Abstract
Background: Frequent dairy consumption in childhood has been related to higher growth-hormone concentrations that may affect mammary gland and pubertal development.
Objective: We evaluated the relation of dairy intake to breast composition at Tanner stage 4 and age at menarche.
Design: A total of 515 Chilean girls are included in the Growth and Obesity Cohort Study. The subjects have been followed longitudinally since they were 3–4 y old (from 2006 to the present). Starting in 2013, diet was assessed every 6 mo via a 24-h recall. The breast fibroglandular volume (FGV) was measured with the use of dual-energy X-ray absorptiometry at Tanner stage 4. The date of menarche was reported every 6 mo. Our analysis included 290 girls with data on prospective diet and breast composition and 324 girls with data on prospective diet and age at menarche.
Results: The mean ± SD breast FGV and percentage of fibroglandular volume (%FGV) (i.e., FGV divided by total breast volume times 100) at Tanner stage 4 was 81.7 ± 32.2 cm3 and 42.0% ± 16.7%, respectively. Only sweetened, artificially flavored milk-based drinks were associated with the %FGV with girls who consumed >125 g/d having a %FGV that was 4.5% (95% CI: 0.9%, 8.1%) higher than that of girls who consumed none (P-trend = 0.007). Yogurt intake was associated with a lower FGV. Specifically, girls who consumed >125 g yogurt/d had −10.2 cm3 (95% CI: −20.2, −0.3 cm3) less FGV than did girls who consumed no yogurt (P-trend = 0.03). The majority (90.7%) of girls in our cohort attained menarche before the data analyses with a mean ± SD age at menarche of 11.9 ± 0.7 y. In multivariable models, low-fat dairy, low-fat milk, and yogurt intakes were associated with a later age at menarche. In particular, girls who consumed >125 g yogurt/d had menarche, on average, 4.6 mo (95% CI: 1.9, 7.4 mo) later than girls who consumed no yogurt (P-trend = 0.01).
Conclusion: More-frequent consumption of sweetened, artificially-flavored milk-based drinks is associated with a higher %FGV, whereas higher yogurt intake is associated with a lower FGV and delayed age at menarche in Chilean girls.
Keywords: age at menarche, breast composition, dairy, development, puberty
INTRODUCTION
The early onset of puberty is considered an early risk factor for a number of diseases in adulthood including hormone-related cancers (1, 2), a higher risk of all-cause mortality (3), metabolic syndrome (4, 5), and cardiovascular disease (6). In view of these potentially adverse consequences for health in later life, modifiable factors that influence the timing of puberty are of public health importance. Although there is strong genetic heritability of puberty (7), in the past century, the age at menarche and onset of mammary gland development have accelerated by several years, thereby suggesting that environmental characteristics could play a significant role (8, 9).
Although the role of anthropometric measures on pubertal timing has been a matter of intensive discussion (10), roles of other exposures during pubertal development, particularly the diet, have been little explored. Moreover, the studies to date on diet and pubertal development have generally focused on only one pubertal marker, the timing of menarche, which represents a relatively late stage of reproductive development. In adults, breast density (mammographic dense breast tissue) is one of the strongest known risk factors for breast cancer (11, 12). Breast density may be established at the time of breast development (13, 14). Therefore, the assessment of the breast composition during pubertal development is of paramount importance for understanding determinants of breast cancer risk.
In children and adults, frequent dairy consumption, specifically of milk, has been associated with higher endogenous growth hormone, insulin-like growth factor (IGF)11 I, and IGF-binding protein 3 concentrations, which are central regulators of growth and development (15–18). Although the protein in milk could be responsible for higher IGF-I concentrations, meat and other sources of protein do not raise IGF-I concentrations to the same extent as milk does, which suggests that some other factor in milk is stimulating this endogenous IGF-I production (15, 17). Although milk contains bovine IGF-I, which is structurally identical to human IGF-I (19), and rat studies have suggested that there is intact absorption (20), it is generally assumed that IGF-I does not retain bioactivity when consumed orally because of the rapid proteolysis in the upper gut. Milk and dairy products also contain measurable amounts of steroid hormones (21–23), which may influence breast density or pubertal timing (24). Dairy consumption has also been linked to total fat intake, which may, in turn, be associated with breast cancer risk (25). Therefore, the objective of this study was to assess the relations of total and specific types of dairy intake to breast density at Tanner stage 4 and age at menarche in a prospective cohort of Chilean girls.
METHODS
Study design
The Growth and Obesity Cohort Study was initiated in 2006 and included children aged 3 and 4 y who were attending nursery schools of the National Nursery Schools Council Program in the Southeast area of Santiago, Chile (26). Participants were singletons who were born at term (37–42 wk), had a birth weight ≥2500 and <4500 g, and were free from conditions that could affect growth such as food allergies and genetic and metabolic diseases. From 2006 to 2010, the children visited the Institute of Nutrition and Food Technology Health Clinic ≥1 time/y for a physical examination, and in 2011, the frequency of clinic visits increased to every 6 mo. The Growth and Obesity Cohort Study assessments include repeated anthropometric, growth, and maturation assessments as well as biological specimen collections at defined time points over follow-up. Starting in 2013, diet was assessed every 6 mo during the in-person clinic visits with the use of a 24-h recall. More details on recruitment procedures and the study design have been published previously (27). The study protocol was approved by the Ethics Committee of the Institute of Nutrition and Food Technology, University of Chile. Informed consent was obtained from all parents or guardians of children before the start of data collection.
Of the 515 girls who participated in the original cohort, 389 girls who had follow-up information during puberty were considered for in the current study (Supplemental Figure 1). For the analysis of breast density, we further excluded 20 girls with missing breast-density measurements, 6 girls with missing data on diet, and 73 girls who missed a diet assessment before Tanner stage 4, which resulted in an analytic cohort of 290 girls. The girls who were missing diet data or who lacked prospective diet data did not have significantly different breast densities than those of included subjects; however, the girls were younger at their Tanner stage 4 visit (10.4 compared with 11.5 y, respectively). For the analysis of age at menarche, we excluded 10 girls with missing data on diet and 55 girls who missed a diet assessment before menarche, which left a study population of 324 girls. The 10 girls with missing diet data did not have a significantly different age at menarche (11.4 y); however, the 55 girls without prospective diet data did have a younger age at menarche (10.7 y) than that of the included cohort of girls. In total, 287 girls were included in both analyses of breast composition and age at menarche, 3 girls were included only in the analysis of age at menarche, and 37 girls were included only in the analysis of breast composition.
Diet assessment
Dietary intake data were collected by trained dietitians with the use of the USDA multiple-pass method during which girls received cues from the dietitians to help them remember and describe foods that they had consumed over the past 24 h (28). In the in-person interview, subjects were referred to a food atlas that was validated in the Chilean National Dietary Survey, which showed serving sizes of common foods and beverages in glasses, mugs, bowls, and plates (29). Also available were images that showed common condiments and oils in measuring cups, spoons, and dollops with size references. Portion sizes were determined via direct measurements of the portions that were shown in the pictures. The food atlas allowed us to convert the food reported into grams. The USDA Food and Nutrient Database for Dietary Studies was used to calculate nutrient values (30). Chilean mixed dishes that were not found in the USDA Food and Nutrient Database were broken down into their ingredients for linkage (e.g., sopaipilla was broken down into cooked pumpkin, water, corn oil, salt, lard, and wheat flour). Chilean foods that were not found in the USDA Food and Nutrient Database were matched to the food with the closest nutrient profile (e.g., leche asada was matched with “desserts, flan, caramel custard”) as determined by a trained dietitian. During dietary processing, various quality-assurance procedures were conducted to ensure the quality of the data (31). For example, if the description or portion of a food was missing, we assumed it was the most common form or portion of the food in our population. If a girl had multiple 24-h recalls before the outcome of interest, we calculated the cumulative average of each nutrient and food to reduce random-measurement error (32).
Dairy foods were defined as any food or beverages that were produced from the milk of mammals (with the exception of butter). Low-fat dairy was defined as the sum of all dairy foods with <1.5% of calories from fat per serving, and high-fat dairy was defined as the sum of all dairy foods with ≥1.5% of calories from fat per serving. Milk was considered any dairy beverage with ≥75% of milk by volume. Low-fat milk was defined as the sum of powdered and fresh skim- and reduced-fat milk, and high-fat milk was defined as the sum of powdered and fresh whole milk. Mixed dairy drinks were beverages with 30–75% of milk by volume with the second and third most-common ingredients being sugar and artificial flavors, respectively. Total meat intake was the sum of all processed and unprocessed meat including beef, pork, chicken, and turkey (but with the exclusion of fish). Intake of sugar-sweetened beverages included intake of all nondairy beverages with added sugar and included flavored waters, sports beverages, coffee and tea drinks, sodas, and juice.
Dense breast tissue assessment
Starting in 2012, breast development was assessed at the clinical visits via a visual inspection with the use of the Tanner rating scale as well as palpation to differentiate breast tissue from adipose tissue (33–35). A single female dietitian was trained under the supervision of a pediatric endocrinologist on Tanner assessment and achieved a sensitivity and specificity >80% (36). The breast fibroglandular volume (FGV), total breast volume, and percentage of fibroglandular volume (%FGV) were measured in both the left and right breasts, once girls had reached Tanner stage 4, with the use of a dual-energy X-ray absorptiometry scan with a protocol that was designed to quantify breast composition (37). Specifically, each breast was scanned with the use of the Prodigy DXA system software (version 13.6, series 200674; GE Healthcare). The protocol did not require breast compression, and its validity and precision for the measurement of breast density in girls at different Tanner stages have been shown previously (38). Breast composition was derived via a 2-compartment model of adipose and fibroglandular tissues with the use of software developed by J Shepherd and colleagues in the Department of Radiology and Biomedical Imaging, University of California, San Francisco (version 5) (37). The %FGV was defined as the FGV (cubic centimeters) divided by the total breast volume times 100.
A quality-control phantom that contained reference breast-density materials was scanned throughout the study to ensure a stable calibration. We averaged the values of the left and right breasts for all analyses.
Covariates
Weight and height were measured every 6–12 mo during follow-up with the use of standardized techniques by trained personnel as described elsewhere (39). BMI (in kg/m2) was calculated as weight divided by squared height. We estimated BMI for age z scores on the basis of WHO 2007 growth references (40). At an in-clinic study visit, mothers self-reported their highest educational level and their own age at menarche. The mother’s current height and weight were also measured by a trained dietitian. Information on television watching after school and while eating during childhood was collected at age 6 y via a self-report with the following response options: no, sometimes, and yes.
Statistical analysis
Baseline demographics from the 2 analytic cohorts are presented as medians (25th–75th percentiles) if continuous or as a frequency (percentage) if categorical. Whenever possible, we categorized the specific dairy foods into quartiles of intake for the analysis. However, for foods with a high number of nonconsumers, the lowest category was no intake (0 g/d), and the remaining categories were created based on the distribution of intake in our cohort with consideration of standard serving-size amounts (e.g., 125 and 250 g/d) while avoiding having <25 girls in any given category. We used the Kruskal-Wallis test to compare differences in continuous measures across categories of dairy intake, and chi-square tests and an extended Fisher’s exact test (when ≥1 cell count was ≤5) for categorical variables.
For the analysis of cumulative averaged dairy intake and the %FGV and FGV (cubic centimeters), we used a linear regression model. Both the %FGV and FGV were normally distributed in our population, and thus, no transformation of outcome variables was necessary in the analyses. Effect estimates are presented as the absolute difference in the %FGV or FGV for a given category of dairy intake compared with that of the lowest category of dairy intake. For the analysis of cumulative averaged dairy intake and age at menarche, we used a Cox proportional hazards regression model to account for the differing durations of follow-up of the girls and for the failure of some girls to achieve menarche during follow-up. In the analyses, we used a time scale of the calendar time in months from birth to age at menarche, and failing was defined as attaining menarche. If a girl had not reached menarche by the end of follow-up she was censored. For the estimation of failure curves (e.g., the expected proportion of girls who had attained menarche over time) by category of exposure, we set covariates to their mean or most-frequent amounts in the cohort. We evaluated the proportional hazards assumption with the use of a likelihood ratio test; proportional hazards assumptions were met in all of our analyses. Because of the normal distribution of age at menarche in our cohort, we also directly estimated the multivariable-adjusted mean difference (in months) in age at menarche and 95% CIs for categories of exposure with the use of an accelerated failure time model with normal outcome distribution. Tests for trend were conducted with the use of a variable with median intake in each category.
Confounding was assessed with the use of previous knowledge regarding biological relevance as well as descriptive statistics from our study population. Multivariable models for breast density were adjusted for calorie intake (continuous), age at Tanner stage 4 (continuous), intake of sugar-sweetened beverages (quartiles), television watching after school (yes, sometimes, no, or missing), and highest maternal education level (primary school, high school, university level or higher, or missing). Because changes in BMI or height may mediate the associations between dairy intake and breast and pubertal development, we omitted these variables from the initial multivariable model to avoid overadjustment and associated biases (41). However, because of the strong associations between BMI and height with breast density and age at menarche, we also present multivariable models that were further adjusted for these variables. In the analyses of breast density, we used the measured weight and height at Tanner stage 4. For analyses of age at menarche, we used the most-recent measured height and weight before menarche (which was, on average, 194 d prior).
For the analysis of breast composition, we had 80% power to detect a change of 5.3 cm3 for the FGV and a change of 2.8% for the %FGV for a 1-SD change in dairy intake. For the analysis of age at menarche, with the assumption of a dichotomized exposure, we should have been able to detect true HRs of ≤0.68 or ≥1.57. All statistical tests were 2-sided and were performed at the 0.05 level of significance. We used SAS 9.4 software (SAS Institute Inc.) for all analyses.
RESULTS
Of 290 girls in the analysis of dairy intake and breast density, the median (25th–75th percentiles) age at Tanner stage 4 was 11.4 y (10.8–12.0 y) (Table 1). At Tanner stage 4, 30.3% and 13.8% of our girls were overweight (BMI-for-age z score >1; WHO 2007) and obese (BMI-for-age z score >2; WHO 2007), respectively. Overall, 42.4% of girls had completed one 24-h recall, 18.6% of girls had completed two 24-h recalls, 20.3% of girls had completed three 24-h recalls, and 18.6% of girls had completed at least four 24-h recalls before Tanner stage 4. Median dairy intake in our cohort was 258 g/d, which was equal to ∼1 cup (8 fl oz) milk or yogurt/d (237 mL milk or yogurt/d). The largest contributor to total dairy intake was high-fat milk (33.5%), which was followed by yogurt (24.6%), mixed dairy drinks (16.6%), and low-fat milk (13.8%). Other less-frequently consumed dairy products included dairy-based desserts (6.0%), cheese (4.9%), and cream (0.6%). Intakes of the specific dairy foods were not correlated with one another (range of Spearman correlations: from −0.2 for high- and low-fat milk to 0.2 for dairy-based desserts and creams). On average, girls with higher total dairy intake were slightly thinner and younger at Tanner stage 4 than were girls with lower dairy intake; however, there were no other significant differences in demographic and dietary characteristics (data not shown). Demographics were similar for the analysis of dairy intake and age at menarche.
TABLE 1.
Demographics of Chilean girls in the Growth and Obesity Cohort Study who were included in the prospective analysis of diet, breast composition, and age at menarche (2006–2016)
| Outcome cohort |
||
| Breast composition (n = 290 girls) | Age at menarche (n = 324 girls) | |
| Age at breast stage 4 visit, y | 11.4 (10.8, 12.0)1 | — |
| Fibroglandular volume | ||
| % | 38.3 (27.6, 53.7) | — |
| cm3 | 76.8 (58.6, 97.6) | — |
| Breast stage 4 visit | ||
| Weight, kg | 43.3 (38.4, 48.4) | — |
| Height, cm | 149.0 (144.7, 153.7) | — |
| BMI-for-age z score | 0.7 (−0.1, 1.2) | — |
| Girls with menarche, n (%) | — | 294 (90.7) |
| Age at menarche, y | — | 11.9 (11.4, 12.5) |
| Weight before menarche,2 kg | — | 43.1 (38.4, 48.9) |
| Height before menarche, cm | — | 148.7 (144.3, 152.9) |
| BMI-for-age z score before menarche | — | 0.7 (−0.1, 1.2) |
| Mother | ||
| BMI, kg/m2 | 27.5 (24.7, 31.2) | 27.4 (24.6, 31.1) |
| Highest education, n (%) | ||
| Primary school | 46 (15.9) | 50 (15.4) |
| High school | 217 (74.8) | 246 (75.9) |
| University or above | 14 (5.2) | 14 (4.3) |
| Missing | 13 (4.1) | 14 (4.3) |
| Age at menarche, y, n (%) | ||
| ≤11 | — | 51 (15.7) |
| 12 | — | 79 (40.1) |
| 13 | — | 76 (24.4) |
| ≥14 | — | 84 (25.9) |
| Missing | — | 34 (10.5) |
| Television watching after school in childhood, n (%) | ||
| No | 17 (5.9) | 17 (5.2) |
| Sometimes | 13 (4.5) | 14 (4.3) |
| Yes | 248 (85.5) | 280 (86.4) |
| Missing | 12 (4.1) | 13 (4.0) |
| Eating while watching television in childhood, n (%) | ||
| No | 14 (4.8) | 19 (5.9) |
| Sometimes | 132 (45.5) | 142 (43.8) |
| Yes | 131 (45.2) | 149 (46.0) |
| Missing | 13 (4.5) | 14 (4.3) |
| 24-h recalls, n (%) | ||
| 1 | 123 (42.4) | 150 (46.3) |
| 2 | 54 (18.6) | 68 (21.0) |
| 3 | 59 (20.3) | 59 (18.2) |
| 4–6 | 54 (18.6) | 47 (14.5) |
| Intake | ||
| Calories, kcal/d | 1877 (1548, 2237) | 1862 (1537, 2228) |
| From fat, % | 29.6 (25.3, 33.4) | 29.2 (25.1, 32.9) |
| From protein, % | 13.8 (11.8, 15.6) | 13.9 (11.9, 15.9) |
| From carbohydrates, % | 57.8 (54.1, 62.2) | 58.2 (54.2, 62.3) |
| Dairy, g/d | 258 (150, 275) | 261 (146, 375) |
| Meat, g/d | 73.6 (50.0, 111.1) | 73.2 (49.6, 112.0) |
| Sugar-sweetened beverages, mL/d | 284 (188, 429) | 291 (185, 422) |
Median; 25th–75th percentiles in parentheses (all such values).
Median time between weight and height assessment and menarche was 194 d.
Breast composition
The median follow-up time between the first 24-h recall that was reported by a girl and her Tanner stage 4 breast-composition analysis was 209.5 d. The mean ± SD of the FGV and %FGV at Tanner stage 4 was 81.7 ± 32.2 cm3 and 42.0% ± 16.7%, respectively. Although there was a suggestion of a positive association between total dairy intake and the %FGV (P-trend = 0.06), this association became attenuated and nonsignificant after adjustment for BMI and height (P-trend = 0.48) (Table 2). Total dairy intake was not associated with the absolute volume of FGV. When we looked at the specific types of dairy, only mixed dairy drinks were associated with a higher breast density such that girls who consumed 1–125 and >125 g/d had breast densities that were 2.9% (95% CI: −0.4%, 6.2%) and 4.5% (95% CI: 0.9%, 8.1%) higher than those of girls who consumed none (P-trend = 0.007). Only yogurt intake was significantly inversely associated with the absolute volume of dense breast tissue. Specifically, girls who consumed 1–125 and >125 g yogurt/d had, on average, −6.7 cm3 (95% CI: −15.1, 1.6 cm3) and −10.2 cm3 (95% CI: −20.2, –0.3 cm3) less-dense breast tissue than that of girls who consumed no yogurt (P-trend = 0.03).
TABLE 2.
Association between cumulative averaged dairy intake and breast composition at Tanner breast stage 4 in 290 girls in the Growth and Obesity Cohort Study (2006–2016)1
| Fibroglandular volume,2 % |
Fibroglandular volume,2 cm3 |
||||
| Category of intake | Girls, n | Model 1 | Model 2 | Model 1 | Model 2 |
| Total dairy, g/d | |||||
| Q1 (≤149) | 71 | Reference | Reference | Reference | Reference |
| Q2 (150–256) | 74 | 0.2 (−5.3, 5.9) | 0.9 (−2.8, 4.6) | −1.1 (−11.4, 9.1) | −1.0 (−11.1, 9.1) |
| Q3 (257–374) | 73 | 1.6 (−3.7, 7.0) | −0.5 (−4.2, 3.1) | −5.3 (−15.5, 4.8) | −5.0 (−15.0, 5.0) |
| Q4 (≥375) | 72 | 4.7 (−0.7, 10.1) | 1.7 (−2.0, 5.4) | 4.6 (−5.7, 14.8) | 6.0 (−4.1, 16.1) |
| P-trend | — | 0.06 | 0.48 | 0.44 | 0.30 |
| High-fat dairy, g/d | |||||
| Q1 (≤64) | 72 | Reference | Reference | Reference | Reference |
| Q2 (65–174) | 73 | −5.0 (−10.5, 0.4) | 0.3 (−3.5, 4.1) | −1.6 (−12.0, 8.9) | −4.9 (−15.4, 5.6) |
| Q3 (175–261) | 73 | 1.2 (−4.1, 6.6) | 2.7 (−1.0, 6.5) | −1.3 (−11.6, 8.9) | −2.7 (−12.8, 7.4) |
| Q4 (≥262) | 72 | 1.9 (−3.5, 7.2) | 1.6 (−2.1, 5.3) | 5.3 (−4.9, 15.6) | 4.8 (−5.3, 14.9) |
| P-trend | — | 0.19 | 0.28 | 0.27 | 0.25 |
| Low-fat dairy, g/d | |||||
| C1 (0) | 95 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 110 | 1.9 (−2.7, 6.5) | −1.7 (−4.8, 1.5) | −5.6 (−14.3, 3.1) | −4.8 (−13.4, 3.8) |
| C3 (126–249) | 61 | −0.7 (−6.1, 4.6) | −2.3 (−6.0, 1.4) | −9.9 (−20.0, 0.3) | −9.0 (−19.1, 1.1) |
| C4 (≥250) | 24 | 5.0 (−2.4, 12.4) | 1.9 (−7.0, 3.2) | −9.1 (−23.1, 4.9) | −6.4 (−20.5, 7.6) |
| P-trend | — | 0.65 | 0.28 | 0.06 | 0.12 |
| Total milk, g/d | |||||
| C1 (0) | 80 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 91 | 2.1 (−3.0, 7.3) | 0.6 (−2.9, 4.1) | 0.2 (−9.5, 9.9) | 0.3 (−9.3, 9.9) |
| C3 (126–249) | 63 | 2.7 (−2.8, 8.1) | −0.9 (−4.7, 2.9) | −0.7 (−11.1, 9.7) | −0.0 (−10.4, 10.3) |
| C4 (≥250) | 56 | 0.8 (−4.9, 6.4) | −1.5 (−5.3, 2.4) | 4.6 (−6.1, 15.3) | 5.0 (−5.6, 15.6) |
| P-trend | — | 0.71 | 0.30 | 0.53 | 0.45 |
| High-fat milk, g/d | |||||
| C1 (0) | 125 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 79 | 3.0 (−1.8, 7.8) | 1.6 (−1.7, 4.9) | −0.4 (−9.5, 8.7) | 0.5 (−8.6, 9.6) |
| C3 (>125) | 86 | 2.0 (−2.6, 6.6) | −0.1 (−3.2, 3.0) | 2.9 (−5.8, 11.6) | 3.6 (−5.0, 12.2) |
| P-trend | — | 0.36 | 0.98 | 0.53 | 0.42 |
| Low-fat milk, g/d | |||||
| C1 (0) | 206 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 53 | −3.3 (−8.4, 1.8) | −3.9 (−7.4, −0.5) | −5.8 (−15.4, 3.9) | −6.5 (−16.1, 3.0) |
| C3 (>125) | 31 | −2.1 (−8.3, 4.2) | −2.0 (−6.2, 2.2) | 1.3 (−10.5, 13.1) | 0.9 (−10.7, 12.6) |
| P-trend | — | 0.29 | 0.08 | 0.77 | 0.67 |
| Yogurt, g/d | |||||
| C1 (0) | 123 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 110 | 1.9 (−2.5, 6.3) | −1.2 (−4.2, 1.9) | −6.9 (−15.1, 1.4) | −6.7 (−15.1, 1.6) |
| C3 (>125) | 57 | 2.9 (−2.3, 8.1) | −0.3 (−3.9, 3.3) | −12.1 (−21.9, −2.3) | −10.2 (−20.2, −0.3) |
| P-trend | — | 0.25 | 0.77 | 0.01 | 0.03 |
| Mixed-dairy beverage, g/d | |||||
| C1 (0) | 182 | Reference | Reference | Reference | Reference |
| C2 (1–125) | 63 | −1.8 (−6.7, 3.1) | 2.9 (−0.4, 6.2) | 0.3 (−9.0, 9.7) | −1.3 (−10.6, 8.0) |
| C3 (>125) | 45 | 3.9 (−1.5, 9.3) | 4.5 (0.9, 8.1) | 3.0 (−7.2, 13.3) | 2.1 (−8.0, 12.1) |
| P-trend | — | 0.33 | 0.007 | 0.60 | 0.80 |
C, category; Q, quartile.
All values are βs (95% CIs). βs were estimated from a linear regression model that was adjusted for total calorie intake, age at Tanner breast stage 4, sugar-sweetened beverage intake, television watching after school, and highest maternal educational level. Model 2 was adjusted as for model 1 and for BMI-for-age z score and height. For all specific dairy types, βs represent absolute differences in outcomes (percentage of fibroglandular volume and fibroglandular volume) in the second, third, and fourth categories of intake compared with in the first category of intake (the reference category). Tests for trend across categories were conducted with the use of a variable with the median value in each category as a continuous variable.
Age at menarche
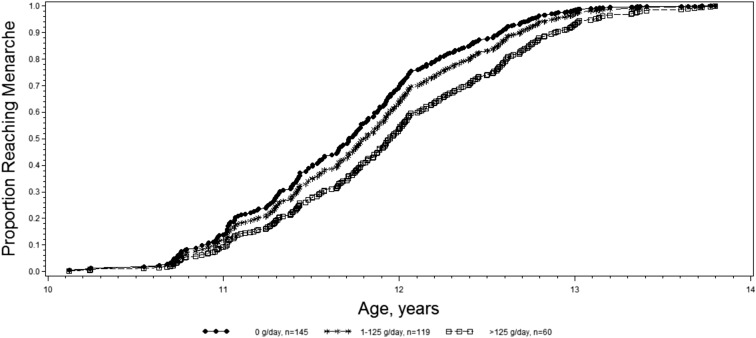
The majority (90.7%) of girls in our analysis of dairy intake and sexual maturation attained menarche by May 2016 with a mean ± SD age at menarche of 11.9 ± 0.7 y. The median follow up time between the first 24-h recall that was reported by a girl and her age at menarche (or the end of follow-up) was 413.5 d. Total dairy intake was not associated with age at menarche; however, low-fat dairy, low-fat milk, and yogurt intakes were all associated with a later age at menarche (Table 3). Girls who consumed 1–125 and >125 g/d of low-fat milk were, on average, 43% (model 2 HR: 0.57; 95% CI: 0.40, 0.82) and 28% (model 2 HR: 0.72; 95% CI: 0.48, 1.07) less likely to attain menarche in the next month, respectively, than were girls who consumed none (P-trend = 0.01). Similarly, girls with intakes of 1–125 and ≥125 g yogurt/d were, on average, 15% (model 2 HR: 0.85; 95% CI: 0.63, 1.13) and 36% (model 2 HR: 0.64; 95% CI: 0.46, 0.91) less likely to attain menarche in the next month, respectively, than were girls who consumed none (P-trend = 0.01) (Figure 1, Table 3). For girls who consumed >125 g yogurt/d, this effect translated into an average delay in menarche of 4.6 mo (95% CI: 1.9, 7.4 mo) compared with girls who consumed no yogurt.
TABLE 3.
Association between cumulative averaged dairy intake and age at menarche in 324 girls in the Growth and Obesity Cohort Study (2006–2016)1
| Model2 |
||||
| Category of intake | Girls, n | 1 | 2 | Difference in age at menarche,3 mo |
| Total dairy, g/d | ||||
| Q1 (≤145) | 81 | 1.0 (reference) | 1.0 (reference) | Reference |
| Q2 (146–260) | 81 | 0.80 (0.57, 1.12) | 0.81 (0.57, 1.14) | 1.7 (−1.0, 4.5) |
| Q3 (261–375) | 82 | 0.75 (0.53, 1.05) | 0.80 (0.56, 1.14) | 2.1 (−0.6, 4.9) |
| Q4 (≥376) | 80 | 0.91 (0.65, 1.28) | 0.96 (0.68, 1.35) | −0.3 (−3.0, 2.5) |
| P-trend | — | 0.67 | 0.96 | — |
| High-fat dairy, g/d | ||||
| Q1 (≤60) | 81 | 1.0 (reference) | 1.0 (reference) | Reference |
| Q2 (61–175) | 81 | 0.60 (0.43, 0.83) | 0.55 (0.39, 0.79) | 5.8 (3.1, 8.5) |
| Q3 (176–260) | 81 | 0.62 (0.44, 0.86) | 0.60 (0.43, 0.85) | 3.7 (1.0, 6.3) |
| Q4 (≥261) | 81 | 0.95 (0.68, 1.32) | 0.93 (0.66, 1.30) | 0.2 (−2.4, 2.9) |
| P-trend | — | 0.80 | 0.78 | — |
| Low-fat dairy, g/d | ||||
| C1 (0) | 112 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 120 | 0.77 (0.57, 1.03) | 0.82 (0.61, 1.11) | 2.1 (−0.2, 4.4) |
| C3 (126–249) | 65 | 0.49 (0.34, 0.70) | 0.46 (0.32, 0.68) | 5.3 (2.5, 8.0) |
| C4 (≥250) | 27 | 0.64 (0.40, 1.02) | 0.66 (0.39, 1.11) | 2.5 (−1.1, 6.2) |
| P-trend | — | <0.001 | <0.001 | — |
| Total milk, g/d | ||||
| C1 (0) | 96 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 101 | 0.60 (0.44, 0.81) | 0.61 (0.44, 0.83) | 3.3 (0.8, 5.8) |
| C3 (126–249) | 64 | 0.63 (0.44, 0.88) | 0.70 (0.49, 0.99) | 2.5 (−0.3, 5.3) |
| C4 (≥250) | 63 | 0.73 (0.51, 1.04) | 0.85 (0.60, 1.22) | −0.1 (−2.8, 2.7) |
| P-trend | — | 0.13 | 0.55 | — |
| High-fat milk, g/d | ||||
| C1 (0) | 147 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 86 | 0.60 (0.45, 0.81) | 0.66 (0.49, 0.90) | 3.1 (0.7, 5.5) |
| C3 (>125) | 91 | 0.77 (0.58, 1.02) | 0.87 (0.65, 1.17) | 0.5 (−1.8, 2.8) |
| P-trend | — | 0.02 | 0.21 | — |
| Low-fat milk, g/d | ||||
| C1 (0) | 235 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 54 | 0.55 (0.39, 0.78) | 0.57 (0.40, 0.82) | 3.8 (1.2, 6.5) |
| C3 (>125) | 35 | 0.73 (0.50, 1.09) | 0.72 (0.48, 1.07) | 0.9 (−2.1, 4.0) |
| P-trend | — | 0.008 | 0.01 | — |
| Yogurt, g/d | ||||
| C1 (0) | 145 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 119 | 0.72 (0.54, 0.95) | 0.85 (0.63, 1.13) | 2.8 (0.6, 5.0) |
| C3 (>125) | 60 | 0.57 (0.41, 0.79) | 0.64 (0.46, 0.91) | 4.6 (1.9, 7.4) |
| P-trend | — | <0.001 | 0.01 | — |
| Mixed-dairy beverage, g/d | ||||
| C1 (0) | 200 | 1.0 (reference) | 1.0 (reference) | Reference |
| C2 (1–125) | 71 | 0.79 (0.58, 1.07) | 0.73 (0.53, 0.99) | 3.9 (1.5, 6.4) |
| C3 (>125) | 53 | 1.41 (1.02, 1.94) | 1.18 (0.84, 1.65) | −1.5 (−4.1, 1.1) |
| P-trend | — | 0.27 | 0.97 | — |
C, category; Q, quartile.
All values are HRs (95% CIs). HRs were estimated from a Cox proportional hazards model that was adjusted for total calorie intake, sugar-sweetened beverage intake, television watching after school, maternal age at menarche, and highest maternal education level. Model 2 was adjusted as for model 1 and for BMI-for-age z score and height at the visit before menarche.
All values are βs (95% CIs). βs were estimated from an accelerated failure time model with a normal-outcome distribution that was adjusted for the same variables in model 2.
FIGURE 1.
Predicted age-at-menarche failure curves in relation to categories of cumulative averaged yogurt intake in the Growth and Obesity Cohort Study (2006–2016). Failure curves were estimated for the average girl in our cohort (i.e., with intake of 1862 kcal/d; with intake of 188–288-mL sugar-sweetened beverages/d (second quartile); who watches television after school; whose mother’s age at menarche was ≥14 y; with a BMI-for-age z score of from −0.1 to 0.7 before menarche (second quartile); and with a height of 144.4–148.7 cm before menarche (second quartile).
DISCUSSION
In this prospective cohort of girls, higher intake of mixed-dairy drinks was associated with a higher breast %FGV, whereas higher yogurt intake was associated with a lower breast FGV and delayed age at menarche. These associations persisted after adjustment for potential important confounders including age and adiposity. Although low-fat milk intake was associated with a later age at menarche, the effect was most pronounced in girls who consumed intermediate amounts of low-fat milk (1–125 g/d) rather than high amounts of low-fat milk (>125 g/d).
The positive association between mixed-dairy beverages, but not fresh milk, and the breast %FGV suggested that sugar, which was a primary component of these drinks, may have been driving this association. In support of this theory are 2 studies in middle-aged women that showed that higher intake of sweet foods was linked to a higher percentage of breast density (42, 43). Biologically, intake of sugar enhances cellular proliferation and migration, induces DNA damage, and increases inflammation (44). Furthermore, an in vitro study on breast tumor cells showed that, although fructose appears to increase cell invasion and migration, glucose seems to increase cell proliferation (45). Excessive sugar consumption is also associated with an increase in the production of insulin that could increase IGF-I (46), which is a growth factor that has mitogenic and antiapoptotic effects on cells (47). Taken together, these observations suggest that intake of sweet items, such as mixed-dairy drinks, could enhance the cellular proliferation in breast tissues and could increase breast density through multiple pathways. Although additional adjustments for total sugar and dairy sugar intake only slightly attenuated the association between mixed-dairy beverages and the %FGV, we lacked information on added sugars that would have been a more informative nutrient to adjust for, particularly for a food group such as dairy in which many natural sugars are present.
To our knowledge, an inverse association between yogurt intake and dense-breast volume has not been previously reported in adults or adolescents, and yet, many previous studies have failed to differentiate between fermented and nonfermented dairy products such as yogurt (48–51). We also failed to find an association between yogurt intake and relative breast density, which suggested that yogurt consumption might affect both dense and nondense mammary gland tissue, thereby potentially lowering the breast size. An accumulating body of epidemiologic and clinical evidence has suggested that yogurt consumption may act beneficially on weight regulation (52); however, our results remained after adjustment for BMI, which suggested that there were other mechanisms. In a nationally representative sample of US children, frequent yogurt consumers had lower insulin resistance and higher insulin sensitivity than infrequent consumers did (53). Because of the link between insulin, IGF-I, and breast density (46, 54), IGF-I could be one mechanism through which yogurt is affecting the volume of dense breast tissue. Although more speculative, there has been increasing evidence that yogurt consumption could lead to advantageous changes in the equilibrium and metabolic activities of the indigenous gut microbiota (55). Moreover, it has been suggested that an increased intestinal microbial diversity influences systemic estrogen-metabolite profiles, specifically favoring a profile with a high ratio of estrogen metabolites to parent estrogen (56), which, in turn, could affect breast density (57).
The relation between milk intake and age at menarche has been studied extensively because of concerns regarding the hormones and growth factors that are endogenous to dairy foods. Although we showed a nonlinear, inverse association between low-fat milk intake and age at menarche, most previous studies have shown null results (48, 50, 51), and a few studies have shown positive associations (49, 58, 59). In concordance with our findings are the results from the Growing Up Today Study, which showed that intake of low-fat milk predicted a modestly older age at menarche (60). Few data exist on the association between yogurt intake and age at menarche; however, the only other study that has investigated this question was conducted in 134 Iranian girls and showed a nonsignificant OR of reaching menarche at ≤12 y of age of 0.72 (95% CI: 0.27, 1.91) when girls who consumed ≥80 g yogurt/d were compared with girls who consumed none (58). The biological mechanism that underlies the association behind yogurt intake and delayed menarche is unknown but could also be related to alterations in IGF-I or estrogen metabolites as previously detailed.
Our study is not without limitations. Although a self-report of diet is subject to measurement errors, the estimation of dietary intake with multiple 24-h recalls in the majority of girls decreased the probability of dietary misclassification. Moreover, because of the prospective design of this study, any dietary misclassification would have been expected to attenuate the observations toward the null. Because girls were asked about their date of menarche every 6 mo, we do not expect that there was substantial error in the reporting of this outcome. Similarly, breast composition was measured in the girls with the use of a previously validated method. Because of the observational nature of this study, residual confounding by other diet and lifestyle factors is possible; however, we tried to reduce this bias by adjusting for intakes of sugar-sweetened beverages and meat, which are the 2 dietary factors that have been most strongly related to the age at menarche in previous studies, and television-watching time as a marker of sedentary behavior. The generalizability of our results to girls of other ethnicities and socioeconomic status is uncertain because all of the girls in our cohort were Chilean and of low-middle socioeconomic status. Finally, girls who were excluded from our analyses because they were missing prospective diet assessments were slightly younger at Tanner stage 4 and at menarche than were included girls, and this difference could have potentially introduced bias. Despite these exclusions, the mean dairy intake, age at Tanner stage 4, and age at menarche in our cohort were comparable with other contemporary Chilean cohorts, thereby suggesting that the girls who were included in our analysis were still representative of public-school children of low-middle socioeconomic status in Santiago (61–63).
Despite these limitations, our study had many strengths including the ability to prospectively study the relation between dairy and measures of puberty and breast development. Adolescence is a critical period when mammary ducts elongate and branch, which may render breasts particularly vulnerable to any effects of diet (64). Therefore, the study of the effects of diet on breast development during adolescence is particularly important and novel. In our analysis of dairy intake and age at menarche, we were also able to differentiate specific types of dairy such as mixed-dairy beverages and yogurt, which turned out to be the foods that were associated with our endpoints of interest. It is possible that the previous null findings regarding the link between adolescent dairy intake and pubertal development or later breast cancer risk were due to a heterogeneous combination of foods that comprised dairy food intake.
In conclusion, higher intake of mixed-dairy drinks is associated with a higher breast %FGV, whereas higher yogurt intake is associated with a lower FGV and delayed age at menarche in Chilean girls. The range of age at menarche is narrow on the population level, and thus, even a small shift in age, such as an average delay of 4.6 mo, may have an impact on health. Because of the strong link between breast composition and age at menarche with later breast cancer risk, our results highlight the potential for early life exposures, such as childhood diet, to affect intermediate endpoints such as breast composition and pubertal development, which may, in turn, influence later health outcomes.
Acknowledgments
The authors’ responsibilities were as follows—AJG: performed the statistical analysis and wrote the manuscript; AJG, AP, DQ, JAS, CC, and KBM: analyzed and interpreted the data; AJG and KBM: had primary responsibility for the final content of the manuscript; AP, DQ, JAS, RU, CC, and KBM: provided critical revision of the manuscript for important intellectual content; AP, DQ, and CC: acquired the data; AP, JAS, RU, CC, and KBM: designed the research plan; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: FGV, fibroglandular volume; IGF, insulin-like growth factor; %FGV, percentage of fibroglandular volume.
REFERENCES
- 1.Bodicoat DH, Schoemaker MJ, Jones ME, McFadden E, Griffin J, Ashworth A, Swerdlow AJ. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res 2014;16:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla C, Lewis SJ, Martin RM, Donovan JL, Hamdy FC, Neal DE, Eeles R, Easton D, Kote-Jarai Z, Al Olama AA, et al. . Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC Med 2016;14:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol 2014;180:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stöckl D, Meisinger C, Peters A, Thorand B, Huth C, Heier M, Rathmann W, Kowall B, Stöckl H, Döring A. Age at menarche and its association with the metabolic syndrome and its components: results from the KORA F4 study. PLoS One 2011;6:e26076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond) 2013;37:1036–43. [DOI] [PubMed] [Google Scholar]
- 6.Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 2009;94:4953–60. [DOI] [PubMed] [Google Scholar]
- 7.Towne B, Czerwinski SA, Demerath EW, Blangero J, Roche AF, Siervogel RM. Heritability of age at menarche in girls from the fels longitudinal study. Am J Phys Anthropol 2005;128:210–9. [DOI] [PubMed] [Google Scholar]
- 8.Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 2008;121(Suppl 3):S172–91. [DOI] [PubMed] [Google Scholar]
- 9.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the copenhagen puberty study. Pediatrics 2009;123:e932–9. [DOI] [PubMed] [Google Scholar]
- 10.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics 2008;121(Suppl 3):S208–17. [DOI] [PubMed] [Google Scholar]
- 11.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, et al. . Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356:227–36. [DOI] [PubMed] [Google Scholar]
- 12.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst 2013;105:1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd N, Martin L, Chavez S, Gunasekara A, Salleh A, Melnichouk O, Yaffe M, Friedenreich C, Minkin S, Bronskill M. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol 2009;10:569–80. [DOI] [PubMed] [Google Scholar]
- 14.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 2010;102:1224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr 2004;58:1211–6. [DOI] [PubMed] [Google Scholar]
- 16.Rich-Edwards JW, Ganmaa D, Pollak MN, Nakamoto EK, Kleinman K, Tserendolgor U, Willett WC, Frazier AL. Milk consumption and the prepubertal somatotropic axis. Nutr J 2007;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev 2002;11:852–61. [PubMed] [Google Scholar]
- 18.Ma J, Giovannucci E, Pollak M, Chan JM, Gaziano JM, Willett W, Stampfer MJ. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst 2001;93:1330–6. [DOI] [PubMed] [Google Scholar]
- 19.Juskevich JC, Guyer CG. Bovine growth hormone: human food safety evaluation. Science 1990;249:875–84. [DOI] [PubMed] [Google Scholar]
- 20.Xian CJ, Shoubridge CA, Read LC. Degradation of IGF-I in the adult rat gastrointestinal tract is limited by a specific antiserum or the dietary protein casein. J Endocrinol 1995;146:215–25. [DOI] [PubMed] [Google Scholar]
- 21.García-Peláez B, Ferrer-Lorente R, Gómez-Ollés S, Fernández-López JA, Remesar X, Alemany M. Technical note: measurement of total estrone content in foods. Application to dairy products. J Dairy Sci 2004;87:2331–6. [DOI] [PubMed] [Google Scholar]
- 22.Pape-Zambito DA, Roberts RF, Kensinger RS. Estrone and 17beta-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci 2010;93:2533–40. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem 1998;62:7–20. [Google Scholar]
- 24.Andersson AM, Skakkebaek NE. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol 1999;140:477–85. [DOI] [PubMed] [Google Scholar]
- 25.Ranganathan R, Nicklas TA, Yang SJ, Berenson GS. The nutritional impact of dairy product consumption on dietary intakes of adults (1995-1996): the Bogalusa Heart Study. J Am Diet Assoc 2005;105:1391–400. [DOI] [PubMed] [Google Scholar]
- 26.Corvalán C, Uauy R, Stein AD, Kain J, Martorell R. Effect of growth on cardiometabolic status at 4 y of age. Am J Clin Nutr 2009;90:547–55. [DOI] [PubMed] [Google Scholar]
- 27.Kain J, Corvalan C, Lera L, Galvan M, Uauy R. Accelerated growth in early life and obesity in preschool Chilean children. Obesity (Silver Spring) 2009;17:1603–8. [DOI] [PubMed] [Google Scholar]
- 28.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. . The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 29.Chilean National Food Survey. Annex: Tables of weight equivalences of the photographic atlas of Chilean foods and Chilean food preparations. Santiago, Chile: Chilean Ministry of Health; 2010 (in Spanish).
- 30. Agriculture Research Service, USDA. National Nutrient Database for Standard Reference release 28. Beltsville (MD): Agricultural Research Service; 2015 [updated 2016 May].
- 31.Anand J, Raper NR, Tong A. Quality assurance during data processing of food and nutrient intakes. J Food Compos Anal 2006;19:586–90. [Google Scholar]
- 32.Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 33.Wu Y, Schreiber GB, Klementowicz V, Biro F, Wright D. Racial differences in accuracy of self-assessment of sexual maturation among young black and white girls. J Adolesc Health 2001;28:197–203. [DOI] [PubMed] [Google Scholar]
- 34.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health 1992;82:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biro FM, Falkner F, Khoury P, Morrison JA, Lucky AW. Areolar and breast staging in adolescent girls. J Pediatr Adolesc Gynecol 1992;5:271–2. [Google Scholar]
- 36.Pereira A, Garmendia ML, Gonzalez D, Kain J, Mericq V, Uauy R, Corvalan C. Breast bud detection: a validation study in the Chilean growth obesity cohort study. BMC Womens Health 2014;14:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd JA, Herve L, Landau J, Fan B, Kerlikowske K, Cummings SR. Clinical comparison of a novel breast DXA technique to mammographic density. Med Phys 2006;33:1490–8. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd JA, Malkov S, Fan B, Laidevant A, Novotny R, Maskarinec G. Breast density assessment in adolescent girls using dual-energy X-ray absorptiometry: a feasibility study. Cancer Epidemiol Biomarkers Prev 2008;17:1709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corvalán C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr 2013;97:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voon NS, Chelliah KK. Is there an influence of dietary habits on breast density as seen on digital mammograms? Asian Pac J Cancer Prev 2011;12:1969–72. [PubMed] [Google Scholar]
- 43.Duchaine CS, Dumas I, Diorio C. Consumption of sweet foods and mammographic breast density: a cross-sectional study. BMC Public Health 2014;14:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Heaney AP. Refined fructose and cancer. Expert Opin Ther Targets 2011;15:1049–59. [DOI] [PubMed] [Google Scholar]
- 45.Monzavi-Karbassi B, Hine RJ, Stanley JS, Ramani VP, Carcel-Trullols J, Whitehead TL, Kelly T, Siegel ER, Artaud C, Shaaf S, et al. . Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int J Oncol 2010;37:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaaks R. [Plasma insulin, IGF-I and breast cancer.] Gynecol Obstet Fertil 2001;29:185–91 (in French). [DOI] [PubMed] [Google Scholar]
- 47.Wang B, Bobe G, LaPres JJ, Bourquin LD. High sucrose diets promote intestinal epithelial cell proliferation and tumorigenesis in APC(Min) mice by increasing insulin and IGF-I levels. Nutr Cancer 2009;61:81–93. [DOI] [PubMed] [Google Scholar]
- 48.Soriguer FJ, Gonzalez-Romero S, Esteva I, Garcia-Arnes JA, Tinahones F, Ruiz de Adana MS, Olveira G, Mancha I, Vazques F. Does the intake of nuts and seeds alter the appearance of menarche? Acta Obstet Gynecol Scand 1995;74:455–61. [DOI] [PubMed] [Google Scholar]
- 49.Wiley AS. Milk intake and total dairy consumption: associations with early menarche in NHANES 1999-2004. PLoS One 2011;6:e14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers IS, Northstone K, Dunger DB, Cooper AR, Ness AR, Emmett PM. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr 2010;13:2052–63. [DOI] [PubMed] [Google Scholar]
- 51.Moisan J, Meyer F, Gingras S. Diet and age at menarche. Cancer Causes Control 1990;1:149–54. [DOI] [PubMed] [Google Scholar]
- 52.Keast DR, Hill Gallant KM, Albertson AM, Gugger CK, Holschuh NM. Associations between yogurt, dairy, calcium, and vitamin D intake and obesity among U.S. children aged 8-18 years: NHANES, 2005-2008. Nutrients 2015;7:1577–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Wang H, Hollis JH, Jacques PF. The associations between yogurt consumption, diet quality, and metabolic profiles in children in the USA. Eur J Nutr 2015;54:543–50. [DOI] [PubMed] [Google Scholar]
- 54.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res 2000;60:3744–8. [PubMed] [Google Scholar]
- 55.Alvaro E, Andrieux C, Rochet V, Rigottier-Gois L, Lepercq P, Sutren M, Galan P, Duval Y, Juste C, Dore J. Composition and metabolism of the intestinal microbiota in consumers and non-consumers of yogurt. Br J Nutr 2007;97:126–33. [DOI] [PubMed] [Google Scholar]
- 56.Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab 2014;99:4632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riza E, dos Santos Silva I, De Stavola B, Bradlow HL, Sepkovic DW, Linos D, Linos A. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2001;10:627–34. [PubMed] [Google Scholar]
- 58.Ramezani Tehrani F, Moslehi N, Asghari G, Gholami R, Mirmiran P, Azizi F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran Lipid and Glucose Study. PLoS One 2013;8:e57696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ku SY, Kang JW, Kim H, Kim YD, Jee BC, Suh CS, Choi YM, Kim JG, Moon SY, Kim SH. Age at menarche and its influencing factors in North Korean female refugees. Hum Reprod 2006;21:833–6. [DOI] [PubMed] [Google Scholar]
- 60.Carwile JL, Willett WC, Wang M, Rich-Edwards J, Frazier AL, Michels KB. Milk consumption after age 9 years does not predict age at menarche. J Nutr 2015;145:1900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amigo H, Vasquez S, Bustos P, Ortiz G, Lara M. Socioeconomic status and age at menarche in indigenous and non-indigenous Chilean adolescents. Cad Saude Publica 2012;28:977–83. [DOI] [PubMed] [Google Scholar]
- 62.Codner E, Unanue N, Gaete X, Barrera A, Mook-Kanamori D, Bazaes R, Avila A, Cassorla F. [Age of pubertal events in Chilean school age girls and its relationship with socioeconomic status and body mass index] Rev Med Chil 2004;132:801–8 (in Spanish). [DOI] [PubMed] [Google Scholar]
- 63.Olivares S, Kain J, Lera L, Pizarro F, Vio F, Moron C. Nutritional status, food consumption and physical activity among Chilean school children: a descriptive study. Eur J Clin Nutr 2004;58:1278–85. [DOI] [PubMed] [Google Scholar]
- 64.Javed A, Lteif A. Development of the human breast. Semin Plast Surg 2013;27:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]