Abstract
Human metapneumovirus (hMPV) has recently been identified as an etiological agent of acute respiratory infections. The hMPV fusion (F) protein has been indicated to be a major antigenic determinant that mediates effective neutralization and protection against hMPV infection. We developed a new immunofluorescence assay (IFA) using Trichoplusia ni (Tn5) insect cells infected with a recombinant baculovirus-expressing hMPV F protein (Bac-F IFA). A total of 200 serum samples from Japanese people 1 month to 41 years old were tested for immunoglobulin G antibodies to hMPV F protein by Bac-F IFA. The results were compared with those of the conventional IFA based on hMPV-infected LLC-MK2 cells (hMPV IFA). The titers obtained by the two IFAs correlated well (correlation coefficient of 0.88), and the concordance of seroreactivities between the two IFAs was 91% (κ = 0.76). For 192 of the 200 serum samples, the titers obtained by the Bac-F IFA were equal to or higher than those obtained by the hMPV IFA. These results indicated that the Bac-F IFA was more sensitive than the hMPV IFA and that the majority of the antibodies detected by the hMPV IFA reacted with the hMPV F protein. The Bac-F IFA is a more reliable, sensitive, and specific method for the detection of hMPV antibodies than is the hMPV IFA.
Human metapneumovirus (hMPV), first isolated in The Netherlands in 2001, is a member of the genus Metapneumovirus of the subfamily Pneumovirinae of the family Paramyxoviridae (25). This subfamily also includes the genus Pneumovirus, which includes respiratory syncytial virus (RSV). hMPV causes upper respiratory tract infection and flu-like illness (3, 21) but is also associated with lower respiratory tract infections such as bronchitis, bronchiolitis, and pneumonia in very young children, elderly persons, and immunocompromised patients (4, 6, 7, 10, 16). hMPV encodes two major surface glycoproteins, the fusion (F) and attachment (G) proteins (24, 25). The F protein promotes fusion of the viral and cell membranes, allowing penetration of the viral ribonucleoprotein into the cell cytoplasm (27), and the G protein mediates virus binding to the cell receptor (11). In RSV infection, the most immunogenic and protective viral antigen is the F protein, which evokes a strong antibody response and is a target for cytotoxic T cells (14, 17, 26).
The baculovirus expression system provides a method for the production of large quantities of biologically active and antigenic eukaryotic proteins for enzyme immunoassays and for immunoblot analysis and immunofluorescence assays (IFAs) (9, 15, 18, 19, 29). In the present study, we expressed the F protein of hMPV in Trichoplusia ni (Tn5) insect cells by a baculovirus system and demonstrated the utility of the recombinant F protein in an IFA.
MATERIALS AND METHODS
Serum samples.
A total of 200 serum samples were obtained at random from Japanese people (1 month to 41 years old) who visited hospitals. All samples were collected after obtaining informed consent from the children's parents or the adults.
Expression of F protein of hMPV in the baculovirus-insect cell system.
A baculovirus expression kit was used to prepare F protein expressed in the baculovirus-insect cell system in accordance with the instructions of the manufacturer (BD PharMingen, San Diego, Calif.). Briefly, the full-length cDNA of F protein from strain JPY88-12 (GenBank accession number AY622381) was amplified by PCR with primers F (5′-GGATCCATGTCTTGGAAAGTGGTGATCATTTTTTC-3′) and R (5′-GCGGCCGCCTAATTATGTGGTATGAAGCCATTGTTTG-3′). (The restriction sites in the primers used for cloning are underlined.) The PCR product was cloned into the BamHI and NotI sites of the pVL1393 baculovirus transfer vector. To generate a recombinant baculovirus, recombinant plasmid pVL1393-F was cotransfected with Baculogold DNA (BD PharMingen) into Sf9 cells. Trichoplusia ni (Tn5) insect cells cultured in Ex-cell 405 medium (JRH Biosciences, Lenexa, Kans.) were infected with the recombinant virus at a multiplicity of infection of 10 virus particles per cell. The cells at 72 h after infection were used as hMPV F protein for IFA and Western blot analysis.
Western blot analysis.
Cells were lysed with sodium dodecyl sulfate (SDS), and the lysate of 105 cell equivalents was subjected to SDS-12% polyacrylamide gel electrophoresis under nonreducing conditions. The separated proteins were electrotransferred onto a nitrocellulose membrane (23). After blocking with 1% bovine serum albumin, hMPV antibody-positive serum (titer of 1:1,280 by hMPV IFA) or hMPV antibody-negative serum (titer of <1:10 by hMPV IFA) at a serum dilution of 1:200 was allowed to bind to the filter and then to react with horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG) polyclonal antibody (Biosource International, Camarillo, Calif.), and the proteins were detected by a chemiluminescence assay method (ECL Western Blotting Detection Reagents; Amersham Pharmacia Biotech, Inc., Piscataway, N.J.).
IFA using the baculovirus-insect cell system (Bac-F IFA).
Tn5 cells infected with the recombinant virus were spotted onto slides. The cell smears were air dried, fixed in acetone for 10 min, and incubated for 30 min at 37°C with serum samples diluted serially, beginning at 1:10. After incubation, the slides were washed three times in phosphate-buffered saline (PBS) for 10 min each time. They were then incubated for 30 min at 37°C with fluorescein isothiocyanate-conjugated rabbit anti-human IgG (Dako, Glostrup, Denmark) at a serum dilution of 1:40. After incubation, they were washed three times in PBS for 10 min each time, air dried, and mounted with PBS-glycerin (1:1). Stained preparations were then examined under a fluorescence microscope. Serum samples that reacted with hMPV F protein at a dilution of more than 1:10 were considered positive for hMPV antibodies. Expression of F protein in Tn5 cells was confirmed by indirect IFA with a guinea pig polyclonal antibody against hMPV, which was a kind gift from Albert D. M. E. Osterhaus, Department of Virology, Erasmus Medical Center. Furthermore, we confirmed that uninfected Tn5 cells did not react with human serum samples.
IFA using hMPV-infected LLC-MK2 cells (hMPV IFA).
hMPV isolate JPS02-76 was inoculated into LLC-MK2 cells. hMPV-infected cells at 2 to 3 weeks after infection were used as hMPV antigen-positive cells for an IFA. The cell smears were air dried, fixed in acetone for 10 min, and incubated for 30 min at 37°C with serially diluted serum samples, beginning at 1:10. After incubation, the slides were washed three times in PBS for 10 min each time. They were then incubated for 30 min at 37°C with fluorescein isothiocyanate-conjugated rabbit anti-human IgG (Dako, Glostrup, Denmark) at a serum dilution of 1:40. After incubation, they were washed three times in PBS for 10 min, air dried, and mounted with PBS-glycerin (1:1). Stained preparations were then examined under a fluorescence microscope. Serum samples that reacted with hMPV at a dilution of more than 1:10 were considered positive for hMPV antibodies (5, 6).
Statistical analyses.
Correlation between titers obtained by the two different IFAs was evaluated by Pearson's correlation coefficient. The regression line was calculated by using the JSTAT 9.2 software. The degree of concordance between the two assays was assessed by using the kappa statistic, which measures the excess of the observed agreement over that expected purely by chance. Kappa (κ) equals 1.0 for perfect agreement and 0 for no agreement beyond chance; values between 0.4 and 0.8 were considered to represent fair to good agreement beyond chance.
RESULTS
Expression of hMPV F protein and Bac-F IFA.
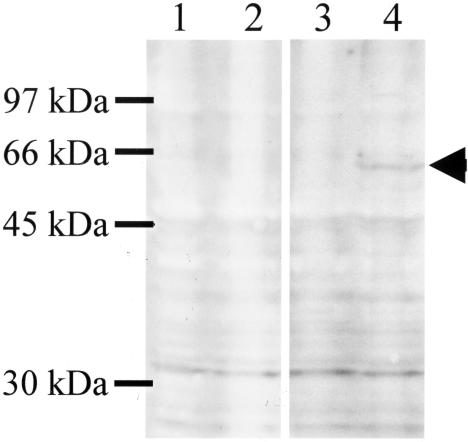
The expected molecular size of the uncleaved and unglycosylated F0 protein of hMPV is 58.5 kDa (8). The observed molecular size of hMPV F protein expressed in the Bac-F-infected Tn5 cells was ∼60 kDa, as determined by Western blot analysis with anti-hMPV F antibody-positive human serum (Fig. 1, lane 4, arrowhead). A representative Bac-F IFA result is shown in Fig. 2. hMPV F protein was present on cell surfaces and in the cytoplasm (Fig. 2A). The positive signal was not detected in intact Tn5 cells by IFA with anti-hMPV F antibody-positive human serum (data not shown).
FIG. 1.
Western blot analysis of hMPV F protein expressed by a baculovirus system. Cell lysates (105 cell equivalents) were prepared from Tn5 cells (lanes 1 and 3) and Bac-F-infected Tn5 cells (lanes 2 and 4). hMPV F protein was detected by using seropositive serum (lanes 3 and 4) and seronegative serum (lanes 1 and 2). The arrowhead indicates the hMPV F protein.
FIG. 2.
Detection of hMPV F protein by Bac-F IFA using seropositive serum (titer of 1:10,240 by hMPV IFA) (A) and seronegative serum (titer of <1:10 by hMPV IFA) (B) at a serum dilution of 1:40.
Comparison of the results of the two IFAs.
We determined the antibody titers against hMPV F protein by Bac-F IFA, as well as those against whole hMPV protein by hMPV IFA. Results are shown in Fig. 3. The titers obtained by Bac-F IFA and those obtained by hMPV IFA correlated well (correlation coefficient of 0.88). Of the 200 serum samples, all 143 serum samples positive by hMPV IFA were positive by Bac-F IFA. On the other hand, 18 of the 57 serum samples negative by hMPV IFA were positive by Bac-F IFA. The concordance of seroreactivities (titers of more than 10 were regarded as positive, and titers of less than 10 were regarded as negative) between the hMPV and Bac-F IFAs was 91% (κ = 0.76). For 192 of the 200 serum samples, the titers obtained by Bac-F IFA were equal to or higher than those obtained by hMPV IFA. The titers obtained by Bac-F IFA were lower than those obtained by hMPV IFA for only eight serum samples.
FIG. 3.
Relationship between titers measured by the hMPV and Bac-F IFAs in 200 serum samples. The titers obtained by the hMPV and Bac-F IFAs were compared. The values in open circles are the numbers of samples with the indicated titers. The broken lines indicate the cutoffs of the IFAs (serum dilution of 1:10). The solid line is the regression line calculated from the data presented (y = 1.032 × x + 1.44). The calculated r value was 0.88.
DISCUSSION
IFAs using hMPV-infected LLC-MK2 cells or hMPV-infected tertiary monkey kidney cells are widely used and have provided a large volume of serological data (5, 6, 25, 28). However, these conventional IFAs detect antibodies against whole hMPV proteins. In the present study, the specific antibodies against hMPV F protein in human serum were measured by Bac-F IFA. We tentatively used a Bac-F IFA cutoff titer of 1:10 because the hMPV IFA cutoff titer was determined to be 1:10 in our previous study (5, 6). The titers obtained by Bac-F IFA and hMPV IFA correlated well (correlation coefficient of 0.88), and the concordance of seroreactivities between the two IFAs was 91% (κ = 0.76). For 192 of the 200 serum samples, the titers obtained by Bac-F IFA were equal to or higher than those obtained by hMPV IFA. These results indicated that the Bac-F IFA was more sensitive than the hMPV IFA and that the majority of the antibodies detected by the hMPV IFA reacted with the hMPV F protein. Expression of the hMPV F antigen at a high level in a baculovirus expression system could increase the sensitivity for detection of antibodies against hMPV. Therefore, the conventional hMPV IFA might misidentify positive serum samples as false negative. Furthermore, it takes a few weeks to prepare the cells for the conventional hMPV IFA. Therefore, the Bac-F IFA is a useful alternative to the hMPV IFA.
The hMPV IFA-positive serum samples reacted weakly with the F protein of hMPV separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane, as shown in Fig. 1, suggesting that they were probably directed to conformational-type epitopes of the F protein. The antigenicity and immunogenicity of the F protein of RSV are also highly dependent on its three-dimensional structure (12).
Sequence analyses of hMPV isolates indicated that there are two major genetic lineages of hMPV (1). The predicted amino acids of hMPV F protein in these two lineages are highly conserved (2, 8). In our previous study, the hMPV IFA titers of human serum samples tested by LLC-MK2 cells infected with one lineage of hMPV (group 1, JPS03-178) were the same as those of human serum samples tested by cells infected with another lineage (group 2, JPS02-76) (5, 6). Recently, the hMPV F protein has been indicated to be a major antigenic determinant that mediated effective neutralization and protection against both lineages of hMPV in hamsters, chimpanzees, and rhesus macaques (13, 20, 22). Therefore, the Bac-F IFA system made with the recombinant hMPV F protein of one lineage (group 1, JPY88-12) could also detect IgG antibodies against the hMPV F protein of the other lineage (group 2). Detection of a specific antibody against the hMPV F protein by Bac-F IFA should be a useful means for investigating protective immunity.
We conclude that the availability of large quantities of baculovirus-expressed hMPV F protein offers an opportunity to use this recombinant protein as a diagnostic reagent in the IFA and to study antigenic and immunogenic characteristics of the F protein. Further study is needed to determine the false positivity or false negativity rate in the Bac-F IFA by measuring the antibody titers in the acute and convalescent phases after primary hMPV infection.
Acknowledgments
This study was partially supported by a grant-in-aid for exploratory research (14657179 [2002]) from the Ministry of Education, Culture, Sports, Science and Technology and by a grant-in-aid for the 21st Century Center of Excellence for Zoonosis Control from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
We thank Kunihiko Kobayashi, professor emeritus of Hokkaido University, for giving us suggestions and Stewart Chisholm for proofreading the manuscript.
REFERENCES
- 1.Bastien, N., S. Normand, T. Taylor, D. Ward, T. C. Peret, G. Boivin, L. J. Anderson, and Y. Li. 2003. Sequence analysis of the N, P, M and F genes of Canadian human metapneumovirus strains. Virus Res. 93:51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 315:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect. Dis. 186:1330-1334. [DOI] [PubMed] [Google Scholar]
- 4.Cane, P. A., B. G. van den Hoogen, S. Chakrabarti, C. D. Fegan, and A. D. Osterhaus. 2003. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 31:309-310. [DOI] [PubMed] [Google Scholar]
- 5.Ebihara, T., R. Endo, H. Kikuta, N. Ishiguro, H. Ishiko, M. Hara, Y. Takahashi, and K. Kobayashi. 2004. Human metapneumovirus infection in Japanese children. J. Clin. Microbiol. 42:126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebihara, T., R. Endo, H. Kikuta, N. Ishiguro, M. Yoshioka, X. Ma, and K. Kobayashi. 2003. Seroprevalence of human metapneumovirus in Japan. J. Med. Virol. 70:281-283. [DOI] [PubMed] [Google Scholar]
- 7.Freymouth, F., A. Vabret, L. Legrand, N. Eterradossi, F. Lafay-Delaire, J. Brouard, and B. Guillois. 2003. Presence of the new human metapneumovirus in French children with bronchiolitis. Pediatr. Infect. Dis. J. 22:92-94. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro, N., T. Ebihara, R. Endo, X. Ma, H. Kikuta, H. Ishiko, and K. Kobayashi. 2004. High genetic diversity of the attachment (G) protein of human metapneumovirus. J. Clin. Microbiol. 42:3406-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaaskelainen, A., X. Han, M. Niedrig, A. Vaheri, and O. Vapalahti. 2003. Diagnosis of tick-borne encephalitis by a mu-capture immunoglobulin M-enzyme immunoassay based on secreted recombinant antigen produced in insect cells. J. Clin. Microbiol. 41:4336-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jartti, T., B. van den Hoogen, R. P. Garofalo, A. D. Osterhaus, and O. Ruuskanen. 2002. Metapneumovirus and acute wheezing in children. Lancet 360:1393-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine, S., R. Klaiber-Franco, and P. R. Paradiso. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521-2524. [DOI] [PubMed] [Google Scholar]
- 12.Lopez, J. A., R. Bustos, C. Orvell, M. Berois, J. Arbiza, B. Garcia-Barreno, and J. A. Melero. 1998. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J. Virol. 72:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacPhail, M., J. H. Schickli, R. S. Tang, J. Kaur, C. Robinson, R. A. Fouchier, A. D. Osterhaus, R. R. Spaete, and A. A. Haller. 2004. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 85:1655-1663. [DOI] [PubMed] [Google Scholar]
- 14.Olmsted, R. A., N. Elango, G. A. Prince, B. R. Murphy, P. R. Johnson, B. Moss, R. M. Chanock, and P. L. Collins. 1986. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc. Natl. Acad. Sci. USA 83:7462-7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastey, M. K., and S. K. Samal. 1998. Baculovirus expression of the fusion protein gene of bovine respiratory syncytial virus and utility of the recombinant protein in a diagnostic enzyme immunoassay. J. Clin. Microbiol. 36:1105-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelletier, G., P. Dery, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8:976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pemberton, R. M., M. J. Cannon, P. J. Openshaw, L. A. Ball, G. W. Wertz, and B. A. Askonas. 1987. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J. Gen. Virol. 68(Pt. 8):2177-2182. [DOI] [PubMed] [Google Scholar]
- 18.Pereira, R. F., W. N. Paula, C. Cubel Rde, and J. P. Nascimento. 2001. Anti-VP1 and anti-VP2 antibodies detected by immunofluorescence assays in patients with acute human parvovirus B19 infection. Mem. Inst. Oswaldo Cruz 96:507-513. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Martinez, D., J. L. Patton, J. A. Stewart, and P. E. Pellett. 1995. Detection of Epstein-Barr virus-specific antibodies by means of baculovirus-expressed EBV gp125. J. Virol. Methods 52:145-153. [DOI] [PubMed] [Google Scholar]
- 20.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, J. M. Riggs, S. R. Surman, E. Amaro-Carambot, J. M. McAuliffe, W. R. Elkins, M. St Claire, P. L. Collins, and B. R. Murphy. 2004. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 78:6927-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockton, J., I. Stephenson, D. Fleming, and M. Zambon. 2002. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg. Infect. Dis. 8:897-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, R. S., J. H. Schickli, M. MacPhail, F. Fernandes, L. Bicha, J. Spaete, R. A. Fouchier, A. D. Osterhaus, R. Spaete, and A. A. Haller. 2003. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J. Virol. 77:10819-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Hoogen, B. G., T. M. Bestebroer, A. D. Osterhaus, and R. A. Fouchier. 2002. Analysis of the genomic sequence of a human metapneumovirus. Virology 295:119-132. [DOI] [PubMed] [Google Scholar]
- 25.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh, E. E., C. B. Hall, M. Briselli, M. W. Brandriss, and J. J. Schlesinger. 1987. Immunization with glycoprotein subunits of respiratory syncytial virus to protect cotton rats against viral infection. J. Infect. Dis. 155:1198-1204. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, E. E., and J. Hruska. 1983. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J. Virol. 47:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf, D. G., Z. Zakay-Rones, A. Fadeela, D. Greenberg, and R. Dagan. 2003. High seroprevalence of human metapneumovirus among young children in Israel. J. Infect. Dis. 188:1865-1867. [DOI] [PubMed] [Google Scholar]
- 29.Zhu, L., R. Wang, A. Sweat, E. Goldstein, R. Horvat, and B. Chandran. 1999. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8-infected BCBL-1 cells. Virology 256:381-392. [DOI] [PubMed] [Google Scholar]