Abstract
A dipstick dye immunoassay (DDIA) was developed to detect immunoglobulin G (IgG) or IgM antibodies of toxoplasmosis infection in humans. The assays employ a blue colloidal dye particles (D-1) conjugated to sheep anti-human IgG and rabbit anti-human IgM as the visualizing agents and a soluble antigen of tachyzoites of Toxoplasma gondii strain RH (TSA) as the detective antigen. The mixture of dye-labeled anti-human antibody-special human antibody was captured by TSA onto a nitrocellulose membrane dipstick by means of immunochromatography. The assays are rapid (the whole test can be completed within 15 min), simple, and cheap, and they don't require any equipment. They are sensitive and specific for the detection of anti-Toxoplasma IgG or IgM antibodies and generally agree closely with the results from the enzyme-linked immunosorbent assay. The assays are especially suitable for field applications.
Toxoplasma gondii infection is widespread in humans, although its prevalence varies widely from place to place. In the United States and the United Kingdom, it is estimated that 16 to 40% of the population are infected, whereas in Central and South America and continental Europe, estimates of infection range from 50 to 80% (4). Most infections in humans are asymptomatic but the parasite can produce devastating disease. In pregnancy, infection can result in congenital infection with severe sequelae or late-onset eye disease, and it is a frequent cause of encephalitis in severely immunosuppressed patients with AIDS (1, 12). Toxoplasmosis is also a serious complication following organ transplantation (2). In addition to being a major source of infection for humans, it is also of considerable importance in domestic animals and is responsible for abortions in sheep and swine (16). Therefore, there is an urgent need to develop an effective diagnostic kit and vaccine.
For clinical purposes, toxoplasmosis can be divided for convenience into five infection categories: (i) those acquired by immunocompetent patients, (ii) those acquired during pregnancy, (iii) those acquired congenitally, (iv) those acquired by or reactivated in immunodeficient patients, and (v) ocular infections. In any category, clinical presentations are not specific for toxoplasmosis, and a wide differential diagnosis must be considered. Furthermore, methods of diagnosis and their interpretations may differ for each clinical category.
Diagnosis of T. gondii infection or toxoplasmosis in humans is made by biological, serological, histological, or molecular methods or by some combination of these. Clinical signs of toxoplasmosis are nonspecific and are not sufficiently characteristic for a definite diagnosis. In fact, toxoplasmosis mimics several other infectious diseases. Detection of T. gondii antibodies (mainly immunoglobulin G [IgG] and IgM) in patients may aid diagnosis. IgG antibodies usually appear within 1to 2 weeks of acquisition of the infection, peak within 1 to 2 months, decline at various rates, and usually persist for life (6, 8). IgM antibodies may appear earlier and decline more rapidly than IgG antibodies, so the detection of IgG antibodies may be helpful for diagnosis of chronically infected patients, if IgM antibodies are negative. An IgM test is still used by most laboratories to determine if a patient has been infected recently or in the distant past; because of the hurdles posed in interpreting a positive IgM test result, confirmatory testing should always be performed (3, 9, 17).
There are numerous serological procedures available for the detection of humoral antibodies; these include the Sabin-Feldman dye test, the indirect hemagglutination assay, the indirect fluorescent antibody assay, the direct agglutination test, the latex agglutination test, the enzyme-linked immunosorbent assay (ELISA), and the immunosorbent agglutination assay test (13). Most of these immunodiagnostic tests are not easy to apply in the field, e.g., the ELISA or the indirect fluorescence antibody assay, since these techniques require special equipment and reagents. Performing any of these tests even in the laboratory generally takes time, sometimes with overnight incubation steps; otherwise, enzyme reagents would need a cold chain for delivery. In such situations, a rapid, simple, and inexpensive colorimetric assay with robust reagents and no instrumentation could have many diagnostic applications.
In this study, the dipstick dye immunoassay (DDIA) for detection of IgG or IgM antibodies of human toxoplasmosis was developed, sheep anti-human IgG or rabbit anti-human IgM conjugated with a colloidal dye produced in China served as the color-detecting reagents, and a soluble antigen of tachyzoites of T. gondii strain RH (TSA) on a nitrocellulose paper (NCP) membrane dipstick was used as the capture antigen. The DDIA assay for the detection of IgG or IgM antibodies of human toxoplasmosis was found to be rapid, simple, cheap, and effective.
MATERIALS AND METHODS
Serum samples.
Twenty-five serum samples were provided by Jack S. Remington, Toxoplasma Serology Laboratory, Palo Alto Medical Foundation Research Institute. Each of these sera was positive for both IgG (detected by the Sabin-Feldman dye test) and IgM (detected by IgM-ELISA), with different titers ranging from 1:128 to >1:16,000 (IgG) and different absorbance values ranging from 2.9 to 9.1 (IgM). Fifty serum samples negative for T. gondii were obtained from healthy subjects in the central blood station, Wuxi, China. Each serum sample was confirmed by ELISA to be negative for both anti-T. gondii IgG and IgM antibodies. A total of 172 serum samples were obtained from a group carrying out an epidemiological investigation of toxoplasmosis by serological methods. All sera were stored in aliquots at −20°C.
Antibodies for labeling and quality control.
Sheep anti-human IgG (IgG fragment of antiserum) was obtained from the Sino-American Biotech Company; the concentration of protein was 5 mg/ml. Rabbit anti-human IgM (IgG fragment of antiserum) (Sigma) was purchased from the Beijing Superior Chemical & Instruments Co., Ltd.; the protein concentration was 4 mg/ml. Sheep anti-rabbit IgG and rabbit anti-sheep IgG were purchased from the Beijing Biodee Biotech Co., Ltd.; the protein concentration of each was 2 mg/ml.
Antigen preparation.
The original inocula of T. gondii RH strain were peritoneal exudates from infected mice maintained in our laboratory. The exudates selected contained >108 Toxoplasma tachyzoites/ml. Each mouse was inoculated with 0.2 ml of the exudates, the mice were sacrificed 3 days later, and the peritoneal exudates were collected. The exudates were washed three times with physiological saline. The exudates were then filtered through a glass fiber column and then through a CF-11 cellulose column. The purified Toxoplasma tachyzoites were resuspended in phosphate-buffered saline (PBS), and freeze-thawed five times in a refrigerator at −20°C and a 37°C water bath. Exudates were sonicated and centrifuged, and the supernatant was collected as a soluble antigen of Toxoplasma tachyzoites (TSA). The concentration of TSA was 1.2 mg/ml as measured by a spectrophotometer, and then the concentration of TSA was adjusted to 1 mg/ml with physiological saline.
Colloidal dye preparation.
The colloidal dye (D-1) produced in China was used as described previously (18). The stock colloidal dye D-1 suspension was prepared as described by Snowden and Hommel (15). Briefly, 0.5 g of D-1 dye was suspended in 30 ml of deionized water and stirred overnight at room temperature. The suspension was washed six times by centrifugation at 20,000 × g for 30 min at room temperature, and the pellet was resuspended in an equal volume of double-distilled water. Aggregated colloidal particles were removed by low-speed centrifugation (500 × g; 30 min). This stock colloidal dye solution, with 0.01% thimerosal added as a preservative, was stored at 4°C.
Labeling sheep anti-human IgG or rabbit anti-human IgM with colloidal dye D-1.
A total of 5 ml of prepared dye suspension was mixed with 100 μl of sheep anti-human IgG (5 mg/ml) or 125 μl of rabbit anti-human IgM (4 mg/ml). The mixture was incubated overnight at room temperature. Then, bovine serum albumin was added at a final concentration of 5% to the mixture for another 2 h, after which it was centrifuged at 18,000 rpm for 30 min. The pellet was washed twice with PBS and dispersed in 5 ml of PBS with 0.01% thiomersol.
Dipstick preparation.
NCP membranes were purchased from Millipore (Bedford, Mass.). The dipsticks were prepared from NCP (3.0 by 0.3 cm). At the top and bottom of the dipstick, paper pads of 2.0 and 0.5 cm, respectively, were attached. At 1.0 cm from the bottom of the NCP, a band of TSA (about 3 μl per each band, at a concentration of 1 mg/ml) was applied as the detection band. At sites that were 1.0 cm from the top, another band of rabbit anti-sheep IgG (for IgG detection) or sheep anti-rabbit IgG (for IgM detection) was added as a control band.
IgG-DDIA and IgM-DDIA.
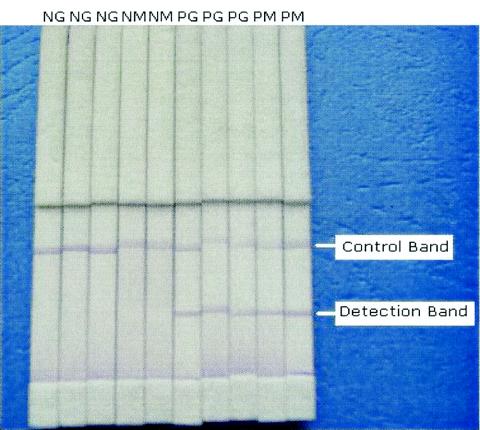
The IgG-DDIA kit included the sheep anti-human IgG conjugated with dye, prepared dipsticks (the control band was rabbit anti-sheep IgG), and cups, while the IgM-DDIA kit included the rabbit anti-human IgM conjugated with dye, prepared dipsticks (the control band was sheep anti-rabbit IgG), and cups. The detection procedure for the IgG-DDIA and IgM-DDIA was as follows: 10 μl of serum sample was placed in a small plastic cup (0.6 by 1.0 cm), and 50 μl of antibody-conjugated dye was added and mixed. The prepared dipstick was inserted in the cup for 5 to 15 min at room temperature. If both the detection band and the control band were colored purple-blue, the sample was recorded as positive. If the control band was colored purple-blue but the detection band was not colored, it was recorded as negative. If neither band was colored, the test reagents were assumed to be invalid (Fig. 1).
FIG. 1.
Examples of positive and negative results of IgG-DDIA and IgM-DDIA. NG, negative IgG-DDIA; NM, negative IgM-DDIA; PG, positive IgG-DDIA; PM, positive IgM-DDIA.
Sample detection with SERION ELISA classic T. gondii IgG and IgM kits.
SERION ELISA classic T. gondii IgG and IgM kits (virion/serion, Wurzburg, Germany) were purchased from the Wuxi Donglin Science and Technology Development Co., Ltd. The detection procedure was carried out according to the manufacturer's instructions for the IgG and IgM kits.
Stability of DDIA.
The stability of the dye-antibody conjugation was monitored as follows: the dye-antibody conjugation was stored at 4°C and checked after 1, 2, and 6 months and at room temperature after 1, 2, 3, and 4 weeks. The stability of the dipstick was monitored as follows: the dipstick was placed at in a refrigerator at 4°C for 6 months and at room temperature for 4 weeks.
RESULTS
Sensitivity and specificity.
Twenty-five serum samples that were positive for both IgG and IgM were used to test the sensitivity of the IgG-DDIA and IgM-DDIA; 50 serum samples from healthy subjects were also used to detect specificity (Table 1). The sensitivity and specificity of IgG-DDIA were 100 and 96%, respectively; the sensitivity and specificity of IgM-DDIA were 100 and 94%, respectively.
TABLE 1.
Sensitivity and specificity test of IgG- and IgM-DDIAa
| Serum sample | No. of samples | IgG-DDIA
|
IgM-DDIA
|
||
|---|---|---|---|---|---|
| Pos | Neg | Pos | Neg | ||
| Positive | 25 | 25 | 0 | 25 | 0 |
| Normal | 50 | 2 | 48 | 3 | 47 |
Pos, positive; Neg, negative.
Comparison between DDIA and ELISA.
A total of 172 serum samples obtained from an epidemiological study of toxoplasmosis were used to compare DDIA and ELISA (Table 2). The total rate of correspondence (i.e., the rate of positive correspondence and the rate of negative correspondence) for IgG detection between IgG-DDIA and IgG-ELISA was 97.7% (86 of 88 samples), 100% (52 of 52 samples), and 94.4% (34 of 36 samples), respectively. For IgM detection, the total rate of correspondence (the rate of positive correspondence and the rate of negative correspondence) between IgM-DDIA and IgM-ELISA was 97.6% (82 of 84 samples), 100% (35 of 35 samples), and 95.9% (47 of 49 samples). There was no significant difference between the two assays (P > 0.05).
TABLE 2.
Comparison of detection results obtained by DDIA and ELISA
| Results by IgG- or IgM-DDIA (n) | No. of samples with the following result by alternative kitsa
|
|
|---|---|---|
| Pos | Neg | |
| IgG-DDIA | ||
| Pos (54) | 52 | 2 |
| Neg (34) | 0 | 34 |
| IgM-DDIA | ||
| Pos (37) | 35 | 2 |
| Neg (47) | 0 | 47 |
The serum samples were tested by SERION ELISA classic T. gondii IgG or IgM kit, respectively. Pos, positive; Neg, negative.
Stability of DDIA.
The dye-labeled antibodies can be stored at 4°C for 6 months and at room temperature for 2 weeks without loss of activity. The dipstick kept its detective capacity after storage at 4°C for at least 6 months and at room temperature for 2 weeks.
DISCUSSION
The diagnosis of T. gondii infection or toxoplasmosis can be established by serologic tests, PCR, histological examination, or isolation of the parasite. T. gondii infection can be asymptomatic, and the clinical manifestations of patients with symptomatic toxoplasmosis are protean and nonspecific. The choice of the appropriate diagnostic method(s) and its (their) interpretation may differ for each clinical category. This study describes the rapid, simple, and inexpensive serodiagnostic tests for detection of IgG and IgM antibodies to human toxoplasmosis. The sensitivity and specificity were similar to those produced by ELISA (SERION ELISA classic T. gondii IgG and IgM kits), but the DDIA technique was more rapid and simpler to carry out, taking just 5 to 15 min and not requiring special equipment.
In the past, a number of modifications to the ELISA have been described in efforts to produce a more field-applicable assay format. The dot immunobinding assay, using an NCP membrane as a test matrix, is becoming widely used in simple qualitative research applications (11, 14). Colloidal gold-labeled antibodies are also used in dot blot assays to avoid use of the sometimes-problematic enzyme-labeled detecting antibodies (7, 10). Recently, some colloidal dye particles were screened to label antigen or antibody for the detection of antibody or antigen (5, 15, 19); compared with gold or enzyme, the colloidal dye is cheaper or easier to preserve. The colloidal dye particle technique utilizes the concepts of ELISA, dot blot assays, colloidal dye-labeled antigen or antibody, and immunochromatography to produce an inexpensive, robust, NCP-based dipstick test for antibody or antigen detection. Since it requires no instrumentation for qualitative detection of antigen or antibody, it has many potential field applications.
Previously, our laboratory used schistosome antigen conjugated with a colloidal dye produced in China to detect antibody on a NCP membrane dipstick based on immunochromatography. The DDIA for detecting antibody in patients with schistosomiasis showed very high sensitivity, specificity, and positive predictive value. The assay for detection of schistosomiasis in areas where the disease is endemic was found to be rapid, simple, cheap, and effective (19).
In this study, we successfully applied the blue colloidal dye particles (D-1) to label sheep anti-human IgG or rabbit anti-human IgM to detect IgG or IgM antibodies to human toxoplasmosis. To demonstrate the use of the IgG-DDIA and IgM-DDIA, 25 IgG- and IgM-positive serum samples and 50 serum samples from healthy subjects were tested and resulted in high sensitivity and good specificity. In comparing the two tests, there was no significant difference in sensitivity and specificity, but the DDIA was faster and much easier to perform than the commercial ELISA kit. The preparation and use of dye-antibody conjugations is similar to or even simpler than antibody-colloidal gold particle probes. The dye-labeled antibodies can maintain reactivity for at least 6 months when stored at 4°C in a liquid suspension, and the dipstick can also keep its detective capacity after storage at 4°C for at least 6 months.
It is important to mention that the presented data are only preliminary, but they give us a first impression about the suitability of such a test system for routine diagnostics. In other words, the DDIA which utilize sheep anti-human IgG or rabbit anti-human IgM-colloidal dye conjugates as the agents visualized could also be used for detection of specific antibodies in other infectious diseases, if there were specific detective antigens.
Acknowledgments
This research was supported by the Jiangsu Provincial Bureau of Health, People’s Republic of China (grant H2006), the Joint Research Management Committee (JRMC), Ministry of Health, People’s Republic of China, and WHO/TDR (grant J8-21).
REFERENCES
- 1.Araujo, F. G., and J. S. Remington. 1987. Toxoplasmosis in immunocompromised patients. Eur. J. Clin. Microbiol. 6:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, D., F. Foudrinier, I. Villena, J. M. Pinon, M. F. Biava, and E. Renoult. 1996. PCR for diagnosis and follow-up of two cases of disseminated toxoplasmosis after kidney grafting. J. Clin. Microbiol. 34:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobic, B., D. Sibalic, and O. Djurkovic-Djakovic. 1991. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary Toxoplasma infection. Gynecol. Obstet. Investig. 31:182-184. [DOI] [PubMed] [Google Scholar]
- 4.Dubey, J. P., and C. P. Beattie. 1988. Toxoplasmosis of animals and man. CRC Press, Boca Raton, Fla.
- 5.Eliades, P., E. Karagouni, I. Stergiatou, and B. Miras. 1998. A simple method for the serodiagnosis of human hydatid disease based on a protein A/colloidal dye conjugate. J. Immunol. Methods 218:123-132. [DOI] [PubMed] [Google Scholar]
- 6.Hedman, K., M. Lappalainen, I. Seppala, and O. Makela. 1989. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-739. [DOI] [PubMed] [Google Scholar]
- 7.Hus, Y. 1984. Immunogold for detection of antigen on nitrocellulose paper. Anal. Biochem. 142:221-225. [DOI] [PubMed] [Google Scholar]
- 8.Jenum, P. A., B. Stray-Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G activity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liesenfeld, O., C. Press, J. G. Montoya, R. Gill, J. L Isaac-Renton, K. Hedman, and J. S. Remington. 1997. False-positive results in immunoglobulin M (IgM) Toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J. Clin. Microbiol. 35:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moeremans, M, G. Daneels, and A. VanDijck. 1984. Sensitive visualization of antigen-antibody reactions in dot and blot immune overlay assays with immunogold and immunogold/silver staining. J. Immunol. Methods 74:353-360. [DOI] [PubMed] [Google Scholar]
- 11.Pappas, M., R. Hajkowski, and W. Hockmeyer. 1983. Dot enzyme-linked immunosorbent assay (dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J. Immunol. Methods 64:205-214. [DOI] [PubMed] [Google Scholar]
- 12.Pinon, J. M., C. Chemla, I. Villena, F. Foundrinier, D. Aubert, D. Puygauthier-Toubas, B. Leroux, D. Dupouy, C. Quereux, M. Talmud, T. Trenque, G. Potron, M. Pluot, G. Remy, and A. Bonhomme. 1996. Early neonatal diagnosis of congenital toxoplasmosis: value of comparative enzyme-linked immunofiltration assay immunological profiles and anti-Toxoplasma gondii immunoglobulin M (IgM) or IgA immunocapture and implication for postnatal therapeutic strategies. J. Clin. Microbiol. 34:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remington, J. S., R. McLeod, P. Thulliez, and G. Desmonts. 2000. Toxoplasmosis, p. 205-346. In J. S. Remington and J. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders, Philadelphia, Pa.
- 14.Scott, D. 1983. Immunoblotting and dot blotting. J. Immunol. Methods 119:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snowden, K., and M. Hommel. 1991. Antigen detection immunoassay using dipsticks and colloidal dyes. J. Immunol. Methods 140:57-65. [DOI] [PubMed] [Google Scholar]
- 16.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animal to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson, M., J. S. Remington, C. Clavet, G. Varney, C. Press, and D. Ware. 1997. Evaluation of six commercial kits for detection of human immunoglobulin M antibodies to Toxoplasma gondii. J. Clin. Microbiol. 35:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu, Y., C. Yu, X. Yin, and Y. Liu. 1995. Establishment and preliminary application of dot colloidal dye immunoassay for antibody detection in patients of schistosomiasis japonica. Chin. J. Schistosomiasis Control 7:214-218. [Google Scholar]
- 19.Zhu, Y., W. He, Y. Liang, M. Xu, C. Yu, W. Hua, and Q. Chao. 2002. Development of a rapid, simple dipstick dye immunoassay for schistosomiasis diagnosis. J. Immunol. Methods 266:1-5. [DOI] [PubMed] [Google Scholar]