCs. Data shown are the means ± standard errors of the means (error bars) from three independent experiments. (b) OPN production by LPS- or CD40L-stimulated monocyte-derived macrophages (Mo-Mφ). To obtain Mo-Mφ, human monocytes were incubated with M-CSF (104 U/ml) for 120 h. After incubation, these cells were CD14 positive and had a great deal of cytoplasm (data not shown). Activated Mo-Mφ were obtained by stimulation with LPS (1 μg/ml) or CD40L (1 μg/ml). After 48 h, the supernatant was analyzed for OPN levels. Activated Mo-Mφ, especially CD40L-stimulated Mo-Mφ, synthesized more OPN than did unstimulated Mo-Mφ. Results shown are the means ± standard errors of the means (error bars) from three independent experiments. Values that were significantly different (P < 0.05) from the control value (asterisk) are indicated.
Activated macrophages are known to produce OPN (23). We generated monocyte-derived macrophages, and activated macrophages were obtained by stimulation with LPS (1 μg/ml) and CD40L (1 μg/ml) as described above for activated DCs. OPN production increased significantly in activated macrophages (Fig. 4b). These results suggest that OPN production during maturation or activation is regulated differently in DCs and macrophages.
OPN may be involved in DC viability and differentiation from monocytes.
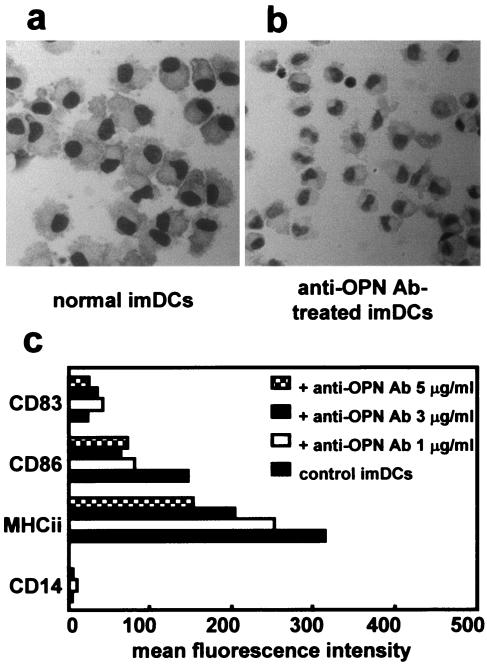
In this study, we observed production of OPN by human monocytes and Mo-DCs. Because OPN production was increased during differentiation from monocytes to immature DCs, we hypothesized that OPN enhances differentiation and maturation of Mo-DC, especially during the early stage of differentiation. To examine the function of OPN, purified CD14+ monocytes were cultured with GM-CSF (800 U/ml) and IL-4 (500 U/ml) with or without a neutralizing OPN MAb at concentrations of 1, 3, and 5 μg/ml. Mo-DCs from cultures with or without anti-OPN Ab lost CD14 surface antigen, indicating that the cells had differentiated from monocytes to immature DCs. Looking at the morphology of the cells, the cells appeared to have differentiated into immature DC-like cells even when cultured with anti-OPN Ab. Immature DCs treated with 3 or 5 μg of anti-OPN Ab per ml were smaller than control immature DCs; however, they still possessed morphological features characteristic of immature DCs and differed in appearance from the 5-day culture of monocytes used as a control (Fig. 5a and b). There were no morphological differences between immature DCs treated with anti-OPN Ab (1 μg/ml) and untreated immature DCs. During incubation with or without anti-OPN Ab, cell viability was maintained at over 80%. We then analyzed expression of HLA-DR, CD83, and CD86 by immature DCs treated with anti-OPN Ab or not treated with the Ab. As expected, immature DCs did not express CD83, which is a reliable marker of DC maturation. Surface expression of CD86 and HLA-DR was lower in immature DCs treated with anti-OPN Ab than in untreated control immature DCs (Fig. 5c).
FIG. 5.
OPN mediates differentiation from monocytes to immature DCs. To obtain immature DCs and immature DCs treated with anti-OPN antibody, monocytes were cultured with GM-CSF (800 U/ml) and IL-4 (500 U/ml) in the presence or absence of anti-OPN Ab (1, 3, or 5 μg/ml) for 120 h. Cells were collected, cytocentrifuged, stained with Diff-Quick, and morphological changes were observed by light microscopy. Surface expression of HLA-DR (MHCii), CD83, and CD86 on DCs generated in the presence of anti-OPN Ab (1, 3, or 5 μg/ml) were analyzed by flow cytometry. (a) Morphology of immature DCs (imDCs) (Diff-Quick staining) (original magnification, ×400). (b) Morphology of immature DCs generated with 5 μg of anti-OPN Ab per ml (Diff-Quick staining) (original magnification, ×400). Immature DCs treated with anti-OPN Ab were smaller than control immature DCs. (c) Surface expression of HLA-DR, CD83, and CD86 on immature DCs and immature DCs treated with anti-OPN Ab. Immature DCs treated with anti-OPN Ab produced lower levels of HLA-DR and CD86 than control immature DCs. The inhibitory effect of anti-OPN Ab on DC differentiation occurred in a dose-dependent manner. Results are representative of three independent experiments.
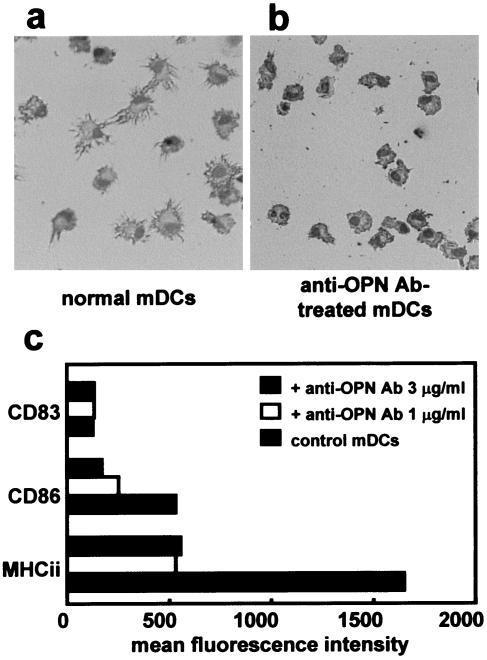
To examine whether OPN mediates maturation of immature DCs to mature DCs, final maturation was induced by stimulation with LPS (1 μg/ml) for 2 days in the presence or absence of anti-OPN Ab (Fig. 6). Maturation and activation were evaluated on the basis of morphology and expression of HLA-DR, CD83, and CD86. LPS stimulation without anti-OPN Ab caused immature DCs to mature (Fig. 6a). Stimulation in the presence of anti-OPN Ab caused immature DCs to develop poor dendrites and little cytoplasm (Fig. 6b). HLA-DR and CD86 expression was lower in maturing DCs not treated with anti-OPN Ab than in mature control DCs (Fig. 6c). CD83 expression was higher in all viable mature DCs than in immature DCs, and there was no significant difference in the mean fluorescence level of CD83 in control untreated DCs and DCs treated with anti-OPN Ab. These results suggest that final maturation and activation as judged by increased expression of CD86 and HLA-DR were inhibited by anti-OPN Ab. Even 1 μg of anti-OPN Ab per ml showed a sufficient inhibitory effect. Furthermore, DCs exposed to 5 μg of anti-OPN Ab per ml contracted, and more than 50% of the cells underwent apoptosis.
FIG. 6.
OPN mediates maturation from immature DCs to mature DCs. To induce DC maturation, immature DCs were incubated with LPS (1 μg/ml) for another 48 h with or without anti-OPN Ab (1, 3, or 5 μg/ml). (a) Control mature DCs (mDCs) obtained by stimulation with LPS (1 μg/ml) acquired the morphology characteristic of DCs. (b) Maturing DCs treated with anti-OPN (1 μg/ml) Ab also showed dendritic morphology. However, the majority of cells remained in the immature state as judged by morphology. (c) Changes in surface expression of HLA-DR (MHCii), CD83, and CD86 on DCs after maturation. Mature DCs expressed high levels of MHC class II and CD86, whereas maturing DCs treated with anti-OPN Ab expressed lower levels of HLA-DR and CD86, whereas the inhibitory effect of anti-OPN Ab on DC maturation was dose dependent. Results are representative of three independent experiments.
Effects of OPN on viability and survival of DCs.
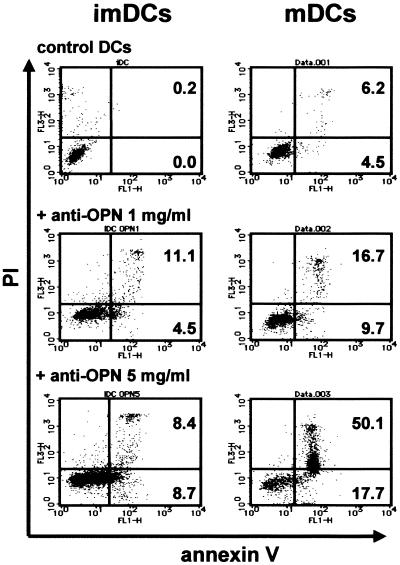
Several reports have suggested that OPN may play a role in cell survival. OPN has been shown to inhibit apoptosis in smooth muscle cells, endothelial cells (14), epithelial cells (22), and pro-B cells (28). In one study, LPS stimulation caused DCs treated with anti-OPN Ab (5 μg/ml) to contract, and cell recovery was low. However, the roles of OPN in regulation of survival and death of DCs have not been elucidated. We attempted to determine whether OPN is involved in the viability of DCs with annexin V and PI staining. The effects of anti-OPN Ab on survival of monocytes, immature DCs, and LPS-stimulated mature DCs were examined. Annexin V staining revealed that anti-OPN Ab at concentrations of 1 and 5 μg/ml significantly reduced the viability of monocytes from more than 90 to 80% (data not shown). As shown in Fig. 7, immature DCs treated with anti-OPN Ab (1 and 5 μg/ml) underwent apoptosis at a rate of 15 to 20%. More than 50% of mature DCs treated with a high dose of anti-OPN Ab and LPS underwent apoptosis; only 16.7% of cells underwent apoptosis at an Ab concentration of 1 μg/ml. These results suggest that OPN is necessary for DC maturation and survival.
FIG. 7.
Effect of OPN on apoptosis of DCs. Immature DCs (imDCs) generated from monocytes incubated in the presence or absence of anti-OPN Ab (1, 3, or 5 μg/ml) were collected on day 5 and analyzed for apoptosis by annexin V and PI staining. Anti-OPN Ab treatment increased apoptosis of immature DCs from 0.2% of immature control DCs to 8.4% of immature DCs treated with Ab (5 μg/ml). Immature DCs were collected and resuspended in new CM in the presence or absence of anti-OPN Ab and stimulated with LPS (1 μg/ml) for 48 h to obtain mature DCs (mDCs), and then mature DCs were collected and analyzed for apoptosis by annexin V and PI staining. Approximately 6.2% of induced mature DCs induced by LPS stimulation underwent apoptosis. Anti-OPN Ab treatment raised the rate of apoptosis to 50.1% in a dose-dependent manner. The results shown were obtained from a single experiment and are representative of three independent experiments that gave similar results.
DISCUSSION
OPN is a phosphorylated acidic glycoprotein that is expressed in a variety of tissues as a component of the ECM. Expression of OPN by many types of cells, including macrophages, T cells, NK cells, endothelial cells, smooth muscle cells, and epithelial cells, has been reported (7, 24, 26). Several studies have suggested that OPN plays a role in regulation of inflammatory cell accumulation at sites of inflammation and repair (23). OPN expression has been reported under both physiological and pathological conditions, such as tuberculosis (20) and sarcoidosis (24). OPN-knockout mice develop inadequate antimicrobial immunity to a broad range of pathogens. One study revealed that these mice have defects in their ability to clear Listeria monocytogenes after systemic infection (3), and another showed that these mice have increased susceptibility to infection by mycobacteria (21). O'Regan and colleagues (25) reported abnormal granuloma formation in the lungs of OPN-deficient mice. Furthermore, OPN expression in humans also contributes to resistance to mycobacterial infection (19). Therefore, OPN not only participates in the maintenance or reconfiguration of tissue integrity during inflammatory processes but also plays a role in cell-mediated immunity. However, the precise role of OPN in immune responses is still unclear.
DCs play a crucial role during the initiation and regulation of immune responses. They are essential for the containment of infections that induce cellular immune responses (12, 27, 33, 36). We recently reported that DCs play a key role in the initiation of cell-mediated immune granuloma formation (12).
Recently, Ahn and colleagues (1) reported that monocyte-derived DCs express the OPN gene. Both DCs and OPN appear to be indispensable for granulomatous inflammation; however, there is little information concerning the interaction between OPN and DCs. Monocytes are precursors of myeloid DCs and are recruited to the sites of inflammation where they differentiate into DCs or macrophages. In the present study, we found that OPN is synthesized by monocytes as well as by immature and mature Mo-DCs. Secreted OPN enhances differentiation and maturation of DCs from monocytes to mature DCs with high levels of expression of MHC class II and costimulatory molecules that are necessary for antigen presentation. Furthermore, OPN is important in DC survival. On the basis of these findings, we believe that OPN has an indispensable role in differentiation and survival of DCs. The abnormal cell-mediated immunity and antimicrobial immunity in OPN-deficient mice may be due to impaired function of DCs that failed to mature fully and could not be activated.
The signaling pathways involving OPN are not well understood. However, both RGD-dependent (e.g., αVβ3 integrin) and -independent (e.g., CD44) signaling pathways can serve as receptors for OPN. Although CD44 is a major receptor for hyaluronan (2), it also acts as a receptor for OPN and has multiple bob-RGD binding sites (13). One study showed that anti-CD44 Ab interfered with OPN binding to CD44 on the surfaces of DCs, which partially impaired function and maturation of DCs (11, 32, 35). Lin and colleagues (17) reported that OPN contributes to the survival-promoting activities of cytokines, such as GM-CSF and IL-3, and its signaling pathway occurs through the interaction between CD44 and OPN. We confirmed that anti-CD44 Ab treatment induced apoptosis of Mo-DCs, as observed with anti-OPN Ab-treated DCs (data not shown). For Mo-DCs, the interaction between CD44 and OPN may be important for survival.
It was reported that OPN can inhibit apoptosis of endothelial cells (14), epithelial cells (22), and pro-B cells (28). These reports suggest that OPN acts as a cell survival factor and protects cells from apoptosis. In the present study, we clearly showed that Mo-DCs undergo apoptosis easily in the absence of OPN. Our results are consistent with previous findings for several other cell types (14, 22, 28), suggesting that OPN might be a fundamental factor for cell survival regardless of cell lineage.
DCs undergo apoptosis after finishing antigen presentation, which may be the physiological means of terminating the immune response and preventing prolonged activation of T cells to avoid excessive inflammation (6). Our data suggest that maturation of DCs in response to various factors reduces production of OPN, which may promote apoptosis of DCs. The role of locally synthesized OPN in the survival of DCs appears to be beneficial for maintaining homeostasis at the inflammation site.
In summary, we showed that human Mo-DCs synthesize OPN and that OPN acting in an autocrine and/or paracrine manner contributes to maturation and activation of DCs. Furthermore, OPN promotes survival of DCs as it does for other inflammatory cells. Further characterization of OPN function and the mechanisms of interaction between OPN and inflammatory cells, including DCs, may improve our understanding of inflammatory processes.
Acknowledgments
This work was supported by a grant-in-aid for interstitial lung diseases from the Ministry of Health, Labour and Welfare, Japan.
REFERENCES
- 1.Ahn, J. H., Y. Lee, C. Jeon, S. J. Lee, B. H. Lee, K. D. Choi, and Y. S. Bae. 2002. Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood 100:1742-1754. [PubMed] [Google Scholar]
- 2.Aruffo, A., I. Stamenkovic, M. Melnick, C. B. Underhill, and B. Seed. 1990. CD44 is the principal cell surface receptor for hyaluronate. Cell 61:1303-1313. [DOI] [PubMed] [Google Scholar]
- 3.Ashkar, S., G. F. Weber, V. Panoutsakopoulou, M. E. Sanchirico, M. Jansson, S. Zawaideh, S. R. Rittling, D. T. Denhardt, M. J. Glimcher, and H. Cantor. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860-864. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Behrend, E. I., A. M. Craig, S. M. Wilson, D. T. Denhardt, and A. F. Chambers. 1994. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 54:832-837. [PubMed] [Google Scholar]
- 6.Bertho, N., B. Drenou, B. Laupeze, C. L. Berre, L. Amiot, J.-M. Grosset, O. Fardel, D. Charron, N. Mooney, and R. Fauchet. 2000. HLA-DR-mediated apoptosis susceptibility discriminates differentiation stages of dendritic/monocytic APC. J. Immunol. 164:2379-2385. [DOI] [PubMed] [Google Scholar]
- 7.Brown, L. F., B. Berse, L. Van de Water, A. Papadopoulos-Sergiou, C. A. Perruzzi, E. J. Manseau, H. F. Dvorak, and D. R. Senger. 1992. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol. Biol. Cell 3:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiocchetti, A., M. Indelicato, T. Bensi, R. Mesturini, M. Giordano, S. Sametti, L. Castelli, F. Bottarel, M. C. Mazzarino, L. Garbarini, F. Giacopelli, G. Valesini, C. Santoro, I. Dianzani, U. Ramenghi, and U. Dianzani. 2004. High levels of osteopontin associated with polymorphisms in its gene are a risk factor for development of autoimmunity/lymphoproliferation. Blood 103:1376-1382. [DOI] [PubMed] [Google Scholar]
- 9.Denhardt, D. T., M. Noda, A. W. O'Regan, D. Pavlin, and J. S. Berman. 2001. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 107:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Saint-Vis, B., J. Vincent, S. Vandenabeele, B. Vanbervliet, J. J. Pin, S. Ait-Yahia, S. Patel, M. G. Mattei, J. Banchereau, S. Zurawski, J. Davoust, C. Caux, and S. Lebecque. 1998. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 9:325-336. [DOI] [PubMed] [Google Scholar]
- 11.Haegel-Kronenberger, H., H. de la Salle, A. Bohbot, F. Oberling, J.-P. Cazenave, and D. Hanau. 1998. Adhesive and/or signaling functions of CD44 isoforms in human dendritic cells. J. Immunol. 161:3902-3911. [PubMed] [Google Scholar]
- 12.Iyonaga, K., K. M. McCarthy, and E. E. Schneeberger. 2002. Dendritic cells and the regulation of a granulomatous immune response in the lung. Am. J. Respir. Cell Mol. Biol. 26:671-679. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri, Y. U., J. Sleeman, H. Fujii, P. Herrlich, H. Hotta, K. Tanaka, S. Chikuma, H. Yagita, K. Okumura, M. Murakami, I. Saiki, A. F. Chambers, and T. Uede. 1999. CD44 variants but not CD44s cooperate with β1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 59:219-226. [PubMed] [Google Scholar]
- 14.Khan, S. A., C. A. Lopez-Chua, J. Zhang, L. W. Fisher, E. S. Sorensen, and D. T. Denhardt. 2002. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J. Cell. Biochem. 85:728-736. [DOI] [PubMed] [Google Scholar]
- 15.Koguchi, Y., K. Kawakami, K. Uezu, K. Fukushima, S. Kon, M. Maeda, A. Nakamoto, I. Owan, M. Kuba, N. Kudeken, M. Azuma, S. Yara, T. Shinzato, F. Higa, M. Tateyama, J.-I. Kadota, H. Mukae, S. Kohno, T. Uede, and A. Saito. 2003. High plasma osteopontin level and its relationship with interleukin-12-mediated type 1 T helper cell response in tuberculosis. Am. J. Respir. Crit. Care Med. 167:1355-1359. [DOI] [PubMed] [Google Scholar]
- 16.Komuro, I., N. Keicho, A. Iwamoto, and K. S. Akagawa. 2001. Human alveolar macrophages and granulocyte-macrophage colony-stimulating factor-induced monocyte-derived macrophages are resistant to H2O2 via their high basal and inducible levels of catalase activity. J. Biol. Chem. 276:24360-24364. [DOI] [PubMed] [Google Scholar]
- 17.Lin, Y.-H., C.-J. Huang, J.-R. Chao, S.-T. Chen, S.-F. Lee, J. J.-Y. Yen, and H.-F. Yang-Yen. 2000. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol. Cell. Biol. 20:2734-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzali, M., T. Kipari, V. Ophascharoensuk, J. A. Wesson, R. Johnson, and J. Hughes. 2002. Osteopontin—a molecule for all seasons. QJM 95:3-13. [DOI] [PubMed] [Google Scholar]
- 19.Nau, G. J., G. L. Chupp, J.-F. Emile, E. Jouanguy, J. S. Berman, J.-L. Casanova, and R. A. Young. 2000. Osteopontin expression correlates with clinical outcome in patients with mycobacterial infection. Am. J. Pathol. 157:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nau, G. J., P. Guilfoile, G. L. Chupp, J. S. Berman, S. J. Kim, H. Kornfeld, and R. A. Young. 1997. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 94:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nau, G. J., L. Liaw, G. L. Chupp, J. S. Berman, B. L. M. Hogan, and R. A. Young. 1999. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect. Immun. 67:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ophascharoensuk, V., C. M. Giachelli, K. Gordon, J. Hughes, R. Pichler, P. Brown, L. Liaw, R. Schmidt, S. J. Shankland, C. E. Alpers, W. G. Couser, and R. J. Johnson. 1999. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 56:571-580. [DOI] [PubMed] [Google Scholar]
- 23.O'Regan, A., and J. S. Berman. 2000. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 81:373-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Regan, A. W., G. L. Chupp, J. A. Lowry, M. Goetschkes, N. Mulligan, and J. S. Berman. 1999. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J. Immunol. 162:1024-1031. [PubMed] [Google Scholar]
- 25.O'Regan, A. W., J. M. Hayden, S. Body, L. Liaw, N. Mulligan, M. Goetschkes, and J. S. Berman. 2001. Abnormal pulmonary granuloma formation in osteopontin-deficient mice. Am. J. Respir. Crit. Care Med. 164:2243-2247. [DOI] [PubMed] [Google Scholar]
- 26.Pollack, S. B., P. A. Linnemeyer, and S. Gill. 1994. Induction of osteopontin mRNA expression during activation of murine NK cells. J. Leukoc. Biol. 55:398-400. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 28.Rittling, S. R., and D. T. Denhardt. 1999. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp. Nephrol. 7:103-113. [DOI] [PubMed] [Google Scholar]
- 29.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685-711. [DOI] [PubMed] [Google Scholar]
- 32.Termeer, C., H. Johannsen, T. Braun, A. Renkl, T. Ahrens, R. W. Denfeld, M. B. Lappin, J. M. Weiss, and J. C. Simon. 2001. The role of CD44 during CD40 ligand-induced dendritic cell clustering and maturation. J. Leukoc. Biol. 70:715-722. [PubMed] [Google Scholar]
- 33.Tsuchiya, T., K. Chida, T. Suda, E. E. Schneeberger, and H. Nakamura. 2002. Dendritic cell involvement in pulmonary granuloma formation elicited by bacillus Calmette-Guerin in rats. Am. J. Respir. Crit. Care Med. 165:1640-1646. [DOI] [PubMed] [Google Scholar]
- 34.Weiss, J. M., A. C. Renkl, C. S. Maier, M. Kimmig, L. Liaw, T. Ahrens, S. Kon, M. Maeda, H. Hotta, T. Uede, and J. C. Simon. 2001. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J. Exp. Med. 194:1219-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss, J. M., J. Sleeman, A. C. Renkl, H. Dittmar, C. C. Termeer, S. Taxis, N. Howells, M. Hofmann, G. Kohler, E. Schopf, H. Ponta, P. Herrlich, and J. C. Simon. 1997. An essential role for CD44 variant isoforms in epidermal Langerhans cell and blood dendritic cell function. J. Cell Biol. 137:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoneyama, H., K. Matsuno, Y. Zhang, M. Murai, M. Itakura, S. Ishikawa, G. Hasegawa, M. Naito, H. Asakura, and K. Matsushima. 2001. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193:35-49. [DOI] [PMC free article] [PubMed] [Google Scholar]