Abstract
In rodent models, acoustic exposure too modest to elevate hearing thresholds can nonetheless cause auditory nerve fiber deafferentation, interfering with the coding of supra-threshold sound. Low-spontaneous rate nerve fibers, important for encoding acoustic information at supra-threshold levels and in noise, are more susceptible to degeneration than high-spontaneous rate fibers. The change in auditory brainstem response (ABR) wave-V latency with noise level has been shown to be associated with auditory nerve deafferentation. Here, we measured ABR in a forward masking paradigm and evaluated wave-V latency changes with increasing masker-to-probe intervals. In the same listeners, behavioral forward masking detection thresholds were measured. We hypothesized that 1) auditory nerve fiber deafferentation increases forward masking thresholds and increases wave-V latency and 2) a preferential loss of low-SR fibers results in a faster recovery of wave-V latency as the slow contribution of these fibers is reduced. Results showed that in young audiometrically normal listeners, a larger change in wave-V latency with increasing masker-to-probe interval was related to a greater effect of a preceding masker behaviorally. Further, the amount of wave-V latency change with masker-to-probe interval was positively correlated with the rate of change in forward masking detection thresholds. Although we cannot rule out central contributions, these findings are consistent with the hypothesis that auditory nerve fiber deafferentation occurs in humans and may predict how well individuals can hear in noisy environments.
Keywords: Auditory brainstem response, Forward masking, cochlear synatopathy, hidden hearing loss
1. Introduction
Listening in everyday acoustic scenes can be challenging, even for listeners who have audiometrically normal hearing thresholds (NHT). Many perceptual attributes of natural sounds (e.g. timbre, location) rely on reliable coding of temporal information. Acoustic environments typically contain competing sound sources and reverberant energy which degrade the temporal structure of the sound reaching a listener’s ear. This degradation may render spatial information about a single source diffuse and make speech less intelligible (Stellmack et al., 2010; Jørgensen and Dau, 2011).
The convergence of multiple auditory nerve fibers (ANFs) is vital in multi-source acoustic scenes as it underlies the enhancement in the fidelity of temporal coding at higher nuclei along the auditory pathway (Joris et al., 1994). A reduction in the ANF population reduces this temporal coding fidelity. Growing evidence in animal models has demonstrated that acoustic overexposure and early aging can damage afferent synapses without elevating thresholds in quiet (Kujawa and Liberman, 2009; Furman et al., 2013; Liberman and Liberman, 2015; Schmiedt et al., 1996; Makary et al., 2011; Sergeyenko et al., 2013). Such damage is invisible to traditional clinical tests. ANFs with low-spontaneous rates (low-SR: SR < 20 spikes/s) are particularly susceptible to deafferentation (i.e., reduction of the AN population) (Schmiedt et al., 1996; Furman et al., 2013; Bourien et al., 2014; Liberman and Liberman, 2015). Model simulations and data suggest that ANF deafferentation, often referred to as cochlear synaptopathy, degrades temporal coding (Lopez-Poveda and Barrios, 2013; Shaheen et al., 2015; Chambers et al., 2016). The degradation of temporal encoding of sound likely leads to deficits in sound localization and stream segregation, resulting in perceptual difficulties when trying to understand speech in challenging acoustic settings (Ruggles et al., 2011). Furthermore, compared to high-spontaneous rate (high-SR) ANFs, low-SR fibers are more robust to masking (Costalupes et al., 1984; Costalupes, 1985; Young and Barta, 1986) and more strongly synchronized to amplitude-modulations in the stimulus at moderate to high sound levels (Joris and Yin, 1992). Thus, a selective low-SR synaptopathy would further increase the likelihood of perceptual difficulties in processing supra-threshold sound, even though it likely has no effect on thresholds.
Indeed, NHT listeners show large individual differences in behavioral measures of temporal coding, such as interaural timing difference and amplitude modulation sensitivity. These measures correlate with physiological brainstem measures affected by ANF deafferenation (Plack et al., 2014; Bharadwaj et al., 2015; Mehraei et al., 2016). Although the evidence is not conclusive, some studies have shown that greater noise exposure seems to correspond to a smaller amplitude of the AN-generated ABR wave-I (Stamper and Johnson, 2015a,b; Liberman et al., 2016), consistent with the effects of cochlear synaptopathy on ABR wave-I in rodents. Still, others do not find this link (Prendergast et al., 2016; Guest et al., 2016). The evidence in these studies suggests that synaptopathy may underlie the variations in hearing ability among NHT listeners. A test that quantifies cochlear synaptopathy and relates this impairment to speech intelligibility will have significant implications for human health and will influence how we test for this previously unknown form of noise-induced hearing loss in humans.
A recent study suggests that cochlear synaptopathy affects the latency change of ABR wave-V in noise in mice (Mehraei et al., 2016). The same study shows that in both humans and mice, the ABR wave-V latency in noise is related to ABR wave-I amplitude and that the individual differences in the size of the effect of noise on human ABR wave-V latency was a significant predictor of a measure of temporal processing. ABR wave-V is a robust clinical measure in humans and can be recorded at low stimulus levels and in background noise. Thus, it may be a good candidate for a potential measure of cochlear synaptopathy.
Here, we investigated whether ABR wave-V latency in non-simultaneous masking is affected by cochlear synaptopathy in young NHT listeners and if so, whether we can tease apart the selective loss of low-SR ANFs. We focused on the recovery of the ABR wave-V latency in forward masking, where the response to a stimulus (probe) is decreased by the presence of a preceding stimulus (masker). Forward masking has been assumed to arise in part because of depletion of synaptic vesicles by the masker, limiting the number of vesicles available to respond to the probe at the level of the AN (Harris and Dallos, 1979). The preceding masker not only reduces the AN response/ABR wave-I amplitude but increases the ABR wave-V latency as illustrated in Fig. 1B and C. As the delay between the masker and the probe increases, the ABR wave-I amplitude and the ABR wave-V latency recover to control (i.e., no preceding masker). It might be possible to disentangle the selective loss of low-SR ANFs using this measure because of the systematic difference in the adaptive properties of low vs. high-SR ANFs. Specifically, animal studies show that low-SR fibers have a longer recovery time to prior stimulation (>100ms) than that of high-SR fibers (<100ms) (Relkin and Doucet, 1991). There is evidence of low- and high-SR contribution to the recovery of the compound action potential (CAP)/ABR wave-I amplitude from forward masking in humans and animals (Relkin and Doucet, 1995; Murnane et al., 1998). The recovery of the CAP can be modeled by two separate exponential functions that characterize the fast and slow component of the recovery time course. A selective loss of low-SR fibers with age has been shown to yield a faster recovery of the CAP (Schmiedt et al., 1996).
Figure 1.
Forward masking is defined as a decreased probe response, depicted by a reduced CAP (B), following a preceding masker. At short masker to probe intervals (MPIs), the ABR wave-V latency is delayed relative to the control (no preceding masker) as illustrated here in A and C. As the gap between the masker and the probe increases, the ABR wav-I amplitude increases (B) and wave-V latency decreases (C). Preferential deafferentation of low-SR fibers (dashed line in B and C) would hypothetically cause a faster recover of the ABR-I amplitude (B) and wave-V latency (C).
Although changes to CAP/ABR wave-I amplitude in forward masking could prove useful in teasing apart the loss of low-SR fibers, they are difficult to obtain and quantify reliably in humans. Thus, we focused on the change of the ABR wave-V latency in forward masking as a function of the masker to probe interval (MPI). In forward masking, as the probe-elicited ABR wave-I amplitude increases with increasing MPI, the wave-V latency subsequently decreases (Kramer and Teas, 1982; Burkard and Hecox, 1987; Boettcher et al., 1996; Walton et al., 1999). We propose that two distinct properties of low- vs. high-SR fibers affect forward masking ABR: 1) the difference between low- and high-SR fibers’ recovery time to prior stimulation and 2) the resistance of low-SR fibers to noise. We hypothesize that the low-SR contribution to forward masking slows down the recovery of the ABR wave-I amplitude and subsequently affects the recovery of wave-V latency, as illustrated in Fig. 1C. Furthermore, the contribution of low-SR fibers may also affect the absolute shift in wave-V latency and thresholds in forward masking. We hypothesize that at small MPIs, the absolute wave-V latency shift and behavioral thresholds may be larger with low-SR synaptopathy (Fig. 1C) because high-SR fibers may not respond with good fidelity following a suprathreshold noise masker. Deafferentation of low-SR fibers may yield a faster recovery of the probe-elicited ABR wave-I amplitude and thereby produce a larger decrease in ABR wave-V latency with increasing MPI (Fig. 1B and C). Indeed, there is some evidence that older NHT listeners and aged animals have greater wave-V latency in forward masking at short MPIs, in line with this hypothesis (Boettcher et al., 1996; Walton et al., 1999). In addition, relative to high-SR fibers, low-SR fibers have higher thresholds, larger dynamic ranges, smaller effective response areas (narrower bandwidths), and are better able to preserve timing information and amplitude modulation (see review in Schmiedt et al., 1996, (Schmiedt et al., 1996)). These characteristics may provide low-SR fibers with an increased resistance to the effects of masking. Loss of low-SR fibers may thus increase perceptual forward masking detection thresholds. These hypothesized effects should be strongest at short MPIs where there is contribution from both high- and low-SR fibers.
In a cohort of young NHT listeners, we measured ABRs in forward masking. To determine whether differences in ABR wave-V latency predict perceptual measures related to temporal coding and speech intelligibility in noise, we chose to measure forward masking behavioral thresholds, a correlate of speech-recognition in interrupted noise (Dubno et al., 2003). Moreover, in an effort to better understand the effects of forward masking in the AN, we present AN model simulations of forward masking. We hypothesize that low-SR synaptopathy in the model should 1) yield a larger reduction of the CAP at short MPIs compared to at long MPIs and 2) cause the CAP to recover more quickly with increasing MPI. We propose that, compared to when there is no deafferentiation, the effects of low-SR fiber synaptopathy at the level of the AN will translate to 1) a larger shift in ABR wave-V latency at short MPIs compared to longer MPIs and 2) an increase in the shift of the ABR wave-V latency with MPI.
2. Experimental Methods
2.1. Apparatus
All measures were obtained with subjects seated in an acoustically and electrically shielded booth (double-walled IAC booth, Lyngby, Denmark). For passive forward masking ABR measures, subjects watched a silent, captioned movie of their choice, ignoring the acoustic stimuli. A desktop computer outside the booth controlled all aspects of the experiment, including triggering, sound delivery and storing data. The stimuli were presented via Fireface UCX (RME, Haimhausen Germany) and triggers were sent from a RME ADI-8 trigger box (RME, Haimhausen Germany). A headphone driver presented sound through ER-2 insert phones (Etymotic, Elk Grove Village, IL). All sounds were digitized at a sampling rate of 44.1kHz. For the behavioral experiments, subjects responded using a touch screen in the booth. All tests were measured in the left ear with the exception of one subject.
2.2. Subjects
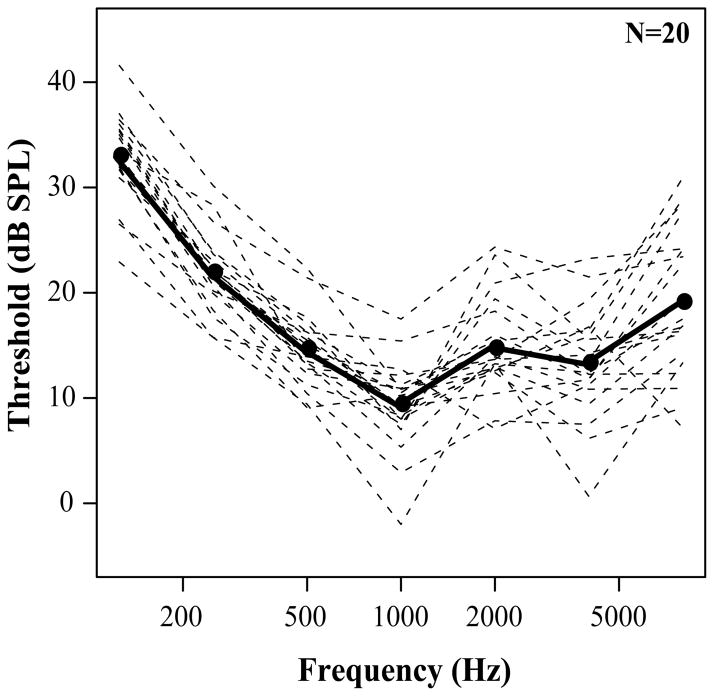
Twenty (four female) subjects, aged from 20–30 years (mean=25.26), were recruited from Technical University of Denmark in Lyngby, Denmark. All participants had pure-tone thresholds better than 20 dB hearing level (HL) in the tested ear at octave frequencies between 0.25 and 8 kHz, as shown in Fig. 2. Subjects provided informed consent in accordance with protocols established at Technical University of Denmark.
Figure 2.
Pure-tone thresholds expressed in dB SPL for each participant. Solid line represents mean threshold at each tested frequency. Dashed lines depict individual pure-tone thresholds.
2.3. Pure-tone Thresholds
Pure-tone thresholds were measured using a three-interval alternative forced choice task (AFC; the psychophysical-measurement package for MATLAB, University of Oldenburg, Germany). Thresholds were collected in the tested ear at octave frequencies between 0.125 and 8 kHz. The duration of the tones was 100 ms. On each trial, the presentation of each interval was indicated visually by highlighting the interval button on the screen. Listeners were asked to identify which of the three intervals contained the pure-tone signal. Intervals were separated by 201 ms and trials were separated by 660 ms. A non-parametric, 2-down 1-up adaptive procedure was used to obtain thresholds (Levitt, 1971). The pure-tone level started at 50 dB sound pressure level (SPL) and was reduced by 5, 2, and then 1 dB in the tracking procedure to reach threshold. The step size was changed after each upper reversal. Threshold was defined as the mean level at the last six reversals. This measure was repeated twice for each tone. If thresholds differed by more than 10 dB across repetitions, an additional threshold was measured. The threshold that was a standard deviation away from the mean of the three repetitions was not used in the analysis.
2.4. Forward masking behavioral experiment
Forward masking detection thresholds were also measured using the AFC package. A 100 ms long broadband noise masker was presented at 35 and 70 dB SPL. The noise was ramped with a 20 ms cos2 rise-decay to minimize the use of onset cues. The bandwidth of the noise was limited by the sampling frequency (i.e., 44.1 kHz) and the frequency response of the ear phones. A flat-spectrum, broadband, “synchronized” chirp spanning the frequency range of 0.08–20 kHz was used as the probe (Dau et al., 2000). This chirp is designed to account for the group delay observed in the traveling wave along the cochlea by first presenting low- and then high-frequency components in time (Dau et al., 2000). As illustrated in Fig. 1, the probe was presented following the masker at MPI (i.e. offset of masker to onset of probe) of 20, 40, 72, 132, 168, and 201 ms. On each trial, the masker followed by the probe was presented randomly in one of three intervals. The other two intervals contained only the masker. Listeners were asked to identify the interval in which the probe was present. For each MPI and masker level condition, the probe level was varied to obtain detection threshold. The probe level started at 70 dB peak equivalent (pe) SPL and was adaptively changed using the same step size as in the pure-tone threshold procedure. The conditions were randomly presented in blocks and two repetitions of each condition were implemented. A third repetition was acquired if thresholds differed by more than 10 dB. Any threshold that was one standard deviation away from the mean across three repetitions was discarded.
Additionally, chirp thresholds (i.e., without a preceding masker) were measured using the same experimental design. The subjects were asked to identify the chirp in one of three intervals on each trial. The chirp level started at 50 dB peSPL and was varied adaptively similar to the other experiments in this study.
2.5. Forward masking ABR
Forward masking ABRs were recorded using a five-channel EEG system (Biosemi Active II system, Amsterdam, Netherlands). The five channel configuration included channels, Pz, Cz, Fz on the 32-channel cap along with the left and right mastoids. ABRs were measured using the same masker and probe as in the forward masking behavioral task. However, in contrast to the forward masking detection task, the probe level was fixed at 90 dB peSPL to elicit a strong response from low- and high-SR fibers. A repetition rate of 2 Hz, measured from the onset of the masker in the previous trial to the onset of the masker in the current trial, was used to limit effects of adaptation and fatigue in the ANFs. In addition, a 20 ms jitter was introduced in the repetition rate to avoid the accumulation of any stationary interference including the 50 Hz power-line noise.
Forward masking ABRs were recorded for MPIs of 20, 40, and 201 ms at two different masker levels (35 and 70 dB SPL) yielding a total of six stimulus conditions. 1500 trials were presented in random order per stimulus condition. Additionally, ABRs to the chirp alone (without a preceding masker) were recorded as the control condition using the same chirp level and repetition rate. The recording session took approximately 2 hours. The channels were referenced to the average of the mastoid channels. The ABR wave-V was best identified using the Cz to average mastoids; thus, this configuration was used for wave-V latency analysis.
The recorded data, sampled at 16.384 kHz, were filtered between 0.1 and 2 kHz. Power line noise (50 Hz and harmonics) was removed by applying Thomson’s regression method as implemented in the Chronux toolbox (Bokil et al., 2010). The filtered data were then time-epoched from −10 to 10 ms relative to the offset of the chirp. Bad trials were removed by analyzing the distribution of the overall amplitude across trials. For each subject, the number of good trials retained for analysis was equalized across conditions. Peak latency of wave-V was identified using visual overlay cursors on a computer screen. The latency peak was confirmed using an automated procedure where the max peak was defined in the ABR. The change in wave-V latency was defined as the difference in the wave-V latency across tested MPIs.
2.6. Forward masking recovery function
As seen in past studies, the forward-masking recovery function consists of a fast and slow time component and can be modeled as a sum of two exponential functions (Relkin and Doucet, 1995; Murnane et al., 1998). In the forward masking detection task, the fast component was defined as thresholds for MPI< 72 ms. In our forward masking ABR, the fast component of the wave-V latency recovery function was defined by considering measurements for MPI< 40 ms as we did not record ABR at a 72 ms gap. No significant differences in detection thresholds at 132, 168 and 201 ms MPI were found (using t-tests); thus, these MPIs defined the slow component for the forward-masking function. In both experiments, the fast component of the forward masking recovery function likely had contributions from both SR fibers groups because of the high presentation level of the chirp.
To derive a single metric of forward masking, for each listener, detection thresholds at MPIs between 20–72 ms were fitted using a power law function . This fit yielded a single exponential constant for the fast component of the forward masking recovery function that describes how quickly the forward masking detection thresholds decreased with MPI. We opted to compare this fit to the change in peak ABR wave-V latency from 20 to 40 ms MPI because we were limited to the few MPI conditions measured.
2.7. Forward masking simulations in the auditory nerve
A transmission line auditory model (Verhulst et al., 2015) was used for the simulations as it captures the across-frequency mechanics of the cochlea, important for broadband signals. Details of the model can be found in Verhulst et al. (2015) (Verhulst et al., 2015). In this model, the stimulus pressure passes through a 4 kHz low-pass and 0.6 kHz high-pass filter with a pass-band gain that matches those of the human middle ear transfer function (Puria, 2003). The filtered stimulus enters a nonlinear transmission-line model of the cochlear partition (Verhulst et al., 2012), after which basilar membrane velocity is translated to inner hair cell (IHC) bundle deflection. The deflections at each characteristic frequency (CF) are passed through a compressive nonlinear function and a second-order 1 kHz cutoff low-pass filter to account for the degradation of temporal fine structure phase locking. The IHC output drives the model for the IHC-AN synapse, which is described by the time-varying three-store diffusion model of Westerman and Smith (1988, Westerman and Smith (1988)). AN thresholds were rendered SR dependent in the model, which yielded AN thresholds that were 20 dB higher for a low-SR fiber of 1 sp/s than for a high-SR fiber of 100 sp/s, in agreement with cat AN recordings (Liberman, 1978). Further, to capture the onset responses of different fiber population types to repeated stimuli, the ratio between the amplitudes of the rapid and short-term exponentials in the simulated instantaneous firing rate was set to the fibers’ SR. This yields slower recovery for low-SR fibers than for high-SR fibers without influencing the instantaneous firing rate amplitude (Verhulst et al., 2015).
To simulate forward masking, a 100 ms broadband noise followed by a “synchronized” flat-spectrum chirp was used (Dau et al., 2000). The masker (i.e., broadband noise) was presented at 70 dB SPL while the chirp level was kept constant at 90 dB peSPL. The chirp contained frequency components ranging from 0.08–20 kHz. Further, the simulations were implemented using MPIs of 0, 10, 20, 40, 100, 500, 1000 ms with a control condition without a preceding masker. All stimuli were generated at a 100 kHz sampling rate.
The response of the different SR fibers in the model were weighted at each CF according to the known population size. Here, we assigned 47% of the population of ANFs as low-SR (this includes medium-SR) and the remaining 53% as high-SR ANFs. This distribution is based on the ANF recordings of Furman et al. (2013) in guinea pigs (Furman et al., 2013). At each CF, the weighted instantaneous firing rates of AN fibers are summed to yield the CAP.
To examine whether peripheral change affects the forward-masking time course of the AN response, we simulated hearing impairment (HI) in the model by reducing the compression and broadening the tuning of the cochlear channels. In this model, compression and cochlear tuning are linked via the basilar membrane (BM) admittance pole. This pole controls the value of the damping, stiffness, and feedback terms at low stimulus levels. This means that higher pole values will yield more damping and wider cochlear filters. To simulate the loss of cochlear compression, the BM admittance pole was changed from a value of 0.06 to a value of 0.1, leading to auditory filters with a Q equivalent rectangular bandwidth (ERB) of 5.5 (Verhulst et al., 2012). As changing this pole affects both the stiffness and damping of the BM, widened auditory filters are accompanied by overall lower BM displacement levels than would be obtained for filters with higher QERB values.
2.8. Statistical tests
Unless otherwise specified, statistical inference was performed by fitting linear regression models to the data and adopting a model comparison approach (Baayen et al., 2008). Fixed-effects terms were included for the various experimental factors whereas subject-related effects were treated as random. In order to not over-parameterize the random effects, models were compared with and without each term using the Akaike information criterion (Pinheiro and Bates, 2000). All model coefficients and covariance parameters were estimated using restricted maximum likelihood as implemented in the lme4 library in R. An F approximation for the type-II scaled Wald statistic was employed to make inferences about the fixed effects (Kenward and Roger, 1997): this approximation is more conservative in estimating Type I error than the Chi-squared approximation and performs well even with complex random-effects covariance structures (Schaalje et al., 2002). The p-values and F-statistics based on this approximation are reported.
3. Results
3.1. Forward masking ABR and detection thresholds
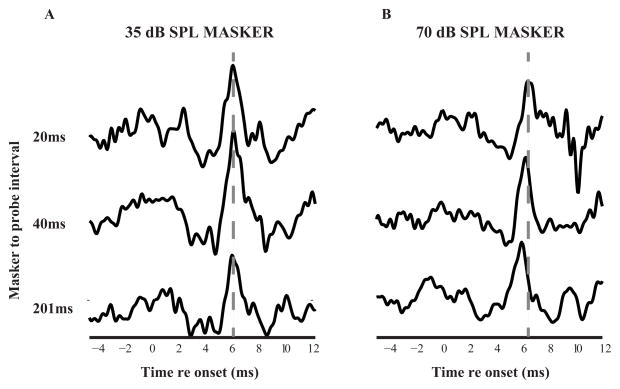
Fig. 3 shows a sample ABR recording from one subject. When the masker is presented at 35 dB SPL (Fig. 3A), wave-V latency changes very little, if at all, with increasing MPI. However, at a masker level of 70 dB SPL (Fig. 3B), there is a monotonic decrease of the wave-V latency with increasing MPI. The ABR wave-V latency and behavioral results for the two masker levels for all subjects are summarized in Fig. 4. Each line represents an individual listener. All data are normalized to the control (i.e., condition with preceding noise-condition without preceding noise) to reduce differences in external factors, like head geometry and gender, that can affect detection thresholds and ABR. Peak ABR wave-V latency (Fig. 4A and C) results are quantified as the amount of latency shift relative to the control condition (without the preceding masker). Similarly, the forward masking detection thresholds (Fig. 4B and D) are represented as the shift in thresholds in the presence of a preceding masker vs. the condition without a masker. Control ABRs could not be measured in four of the subjects due to subject availability. Hence, the data from only 16 subjects are shown.
Figure 3.
Sample ABR recording in forward masking for a subject at both 35 dB (left panel) and 70 dB SPL (right panel) masker level.
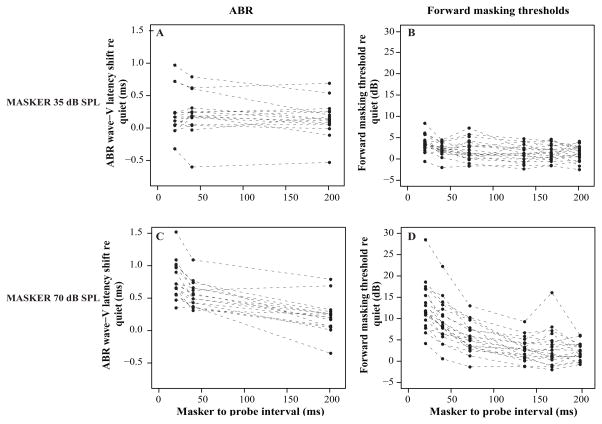
Figure 4.
ABR wave-V latency shifts relative to control in the presence of a 35 dB SPL (A) and 70 dB SPL (C) preceding masker as a function of MPI. Forward masking detection thresholds relative to control with a 35 dB SPL (B) and 70 dB SPL (D) masker at different MPIs. ABR wave-V latency is defined as the peak latency of the wave. Here, this latency is plotted as the shift in peak timing relative to the control condition, in which there is no preceding masker. Similarly, forward masking thresholds are shown as the amount of forward masking relative to thresholds found in the absence of a preceding masker (control). Each line presents one individual listener.
Across all subjects, at a masker level of 35 dB SPL, little effect of forward masking was observed on either the ABR wave-V latency (Fig. 4A) or forward masking thresholds (a level at which low-SR contributions to the response are likely modest; Fig. 4B). In contrast, we observed a strong effect of forward masking in the ABR and detection thresholds when the masker level was increased to 70 dB SPL (a level at which low-SR contributions to the response should be relatively strong; Fig. 4C–D). At this level, large individual differences were observed in the ABR wave-V latency shift and the detection thresholds, especially at MPIs < 72 ms. Further, the forward-masking recovery times for the ABR wave-V latency and detection thresholds varied greatly from subject to subject.
3.2. The relationship between ABR wave-V latency and behavioral detection thresholds
To evaluate the relative contribution of different factors to the behavioral forward masking threshold recovery function at short MPIs ( < 72 ms), a linear regression model was implemented. The model included gender, age and the shift of the ABR wave-V peak latency from 20 to 40 ms MPI as predictor terms. An interaction term between age and ABR wave-V latency shift from 20 to 40 ms was also included as a predictor variable in the model. Additionally, we wanted to examine the contribution of the pure-tone (PT) thresholds measured for the seven tested frequencies since broadband stimuli were used to elicit the ABR. However, these thresholds were correlated with each other. To disentangle their respective contribution and de-correlate these variables, principle component analysis was used. The first two components of this analysis, which accounted for roughly 75% of the variance in the pure-tone thresholds, were also used as predictor terms in the model. An F approximation for the type-II scaled Wald statistic yielded a main effect of ABR wave-V latency shift from 20 to 40 ms MPI as a significant predictor for the forward masking threshold recovery function at short MPIs [F(1,12)=5.328, p=0.039]. Other predictors were not significant (Age: [F=0.0003, p=0.987], Gender: [F=0.2863, p=0.602], PT component 1: [F=0.3669, p=0.556], PT component 2: [F=0.3896, p=0.544], Age*ABR shift: [F=0.048, p=0.536]).
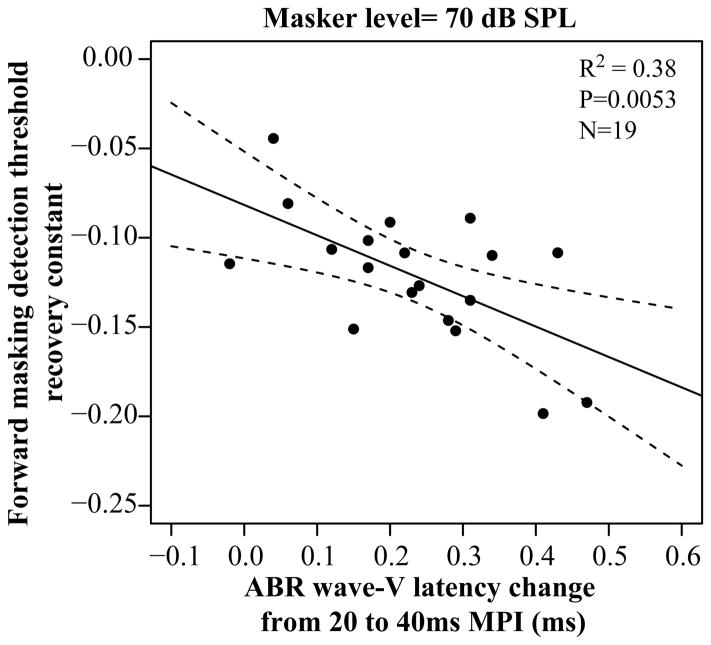
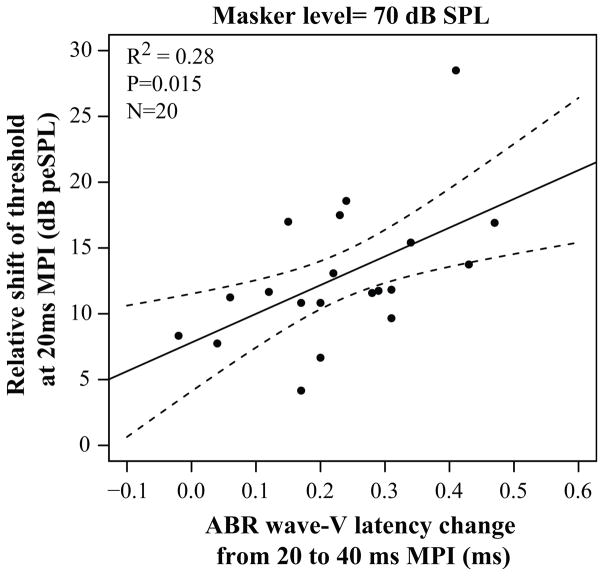
As shown in Fig. 5, there was a significant relationship between the amount of change in ABR wave-V latency and forward masking detection thresholds at short MPIs [r=0.6164, p=0.0053]. Listeners with a larger decrease in wave-V latency from 20 to 40 ms MPI also exhibited a greater decrease in forward masking detection thresholds at short MPIs. This relationship remained significant when simply comparing the change in ABR wave-V latency to the change in forward masking detection thresholds from 20 to 40 ms MPI [r=0.6612, p=0.005].
Figure 5.
Comparison of the change in ABR wave-V latency from 20 to 40 ms MPI and the recovery of the forward masking thresholds at MPI< 72ms. As indicated by the arrow, a more negative time constant depicts a faster recovery/change in forward masking detection thresholds with MPI.
Further, the listeners with a larger decrease in wave-V latency from 20 to 40 ms MPI were affected more by a preceding masker at the 20 ms MPI (i.e., they had higher thresholds at this short MPI, Fig. 6) [r=0.5348, p=0.0151]. Interestingly, the listeners who were affected more by the preceding masker at 20 ms MPI also showed greater shifts in detection thresholds at the longest tested MPI (201 ms) relative to the control condition [r=0.6, p=0.0052, N=20]. Similarly, in the ABR measurements listeners with a larger delay in wave-V latency at 20 ms MPI also exhibited a larger decrease in wave-V latency from 20 to 40 ms MPI.
Figure 6.
The change in ABR wave-V latency from 20 to 40 ms MPI was also a significant predictor of the amount of masking on forward masking thresholds at a 20 ms MPI. The larger the decrement of forward masking thresholds at a 20 ms MPI (higher threshold), the larger the change in ABR wave-V latency.
3.3. Auditory nerve simulations
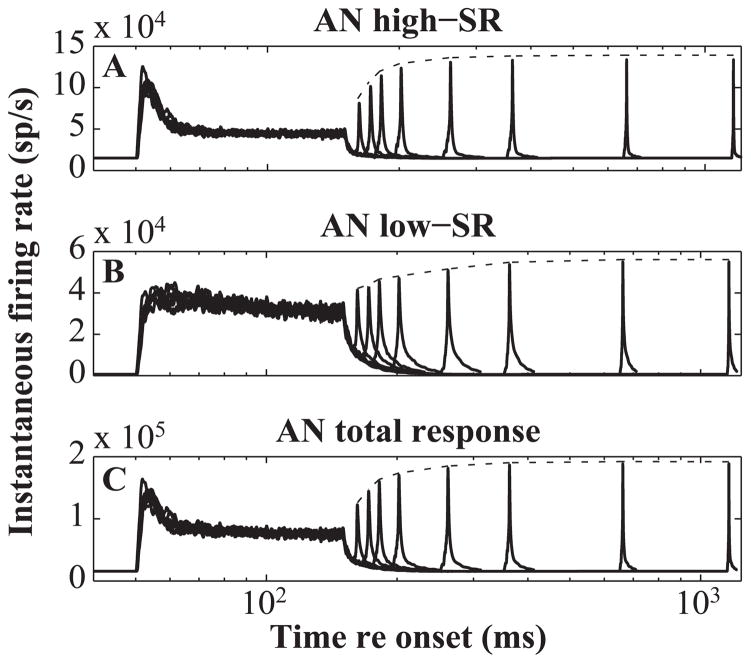
Fig. 7 presents the AN model response in forward masking for high-SR fibers only (A), low-SR fibers only (B) and all fibers combined (C). Consistent with physiological studies, the probe-elicited AN response of the high-SR fibers grows more rapidly with increasing MPI than does the low-SR fibers (Relkin and Doucet, 1991). Additionally, the low-SR response is less affected by a preceding noise than the high-SR response (Fig. 7A, B). The combined AN response (Fig. 7C) tends to be dominated by the high-SR response because the high-SR fibers have a much higher firing rate, overall, than the low-SR fibers. Notice that the AN response is almost fully recovered by 200 ms MPI, consistent with our forward masking experimental data, which generally show a full recovery of the wave-V latency and detection thresholds by 201 ms.
Figure 7.
Simulated forward masking responses of high-SR (A), low-SR (B), and combined (C) ANFs. As MPI increases, the AN probe response increases faster in the high-SR ANFs than in the low-SR ANFs.
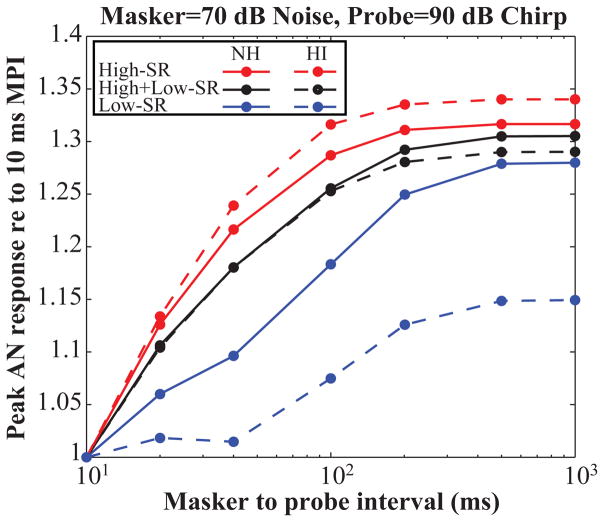
Fig. 8 shows the relative growth of the AN response for the different sets of fibers (i.e. solid lines). When there is a selective loss of low-SR fibers, shown in red, the growth of the AN probe response is relatively faster than for the combined response including both low- and high- SR fibers. In contrast, high-SR synaptopathy, depicted in blue, yields a slower recovery of the AN response.
Figure 8.
Simulated probe-elicited CAP as a function of MPI for different SR fibers for NH (solid lines) and HI (dashed lines) model. The CAP is normalized to the response at a 10ms MPI.
To examine whether peripheral changes affect the forward-masking time course of the AN response, we simulated hearing impairment (HI) in the model by reducing the compression and broadening the tuning of the cochlear channels. This was motivated by studies that have used changes of forward masking detection thresholds as a measure of cochlear compression (Plack and Oxenham, 1998). Shown by the dashed traces in Fig. 8, the reduced cochlear compression and the broader tuning in the HI model accentuate the SR-dependent differences in the CAP growth with MPI: the high-SR fiber CAP grows more quickly whereas the CAP growth of the low-SR fibers is reduced relative to the NH model. However, the combined response of these fibers does not significantly differ from that of the NH model.
4. Discussion
Growing evidence suggests that a portion of the individual variation in humans with NHTs measured both behaviorally and electrophysiologically can be explained by cochlear synaptopathy (Stamper and Johnson, 2015a, b; Bharadwaj et al., 2015; Mehraei et al., 2016; Liberman et al., 2016). The purpose of this study was to investigate whether changes in ABR wave-V latency in forward masking may reflect ANF loss, especially loss of low-SR fibers. This study was motivated by our previous work, where we showed the effects of cochlear synaptopathy on ABR wave-V latency in noise. Here, we aimed to tease apart the selective loss of low-SR ANFs using forward masking by exploiting the differences in the adaptive properties of low and high-SR fibers. The results showed that, in young NHT listeners, there are large individual differences in the recovery of the ABR wave-V latency and detection thresholds in forward masking. We found that individual differences in how ABR wave-V latency changes with increasing MPI are related not only to differences in the recovery of behavioral forward masking detection thresholds but also to how much a listener’s detection threshold is affected by a preceding masker. Listeners with poorer forward masking detection thresholds exhibited a larger change of the ABR wave-V latency as a function of MPI, consistent with our hypothesis that cochlear synaptopathy accounts for some of the individual differences we observed.
4.1. Forward masking and cochlear synaptopathy
On a single-unit level, animal studies have shown differences in how forward masking affects low- and high-SR fibers (Relkin and Doucet, 1991; Furman et al., 2013). Generally, fibers with less than 100 ms recovery time have high-SRs and fibers with greater than 100 ms recovery time have mostly low-SRs (Furman, 2013). The clear separation between SR types would suggest that contributions from different SR types should be separable with gross physiology such as ABR or CAP. Consistent with this view, there is evidence that both low and high-SR fibers contribute to the recovery of ABR wave-I/CAP (Relkin and Doucet, 1995; Murnane et al., 1998).
Loss of or damage to low-SR fibers may 1) increase the effects of masking at very short masker-probe intervals and 2) yield a faster recovery of the AN population response (i.e., ABR wave-I/CAP). Indeed, previous work has shown a faster recovery of the CAP in forward masking with deafferentation of low-SR fibers (Schmiedt et al., 1996). Further, a recent study of noise exposure showed larger effects of forward masking in single-unit recordings in noise exposed animals (Song et al., 2016). Specifically, it was demonstrated that the probe-to-masker response ratio was significantly reduced at short MPIs in both low- and high-SR fibers after noise exposure. Additionally, this ratio changed more with MPI in noise-exposed than in unexposed, control ANFs. These effects were especially pronounced in low-SR ANFs. Although the stimulus configuration in this study was different than what was used in the current experiments (i.e., click pairs), the patterns we observed here are consistent with these earlier studies of cochlear synaptopathy and may be due to noise exposure.
Although we could not obtain reliable recordings of ABR wave-I/CAP in forward masking in this study, our modeling results suggest that there are individual differences in the CAP forward masking recovery function based on the individual differences in the ABR wave-V latency forward masking function. Changes at the level of the AN affect the timing of later auditory pathways, as shown in Mehraei et al. (2016) in humans and animals (Mehraei et al., 2016). Therefore, a greater shift in wave-V latency at short MPIs would translate to increased masking of the CAP at these short intervals, potentially due to the deafferentation or damage of low-SR fibers. Additionally, the large decrease in wave-V latency with increasing MPI suggests a faster recovery of the CAP, consistent with synaptopathy of slowly recovering low-SR fibers. Future work should focus on simultaneously measuring ABR wave-I with wave-V in forward masking paradigms. Recording wave-I will allow for direct comparison of changes in ABR peak amplitude emanating from the auditory nerve (wave-I) and the brainstem (wave-V). This will help to verify that the present results are due to changes in the auditory periphery rather than from more central auditory processing regions.
4.2. Effects of forward masking on ABR wave-V latency
An increase of wave-V latency at short forward-masking intervals was observed in this study. As the forward-masker interval increased, the wave-V latency systematically decreased, almost reaching baseline latency by 201 ms MPI in all but two subjects. A potential reason why the wave-V latency did not fully recover to baseline may be because of slight differences in the stimulus repetition rate of the chirp in the forward masking vs. the control experiment; repetition rate is known to affect the ABR wave-V latency (Burkard and Voigt, 1989; Burkard et al., 1996; Burkard and Sims, 2001): the inter-stimulus interval in the forward masking experiment was defined from onset to onset of the masker. Although this was fixed to 2 Hz, the chirp presentation rate varied slightly as the MPI differed from trial to trial. In contrast, the repetition rate of the chirp in the control experiment was fixed to 2 Hz. Furthermore, potential differences in high-frequency sensitivity (i.e., > 8000 Hz) may also contribute to the recovery rate of the wave-V latency: damage in this region would shift the peak response to more apical cochlear regions along the basilar membrane, thereby introducing a delay. These potential differences in high-frequency cochlear sensitivity may be linked to noise-induced cochlear synaptopathy: recent human studies show a link between noise exposure history and elevated high-frequency thresholds (Liberman et al., 2016; Prendergast et al., 2016). Thus, although our individual differences are likely related to differences in high-frequency thresholds, the high-frequency threshold differences may be due to noise exposure that also leads to synaptopathy of the nerve and eventually hair cell damage.
The pattern of recovery of the ABR wave-V latency observed here is consistent with previous reports in humans (Lasky and Allen, 1982; Kramer and Teas, 1982; Burkard and Hecox, 1987; Lasky, 1993; Walton et al., 1999), gerbils (Boettcher et al., 1996) and mice, using tone-burst maskers and probes (Walton et al., 1995), noise-burst maskers and probes (Boettcher et al., 1996), and noise-burst maskers and click probes (Lasky and Allen, 1982; Kramer and Teas, 1982; Burkard and Hecox, 1987; Lasky, 1993; Walton et al., 1999). As forward-masking recovery time appears to depend on the acoustic characteristics of the probe and masker (Lasky, 1993), a comparison across these different studies of forward masking recovery times is not undertaken here. Although our choice of masker intensities and duration have an affect on wave-V latency, these acoustic characteristics were kept fixed across subjects in the ABR experiment, allowing us to make direct comparisons across experiments here. Indeed, previous work has shown that human ABR recovery of peak latency to click stimuli from a noise forward masker is similar for a range of probe and masker levels as long as the relative level of the probe to the masker is held constant (Lasky and Allen, 1982).
The greater wave-V latency shifts that we observed in the subjects who performed relatively poorly in the forward masking detection task are consistent with results from studies of aging in humans (Walton et al., 1999) and animals (Boettcher et al., 1996; Walton et al., 1995). Walton et al. (1999) reported greater wave-V latency shifts at short MPI in older listeners with NHTs relative to the young NHT group. This effect was observed only in high-frequency regions, where animal studies suggest greater low-SR fiber innervations (Temchin et al., 2008). The greater wave-V latency shifts at short MPIs in the older NHT listeners subsequently yielded larger changes in wave-V latency with MPI compared to the young listeners (Walton et al., 1999).
Boettcher et al. (1996) reported that aged gerbils showed greater ABR wave-IV latency shifts than young-adult gerbils in forward masking paradigms. They argued that the prolongation of ABR wave-IV latency in aged gerbils was the result of changes in the brain of aged animals and not due to peripheral changes, because the CAP showed no age-dependent change in recovery rate. Similarly, middle-aged mice demonstrated greater wave-V latency in forward masking conditions while the CAP showed no such age-dependent latency shift (Walton et al., 1995). This is in contrast with previous work and our current hypothesis that aging, similar to noise exposure, leads to a selective deafferenation of low-SR fibers. It may be that in both of these previous studies, the aged animals had cochlear hearing loss and thus, the peripheral changes may have masked the effects of cochlear synaptopathy on the CAP recovery rate.
4.3. Forward masking detection thresholds
In our cohort of young, NHT subjects, individual differences in how ABR wave-V latency changes with increasing MPI are related not only to differences in the recovery of behavioral forward masking detection thresholds (Fig. 5), but also to how much a listener’s detection threshold is affected by a preceding masker at a short MPI (i.e., cost, Fig. 6). The change in detection thresholds in forward masking is linked to the effect of a preceding masker (“cost”) at short MPIs; greater shifts in detection thresholds at 20 ms MPI were accompanied by a larger change in the forward masking detection thresholds as a function of MPI. Although this may suggest a faster recovery, our analysis suggests that this effect of a preceding masker is consistent across MPIs: if listeners were hurt more by a preceding masker at 20 ms, they also showed larger thresholds at 201 ms MPI. This is similar to previous work on older NHT listeners (Dubno et al., 2002, 2003; Grose et al., 2016) who showed larger effects of forward masking relative to young controls.
Listeners with prolonged forward masking may have a reduced benefit in recognizing speech in a fluctuating masker because of increased forward masking of the speech snippets that occur during the masker minima. Dubno et al. (2003) found an association between speech intelligibility in an interrupted masker and forward masking thresholds. Similarly, another study showed higher speech recognition thresholds and increased forward masking in older listeners (Gifford et al., 2007). Speech recognition could not be measured in this study because subjects were not of the same native language. Nonetheless, the prolonged forward masking thresholds and wave-V latencies observed here in our young NHT listeners may be predictive of speech performance in noisy environments.
The largest individual differences observed here in the psychophysical forward masking measure were at the shortest MPIs. This may reflect the importance of contributions of different SR fibers: at a very short MPI, the probe may be encoded more robustly by low-SR fibers as their response is relatively robust in the presence of masking noise (Costalupes et al., 1984). A loss of these fibers would presumably increase forward masking detection thresholds. Indeed, a model of the auditory periphery closely approximates human psychophysical forward masking data when high- and low-SR types are combined (Meddis and O’Mard, 2005). Additionally, because the low-SR fibers are believed to be a major input to the olivocochlear reflex (Liberman, 1988; Nayagam et al., 2011), which serves an “antimasking” role (Kawase et al., 1993), a loss of these fibers should cause deficits in experiments such as forward masking.
4.4. Other neural correlates of forward masking
Although it may be tempting to explain the forward masking effects in this study in terms of the adaption properties of the AN, there are other neural factors that significantly contribute to the effects seen in forward masking. For instance, forward masking can be observed in cochlear implant patients where hair cell physiology may not be relevant (Lim et al., 1989). Indeed, there is evidence that forward masking thresholds may be related to central (i.e. post-AN) adaptation/integration or in central detection efficiency, rather than individual differences at the level of the AN (Turner et al., 1994). Specifically, the inhibitory networks of the brainstem may significantly contribute to forward masking. It is known that the efferent inhibitory mechanisms, for instance, influence the cochlear nucleus (CN) response to forward masking (Shore, 1998). By recording forward suppression in marmoset inferior colliculus (IC) neurons, Nelson et al. (Nelson et al., 2009) suggested that psychophysical forward masking may arise from forward masking at the level of the IC and may involve inhibitory mechanisms from within the IC or from earlier auditory processing stages (Nelson et al., 2009). Our results may indeed be explained by differences in inhibition at the IC level, especially since the ABR wave-V is generated there (Møller and Jannetta, 1985; Melcher and Kiang, 1996). Increased inhibition in the IC following the masker may reduce the neural response to the probe. This may in turn yield higher thresholds and a delayed neural response, which may be reflected in the wave-V latency.
A more recent study has shown that forward-masking suppression in the CN of the guinea-pig closely resembles forward-masking threshold shifts observed in humans (Winter et al., 2014)). Data from CN lends support to the idea that different ANFs differ in their rate of recovery from forward masking. Specifically, Winter et al. found that low-SR neurons in the CN took longer to recover from forward masking than high-SR neurons, just as in AN studies (Winter et al., 2014). Furthermore, model simulations also suggest that a model of neural adaptation can predict forward masking thresholds (Dau et al., 1996a, b; Oxenham, 2001; Meddis and O’Mard, 2005; Jepsen et al., 2008). Nevertheless, although the results here may stem from post-AN sites, it is reasonable to believe that damage and or synaptoapthy in the AN will affect these central sites responsible for forward masking.
5. Conclusion
Young NHT listeners from the general population vary greatly in their sensitivity to temporal structure in forward masking both perceptually and in the ABR. We find that changes in the ABR wave-V latency in forward masking are related to individual differences in forward masking detection thresholds, a correlate of speech intelligibility in noise. The results may be consistent with the differences in the fidelity with which temporal features are encoded by in very early levels of the neural pathway. However, we cannot confidently rule out the influence of more central sites. Additional experiments are needed to investigate the relationship between forward masking ABR wave-V latency and measures of the auditory nerve.
Highlights.
Auditory brainstem response (ABR) wave-V latency was measured in forward masking
Individual differences in forward masking ABR and detection thresholds were large
Forward masking wave-V latency shifts predict forward masking behavioral thresholds
Model simulations suggest that auditory-nerve loss affects ABR in forward masking
Acknowledgments
This work was supported by National Institute of Deafness and Communication Disorders (R01 DC013825, B. G. Shinn-Cunningham), Erasmus Mundus Auditory Cognitive Neuroscience grant (G. Mehraei) and the Oticon foundation for Centre of Excellence for Hearing and Speech Sciences (T. Dau).
Abbreviations
- ABR
Auditory brainstem response
- NHT
Normal hearing threshold
- AN
Auditory Nerve
- ANFs
Auditory nerve fibers
- low-SR
Low-spontaneous discharge rate
- high-SR
High-spontaneous discharge rate
- MPI
Masker-to-probe interval
- CAP
Compound action potential
- IHC
Inner haircell
- CF
Characteristic frequency
- CN
Cochlear nucleus
- IC
Inferior colliculus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem and Lang. 2008;59(4):390–412. [Google Scholar]
- Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham B. Individual Differences Reveal Correlates of Hidden Hearing Deficits. J Neurosci. 2015;35(5):2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher F, Mills J, Swedloff J, Holley B. Auditory evoked potentials in aged gerbils: responses elicited by noises separated by a silent gap. Hear Res. 1996;102(1):167–178. doi: 10.1016/s0378-5955(96)90016-7. [DOI] [PubMed] [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: a platform for analyzing neural signals. Journal of neuroscience methods. J Neurosci Meth. 2010;192(1):146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Wang J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophys. 2014;112(5):1025–1039. doi: 10.1152/jn.00738.2013. [DOI] [PubMed] [Google Scholar]
- Burkard R, Hecox K. The effect of broadband noise on the human brain-stem auditory evoked response. III. Anatomic locus. J Acoust Soc Am. 1987;81:1050–1063. doi: 10.1121/1.394677. [DOI] [PubMed] [Google Scholar]
- Burkard R, McGee J, Walsh E. Effects of stimulus rate on the feline brain-stem auditory evoked response during development. I. Peak latencies. J Acoust Soc Am. 1996;100(1) doi: 10.1121/1.416209. [DOI] [PubMed] [Google Scholar]
- Burkard R, Sims D. The Human Auditory Brainstem Response to High Click Rates: Aging Effects. Am J Audiol. 2001;10:53–61. doi: 10.1044/1059-0889(2001/008). [DOI] [PubMed] [Google Scholar]
- Burkard R, Voigt H. Stimulus dependencies of the gerbil brainstem auditory evoked response BAER. I. Effects of click level, rate and polarity. J Acoust Soc Am. 1989;85:2514–2525. doi: 10.1121/1.397746. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron. 2016;89(4):867–879. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA. Representation of tones in noise in the responses of auditory nerve fibers in cats. I. Comparison with detection thresholds. J Neurosci. 1985;5(12):3261–3269. doi: 10.1523/JNEUROSCI.05-12-03261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51(6):1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Dau T, Püschel D, Kohlrausch A. A quantitative model of the “effective” signal processing in the auditory system. i. model structure. The Journal of the Acoustical Society of America. 1996a;99(6):3615–3622. doi: 10.1121/1.414959. [DOI] [PubMed] [Google Scholar]
- Dau T, Püschel D, Kohlrausch A. A quantitative model of the “effective” signal processing in the auditory system. ii. simulations and measurements. The Journal of the Acoustical Society of America. 1996b;99(6):3623–3631. doi: 10.1121/1.414960. [DOI] [PubMed] [Google Scholar]
- Dau T, Wenger O, Mellert V, Kollmeier B. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J Acoust Soc Am. 2000;107(3):1530–1540. doi: 10.1121/1.428438. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. J Acoust Soc Am. 2002;111(6):2897–2907. doi: 10.1121/1.1480421. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, Ahlstrom JB. Recovery from prior stimulation: masking of speech by interrupted noise for younger and older adults with normal hearing. J Acoust Soc Am. 2003;113(4):2084–2094. doi: 10.1121/1.1555611. [DOI] [PubMed] [Google Scholar]
- Furman AC. PhD thesis. Massachusetts Institute of Technology; 2013. Primary neuronal degeneration in the guinea pig effects on spontaneous rate-type. [Google Scholar]
- Furman AC, Kujawa SG, Liberman CM. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(3):577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Bacon SP, Williams EJ. An examination of speech recognition in a modulated background and of forward masking in younger and older listeners. J Speech Lang Hear Res. 2007;50(4):857–864. doi: 10.1044/1092-4388(2007/060). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Menezes DC, Porter HL, Griz S. Masking period patterns and forward masking for speech-shaped noise: Age-related effects. Ear and hearing. 2016;37(1):48–54. doi: 10.1097/AUD.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hearing Research. 2016 doi: 10.1016/j.heares.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Dallos P. Forward masking of auditory nerve fiber responses. J Neurphys. 1979;42(4):1083–1107. doi: 10.1152/jn.1979.42.4.1083. [DOI] [PubMed] [Google Scholar]
- Jepsen ML, Ewert SD, Dau T. A computational model of human auditory signal processing and perception. J Acoust Soc Am. 2008;124(1):422–438. doi: 10.1121/1.2924135. [DOI] [PubMed] [Google Scholar]
- Jørgensen S, Dau T. Predicting speech intelligibility based on the signal-to-noise envelope power ratio after modulation-frequency selective processing. J Acoust Soc Am. 2011;130(3):1475–1487. doi: 10.1121/1.3621502. [DOI] [PubMed] [Google Scholar]
- Joris PX, Carney LH, Smith PH, Yin T. Enhancement of neural synchronization in the anteroventral cochlear nucleus I. Responses to tones at the characteristic frequency. J Neurophysiol. 1994;71:1022–1022. doi: 10.1152/jn.1994.71.3.1022. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin T. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am. 1992;91(1):215–232. doi: 10.1121/1.402757. [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, CML Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurphys. 1993;70(6):2533–2549. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997:983–997. [PubMed] [Google Scholar]
- Kramer S, Teas D. Forward masking of auditory nerve (N1) and brainstem (wave V) responses in humans. J Acoust Soc Am. 1982;72(3):795–803. doi: 10.1121/1.388186. [DOI] [PubMed] [Google Scholar]
- Kujawa S, Liberman M. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky R. The effect of forward masker duration, rise/fall time, and integrated pressure on auditory brain stem evoked responses in human newborns and adults. Ear and Hearing. 1993;14(2):95–103. doi: 10.1097/00003446-199304000-00004. [DOI] [PubMed] [Google Scholar]
- Lasky R, Allen RL. emporal masking of auditory evoked brainstem responses in human newborns and adults. Hear Res. 1982;6(3):315–334. doi: 10.1016/0378-5955(82)90063-6. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(2):467–477. [PubMed] [Google Scholar]
- Liberman CM. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63(2):442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman CM. Physiology of cochlear efferent and afferent neurons: direct comparisons in the same animal. Hear Res. 1988;34(2):179–191. doi: 10.1016/0378-5955(88)90105-0. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Liberman CM. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16(2):205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PloS one. 2016;11(9):e0162726. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Yit C, Graeme CM. Forward masking patterns produced by intracochlear electrical stimulation of one and two electrode pairs in the human cochlea. J Acoust Soc Am. 1989;86(3):971–980. doi: 10.1121/1.398732. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios P. Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Front Neurosci. 2013:7. doi: 10.3389/fnins.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman CM, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddis R, O’Mard L. A computer model of the auditory-nerve response to forward-masking stimuli. J Acoust Soc Am. 2005;117(6):3787–3798. doi: 10.1121/1.1893426. [DOI] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. The Journal of Neuroscience. 2016;36(13):3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher J, Kiang N. Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear Res. 1996;93:52–71. doi: 10.1016/0378-5955(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ. Neural generators of the auditory brainstem response. The auditory brainstem response. J Acoust Soc Am. 1985:13–31. [Google Scholar]
- Murnane OD, Prieve BA, Relkin EM. Recovery of the human compound action potential following prior stimulation. Hear Res. 1998;124:182–189. doi: 10.1016/s0378-5955(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Nayagam B, Muniak M, Ryugo D. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res. 2011;278(1):2–20. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Zachary MS, DYE Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Acoust Soc Am. 2009;29(8):2553–2562. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ. Forward masking: Adaptation or integration? The Journal of the Acoustical Society of America. 2001;109(2):732–741. doi: 10.1121/1.1336501. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. Springer-Verlag; New York, NY: 2000. [Google Scholar]
- Plack C, Baker D, Prendergast G. Perceptual consequences of ”hidden” hearing loss. Trends Hear. 2014:18. doi: 10.1177/2331216514550621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack C, Oxenham AJ. Basilar-membrane nonlinearity and the growth of forward masking. J Acoust Soc Am. 1998;103(3):1598–1608. doi: 10.1121/1.421294. [DOI] [PubMed] [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Léger A, Hall DA, Heinz MG, Plack CJ. Effects of noise exposure on young adults with normal audiograms i: Electrophysiology. Hearing Research. 2016 doi: 10.1016/j.heares.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puria S. Measurements of human middle ear forward and reverse acoustics: Implications for otoacoustic emissions. J Acoust Soc Am. 2003;113(5):2773–2789. doi: 10.1121/1.1564018. [DOI] [PubMed] [Google Scholar]
- Relkin EM, Doucet JR. Recovery from prior stimulation. I: Relationship to spontaneous firing rates of primary auditory neurons. Hear Res. 1991;55(2):215–222. doi: 10.1016/0378-5955(91)90106-j. [DOI] [PubMed] [Google Scholar]
- Relkin EM, Doucet JR. Recovery of the compound action potential following prior stimulation: evidence for a slow component that reflects recovery of low spontaneous-rate auditory neurons. Hear Res. 1995;83(1):183–189. doi: 10.1016/0378-5955(95)00004-n. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham B. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci U S A. 2011:1–6. doi: 10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaalje BG, Mcbride JB, Fellingham GW. Adequacy of approximations to distributions of test statistics in complex mixed linear models. J Agricult, Biol, Environ Stats. 2002;7(4):512–524. [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76(4):2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen LA, Valero MD, Liberman MC. Towards a diagnosis of cochlear neuropathy with envelope following responses. Journal of the Association for Research in Otolaryngology. 2015;16(6):727–745. doi: 10.1007/s10162-015-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. Influence of centrifugal pathways on forward masking of ventral cochlear nucleus neurons. J Acoust Soc Am. 1998;104(1):378–389. doi: 10.1121/1.423294. [DOI] [PubMed] [Google Scholar]
- Song Q, Shen P, Li X, Shi L, Liu L, Wang J, Yu Z, Stephen K, Aiken S, Yin S, et al. Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Scientific reports. 2016:6. doi: 10.1038/srep25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper G, Johnson T. Auditory Function in Normal-Hearing, Noise-Exposed Human Ears. Ear Hear. 2015a doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Letter to the editor: Examination of potential sex influences in stamper, gc, & johnson, ta (2015). auditory function in normal-hearing, noise-exposed human ears, ear hear, 36, 172–184. Ear and hearing. 2015b;36(6):738–740. doi: 10.1097/AUD.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellmack MA, Byrne AJ, Viemeister NF. Extracting binaural information from simultaneous targets and distractors: Effects of amplitude modulation and asynchronous envelopes. J Acoust Soc Am. 2010;128(3):1235–1244. doi: 10.1121/1.3466868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temchin A, Nola R, Ruggero M. Threshold tuning curves of chinchilla auditory nerve fibers. II. Dependence on spontaneous activity and relation to cochlear nonlinearity. J Neurophys. 2008;100(5):2899–2906. doi: 10.1152/jn.90639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CW, Relkin EM, Doucet J. Psychophysical and physiological forward masking studies: Probe duration and rise-time effects. The Journal of the Acoustical Society of America. 1994;96(2):795–800. doi: 10.1121/1.410317. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Bharadwaj HM, Mehraei G, Shera CA, Shinn-Cunningham BG. Functional modeling of the human auditory brainstem response to broadband stimulation. J Acoust Soc Am. 2015;138(3):1637–1659. doi: 10.1121/1.4928305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Dau T, Shera C. Nonlinear time-domain cochlear model for transient stimulation and human otoacoustic emission. J Acoust Soc Am. 2012;136(6):3842–3848. doi: 10.1121/1.4763989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J, Orlando M, RB Auditory brainstem response forward-masking recovery functions in older humans with normal hearing. Hear Re. 1999;127(1):86–94. doi: 10.1016/s0378-5955(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina R, Meierhans L. Sensorineural hearing loss alters recovery from short-term adaptation in the C57BL/6 mouse. Hear Re. 1995;88(1):19–26. doi: 10.1016/0378-5955(95)00093-j. [DOI] [PubMed] [Google Scholar]
- Westerman L, Smith R. A diffusion model of the transient response of the cochlear inner hair cell synapse. J Acoust Soc Am. 1988;83(6):2266–2276. doi: 10.1121/1.396357. [DOI] [PubMed] [Google Scholar]
- Winter IM, Itatani N, Bleeck S, Ingham N. Enhancement of forward suppression begins in the ventral cochlear nucleus. J Acoust Soc Am. 2014;135(4):2347. doi: 10.1016/j.brainres.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ED, Barta PE. Rate responses of auditory nerve fibers to tones in noise near masked threshold. J Acoust Soc Am. 1986;79(2):426–442. doi: 10.1121/1.393530. [DOI] [PubMed] [Google Scholar]