Here, we review the experiences of the pneumococcal vaccine community in the adoption of cell lines for bioassays. We have drawn upon successes and failures in the development of in vitro functional assays reported by the international vaccine community, descriptions of the cell line by its originators, and studies by the leukemia research community into cell differentiation. Bioassays are increasingly based on cell lines, since they offer various advantages over using whole animals, including simplicity of care, ease of standardization, and low cost. For example, an in vitro toxin neutralization assay is used to determine the capacity of anti-diphtheria toxin antibodies to neutralize the cytopathic effect of this toxin on Vero cells (61). The capacity of pneumococcal antibodies to opsonize bacteria in vitro for phagocytes is an important measure of the protective immunity induced with a pneumococcal vaccine. Studies of pneumococcal vaccines have revealed various issues important in selecting cell lines used in the bioassay. This review discusses the lessons relevant to the adoption of cell lines for bioassays, which were learned from pneumococcal vaccine studies.
Streptococcus pneumoniae is an important bacterial pathogen responsible for sepsis, meningitis, pneumonia, and otitis media (2). Antibodies to pneumococcal capsular polysaccharide (PS) protect the host by opsonizing pneumococci for phagocytosis by granulocytes and macrophages, and this opsonizing potential has also been associated with vaccine-induced immunoprotection (6, 14, 26, 47, 78). Although the association between vaccine-induced antibody concentration as measured by enzyme-linked immunosorbent assay (ELISA) and antibody function (opsonophagocytosis) has been established for children participating in three different vaccine efficacy trials of a pneumococcal conjugate vaccine (47), many studies have shown that antibodies detected by ELISA may lack specificity (20, 22, 62, 96). An ELISA modified to decrease nonspecificity is, however, routinely performed with good specificity (91).
Given that the ability of granulocytes to opsonize pneumococci is the key measure of vaccine-induced immunoprotection, diverse in vitro methods of measuring opsonic capacities of antibodies have been devised; these assays are termed opsonophagocytic assays (OPAs). The most established OPA is the opsonophagocytic killing assay (OPKA), which measures the reduction in the number of viable bacteria in the presence of phagocytes, antibodies, and complement (73). Other OPAs measure the uptake of fluorescent (57) or radiolabeled (87) pneumococci into phagocytes in the presence of antibodies and complement.
All in vitro OPAs require phagocytes, which are commonly obtained from one of two sources. The first source is peripheral blood of normal donors, which provides the most biologically relevant granulocytes but presents several shortcomings. The genetic or clinical status of individual donors will vary, with clear implications for the standardization of an OPA. It is also inconvenient to perform phlebotomy by routine schedules, as donors need to be screened for health conditions and granulocytes must be purified prior to the OPA being performing. In addition, an assay may require a large number of granulocytes, necessitating phlebotomy of large volumes of blood from an individual or smaller volumes pooled from many donors. For these reasons, a promyelocytic cell line has been used to provide phagocytic cells for the OPA. Recently, a number of laboratories have used HL-60 cells, subjected to conditions that promote differentiation towards granulocyte morphology, as phagocytes with varying degrees of success. Here, we review the experience with HL-60 cells and their differentiation into granulocytes for use as effector cells in pneumococcal OPA.
HISTORY OF THE HL-60 CELL LINE
The HL-60 cell line was derived from peripheral blood leukocytes of a 36-year-old Caucasian female with acute promyelocytic leukemia (18). It was among the first long-term suspension cultures of human myeloid leukemic cells to be established and has been extensively characterized during the past decades. The original wild-type HL-60 cell line had several properties of malignant cells and expressed various oncogenes (17). The cells formed tumors in nude mice, predominantly consisting of promyelocytes and myeloblasts (33), and grew as colonies in semisolid medium (methylcellulose and agar), which could be enhanced with various colony-stimulating factors and with increasing passage (33). Consequently, multiple sublines with limited differentiation potential have been developed (10), including lines resistant to chemical inducers of differentiation (43, 58) and eosinophilic sublines incapable of neutrophilic or monocytic differentiation (85). Unless otherwise specified, we will refer to the wild-type HL-60 cells in this review.
There is no reported evidence by the originators of HL-60 (33), the American Type Culture Collection (ATCC) (Rockville, Md.), or the European Collection of Cell Cultures (ECACC) (Porton Down, United Kingdom) that HL-60 cells are infected by pathogenic viruses including Epstein-Barr virus, cytomegalovirus, or herpes simplex virus. The Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) also reports that the HL-60 cell line (DSMZ ACC 3) is negative for Epstein-Barr virus, hepatitis B virus, hepatitis C virus, human herpes virus 8, human immunodeficiency virus, and human T-cell leukemia virus types 1 and 2. HL-60 has recently been reported to contain a sequence similar to that of the human endogenous retrovirus H/F-expressing cell line Reh (ATCC CRL-8286) (70), although there is currently no evidence that human endogenous retroviruses are expressed.
Consistent with the origins of HL-60, cytological studies have found HL-60 cells to be myeloblastic or promyelocytic. In culture, cells are ovoid or round but occasionally express pseudopods and are heterogeneous in size (9 to 25 μm in diameter). HL-60 cells have large round nuclei, which are occasionally binucleate with distinct margins, fine chromatin, and two to four nucleoli (33, 85). The cytoplasm is deeply basophilic with prominent multiple azurophilic granules. Cells stain positively with periodic acid-Schiff reagent and occasionally for acid phosphatase, characteristics of mature in vivo granulocytes. HL-60 cells do not, however, express alkaline phosphatase or α-naphtol AS-D acetate (nonspecific) esterase, characteristic of in vivo-derived neutrophilic granulocytes or myeloid cells, and some cells can resemble megakaryocytes and erythrocyte precursors (17).
DIFFERENTIATION OF HL-60
Critical to the successful adoption of a cell line for bioassay is the reproducibility of the function of the cell within the assay itself. In the OPA, the effector cell must first be induced to differentiate to a phagocyte. Size, morphology, and the extent of heterogeneity among cultured HL-60 cells suggest that they may spontaneously differentiate in vitro (18). Approximately 5 to 10% of HL-60 cells appear to be mature myelocytes; many resemble mononuclear phagocytes, metamyelocytes, banded cells, or fully segmented neutrophils. Similarly, about 5 to 10% of cultured HL-60 cells have properties of differentiated granulocytes, such as phagocytic ability and the ability to respond to chemotactic peptides (e.g., N-formylmethionyl-leucine) (33). The effector HL-60 cells for an OPA can also be regulated by induction of differentiation towards three distinct myeloid cell lineages (monocytic, eosinophilic, and granulocytic), depending on the environmental conditions and the chemical inducers used (17, 66).
The multipotentiality of this cell line to differentiate into various cell lineages has been a research subject since its discovery. Environmental conditions such as pH and multiple chemical inducers can greatly facilitate the differentiation of HL-60 cell lines into various myeloid lineages (17, 19, 59, 66, 74). These chemicals generally arrest the cell cycle at the time of induction of differentiation. For example, sodium butyrate induces monocytic differentiation (8) and N,N-dimethylformamide (DMF) and other polar compounds induce granulocytic differentiation (19). Additional inducers such as retinoic acid, dimethyl sulfoxide (DMSO), and dibutryl cyclic AMP have been also reported to induce granulocytic differentiation. Many factors can affect optimal differentiation; conditions for differentiation depend primarily on the concentration of the inducer, the time of exposure, and the relative proportion of cells in different segments of the cell cycle (17, 19). Continuous exposure to these inducers is not necessary (74); even a short exposure may be sufficient (82).
The observations of spontaneous differentiation, selection of sublines, and changes in colony-forming tendencies with increasing passage (33) enforce the requirement for robust control of the cell line and its processes. Bioassays by their very nature require standardization, and unnecessary variation must be avoided. Therefore, for a standardized bioassay it is critical to standardize and optimize differentiation conditions to provide reproducible yields of granulocytes suitable for use as effector cells within the OPA. Optimized conditions have been described by various authors for differentiation into neutrophils and these include 1.25% (vol/vol) of DMSO in a period of 5 to 7 days (10, 19), 100 mM DMF in a period of 5 days (57, 72, 73), or 0.1 μM all-trans-retinoic acid (ATRA) in a period of 5 days (11). In some cases, comparisons between inducers indicate they can be synergistic and are more effective when they are used in combination (11, 13). For instance, HL-60 cells widely available in Europe (ECACC 98070106) differentiate into functional neutrophils better when they are exposed to a mixture of ATRA, 1α,25 dihydroxyvitamin D3 (vitamin D3), and granulocyte colony-stimulating factor (29).
SOURCES OF HL-60 CELL LINES, WILD TYPE, AND SUB-LINES
Reflecting its versatility and long history, the wild-type HL-60 cell line and its various sublines can be obtained from multiple sources (Table 1). The wild type (ATCC CCL-240) is available from ATCC, whose stock was obtained at passage 8 and is distributed at passage 21. The main European source of HL-60 (ECACC 98070106) was deposited at ECACC by C. Bunce of the University of Birmingham, Birmingham, United Kingdom, and it is believed that the original seed stock was a gift from the originator. The seed stock (DSMZ ACC 3) held at DSMZ, was deposited by E. Porfiri of the Royal Free Hospital, London, United Kingdom. Additional European sources include the Interlab Cell Line Collection, Genoa, Italy (ICLC HTL95010), whose deposit was obtained from ECACC, and Istituto Zooprofilattico Sperimentale, Brescia, Italy (IZSBS BS TCL25), which is restricted to Italian researchers. ATCC cell lines are also distributed under license through LGC, Ltd., London, United Kingdom. Although exact details regarding the passage level of the original deposits and passage number banked are difficult to determine, it is likely that the original seed stocks of HL-60 deposited by researchers other than the originating laboratory and retained at ECACC and DSMZ are at a higher passage level than those at ATCC. On-line catalogue collections of HL-60 cells may be found at several sources (3, 23, 27, 44).
TABLE 1.
Selection of HL-60 and derived sublines available from catalogue culture collections
| Collectiona | Name of line | Identifier | Traits, commentsb |
|---|---|---|---|
| ATCC | HL-60 | CCL-240 | Wild-type HL-60 |
| ATCC | Clone 15 HL-60 | CRL-1964 | Undergoes eosinophilic differentiation when treated with butyric acid; established from a clone of HL-60 (ATCC CCL-240) grown at elevated pH (pH 7.6-7.8) for 2 months |
| ATCC | HL-60/MX1 | CRL-2258 | Selected for mitoxantrone resistance from HL-60 (ATCC CCL-240) |
| ATCC | HL-60/MX2 | CRL-2257 | Selected for mitoxantrone resistance from HL-60 (ATCC CCL-240) |
| ECACC | HL-60 | 98070106 | Wild type; 10% spontaneously differentiated; proportion enhanced by polar-planar compounds (e.g., DMSO, butyrate, hypoxanthine, TPA, actinomycin D, retinoic acid) |
| ECACC | Eos-HL-60 | 96100920 | Variant of HL-60 (ECACC 85011431); although capable of reverting to the parental phenotype, maintains a high degree of eosinophil differentiation |
| ECACC | HL60 15-12 | 88120805 | Capable of chemical differentiation towards neutrophils or monocytes and if culture medium becomes acidic |
| ECACC | HL60 Ast.3 | 88120801 | Variant of HL-60 (ECACC 85011431) capable of differentiating into neutrophils by induction with 1.75% DMSO |
| ECACC | HL60 Ast.4 | 88120802 | Variant of HL-60 (ECACC 85011431) where 50 nM TPA results in limited basophilic differentiation and no differentiation towards monocyte lineage |
| ECACC | HL60 M2 | 88120803 | Expansion of subclones of HL-60 with inherent restricted capacity for neutrophil differentiation |
| ECACC | HL60 M4 | 88120804 | Expansion of subclones of HL-60 with inherent restricted capacity for neutrophil differentiation |
| IFO | HL-60 | IFO50022 | Exhibits more rapid growth than the original HL-60 strain, does not respond to DMSO or TPA for differentiation |
| NIHS (JCRB) | HL60(S) | JCRB0163 | Differentiates to neutrophils or macrophages by tumor promoters, vitamin D3, or cytokines |
| NIHS (JCRB) | HL60RG | JCRB0006 | Subline of the HL60 having faster growth rate but reduced differentiation capability |
NIHS (JCRB), National Institute of Health Sciences (Japanese Collection of Research Bioresources), distributed through the Health Science Research Resources Bank, Tokyo, Japan; IFO, Institute for Fermentation, Osaka, Japan, distributed through the Health Science Research Resources Bank.
TPA, 12-O-tetradecanoyl phorbol-13-acetate.
Subclones of HL-60 with slightly different biological properties are available (Table 1). While some of these sublines can differentiate easily in the direction of monocytes or neutrophils (10), many sublines may not differentiate into phagocytes and thus not be suitable for OPAs. For example, sublines ML60 m2, m4, Sp1, Ast3, and Ast25 require higher concentrations of DMSO to induce neutrophil differentiation (10); clone 15 HL-60 (ATCC CRL-1964) readily undergoes eosinophilic differentiation when treated with butyric acid but does not differentiate well into granulocytes (12) (Table 1). An HL-60 subline resistant to retinoic acid (ATRA) has also been described by Grillier et al. (39) as having late-passage cultures with increased tolerance to DMF and an inability to differentiate with either DMF or ATRA (29).
HL-60 CELL LINE MAINTENANCE AND PASSAGE
HL-60 cells require simple maintenance in vitro and grow as single-cell suspension cultures without the tendency to clump or adhere to plastic or glass (18). Although the cell line's doubling times were originally reported to be 55 to 60 h (18), once it is established, cell density doubles every 24 h in an actively growing culture, although doubling time is about 72 h immediately after recovery from the frozen stock. HL-60 cells may be propagated at 37°C under 5% CO2 in air, in Iscove's modified Dulbecco's medium with 4 mM l-glutamine adjusted to contain 1.5 g of sodium bicarbonate/liter and 20% fetal bovine serum (FBS). Initially, S. J. Collins generated this cell line by growth in RPMI 1640 (Gibco Ltd., Carlsbad, Calif.) supplemented with 15% fetal calf serum (Flow Labs, Irvine, Scotland) and gentamicin (50 μg/ml) (18). Maintenance of HL-60 is possible in RPMI 1640 supplemented with 2 mM l-Glutamine and 10 to 20% heat-inactivated FBS from various sources (HyClone, Logan, Utah; JRH Biosciences, Lenexa, Kans.) (29, 72). Under these conditions, HL-60 cultures can be maintained by diluting the cells with a fresh medium to a density of 105 viable cells/ml when the cell density reaches 106 cells/ml. HL-60 cell lines may even be maintained on serum-free medium as originally described by Breitman et al., (9) or by culture in media such as UltraCulture (BioWhittaker, Walkersville, Md.) with 4 mM l-glutamine adjusted to contain 1.5 g of sodium bicarbonate/liter (G. N. Stacey [NIBSC, Potters Bar, United Kingdom], personal communication). However, these HL-60 cells may differentiate poorly (5, 36) and may require a special mixture of chemicals for differentiation (13).
While the HL-60 cell line is very simple to culture, it is predisposed to differentiate into nonproliferating cells or to a subline. Thus, meticulous attention to its handling, culture, and passage procedures is required. Cell concentration should not be allowed to exceed 106 cells/ml. High pH (pH 7.6 to 8.0) culture conditions can favor the differentiation of HL-60 cells into eosinophils rather than neutrophils (28) and thereby affect the performance of the cells in an OPA. This culture condition may inadvertently arise when the CO2 supply to incubators is interrupted; effects of changes in pH (dissolved CO2) must be considered in the application of HL-60 within a standardized OPA. Romero-Steiner et al. (73) reported difficulty in obtaining differentiated granulocytes for opsonophagocytosis of pneumococci in ATCC CCL-240 at passages above 35. This restriction was resolved by using higher concentrations of FBS (20%) and daily maintenance of the cell line with at least 20% carryover of tissue culture medium from the undifferentiated stock into the differentiation culture aliquot (57, 72). This culture regime may provide a benefit through the transfer in the carryover medium of hepatocyte growth factor-like products naturally secreted by HL-60 cells (67). Distribution of frozen undifferentiated seed stocks to laboratories wishing to perform bioassays is also preferable to shipping live cultures, as this is best practice among tissue culture distributors and minimizes risk from contamination and/or selection pressures. Standardized conditions for freezing of differentiated granulocytes to be used directly in OPA are under investigation (J. Martinez [Centers for Disease Control and Prevention, Atlanta, Ga.], personal communication).
STORAGE OF HL-60 CELLS TO PROVIDE FUTURE SEED STOCKS
Long-term storage of HL-60 cells must be accomplished by cryopreservation in liquid nitrogen of the undifferentiated cell stock at a high density (5 × 106 cells/ml) in RPMI 1640-based medium supplemented with 10% glycerol or DMSO while the cell stock is at low passage. The addition of exogenous cryoprotective compounds such as glycerol or DMSO modulates elevations in salt concentration during freezing (66) and protects cells from osmotic stress (32, 60, 71). Some cryoprotectants (e.g., DMSO) may also induce cellular differentiation (10, 19) and are toxic, reducing cell viability after thawing and requiring rapid removal upon thawing. Cell distributors recommend thawing the 1-ml cell stock within 1 min in a 37°C water bath and immediately adding fresh tissue culture (20 ml) medium to dilute the cryoprotective compound. Alternatively, the cryopreservative can be removed by centrifugation of the thawed cell suspension. If the dilution method is chosen, the medium can be replaced by fresh growth medium after overnight incubation; as cells divide, the volume can be adjusted to expand the cell culture. Differentiation into granulocytes should be attempted once the cells have adjusted to the growth conditions and active cell growth is obtained (i.e., daily doubling of cell numbers). This process takes 2 to 3 weeks, on average (64).
MONITORING IN VITRO DIFFERENTIATION
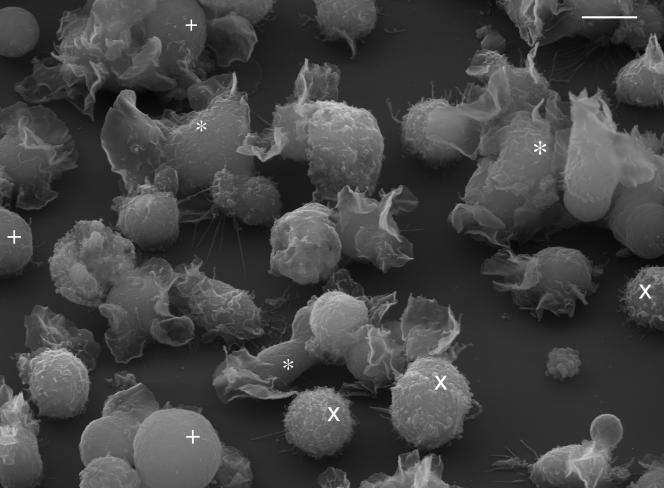
As HL-60 cells differentiate; they cease proliferation, begin to express new genes and molecules, undergo morphological changes, and then die by apoptosis (79). Such changes, including expression of complement receptor 1 (CR1; C3b receptor), have been suggested as indicators of successful differentiation (29, 57, 73) and can be monitored by microscopic, flow cytometric, and molecular techniques. Morphological alterations during differentiation include shrinkage in cell size, decreased nuclear-cytoplasmic ratio, increased nuclear pyknosis and segmentation, decreased cytoplasmic basophilia, replacement of the coarse azurophilic granules with smaller specific granules (17), and alterations in gene and protein expression along macrophage (48, 76) and granulocytic (45) lineages. Undifferentiated HL-60 cells have a generally rounded morphology when viewed by scanning electron microscopy with short- to medium-length pseudopodia and occasional ruffles; they demonstrate clear changes in morphology (increased ruffling and flattening) following differentiation (Fig. 1) (29).
FIG. 1.
Mixed culture of HL-60 cells (ATCC CCL-240) 96 h after treatment with 0.8% DMF, showing differentiated (*) and undifferentiated (×) cells and cells with no clear surface structures (+). Scale bar, 10 μm.
Opsonization involves binding of bacterial serotype-specific antibodies to the PS capsule of pneumococci, which in turn fix complement onto the bacterial surface (90, 93); recurrent pathogenic infections have been associated with natural or causal deficiencies in C3 (31, 83). Thus, successful differentiation of HL-60 for an OPA may be measured by the acquisition of attributes of a phagocyte such as receptor sites, which recognize opsonins. Phagocytic cells, primarily granulocytes, recognize the Fc portions of the bound antibody and the C3b and iC3b complement deposited onto the bacterial surface via specific cell receptors. For the determination of vaccine-induced opsonophagocytic capacity, the primary receptors of interest are the Fcγ types I, II, and III. FcγRI (CD64) is the high affinity receptor for immunoglobulin G1 (IgG1), IgG3, and IgG4; FcγRII (CD32) is the low-affinity receptor for aggregated IgG1, IgG2, and IgG3; and FcγRIII (CD16) is the low-affinity receptor for IgG1 and IgG3. In addition, polymorphisms that result in different binding affinities due to race and genetic lineage have been documented (89). Of particular interest for vaccine-induced antibodies is the FcγRII high responder-low responder described by Sanders et al. and Ernst et al. (25, 77). These differences can be revealed by use of specific monoclonal antibodies or by analysis of the genetic transcripts (69, 89). The presence of the lower-affinity receptors in granulocytes has been associated with lower levels of phagocytosis in the absence of complement (77) and would appear to make this the better option if the assay were to be tailored to be complement independent. Although Fc receptors are important in opsonization, the complement-dependent opsonophagocytic pathway remains central to the clearance of S. pneumoniae in vivo (42, 63); complement receptors for C3b (CR1 and CD35) and iC3b (CR3 and CD11b) are the primary receptors mediating opsonophagocytosis of pneumococci and other encapsulated bacteria (37).
The presence of complement receptors in HL-60 cells and HL-60 granulocytes has been previously documented (4, 29, 46, 73, 75, 89), and their presence may be studied with commercially available antibodies against CD11b (iC3b receptor and CR3 α-chain), CD18 (β-chain of lymphocyte function-associated antigen 1 [LFA-1]) and CD54 (intracellular LFA-1 adhesion molecule 1 [ICAM-1]; ligand for Mac-1). Expression of CD11b, which binds noncovalently to CD18 to form the Mac-1 integrin (15), is a useful marker of granulocytic differentiation and of suitable cell lines for OPA (29, 32, 38, 73). Similarly, the α-chains CD11a and CD11c can bind to CD18 to form two other β2 integrins (LFA-1 family) (1). Because of its simplicity and reproducibility, flow cytometric analysis is the practical way of monitoring the differentiation of HL-60 cells to obtain granulocytes with phagocytic function. CD markers may also be employed to identify monocytes and by deduction neutrophils. For example, CD14 (Leu-M3) is specific for monocytes and macrophages, and CD33 recognizes various myeloid cells, including monocytes and macrophages (8, 24). Differentiation of HL-60 can be readily identified by monitoring the expression of cell surface markers and may therefore be used to quality control the effector cells. Useful markers are summarized in Table 2.
TABLE 2.
Useful markers of cell function and differentiation
| Cell marker | Alternative name | Function and/or comment | Presented in vivo |
|---|---|---|---|
| CD11a | LFA-1; integrin αL subunit | Associates with CD18, up-regulated in inflammation; LFA-1 mediates lymphocyte adhesion by binding ICAM-1 and also binds ICAM-2 and -3 | Leukocytes |
| CD11b | Integrin αM subunit; Mac-1, CR3, C3biR | Associates with CD18, up-regulated in inflammation; this complex serves as a receptor for the iC3b component; Mac-1 also serves as an adhesion molecule for ICAM-1 | Granulocytes, monocytes, NK cells |
| CD11c | Integrin αX subunit; p150/95 | Associates with CD18; up-regulated in inflammation | Granulocytes, monocytes, macrophages, NK cells, T- and B-cell subsets |
| CD16a | Transmembrane form of FcγRIIIA/FcγRIIIB | Low-affinity receptor for aggregated IgG | NK cells and neutrophils |
| CD16b | GPI-linked form of FcγRIIIb | Low-affinity receptor for human IgG | Only expressed on neutrophils (granulocytes) |
| CD18 | Integrin β2 subunit | Associates with CD11a, -b, -c, -d | Leukocytes |
| CD32 | Fc receptor RII | Low-affinity receptor for aggregated IgG | Monocytes, granulocytes, B cells |
| CD35 | CR1; C3b receptor | Binds complement C3b and C4b and enhances phagocytosis | Monocytes, granulocytes, B cells, erythrocytes, dendritic cells, NK cells |
| CD54 | ICAM-1 | Mediates adhesion through the binding of LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) | Endothelial and activated cell types |
| CD71 | T9, transferring receptor | Intracellular adhesion | Proliferating cells, activated T- and B-cells, macrophages |
| CD89 | Fcα receptor R, Fcα-R, IgA Fc receptor, IgA receptor | Binds both monomeric and polymeric forms of either IgA1 or IgA2 at the boundary between the Cd2 and Cd3 domain, induces phagocytosis, degranulation, respiratory burst, and killing of microorganisms | Eosinophils, neutrophils, monocytes, and alveolar macrophages |
| Annexin-5 | Lipocortin 5, endonexin 2, VAC-a, anchorin CII, PAP-1 | Calcium binding protein which interacts with acidic membrane phospholipids; during the early stages of apoptosis, the phosphotidyl serine molecule flips on the cell membrane, exposing an external binding site for annexin-5 | Marker of early, middle, and late cellular apoptosis |
| FMLP receptor | Chemotactic peptide receptor | Binds formyl-Met-Leu-Phe | |
| P2Y11 | ATP receptor | Binding of ATP during cyclic AMP granulocytic differentiation of HL-60 and NB-4 cells | Granulocytic differentiation |
Even after expression of these markers, the relative activity of certain receptors and biochemical pathways can be further modulated (80) as documented in the case of peripheral blood neutrophils (21). Differentiated granulocytes can be further activated by the addition of granulocyte-macrophage colony-stimulating factor (66), C-reactive protein (16), or phorbol 12-myristate 13-acetate to observe the reduction of nitroblue tetrazolium (73) or by coinduction with dexamethasone and DMF to induce higher activity of the chemotactic peptide receptors (80). Differentiated granulocytes have increased levels of protein kinase C and can release arachidonic acid upon stimulation with phorbol 12-myristate 13-acetateand Ca2+ (95). A recent report also indicates that heat-killed pneumococci (serogroup 3) can up-regulate the surface expression of CR3 (CD11b) in a time-dependent fashion prior to uptake of pneumococci by the neutrophils (94).
PERFORMANCE OF HL-60 IN SEVERAL OPAs
HL-60 cells differentiated with DMF into granulocytes were used as phagocytes by Romero-Steiner et al. (73) in a complement-dependent OPKA that measures the capacity of anti-capsule antibodies to opsonize live pneumococci in a single serotype-specific reaction. This assay required the counting the numbers of CFU that remained viable after the granulocytes phagocytosed the target bacteria in vitro. HL-60 granulocytes are used in a 400:1 effector-to-target cell ratio, and the viability of the differentiated cells can be as low as 50% without affecting the opsonophagocytic titer. In this assay, the OPKA titer is defined as the serum dilution with ≥50% killing of the bacterial inoculum (1,000 bacteria/well). This method mimics the opsonophagocytic process occurring in vivo and has been used for many years. Recently, an evaluation of this methodology indicated that this assay has a high degree of reproducibility across multiple laboratories (72).
OPKA has several disadvantages. One is that this method requires tedious colony counting, a difficulty that has hindered wide adoption of this method for testing a large number of samples. Kim et al. overcame the colony-counting problem by colony enhancement with dye and automated colony counting (50). Alternatively, a chromogenic modification has been developed for the faster determination of titers (54). Another disadvantage is that the OPKA has many manual steps and requires at least 40 μl of serum sample to test a single serotype. This problem was resolved by development of a multiplexed OPKA with antibiotic-resistant pneumococci and allowing the simultaneous testing of two serotypes (50, 65). More recently, Bogaert et al. demonstrated that a multiplexed OPKA could handle seven serotypes simultaneously (7). These improvements in the OPKA are likely to increase its adoption and automation, placing greater demands on the provision of phagocytic effector cells and thus the requirement for a comprehensive understanding of the properties and behavior of the HL-60 cell line.
HL-60 differentiated granulocytes have also been used in single and multiplex flow cytometric OPAs for pneumococcus (56, 57). The method relies on phagocytic uptake of fluorescent but killed S. pneumoniae (57) or fluorescent particles coated with pneumococcal capsular PSs by HL-60 granulocytes (55). HL-60 cells are highly efficient at the phagocytosis of fixed pneumococci or PS-coated bead particles in this assay configuration, where the effector-to-target cell ratio is reversed to 1:2 or 1:4. The flow cytometric OPAs provide several advantages. (i) The assay can be semiautomated for rapid analysis of a large number of samples. (ii) It is insensitive to antibiotics in the serum sample, allowing studies of sera derived from individuals treated with antibiotics. (iii) Up to four different targets can be tested simultaneously for OPA titer determination. (iv) It uses smaller numbers of HL-60 phagocytes. (v) This approach may overcome some of the technical difficulties of growing and maintaining consistent target S. pneumoniae. Also, phagocytosis by a specific subset of phagocytes can be determined by tagging phagocytic cells with a cell surface marker of differentiation, e.g., CD11b (29). However, these flow cytometric OPAs have not been extensively implemented or evaluated in a multilaboratory study and only measure binding (and/or phagocytosis) of the bacteria and not killing. Although nonkilling assays have been developed, additional validation is required to show that uptake of beads is equivalent to opsonization, uptake, and killing of bacteria. If a given nonkilling assay was shown to be an efficient surrogate of bacterial killing, there would be potential for its use as a correlate of protection.
HL-60, A PROBLEM CELL LINE?
As HL-60 cells became widely used in many different laboratories as a source of phagocytes, contradictory experiences were reported. A number of laboratories in the United States had varying levels of success in using HL-60 cells for OPA. In contrast, several experienced European laboratories in various countries reported difficulties in achieving reproducible differentiation of HL-60 cells into granulocytes with DMF. Although some European laboratories were successful in using HL-60 (72), these diverse international experiences became a concern in adopting the HL-60 derived granulocytes as the effector cells for pneumococcal OPAs.
A number of hypotheses have been put forward to explain these differences, including differences in the source and quality of serum used in cell cultures to differences between the passage procedures adopted between laboratories. It is likely that the differences between the laboratories in the United States and Europe may actually be intrinsic to the seed source of HL-60 employed. Reports of successful differentiation of HL-60 with DMF are primarily from United States sources, where seed stocks were obtained from ATCC, a cell line deposited at passage 8. In contrast, many of the European seed stocks were sourced from ECACC and were almost certainly derived from an accession of higher passage than those derived directly from ATCC. In addition, it is possible that HL-60 cell lines may have partially differentiated in individual laboratories and therefore become less useful as phagocytes (40). Differences between the responses of two different HL-60 wild types (ATCC CCL-240 and ECACC 98070106) following induction of differentiation with DMF or ATRA have also been observed (29, 30, 34, 49). Thus, for the successful adoption of an OPA based upon HL-60-derived granulocytes, each laboratory must obtain the “correct” HL-60 cell line. It is also critical that source, storage, shipping, maintenance, and differentiation methods are clearly stated in any standardized OPA protocol(s).
ALTERNATIVES TO WILD-TYPE HL-60
Because of the initial conflicting experience with HL-60, several other cell lines were considered that are capable of differentiating into either granulocytic or monocytic lineages (53, 88) by the addition of chemical inducers in a fashion similar to that of HL-60 (Table 3). At the moment, these cell lines have not been evaluated for their use in pneumococcal OPAs to the same extent as HL-60 cells have. One cell line, NB-4, was found to efficiently differentiate into granulocytes (29). As the OPA is designed to determine antibody titer, any functional phagocyte capable of preferentially phagocytosing opsonized pneumococci or PS-coated beads could be used to determine opsonic titers, as long as the OPA titers were comparable to those obtained with the more widely used HL-60 granulocytes (72). However, poorly differentiated cultures or cultures with low viability are likely to be less desirable for use within a standardized assay, as factors which contribute to uncontrolled variations between assays must be controlled if the bioassay is to be considered standardized. In preliminary studies, differentiated NB-4 cells (53) generated opsonic titers comparable to those derived by DMF-differentiated HL-60 cells (ATCC CCL-240) (30, 35). NB-4 cells have a 72- to 96-h differentiation period with opsonic activity after 72 h of differentiation and as early as 48 h after induction of differentiation (30, 35). More-rapid induction of differentiation of the effector cell line (HL-60 or NB-4) may be considered beneficial, as it reduces the total time to perform the bioassay. In addition, a single source of cell line (e.g., NB-4) may be advantageous, as it reduces the potential for variation between different commercially sourced HL-60 cell lines. Another potential advantage for OPA in the absence of exogenous complement is that in contrast to HL-60 which is homozygous for the arginine R131 allele of the low-affinity FcγRII (CD32) receptor, which binds the IgG2 antibody isotope, NB-4 is heterozygous for the point mutation and exhibits both histidine H131 and arginine R131 alleles (34). This difference in receptor affinity may make the NB-4 cell line less complement dependent for use in an OPA.
TABLE 3.
Candidate cell lines
| Cell line | Morphology | Differentiated morphology (reference) |
|---|---|---|
| 3T6 | Fibroblast | Transfected with FcγIIa (84) |
| AML-193 | Myelomonoblast | Granulocyte-monocyte (52) |
| HL-60 | Promyelocyte | Granulocyte-monocyte (18) |
| MHH-225 | Promyelocyte | Granulocyte-monocyte (41) |
| ML-1 and 3 | Myelomonoblast | Granulocyte-monocyte (68) |
| Mono Mac 6 | Promyelocyte | Monocyte (97) |
| NB-4 | Promyelocyte | Granulocyte-monocyte (53) |
| PL-21 | Promyelocyte | Granulocyte-monocyte (51) |
| THP-1 | Promyelocyte | Monocyte (86) |
| U937 | Promyelocyte | Monocyte (81) |
Other approaches for new phagocytes include the genetic engineering of mouse fibroblasts to express molecules necessary for phagocytosis (e.g., CR3 and the Fc receptor). This has been attempted without success with 3T6 fibroblasts transfected with FcγIIa cDNA (92). To overcome difficulties associated with a high percentage of cell death during differentiation (79), monitoring of cell viability of the effector cells is crucial to maintain their efficiency in OPAs. Prevention of cell death following differentiation with bcl-2 transgene manipulations or chemical inhibition of apoptosis could increase effector efficiency and yield. In addition, genes carrying the higher-affinity FcγIIR allele could be introduced to further tailor the specificity of the OPA.
SUMMARY
From a practical point of view, a single source for the HL-60 cell line is needed, as well as standardized culture and differentiation conditions. Suggested conditions for a standardized OPKA protocol are available online (64). This site is updated periodically. Despite confusing experiences in the past, the HL-60 cell line has been extensively characterized and is readily available; its use has been described for the evaluation of pneumococcal vaccine induced anti-capsular antibodies by standardized OPKA, and it remains a good candidate cell line. Perhaps a single distribution center for the cell line may be established to help the pneumococcal research community. In the meantime, any laboratory interested in OPA must obtain the correct HL-60 cell line from a qualified source (e.g., ATCC).
Acknowledgments
We acknowledge the encouragement by E. Griffith, who pushed for this article, G. Carlone, and I. Feavers. We also acknowledge the WHO and NIH, who supported the standardization of OPKA.
The work was supported by NIH (NO1-AI-30021) to M.H.N.
REFERENCES
- 1.Abbas, A. K., A. H. Lichtman, and J. S. Pober (ed.). 1994. Cellular and molecular immunology, 2nd ed., p. 431-435. W. B. Saunders Co., Philadelphia, Pa.
- 2.Alonso deVelasco, A. F. M. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Type Culture Collection. 2004. Electronic catalogue. [Online.] http://www.atcc.org/Home.cfm.
- 4.Atkinson, J. P., and E. A. Jones. 1984. Biosynthesis of human C3b/C4b receptor during differentiation of the HL-60 cell line: identification and characterization of a precursor molecule. J. Clin. Investig. 74:1649-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailly, A., C. Le Page, M. Rauch, and E. Milgrom. 1986. Sequence-specific DNA binding of the progesterone receptor to the uteroglobin gene: effects of hormone, anti-hormone and receptor phosphorylation. EMBO J. 5:3235-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and the Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 7.Bogaert, D., M. Sluijter, R. de Groot, and P. W. M. Hermans. 2004. Multiplex opsonophagocytosis assay (MOPA) is a useful tool for the monitoring of the 7-valent pneumococcal conjugate vaccine. Vaccine 22:4014-4020. [DOI] [PubMed] [Google Scholar]
- 8.Boyd, A. W., and D. Metcalf. 1984. Induction of differentiation in HL-60 leukemic cells: a cell cycle dependent all-or-none event. Leuk. Res. 8:27-43. [DOI] [PubMed] [Google Scholar]
- 9.Brietman, T., S. Collins, and B. Keene. 1980. Replacement of serum by insulin and transferring supports growth and differentiation of the human promyelocytic leukemia cell line, HL-60. Exp. Cell. Res. 126:494-498. [DOI] [PubMed] [Google Scholar]
- 10.Bunce, C. M., A. G. Fisher, D. Toksoz, and G. Brown. 1983. Isolation and characterisation of dimethylsulphoxide resistant variants from the human promyeloid cell line HL60. Exp. Hematol. 11:828-833. [PubMed] [Google Scholar]
- 11.Bunce, C. M., P. J. French, J. Durham, R. A. Stockley, R. H. Michell, and G. Brown. 1994. Indomethacin potentiates the induction of HL60 differentiation to neutrophils, by retinoic acid and granulocyte colony-stimulating factor, and to monocytes, by vitamin D3. Leukemia 8:595-604. [PubMed] [Google Scholar]
- 12.Bunce, C. M., J. M. Lord, A. K. Y. Wong, and G. Brown. 1988. Near-neighbor analysis of variant cell-lines derived from the promyeloid cell line HL-60. Br. J. Cancer 57:559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunce, C. M., L. A. Wallington, P. Harrison, G. R. Williams, and G. Brown. 1995. Treatment of HL60 with various combinations of retinoids and 1 α,25 dihydroxyvitamin D3 results in differentiation towards neutrophils or monocytes or a failure to differentiate and apoptosis. Leukemia 9:410-418. [PubMed] [Google Scholar]
- 14.Butler, J. C., E. D. Shapiro, and G. M. Carlone. 1999. Pneumococcal vaccines: history, current status and future directions. Am. J. Med. 107:69S-76S. [DOI] [PubMed] [Google Scholar]
- 15.Cai, T. Q., S. K. A. Law, H. R. Zhao, and S. D. Wright. 1995. Reversible inactivation of purified leukocyte integrin CR3 (CD11b/CD18, α m β 2) by removal of divalent cations from a cryptic site. Cell Adhes. Commun. 3:399-406. [DOI] [PubMed] [Google Scholar]
- 16.Chi, M., S. Tridandapani, W. Zhong, K. M. Coggeshall, and R. F. Mortensen. 2002. C-reactive protein induces signaling through Fc gamma RIIa on HL-60 granulocytes. J. Immunol. 168:1413-1418. [DOI] [PubMed] [Google Scholar]
- 17.Collins, S. J. 1987. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood 70:1233-1244. [PubMed] [Google Scholar]
- 18.Collins, S. J., R. C. Gallo, and R. E. Gallagher. 1977. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 270:347-349. [DOI] [PubMed] [Google Scholar]
- 19.Collins, S. J., F. W. Ruscetti, R. E. Gallagher, and R. C. Gallo. 1978. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 75:2458-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corey, S. J. and P. M. Rosoff. 1989. Phagocyte activation, p. 25-42. In M. S. Klempner, B. Styrt, and J. Ho (ed.), Phagocytes and disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 22.Coughlin, R. T., A. C. White, C. A. Anderson, G. M. Carlone, D. L. Klein, and J. Treanor. 1998. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine 16:1761-1767. [DOI] [PubMed] [Google Scholar]
- 23.Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. 2004. Electronic catalogue. [Online.] http://www.dsmz.de/dsmzhome.htm.
- 24.Drexler, H. G. 1987. Myeloid-antigen expression in adult acute lymphoblastic-leukemia. N. Engl. J. Med. 317:1156-1157. [DOI] [PubMed] [Google Scholar]
- 25.Ernst, L. K., J. G. J. van de Winkel, I.-M. Chiu, and C. L. Anderson. 1992. Three genes for the human high affinity Fc receptor for IgG (FcγRI) encode four distinct transcription products. J. Biol. Chem. 267:15692-15700. [PubMed] [Google Scholar]
- 26.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, P. H. Makela, S. Lockhart, and M. Ecrola. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 27.European Collection of Cell Cultures. 2004. Electronic catalogue. [Online.] http://www.ecacc.org.uk.
- 28.Fischkoff, S. A., and R. M. Rossi. 1990. Lineage directed HL-60 cell sublines as a model system for the study of early events in lineage determination of myeloid cells. Leuk. Res. 14:979-988. [DOI] [PubMed] [Google Scholar]
- 29.Fleck, R. A., H. Athwal, J. A. Bygraves, D. J. Hockley, I. M. Feavers, and G. N. Stacey. 2003. Optimization of nb-4 and hl-60 differentiation for use in opsonophagocytosis assays. In Vitro Cell. Dev. Biol. Anim. 39:235-242. [DOI] [PubMed] [Google Scholar]
- 30.Fleck, R. A., and M. H. Nahm. 2004. Characterization of effector cells for a standardized opsonophagocytic killing assay (OPKA) with a pneumococcal target, p. 186. In Proceedings of the 4th International Symposium on Pneumococci and Pneumococcal Diseases. KTL, National Public Health Institute, Helsinki, Finland.
- 31.Frank, M. M. 1987. Complement in the pathophysiology of human disease. N. Engl. J. Med. 316:1525-1530. [DOI] [PubMed] [Google Scholar]
- 32.Fuller, B. J. 1987. Low temperature preservation in medicine and veterinary science, p. 432-450. In B. W. W. Grout and G. J. Morris (ed.), The effects of low temperatures on biological systems. Edward Arnold, London, United Kingdom.
- 33.Gallagher, R. S. Collins, J. Trujillo, M. McCredie, M. Ahearn, S. Tsai, R. Metzgar, G. Aulakh, R. Ting, F. Ruscetti, and R. Gallo. 1979. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54:713-733. [PubMed] [Google Scholar]
- 34.Gillett, M., R. Care, R. Fleck, J. Bygraves, and I. Feavers. 2004. Cell line and differentiation, choices in the pneumococcal opsonophagocytic killing assay (OPKA), p. 186. In Proceedings of the 4th International Symposium on Pneumococci and Pneumococcal Diseases. KTL, National Public Health Institute, Helsinki, Finland.
- 35.Gillett, M., R. Care, R. Fleck, J. Bygraves, and I. Feavers. 2004. Establishing a standardized opsonophagocytic killing assay (OPKA) at NIBSC, p. 187. In Proceedings of the 4th International Symposium on Pneumococci and Pneumococcal Diseases. KTL, National Public Health Institute, Helsinki, Finland.
- 36.Goldberg, Y., C. Glineur, J.-C. Gesquière, A. Ricouart, J. Sap, B. Vennström, and J. Ghysdael. 1988. J. Activation of protein kinase C or camp-dependent protein kinase increases phosphorylation of the c-erbA-encoded thyroid hormone receptor and of the v-erbA-encoded protein. EMBO J. 7:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon, D. L., and M. K. Hostetter. 1986. Complement and host defence against microorganisms. Pathology 18:365-375. [DOI] [PubMed] [Google Scholar]
- 38.Gordon, D. L., G. M. Johnson, and M. K. Hostetter. 1986. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J. Infect. Dis. 154:619-626. [DOI] [PubMed] [Google Scholar]
- 39.Grillier, I., T. Umiel, E. Elstner, S. J. Collins, and H. P. Koeffler. 1997. Alterations of differentiation, clonal proliferation, cell cycle progression and bcl-2 expression in RAR alpha-altered sublines of HL-60. Leukemia 11:393-400. [DOI] [PubMed] [Google Scholar]
- 40.Hadjokas, N., C. Bayer, and C. P. Nielson. 1992. Impaired stimulus-response coupling in association with increased growth-rate of HL-60 cells. J. Leukoc. Biol. 52:157-160. [DOI] [PubMed] [Google Scholar]
- 41.Hassan, H. T., E. Petershofem, E. Lux, C. Fonatsch, G. Heil, and M. Freund. 1995. Establishment and characterization of a novel CD34-positive human myeloid leukemia cell line: MHH225 growing in serum-free culture. Ann. Hematol. 71:111-117. [DOI] [PubMed] [Google Scholar]
- 42.Henderson, B., M. Wilson, R. McNab, and A. J. Lax. 1999. The innate immune response, p. 311-353. In B. Henderson, M. Wilson, M.; R. McNab, and A. J. Lax (ed.) Cellular microbiology, bacteria-host interactions in health and disease. John Wiley & Son, Chichester, United Kingdom.
- 43.Huberman. E., C. Weeks, A. Herrmann, M. Callaham, and T. Slaga. 1981. Alterations in polyamine levels induced by phorbol diesters and other agents that promote differentiation in human promyelocytic leukemia-cells. Proc. Natl. Acad. Sci. USA 78:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Interlab Cell Line Collection. 2004. Electronic catalogue [Online.] http://www.iclc.it.
- 45.Itoh, K., K. Okubo, H. Utiyama, T. Hirano, J. Yoshii, and K. Matsubara. 1998. Expression profile of active genes in granulocytes. Blood 92:1432-1441. [PubMed] [Google Scholar]
- 46.Jansen, W. T., M. Väkeväinen-Anttila, H. Käyhty, M. Nahm, N. Bakker, J. Verhoef, H. Snippe, and A. F. Verheul. 2001. Comparison of a classical phagocytosis assay and a flow cytometry assay for assessment of phagocytic capacity of sera from adults vaccinated with a pneumococcal conjugate vaccine. Clin. Diagn. Lab. Immunol. 8:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jódar, L., J. Butler, G. Carlone, R. Dagan, C. Frasch, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 48.Juan, H. F., J. Y. C. Lin, W. H. Chang, C. Y. Wu, T. L. Pan, M. J. Tseng, K. H. Khoo, and S. T. Chen. 2002. Biomic study of human myeloid leukemia cells differentiation to macrophages using DNA array, proteomic, and bioinformatic analytical methods. Electrophoresis 23:2490-2504. [DOI] [PubMed] [Google Scholar]
- 49.Kim, K. H., and J. Y. Seoh. 2004. Phenotypic and functional analysis of HL-60 cells during induction of differentiation for opsonophagocytic assay, p. 194. In Proceedings of the 4th International Symposium on Pneumococci and Pneumococcal Diseases. KTL, National Public Health Institute, Helsinki, Finland.
- 50.Kim, K. H., J. Yu, and M. H. Nahm. 2003. Efficiency of a pneumococcal opsonophagocytic killing assay improved by multiplexing and by coloring colonies. Clin. Diagn. Lab. Immunol. 10:616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubonishi, I., K. Machida, K. Niiya, H. Sonobe, Y. Ohtsuki, K. Iwata, and I. Miyoshi. 1984. Establishment of a new peroxidase-positive human myeloid cell line, Pl-21. Blood 63:254-259. [PubMed] [Google Scholar]
- 52.Lange, B., M. Valtieri, D. Santoli, D. Caracciolo, F. Mavilio, I. Gemperlein, C. Griffin, B. Emanuel, J. Finan, P. Nowell, and G. Rovera. 1987. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood 70:192-199. [PubMed] [Google Scholar]
- 53.Lanotte, M., V. Martin-Thouvenin, S. Najman, P. Balerini, F. Valensi, and R. Berger. 1991. NB4, a maturation inducible cell line With t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 77:1080-1086. [PubMed] [Google Scholar]
- 54.Lin, J. S., M. K. Park, and M. H. Nahm. 2001. Chromogenic assay measuring opsonophagocytic killing capacities of antipneumococcal antisera. Clin. Diagn. Lab. Immunol. 8:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez, J., E. Clutterbuck, S. Romero-Steiner, L. L. Wood, and G. M. Carlone. 2002. Development of a multiplexed, bead-based, flow cytometric, opsonophagocytic assay for the simultaneous detection of antibody to multiple Streptococcus pneumoniae serotypes, p. 79-80. In Proceedings of the 3rd International Symposium on Pneumococci and Pneumococcal Diseases. Centers for Disease Control and Prevention, Anchorage, Alaska.
- 56.Martinez, J. E., S. Romero-Steiner, E. Clutterbuck, and G. M. Carlone. 2002. Performance of a multiplexed Streptococcus pneumoniae flow cytometric opsonophagocytic assay, p. 27. In Proceedings of the 3rd International Symposium on Pneumococci and Pneumococcal Diseases. Centers for Disease Control and Prevention, Anchorage, Alaska.
- 57.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for the measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mascioli, D. W., and R. D. Estensen. 1984. Analysis of human promyelocytic leukemia-cell (HL60) variants insensitive to phorbol ester tumor promoters. Cancer Res. 44:3280-3285. [PubMed] [Google Scholar]
- 59.Mata-Greenwood, E., A. Ito, H. Westenburg, B. Cui, R. G. Mehta, A. D. Kinghorn, and J. M. Pezzuto. 2001. Discovery of novel inducers of cellular differentiation using HL-60 promyelocytic cells. Anticancer Res. 21:1763-1770. [PubMed] [Google Scholar]
- 60.Mazur, P. 1970. Cryobiology: the freezing of biological systems. Science 168:939-949. [DOI] [PubMed] [Google Scholar]
- 61.Miyamura, K., E. Tajiri, A. Ito, R. Murata, and R. Kono. 1974. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using VERO cells. II. Comparison with the rabbit skin method and practical application for seroepidemiological studies. J. Biol. Stand. 2:203-209. [DOI] [PubMed] [Google Scholar]
- 62.Musher, D. M., D. W. Watson, and R. E. Baughn. 1990. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infections? J. Infect. Dis. 161:736-740. [DOI] [PubMed] [Google Scholar]
- 63.Musher, D. M., A. J. Chapman, A. Goree, S. Jonsson, D. E. Briles, and R. E Baughn. 1986. Natural and vaccine related immunity to Streptococcus pneumoniae: prevalence, persistence, and response to re-vaccination. J. Infect. Dis. 154:245-256. [DOI] [PubMed] [Google Scholar]
- 64.Nahm, M. H. (ed.) 2004. Opsonophagocytosis protocol [Online.] http://www.vaccine.uab.edu.
- 65.Nahm, M. H., D. E. Briles, and X. Yu. 2000. Development of a multi-specificity opsonophagocytic killing assay. Vaccine 18:2768-2771. [DOI] [PubMed] [Google Scholar]
- 66.Nauseef, W. M. 1989. Ontogeny of phagocytes, p. 1-23. In M. S. Klempner, B. Styrt, and J. Ho (ed.), Phagocytes and disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 67.Nishino, T., N. Kaise, Y. Sindo, N. Nishino, T. Nishida, S. Yasuda, and Y. Masui. 1991. Promyelocytic leukemia-cell line, HL-60, produces human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 181:323-330. [DOI] [PubMed] [Google Scholar]
- 68.Ohyashiki, K., J. H. Ohyashiki, and A. A. Sandberg. 1986. Cytogenetic characterization of putative human myeloblastic leukaemia cell lines (ML-1, -2, and -3): origin of the cells. Cancer Res. 46:3642-3647. [PubMed] [Google Scholar]
- 69.Osborne, J. M., G. W. Chacko, J. T. Brandt, and C. L. Anderson. 1994. Ethnic variation in frequency of an allelic polymorphism of human Fc gamma RIIa determined with allele specific oligonucleotide probes. J. Immunol. Methods 173:207-217. [DOI] [PubMed] [Google Scholar]
- 70.Patzke, S., M. Lindeskog, E. Munthe, and H. C. Aasheim. 2002. Characterization of a novel human endogenous retrovirus, HERV-H/F, expressed in human leukemia cell lines. Virology 303:164-173. [DOI] [PubMed] [Google Scholar]
- 71.Polge, C., A. U. Smith, and A. S. Parkes. 1949. Revival of spermatazoa after vitrification and dehydration at low temperatures. Nature 164:666. [DOI] [PubMed] [Google Scholar]
- 72.Romero-Steiner, S., C. Frasch, N. Concepcion, D. Goldblatt, H. Käyhty, M. Väkeväinen, C. Laferriere, D. Wauters, M. H. Nahm, M. F. Schinsky, B. D. Plikaytis, and G. M. Carlone. 2003. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 10:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero-Steiner, S., D. Libutti, L B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rovera, G., D. Santoli, and C. Damsky. 1979. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with phorbol diester. Proc. Natl. Acad. Sci. USA 76:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryynänen, M., K. Jousimies, N. Nikkanen, H. Käyhty, and N. Ekström. 2004. Multiplexed and single-colour flow cytometric opsonophagocytosis assays for detection of functional pneumococcal antibodies in infant sera, p. 230. In Proceedings of the 4th International Symposium on Pneumococci and Pneumococcal Diseases. KTL, National Public Health Institute, Helsinki, Finland.
- 76.Salvi, H., Y. Aalto, B. Nagy, S. Knuutila, and S. Pakkala. 2002. Gene expression analysis of 1,25(OH)2D3-dependent differentiation of HL-60 cells: a cDNA array study. Br. J. Hematol. 118:1065-1070. [DOI] [PubMed] [Google Scholar]
- 77.Sanders, L. A. M., J. G. J. van de Winkel, G. T. Rijkers, M. M. Voorhorst-Orgink, M. de Haas, P. J. A. Capel, and B. J. M. Zegers. 1994. Fcγ receptor IIa (CD 32) heterogeneity in patients with recurrent bacterial respiratory tract infections. J. Infect. Dis. 170:854-861. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro, E. D., A. T. Berg, R Austrian, D. Schroeder, V. Parcells. A. Margolis. R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 79.Shellhaas, J. L., and S. H. Zuckerman. 1995. In vitro detection of apoptotic stimuli by use of the HL-60 myeloid leukemic cell line. Clin. Diagn. Lab. Immunol. 2:598-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skubitz, K. M., Y.-S. Zhen, and J. T. August. 1982. Dexamethasone synergistically induces chemotactic peptide receptor expression in HL-60 cells. Blood 59:586-593. [PubMed] [Google Scholar]
- 81.Sundstrom, C., and K. Nilsson. 1976. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17:565-577. [DOI] [PubMed] [Google Scholar]
- 82.Tarella, C., D. Ferrero, E. Gallo, G. L. Pagliardi, and F. W. Ruscetti. 1982. Induction of differentiation of HL-60 cells by dimethylsulfoxide: evidence for a stochastic model not linked to the cell division cycle. Cancer Res. 42:445-449. [PubMed] [Google Scholar]
- 83.Tellado, J. M., and N. V. Christou. 1993. Activation state of polymorphonuclear leukocytes in surgical patients: characterization of surface receptor expression. Surgery 113:624-630. [PubMed] [Google Scholar]
- 84.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomonaga, M., D. W. Golde, and J. C. Gasson. 1986. Biosynthetic (recombinant) human granulocyte-macrophage colony-stimulating factor: effect on normal bone marrow and leukemia cell lines. Blood 67:31-36. [PubMed] [Google Scholar]
- 86.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 87.Väkeväinen, M., W. Jansen, E. Saeland, I. Jonsdottir, H. Snippe, A. Verheul, and H. Käyhty. 2001. Are opsonophagocytic activities of antibodies in infant sera measured by different pneumococcal phagocytosis assays comparable? Clin. Diagn. Lab. Immunol. 8:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valtieri, M., G. Boccoli, U. Testa, C. Barletta, and C. Peschle. 1991. Two-step differentiation of AML-193 leukemic line: terminal maturation is induced by positive interaction of retinoic acid with granulocyte colony-stimulating factor (CSF) and vitamin D3 with monocyte CSF. Blood 77:1804-1812. [PubMed] [Google Scholar]
- 89.Van de Winkel, J. G. J., and C. L. Anderson. 1991. Biology of human immunoglobulin G Fc receptors. J. Leukoc. Biol. 49:511-524. [DOI] [PubMed] [Google Scholar]
- 90.Vidarsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]
- 91.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and N. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiener, E., R. A. Dellow, F. Mawas, and C. H. Rodeck. 1996. Role of Fc gamma RIIa (CD32) in IgG anti-RhD-mediated red cell phagocytosis in vitro. Transfus. Med. 6:235-241. [DOI] [PubMed] [Google Scholar]
- 93.Wilkestein, J. A., M. R. Smith, and H. S. Shin. 1975. The role of C3 as an opsonin in the early stages of infection. Proc. Soc. Exp. Biol. Med. 149:397-401. [DOI] [PubMed] [Google Scholar]
- 94.Williams, J. H., Jr., M. V. Pahl, D. Kwong, J. Zhang, D. Hatakeyama, K. Ahmad, M. Naderi, M. Kim, and N. Vaziri. 2003. Modulation of neutrophil complement receptor 3 expression by pneumococci. Clin. Sci. 104:615-625. [DOI] [PubMed] [Google Scholar]
- 95.Xing, M., P. L. Wilkins, B. K. McConnell, and R. Mattera. 1994. Regulation of phospholipase A2 activity in undifferentiated and neutrophil-like HL-60 cells. J. Biol. Chem. 269:3117-3124. [PubMed] [Google Scholar]
- 96.Yu, X., Y. Sun, C. Frasch, N. Concepcion, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zieglerheitbrock, H. W. L, E. Thiel, A. Futterer, V. Herzog, A. Wirtz, and G. Riethmuller. 1988. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 41:456-461. [DOI] [PubMed] [Google Scholar]