Abstract
We investigated antibody responses against pneumococci of serotypes 6B, 14, and 23F in 56 children and adolescents with perinatal human immunodeficiency virus (HIV) infection who were vaccinated with 7-valent pneumococcal conjugate vaccine. Overall immune responses differed greatly between serotypes. Correlation coefficients between immunoglobulin G (IgG) measured by enzyme-linked immunosorbent assay (ELISA) and functional antibodies measured by a flow cytometry opsonophagocytosis assay (OPA) varied with serotype and time points studied. After 3 months of administering a second PCV7 dose we got the highest correlation (with significant r values of 0.754, 0.414, and 0.593 for serotypes 6B, 14, and 23F, respectively) but no significant increase in IgG concentration and OPA titers compared to the first dose. We defined a responder to a serotype included in the vaccine with two criteria: frequency of at least twofold OPA and ELISA increases for each serotype and frequency of conversion from negative to positive OPA levels. Responders varied from 43.9% to 46.3%, 28.5% to 50.0%, and 38.0% to 50.0% for serotypes 6B, 14, and 23F, respectively, depending on the response criterion. The present research highlights the importance of demonstrating vaccine immunogenicity with suitable immunological endpoints in immunocompromised patients and also the need to define how much antibody is required for protection from different serotypes, since immunogenicity differed significantly between serotypes.
The first pneumococcal conjugate vaccine serotype against invasive pneumococcal disease, Wyeth's 7-valent Prev(e)nar (PCV7), was licensed in Spain in June 2001. This vaccine significantly reduces the incidence of invasive pneumococcal disease in children and adults (2, 26). The Vaccines Advisory Committee of the Spanish Society of Pediatrics recommends vaccination with PCV7 in immunocompromised children of all ages, specifically with two doses in children older than 2 years of age (1, 3).
Efficacy trial data of pneumococcal vaccination for human immunodeficiency virus (HIV)-infected patients from prospective studies are not feasible. This drawback highlights the need for further immunogenicity studies to assess the impact of vaccination in immunocompromised patients. Enzyme-linked immunosorbent assay (ELISA) has been widely used to establish correlates of protection in the evaluation of sera of vaccinees (19). However, establishing effectiveness in preventing pneumococcal disease based on serological criteria is hindered by the absence of an ideal serological correlate of protection in vitro. The 3rd International Symposium on Pneumococci and Pneumococcal Diseases (Anchorage, Alaska, 2002) recommended that opsonophagocytic assays (OPAs) should be used to supplement ELISA antibody concentration measurements (11). Clinical and mouse protection data for the selected serotypes for which this assay works indicate that the opsonophagocytosis assay provides the best basis for protection (12, 24). Measuring OPA titers has revealed low functional activity of conjugate vaccine-induced antibodies in bone marrow transplant recipients (18) and different responses to conjugate and plain polysaccharide vaccines in children and adults with sickle cell disease (25).
The present study aimed to investigate PCV7 immunogenicity in pediatric patients with perinatal HIV infection by assessing antibody response measured by ELISA, the functional capacity of the antibody response measured by OPA, and the antibody response after one and two doses of vaccine. We focused on the analysis of OPA responders within the framework of ELISA consensus threshold antibody levels based on efficacy trials in healthy individuals and on the serotype-specific differences reported here. Our study involved a cohort of 56 HIV-infected children and adolescents attending the Pediatric Clinic Unit of 12 de Octubre Hospital (Madrid, Spain).
MATERIALS AND METHODS
Patients and pneumococcal vaccination.
Patients attending the Pediatric Clinic specializing in AIDS and HIV care at 12 de Octubre Hospital (Madrid, Spain) were selected for the present study by their fulfillment of the following inclusion criteria for obtaining a homogeneous cohort: (i) the patient was older than 2 years and had received the 23-valent polysaccharide vaccine (PneumovaxII; Aventis Pasteur MSD) at least 1 year earlier and (ii) the patient was undergoing highly active antiretroviral therapy and the mode of HIV transmission had been perinatal; (iii) the patient was not known to have had any opportunistic infection at the time of the present vaccination. All patients received two doses of 7-valent conjugate vaccine (PCV7; Prev(e)nar, Wyeth-Lederle vaccines, Pearl River, N.Y.) separated by a 2-month interval. The demographic characteristics of these patients are summarized in Table 1.
TABLE 1.
Demographic characteristics, pneumococcal vaccination, and surrogate markersa
| Parameter | |
|---|---|
| No. of patients | 56 |
| Age (yr): mean (SD); median (range) | 10.8 (4); 11 (3-19) |
| Gender (no. of patients) | |
| Male | 29 |
| Female | 27 |
| Age (yr) at 23-valent PPS vaccination: mean (SD); median (range) | 9.3 (4); 9 (1-18) |
| Lag (mo) between 23-valent PPS vaccination and 7-valent conjugate vaccination: median (range) | 18 (11-84) |
| % CD4/CD8 (median; mean; SD) | 29/37; 29/38; 7/10 |
| Immune category before HAART (no. of patients) | |
| 1 (no suppression) | 18 |
| 2 (moderate suppression) | 12 |
| 3 (severe suppression) | 26 |
| Clinical category before HAART (no. of patients) | |
| N (asymptomatic) | 0 |
| A (mildly symptomatic) | 27 |
| B (moderately symptomatic) | 15 |
| C (severely symptomatic) | 14 |
PPS, polysaccharide; HAART, highly active antiretroviral therapy. Categories are those defined by the Centers for Disease Control and Prevention in their guidelines for the use of antiretroviral agents in pediatric HIV infection.
Blood samples and CD4/CD8 cell counts.
Blood was drawn immediately before the first PCV7 dose and immediately before and 3 months after the second PCV7 dose. Serum was separated from whole-blood samples by centrifugation and stored at −20°C. Heparin-anticoagulated blood samples obtained immediately before vaccination were processed for measurement of CD4+ and CD8+ counts by flow cytometry (FACSCalibur, Becton Dickinson).
Specific anticapsular antibody determination.
ELISA was performed as previously described (4, 21) with minor modifications to determine the concentration of specific anticapsular IgG to pneumococcal serotypes 6B, 14, and 23F. Briefly, serum reference standard assay, quality-control specimens, and test specimens were absorbed with a 1:50 dilution of 10 μg/ml of cell wall polysaccharide (Statens Seruminstitut, Copenhagen, Denmark). ELISA microtiter plates (Nunc Maxisorp, PGC Scientifics Corp., Gaithersburg, Md.) were coated with 0.5 μg of purified polysaccharide antigen (American Type Culture Collection, Manassas, Va.). Every plate included paired pre- and postvaccination samples, our own laboratory reference standard serum with calculated concentrations of IgG to a specific polysaccharide (based on cross-standardization with the 89-SF serum reference-assay standard, kindly provided by Carl E. Frasch, Centers for Biologics, Rockville, Md.), four quality controls, and a negative serum specimen that contained no IgG to the individual polysaccharide studied.
Pneumococcal strains.
The pneumococcal serotypes selected for the OPA included the least immunogenic serotypes (6B and 23F) and serotype 14 (20). These three serotypes are thought to be the most prevalent in invasive pneumococcal disease among HIV-infected children (13). Pneumococcal strains were obtained from our collection (Reference Laboratory of Pneumococci, Centro Nacional de Microbiología, Madrid, Spain) and serotyped as previously described (8).
Opsonophagocytosis activity titers.
Flow cytometric OPA for each of the three serotypes was performed largely as previously described but with minor modifications (10). Three strains of serotypes 6B, 14, and 23F were grown thrice consecutively to log phase in Todd-Hewitt medium supplemented with 1% of decomplemented human pooled serum to ensure a high level of encapsulation. This step was critical for measuring specific anticapsular antibodies (17). Subsequently, strains were heat inactivated and fluorescein isothiocyanate labeled. Human pooled serum was depleted of IgG by protein G affinity chromatography and used as a complement source. In optimizing experiments, the addition of complement did not significantly change OPA titers relative to those of corresponding non-heat-inactivated strains. This ensured a high degree of encapsulation of the strains used in the assay.
Human polymorphonuclear leukocytes (PMNs) were isolated from the blood of healthy, Fcγ receptor-typed volunteers (9) by Ficoll-Histopaque gradient centrifugation. Sera from vaccinees were heated (30 min at 56°C) to inactivate the complement before it was assayed. Samples of 2.5 × 106 pneumococci and three serial fivefold dilutions starting with a 1:10-diluted serum sample were added to each of the 96 wells of a microtiter plate (ratio of PMN to bacteria = 1:20). After 30 min of opsonization at 37°C on a microtiter plate shaker, 1.25 × 105 freshly isolated PMNs were added per well and phagocytosis was carried out for 30 min at 37°C with vigorous shaking. After three washing steps with ice-cold bovine serum albumin-Hanks' balanced salt solution, cells were transferred to fluorescence-activated cell sorter tubes, immediately fixed with paraformaldehyde (2%) in phosphate-buffered saline, and analyzed in a flow cytometer (FACSCalibur; Becton Dickinson). The percentage of fluorescein isothiocyanate-positive PMNs was used as a measure of the phagocytic activity of the serum. Results are expressed as the log10 of the phagocytosis titer, which is the reciprocal of the serum dilution yielding 25% fluorescein isothiocyanate-positive PMNs. A positive control serum was included on each plate to control the reproducibility of the assay.
Statistical analysis.
All patients were included in the analysis, and data were analyzed with the SPSS 8.0 program. Descriptive statistics (mean, standard deviation, and median) were calculated for all samples. Student's t test for paired samples was used to compare means of variables (prevaccination with postdose 2 and postdose 1 with postdose 2). ELISA and OPA data were correlated using the Pearson's correlation coefficient (r). Chi-square (χ2) contingency tests were used to analyze differences in frequencies of responders and nonresponders for pairs of response criteria. All statistics were regarded as significant for probability values of ≤0.05.
RESULTS
Patients.
Sera from 56 HIV-infected pediatric patients with a mean age of 10.8 years were obtained immediately before vaccination. Follow-up serum samples were obtained from 50 patients 2 months after the first PCV7 dose and from 42 patients 3 months after the second dose. The other 14 patients were excluded since they did not attend on the arranged dates. All patients were followed in study clinics, and standardized criteria were used in their clinical evaluation. Demographic characteristics and immunological data are summarized in Table 1.
OPA titers.
Mean OPA titers are summarized in Table 2. Significant differences were found between prevaccination and postdose 2 OPA titers (6B, t = 4.05, P ≈ 0.000, n = 41; 14, t = 2.669, P = 0.011, n = 42; 23F, t = 2.034, P = 0.048, n = 42). However, no significant differences between the first and second doses were found in any serotype (6B, t = 1.825, P > 0.05, n = 37; 14, t = 1.957, P > 0.05, n = 37; 23F, t = 1.229, P > 0.05, n = 38).
TABLE 2.
Mean antibody responses measured by OPA and ELISA against serotypes 6B, 14 and 23F
| Assay | Result
|
||
|---|---|---|---|
| Prevaccination | Post-dose 1 | Post-dose 2 | |
| Mean OPA titer (SD) | |||
| Serotype 6B | 3.5 (5.9) | 15.7 (14.3) | 12.3 (14.9) |
| Serotype 14 | 31.0 (42.3) | 161.5 (313) | 67.6 (98.7) |
| Serotype 23F | 4.8 (6.0) | 13.5 (14.9) | 9.4 (11.3) |
| Mean IgG concn (SD) | |||
| Serotype 6B | 0.4 (0.8) | 1.4 (1.8) | 1.4 (2.4) |
| Serotype 14 | 1.3 (2.4) | 6.1 (8.5) | 3.9 (4.1) |
| Serotype 23F | 1.2 (4.0) | 2.5 (2.8) | 2.6 (7.3) |
Total IgG concentrations.
Mean IgG concentrations are summarized in Table 2. Significant differences between prevaccination and postdose 2 IgG levels were found (6B, t = 3.124, P = 0.003, n = 41; 14, t = 3.349, P = 0.002, n = 42; 23F, t = 2.052, P = 0.047, n = 42). However, no significant differences between the first and second doses were found except in serotype 6B, which was lower after dose 2 than after dose 1 (6B, t = 2.659, P = 0.012, n = 37; 14, t = 0.771, P > 0.05, n = 37; 23F, t = 1.970, P > 0.05, n = 38).
Correlation between OPA and ELISA.
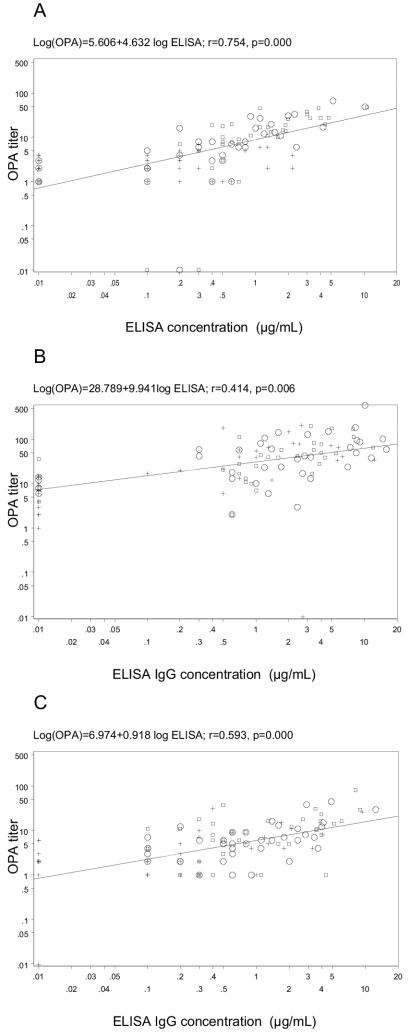
Correlation differed significantly between serotypes and times studied, being higher and significant for each serotype only at 3 months after the administration of dose 2. Considering serotypes 6B, 14, and 23F, postdose 2 r values were 0.754 (P ≈ 0.000), 0.414 (P = 0.006) and 0.593 (P ≈ 0.000), postdose 1 r values were 0.673 (P ≈ 0.000), 0.072 (P > 0.05) and 0.420 (P = 0.002) and, prevaccination r values were 0.216 (P > 0.05), 0.246 (P > 0.05), and 0.544 (P ≈ 0.000), respectively. The distribution of antibody concentrations and OPA titers after one and two doses of PCV7 and prior to vaccination are shown in Fig. 1.
FIG. 1.
Distribution of antibody concentrations (ELISA) and opsonophagocytic assay (OPA) titers prior to vaccination and after one and two doses of 7-valent protein-conjugated vaccine for serotype 6B (A), serotype 14 (B), and serotype 23F (C).
Functional antibodies elicited by the 7-valent conjugate vaccine and relation with IgG concentration measured by ELISA.
We evaluated IgG responses to pneumococcal vaccine with the following criteria: at least twofold OPA and ELISA increases for each serotype (by which we defined a responder to a serotype included in the vaccine) and frequency of conversion from negative to positive OPA levels. OPA cutoff values were equivalent to a geometric mean IgG concentration of vaccine responders from efficacy trial measures of healthy individuals with a single threshold for all serotypes. These were calculated as those corresponding to an ELISA concentration of 0.5 mg/ml determined for each serotype by regression of OPA on ELISA concentration (see Fig. 1). OPA cutoff levels, rounded to the nearest integer, were <4 to ≥4 for serotype 6B, <25 to ≥25 for serotype 14, and <6 to ≥6 for serotype 23F. We used both criteria to maximize the possibility of detecting any potential benefit associated with vaccination.
Responders versus nonresponders.
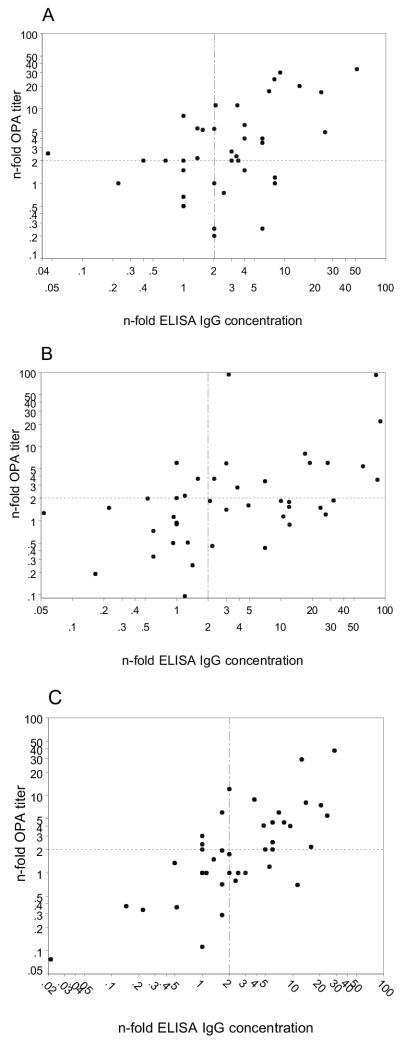
Scatter plots representing the increases in OPA and ELISA after dose 2 for each serotype are shown in Fig. 2. Following the first response criterion, the quadrant analysis revealed that responders were observed in 43.9% (18 of 41, OPA-ELISA mean and standard deviation of 21.3 ± 2.5 and 17.4 ± 3.35, respectively), 28.5% (12 of 42, OPA/ELISA mean of 134.3 ± 5.37 and 159.05 ± 3.61, respectively), and 38.0% (16 of 42, OPA/ELISA mean of 16.12 ± 2.48 and 13.29 ± 3.03, respectively) of cases for serotypes 6B, 14, and 23F, respectively. Nonresponders occurred at frequencies of 31.7% (13 of 41), 33.3% (14 of 42), and 40.4% (17 of 42) for serotypes 6B, 14, and 23F, respectively. OPA-only responders had frequencies of 12.1% (5 of 41), 7.1% (3 of 42), and 7.1% (3 of 42) for serotypes 6B, 14, and 23F, respectively, while those of ELISA-only responders were 12.1% (5 of 41), 30.9% (13 of 42), and 14.2% (6 of 42), respectively.
FIG. 2.
Distribution of serotype-specific vaccine responders as a function of OPA increase and ELISA increase after dose 2. (A) Serotype 6B; (B) serotype 14; (C) serotype 23F. Quadrant lines were drawn at the twofold increase thresholds for the ELISA and opsonophagocytic assays.
Responders versus nonresponders in relation to efficacy trial data.
Employing the second response criterion, 46.3% (19 of 41, OPA-ELISA mean and standard deviation 16.7 ± 1.7 and 17.0 ± 2.5, respectively), 50% (21 of 42, OPA-ELISA mean and standard deviation 37.2 ± 2.9 and 42.9 ± 3.2, respectively), and 50% (21 of 42, OPA-ELISA mean and standard deviation 11.9 ± 2.2 and 11.2 ± 2.7, respectively) of OPA responders were found for serotypes 6B, 14, and 23F, respectively. The rates of response increased to 65.8% (27 of 41), 92.8% (39 of 42), and 69.0% (29 of 42) for serotypes 6B, 14, and 23F, respectively, when values prior to vaccination above the cutoffs were considered. No significant differences in frequencies between both criteria were found (χ2 = 17.7 and P ≈ 0.000, χ2 = 4.2 and P = 0.04, and χ2 = 10.1 and P = 0.001 for serotypes 6B, 14, and 23F, respectively).
Correlation of vaccination response with demographic features and clinical variables.
No correlation was found between response to vaccination and demographic features or clinical characteristics such as immune and clinical category before highly active antiretroviral therapy and CD4 cell counts.
DISCUSSION
The PCV7 vaccine elicited a significant quantitative and qualitative increase in antibody response in AIDS pediatric patients for the three serotypes studied. However, no significant difference between one and two doses of PCV7 was found, indicating that an apparently nonefficient booster immune response is achieved in these patients, as has also been reported in another study in HIV-infected adults (6). In general, antibody concentrations and opsonophagocytic capacity against serotype 14 were higher than they were against 6B and 23F serotypes measured before and after vaccination with one and two vaccine doses. Strikingly, the highest percentage of vaccine responders and the highest correlation between OPA titers and IgG concentrations were observed for serotype 6B. It is of particular note that the correlation differed significantly with serotype and prevaccination and postvaccination serum samples. Those from samples obtained 3 months after the second dose yielded the highest values for all serotypes.
Antibody concentrations in prevaccination serum samples were higher in our pediatric AIDS patients than those reported for healthy U.S. children (2, 22) and corresponding OPA titers were significantly low, except for serotype 14. Poor correlations between OPA titers and IgG concentrations prior to vaccination suggested that a higher level of nonspecific antibodies in the pediatric AIDS patients may account for this difference. An effect of prevaccination with the 23-valent polysaccharide vaccine might lead to higher concentrations, but we have ruled out this explanation in this case since the previous immunization occurred between 11 and 84 months earlier and specific antibodies are known to be lost rapidly after pneumococcal vaccination in HIV-infected patients (16). Total IgG concentrations in AIDS patients higher than in immunocompetent adults were previously reported (7).
Recent developments in the field have included an absorption step with an irrelevant polysaccharide (22F) for the evaluation of antibody concentrations that may influence correlations (5). Feikin et al. reported that IgG ELISAs for serotypes 6B and 23F were more specific for serotype-specific pneumococcal capsular antibodies with 22F polysaccharide absorption by reducing nonspecific antibody binding (the exception being serotype 14). However, correlations between ELISA IgG concentrations and opsonophagocytic titers were not significantly improved and continued to be poorly correlated in the case of prevaccination sera, both with and without 22F absorption (7). In previous work, nonfunctional antibodies failed to be completely removed since a similar reduction in prevaccination and postvaccination antibody levels was observed in control paired samples with a significant OPA rate (data not shown). Moreover, assignments of concentrations from the standard reference serum (89S-F) are calculated without 22F absorption. Taking these matters into account, we decided not to include 22F absorption in these ELISA determinations.
In general, correlations between antibody concentration and opsonophagocytic titer (including all values) in these 56 AIDS patients were poorer than those reported in another study of 12 healthy adults (5) but greater than in a study of 60 renal transplant recipients (15) and another of 33 HIV-infected adults (7). In this regard, the 6B serotype correlation coefficients were more similar to those previously reported in healthy individuals. This suggests that pediatric AIDS patients were better able to produce functional antibodies than HIV-infected adults. The reason for this difference is unclear but might be related to a better response after immunologic restoration with highly active antiretroviral therapy in children than in adults.
Sera from AIDS patients may have higher nonfunctional antibody levels than that from members of the healthy population. This was illustrated when the factors of antibody concentration versus OPA titer were examined by a quadrant analysis of responders (Fig. 2). Ideally, there should be no data points in the upper left and lower right quadrants. As expected, a small number of data points fell in the upper-left quadrant (delimited by values of 2 on the ordinate and abscissa) and a greater percentage, mostly of serotype 14, were distributed in the lower-right quadrant. Our data indicate that although serotype 14 continued to have the highest absolute OPA titers and IgG concentrations, it also had a smallest percentage of responders by the first criterion and, in general, the poorest correlation between OPA titers and IgG concentrations, as was also reported in the study of renal transplant recipients. It is worth noting that the correlation for all serotypes was much greater after the second dose than it was after the first dose, indicating an improvement in the functionality of the antibodies despite no change in the titers for either OPA or IgG. Therefore, booster doses may have a greater effect on the quality of the immune response than on the quantity of the bulk of antipolysaccharide antibodies. Moreover, this observation suggests a relationship between these correlation coefficients and the ability to detect immune responses, since repeating the PCV7 dose improved correlations between OPA and ELISA and made the ELISA values more relevant in terms of protection.
It has been reported that AIDS patients with a CD4 cell count of <500 cells/mm3 have an impaired IgG response to pneumococcal antigens (14, 23). In our study, only one patient had a CD4 cell count of <500, precluding any meaningful analysis of links between the distribution of antibody response and this clinical parameter. No correlation was found between response to vaccination and immune category or clinical category before highly active antiretroviral therapy.
We defined a responder as a patient exhibiting at least a twofold increase in OPA titer and antibody concentration (criterion a). However, the main limitation of these approaches is that clinically relevant cutoff levels (protective efficacy) must be known because the protected or unprotected status of a vaccinee of interest must be determined by criteria independent of the analysis variables. In the World Health Organization workshop on correlates of protection (Anchorage, Alaska, 2002), current scientific opinion based on existing efficacy trial data in children and adults considered the induction of 0.2 μg of postprimary IgG antibody per ml to be a protective threshold against invasive pneumococcal disease, with a single threshold for all serotypes included in the vaccine. As a threshold antibody level should be a more conservative estimate of protection, the geometric mean antibody concentration in PCV7 healthy vaccinees (0.5 μg/ml could be a reasonable threshold value) (2). Subsequently, since opsonophagocytic activity represents qualitative and functional antibody responses, we have considered an additional criterion of response: an OPA titer equal to or greater than the value obtained from the regression curve for each serotype for an IgG concentration of 0.5 μg/ml. Thus, our study reports that a concentrations of 0.5 μg/ml of IgG represented threshold OPA titers of 4, 25, and 6, yielding 46.3, 50.0, and 50.0% OPA responders for serotypes 6B, 14, and 23F, respectively.
We conclude that although significant increases in antibody response have been reported, overall response rates in pediatric HIV infection were generally suboptimal because no booster response was observed after the second dose of PCV7. The present study underlines the need to define how much antibody is needed for protection from different serotypes since analysis of frequency of responders, IgG concentrations, and OPA titers revealed significant differences between serotypes. It also highlights the importance of demonstrating vaccine immunogenicity with suitable endpoints in immunocompromised patients and the difficulties in interpreting serological data.
Acknowledgments
We thank the faculties, technicians, and volunteer blood donors of the Eijkman-Winkler Institute for Microbiology, Infectious Diseases and Inflammation for their technical assistance and the daily supply of PMN leukocytes, A. Avellón of the National Center for Microbiology for help in preparing the statistical analysis, and A. Fenoll of the National Center for Microbiology for providing serotyped clinical strains of Streptococcus pneumoniae.
Financial support was provided by Instituto de Salud Carlos III (grant MPY-1115/03) and Sociedad Española de Microbiología Clínica y Enfermedades Infecciosas (SEIMC). This research was carried out on behalf of the Spanish Network Pneumococcus Study Group (G03/103).
REFERENCES
- 1.Berenguer, J., F. Laguna, J. López-Aldeguer, and S. Moreno. 2000. Prevención de las infecciones oportunistas en pacientes adultos y adolescentes infectados por el virus de la immunodeficiencia humana en la era del tratamiento antirretrovírico de gran actividad. Enferm. Infec. Microbiol. Clin. 18:457-468. [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 3.Comité Asesor de Vacunas de la Asociación Española de Pediatría (Vaccines Advisory Committee of the Spanish Society of Pediatrics). 2002. La enfermedad neumocócica y su prevención. Vacuna neumocócica conjugada heptavalente. An. Esp. Pediatr. 56:79-90. [PubMed] [Google Scholar]
- 4.Concepcion, N. F., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feikin, D. R., C. M. Elie, M. B. Goetz, J. L. Lennox, G. M. Carlone, S. Romero-Steiner, P. F. Holder, W. A. O′Brien, C. G. Whitney, J. C. Butler, and R. F. Breiman. 2002. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 20:545-553. [DOI] [PubMed] [Google Scholar]
- 7.Feikin, D. R., C. M. Elie, M. B. Goetz, J. L. Lennox, G. M. Carlone, S. Romero-Steiner, P. F. Holder, W. A. O′Brien, C. G. Whitney, J. C. Butler, and R. F. Breiman. 2004. Specificity of the antibody response to the pneumococcal polysaccharide and conjugate vaccines in human immunodeficiency virus-infected adults. Clin. Diagn. Lab. Immunol. 11:137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenoll, A., I. Jado, D. Vicioso, A. Pérez, and J. Casal. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990-1996). J. Clin. Microbiol. 36:3447-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen, W. T. M., A. Breukels, H. Snippe, L. A. Sanders, A. F. M. Verheul, and G. T. Rijkers. 1999. Fcgamma receptor polymorphisms determine the magnitude of in vitro phagocytosis of Streptococcus pneumoniae mediated by pneumococcal conjugate sera. J. Infect. Dis. 180:888-891. [DOI] [PubMed] [Google Scholar]
- 10.Jansen, W. T. M., J. Gootjes, M. Zelle, D. V. Madore, J. Verhoef, H. Snippe, and A. F. M. Verheul. 1998. Use of highly encapsulated Streptococcus pneumoniae strains in a flow-cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin. Diagn. Lab. Immunol. 5:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jodar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Kayhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 13.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 14.Kroon, F. P., J. T. van Dissel, E. Ravensbergen, P. H. Nibbering, and R. van Furth. 2000. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine 19:886-894. [DOI] [PubMed] [Google Scholar]
- 15.Kumar, D., C. Rotstein, G. Miyata, D. Arlen, and A. Humar. 2003. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J. Infect. Dis. 187:1639-1645. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen, H., B. Kvinesdal, T. L. Benfield, J. D. Lundgren, and H. B. Konradsen. 1998. Rapid loss of specific antibodies after pneumococcal vaccination in patients with human immunodeficiency virus-1 infection. Scand. J. Infect. Dis. 30:597-601. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen, S. V., U. B. S. Sorensen, and J. Henrichsen. 1993. Antibodies against pneumococcal C-polysaccharide are not protective. Microb. Pathog. 14:299-305. [DOI] [PubMed] [Google Scholar]
- 18.Parkkali, T., M. Väkeväinen, H. Käyhty, T. Ruutu, and P. Ruutu. 2001. Opsonophagocytic activity against Streptococcus pneumoniae type 19F in allogenic BMT recipients before and after vaccination with pneumococcal polysaccharide vaccine. Bone Marrow Transplant. 27:207-211. [DOI] [PubMed] [Google Scholar]
- 19.Plikaytis, B. D., D. Goldblatt, C. E. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puumalainen, T., M. R. Zeta-Capeding, H. Käyhty, M. G. Lucero, K. Auranen, O. Leroy, and H. Nohynek. 2002. Antibody response to eleven-valent tetanus and diphtheria conjugated pneumococcal vaccine in Filipino infants. Pediatr. Infect. Dis. J. 21:309-314. [DOI] [PubMed] [Google Scholar]
- 21.Quataert, S. A., C. S. Kirch, L. J. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Barradas, M. C., D. M. Musher, C. Lahart, C. Lacke, J. Groover, D. Watson, R. Baughn, T. Cate, and G. Crofoot. 1992. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J. Infect. Dis. 165:553-556. [DOI] [PubMed] [Google Scholar]
- 24.Saeland, E., H. Jakobsen, G. Ingolfsdottir, S. Sigurdardottir, and I. Jonsdottir. 2001. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J. Infect. Dis. 183:253-260. [DOI] [PubMed] [Google Scholar]
- 25.Vernacchio, L., S. Romero-Steiner, J. E. Martinez, K. MacDonald, S. Barnard, T. Pilishvili, G. M. Carlone, D. M. Ambrosino, and D. C. Molrine. 2000. Comparison of an opsonophagocytic assay and IgG ELISA to assess responses to pneumococcal polysaccharide and pneumococcal conjugate vaccines in children and young adults with sickle cell disease. J. Infect. Dis. 181:1162-1166. [DOI] [PubMed] [Google Scholar]
- 26.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat; Active Bacterial Core Surveillance of the Emerging Infections Program Network. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]