Abstract
Buruli ulcer disease (BUD), caused by Mycobacterium ulcerans, follows an indolent course of initial progression to ulceration accompanied by extensive tissue damage. It has been suggested that healing disease stages are accompanied by a protective immune response. We hypothesized that interleukin-4 (IL-4)- or IL-10-induced downregulation of Th-1 responses plays a key role in the progression of early BUD and that healing is accompanied by an augmented Th-1 response. Gamma interferon (IFN-γ), IL-4, and IL-10 responses were measured after in vitro stimulation with phytohemagglutinin (PHA) and tuberculin purified protein derivative (PPD) of whole blood from 39 (23 early- and 16 late-stage) BUD patients and 39 healthy control subjects in Ghana. Additionally, 30 patients with active or treated tuberculosis (TB) serving as PPD-responsive positive controls were studied. Early-stage BUD patients produced significantly lower levels of IFN and IFN-γ/IL-4 ratios compared to late-stage BUD patients after PHA stimulation. Compared to that of controls, IFN-γ production after tuberculin stimulation was significantly higher in late-stage but not in early-stage BUD patients (P = 0.009). IL-10 and IL-4 levels did not differ between BUD patients and controls, although active TB patients had significantly higher IL-10 production levels than did treated TB patients. Multivariate analysis showed no confounding factors. In conclusion, Th-1 down regulation in early BUD appears to reverse in later stages of BUD, although an association with IL-10 or IL-4 production does not emerge from our data. Here we show differences in Th-1-type cytokine production between early- and late-stage BUD that might reflect an improved immune defense over time.
Mycobacterium ulcerans infection, commonly known as Buruli ulcer disease (BUD), causes a devastating disease that has emerged in western Africa (21). Its causative agent is a toxin-producing environmental mycobacterium that is endemic in restricted foci throughout the tropical world. BUD starts as a subcutaneous nodule or plaque that eventually ulcerates and may progress until surgical excision or spontaneous healing occurs. Ulcerative disease may lead to permanent disabilities such as contractures, while occasionally limb amputation is necessary. BUD follows an indolent course, with a paucity of inflammatory cells in lesions and predominantly negative M. ulcerans and Mycobacterium bovis purified protein derivative (PPD) skin tests (21). Tuberculin or burulin skin tests typically convert to positive over time (17), and intralesional influx of leukocytes has been reported in late stages, suggesting a change in inflammatory response (4, 8). A cellular Th-1-type immune response with high levels of gamma interferon (IFN-γ) is regarded as crucial for the host defense against mycobacteria (18). Australian BUD patients appear to exhibit Th-1-type anergy, with low IFN-γ production against M. ulcerans and M. bovis, and a Th-2-type (interleukin-4 [IL-4], IL-5, and IL-10) cytokine mRNA pattern (5, 6), suggesting Th-2-mediated Th-1 downregulation. IL-10 can facilitate both Th-2 and Th-1 downregulation and is not exclusively produced by Th-2 cells (3, 19). The cytokine mRNA pattern observed by Gooding et al. could therefore indicate either Th-2 preponderance or IL-10-mediated immunosuppression, necessitating quantitative IL-10 and IL-4 assessment. Active tuberculosis (TB) patients exhibit attenuated Th-1 responses that are more likely to be IL-10 than Th-2 induced (2, 16). Successful chemotherapy is associated with augmented IFN-γ production, clinically detectable approximately 42 days after the onset of treatment (1, 9, 11). Whether BUD is associated with a shift in T-helper subset responses is unclear. Therefore we assessed production of IFN-γ, IL-4, and IL-10 after whole-blood nonspecific (PHA) and tuberculin (PPD) stimulation in Ghanaian subjects with BUD in an active, early disease stage, as well as in late, healing disease stages. Moreover, we included both active and treated TB patients to serve as PPD-responsive positive controls. We developed an incubation method suitable for laboratory facilities in resource-limited environments.
MATERIALS AND METHODS
Patients and controls. (i) BUD patients.
Forty-five BUD patients were included who were diagnosed by experienced clinicians working in the Agogo Presbyterian hospital or the Dunkwa government hospital. These hospitals serve the Ashanti-Akim-North and Upper Denkyira districts of Ghana, where the disease is highly endemic. On the basis of the judgment of photographs, BUD patients were retrospectively divided into early- and late-stage groups by an independent experienced physician (T.S.W.) blind to the cytokine production results. The early-stage group consisted of all patients who either had active disease or had had surgery in the past 42 days. The late-stage group consisted of patients who had been surgically treated >42 days earlier or showed signs of spontaneous healing.
(ii) Healthy volunteers.
Forty-one healthy volunteers age, sex, and community matched to the BUD patients were included as controls. Controls had no history of or close contacts with TB or leprosy.
(iii) Pulmonary TB patients.
Thirty-seven pulmonary TB patients whose diagnosis was confirmed by three successive acid-fast bacillus smear-positive sputum specimens. Twenty-six TB patients on anti-TB treatment for <42 days were assigned to the active group, while 11 patients either cured or on treatment for more than 42 days were considered treated. The institutional review board (ethics committee) in Groningen, The Netherlands, and the institutional review board of the Ministry of Health in Accra, Ghana, approved the protocol. After written informed consent was obtained, peripheral blood samples were collected in heparinized Vacutainers (Becton Dickinson, Eerembodegem-Aalst, Belgium). For all subjects, age, sex, body mass index (BMI, kilograms per square meter), and the presence of a BCG vaccination scar were noted. For BUD patients, the extent of disease was divided into covering more or less than one-fourth of an extremity or a corresponding lesion elsewhere on the body surface. Furthermore, the days after surgical excision and the current or previous use of antimycobacterial chemotherapy were noted.
Antigens.
The antigens used included PHA (murex) dissolved in RPMI 1640 medium (BioWhittaker Europe, with gentamicin at 100 μg/ml) at a final concentration of 1 mg/ml and bovine tuberculin PPD (tuberculin 5000; ID-DLO Lelystad, The Netherlands) dialyzed overnight in phosphate-buffered saline to remove 0.5% phenol and diluted in RPMI 1640 medium to a final concentration of 1 mg/ml.
Culture.
Blood was processed within 2 h. Aliquots (200 μl) of whole blood were diluted 1:5 in 24-well tissue culture plates (Costar, Badhoevedorp, The Netherlands) preloaded with 800 μl of RPMI 1640 medium with or without antigens. Furthermore, 200 μl of whole blood and 800 μl of RPMI 1640 medium were centrifuged for 5 min, and supernatants were recovered immediately and stored along with the remaining serum at −20°C.
Incubation.
Culture plates were either placed at 37°C in 5% CO2 or, by a new incubation method, first placed in a glass container with a burning candle enclosed. The container was then covered air tight with a petrolatum-coated glass lid. When the candle faded, the container was incubated at 37°C. Air tightness was maintained until the end of each incubation period. Supernatants were recovered immediately and stored at −20°C until assayed. Samples stimulated in Ghana were transported to The Netherlands in a polystyrene box containing dry ice. Next, all samples were stored again at −20°C until assay.
IL-10, IFN-γ, and IL-4 assays.
IFN-γ and IL-4 concentrations were determined with the PeliKine Compact IFN-γ- and IL-4 enzyme-linked immunosorbent assay (ELISA) kits in accordance with the guidelines provided by the supplier (Central Laboratory of the Blood Transfusion Service, Amsterdam, The Netherlands). IL-10 concentrations were determined as described previously (14). Optical densities at 450 to 575 nm were measured with SOFTMAX software. Detectability thresholds were determined on the basis of standard curves established with 160 pg of IFN-γ and IL-4 and 80 pg of IL-10. Twofold dilutions were used with IFN-γ for practical reasons. Values from unstimulated cultures were subtracted from stimulated cultures, and negative products were considered nil. After all of the samples were assayed, a random set of samples was remeasured and the variability (mean value [%] = 1/mean × 100) was determined to be less than 25% in 90% of the IL-4, 93% of the IFN-γ, and 92% of the IL-10 measurements.
HIV ELISA and Western blotting.
The presence of anti-human immunodeficiency virus (HIV) antibodies was detected by ELISA. Positive results were confirmed by Western blotting.
Validation of the novel incubation method.
First heparinized blood from two healthy, BCG-vaccinated volunteers was incubated by both the CO2 method and the novel incubation method. Diluted whole blood was incubated for 24, 48, and 72 h with either PHA or PPD at a final concentration of 10 μg/ml. The ideal incubation time for production of IL-10, IL-4, and IFN-γ was determined to be 48 h for PHA-stimulated cultures and 72 h for PPD-stimulated cultures. Between the CO2 and container methods, the concentrations differed 14 to 29% for IFN-γ, 17 to 23% for IL-4, and 2 to 6% for IL-10 with PHA and 5 to 9% for IFN-γ and 0 to 2% for IL-10 with tuberculin. IL-4 concentrations were low or undetectable in PPD-stimulated cultures. Having proved that the novel method for incubation was equal to incubation with CO2 at 5%, we used this novel method for further stimulation experiments in Ghana. Next, blood from one HIV-negative active TB patient was cultured with PPD by incubation for 48, 72, 96, and 168 h. No further increase in IFN-γ and IL-10 concentrations were determined after 72 h of stimulation. IL-4 concentrations again were low or undetectable in all supernatants. All further research was done with tuberculin and PHA at 10 μg/ml for 72 and 48 h, respectively.
Statistical analysis.
Descriptive results of cytokine levels are expressed as medians and interquartile ranges (IQRs). Cytokines were evaluated for an association with disease status (early- versus late-stage BUD or control) with the Kruskal-Wallis test. To be included for further analysis by the Mann-Whitney U test, a P value of ≤0.10 was used; after Bonferroni correction, P < 0.033 was considered statistically significant. The variables extent of lesion, use of chemotherapy, number of days after treatment, BMI, BCG, gender, and age were all evaluated for an association or correlation with the measured cytokine responses with the Pearson χ2 test for categorical data and the Mann-Whitney U test for numerical data; P ≤ 0.05 was considered statistically significant. The cytokines that showed an association with disease status (early- versus late-stage BUD or control) were natural log transformed. In the case of a value of zero, the natural logarithm of the original value plus one was calculated. Variables that showed an association or correlation with any of the measured cytokine responses were included in the multivariate analysis. Multiple linear regression analysis, in which the cytokine concentration was treated as a continuous variable, was performed with the SPSS software package, version 10.0 (SPSS, Chicago, Ill.).
RESULTS
Six BUD patients, four controls, and three TB patients were excluded because of undetectable IFN-γ and IL-10 levels after PHA stimulation, and four TB patients were excluded because of HIV seropositivity, leaving 39 (23 early- and 16 late-stage) BUD patients, 39 healthy controls, and 30 TB patients (20 active and 10 treated) for analysis. Spontaneous production was negligible in unstimulated cultures. IL-4 was not detected in PPD-stimulated supernatants.
BUD patients versus controls.
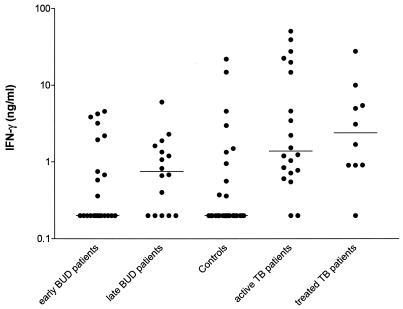
BUD patients had significantly lower PHA-induced and higher PPD-induced IFN-γ production than did controls. No differences were detected in the production of other cytokines or in cytokine ratios in PHA cultures.
Differences between disease stage groups (early versus late) and controls.
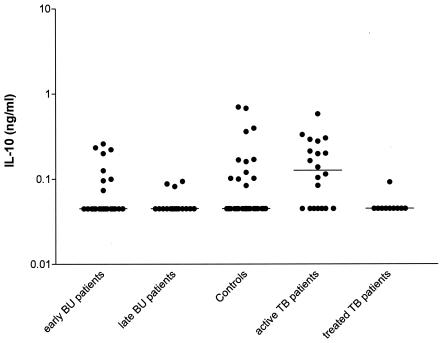
In general, significant differences were detected between groups in IFN-γ production and the IFN-γ/IL-4 ratio after stimulation with PHA and in IFN-γ production after stimulation with PPD (Table 1). Early-stage BUD patients had significantly lower PHA-induced IFN-γ levels than both late-stage BUD patients and controls (P = 0.003 and P = 0.001, respectively, Table 1). Early-stage BUD patients had significantly lower IFN-γ/IL-4 ratios than late-stage BUD patients (P = 0.021), but levels of both BUD groups were comparable to those of controls. PPD-responsive IFN-γ production was comparable between controls and early-stage BUD patients and between early- and late-stage BUD patients. However, late-stage BUD patients produced significantly higher IFN-γ levels than did controls (P = 0.009; Fig. 1 and Table 1). PPD-induced IL-10 production was generally low, and no differences were detected between groups (Table 1 and Fig. 2). IFN-γ production was significantly lower in PPD-stimulated cultures from late-stage BUD patients than in those from both active and treated TB patients (P = 0.0416 and P = 0.0398, respectively). Differences after PPD stimulation could not be accounted for by technical difficulties, as practically all TB patients produced detectable IFN-γ levels.
TABLE 1.
Differences between disease stage groups and controlsa
| Stimulus | Total BUD group (n = 39) | Early BUD (n = 23) | Late BUD (n = 16) | Controls (n = 37) |
P valueb
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Overall groups (Kruskal- Wallis test) | Early vs late | Late vs control | Early vs control | BUD total vs controls | |||||
| IL-10 PHA | 2.12 (1.0-3.7) | 2.0 (1.0-3.7) | 2.5 (1.7-5.8) | 2.8 (1.8-6.0) | 0.136 | 0.10 | |||
| IL-4 PHA | 0.51 (0.41-0.78) | 0.56 (0.41-0.83) | 0.48 (0.40-0.74) | 0.64 (0.43-0.94) | 0.163 | 0.07 | |||
| IFN-γ PHA | 10.4 (6.2-15.8) | 7.4 (4.3-11.3) | 15.2 (9.3-20.9) | 15.5 (7.7-32.5) | 0.001b | 0.003c | 0.771 | 0.001c | 0.02d |
| IFN-γ/IL-4 PHA | 21.0 (9.0-37.8) | 15.9 (4.8-31.0) | 32.9 (18.6-47.0) | 21.5 (9.1-43.1) | 0.072b | 0.021c | 0.323 | 0.069 | 0.48 |
| IFN-γ/IL-10 PHA | 4.96 (2.1-8.1) | 4.0 (1.9-6.4) | 5.0 (2.7-8.7) | 4.0 (1.7-11.0) | 0.541 | 0.53 | |||
| IL-10 tuberculin | 0.00 (0.0-0.08) | 0 (0-0.1) | 0 (0-0) | 0 (0-0.1) | 0.330 | 0.48 | |||
| IFN-γ tuberculin | 0.40 (0.0-1.6) | 0 (0-2.0) | 0.8 (0-1.6) | 0 (0-0.37) | 0.038* | 0.260 | 0.009c | 0.208 | 0.03d |
Values represent medians and IQRs (25th and 75th percentiles) of cytokine production (nanograms per milliliter) and ratios between cytokine concentrations in PHA-stimulated samples. P values represent overall analysis of early- and late-stage Buruli ulcer patients and control participants and comparisons between groups.
Overall group (early stage versus late stage versus controls), P < 0.10 considered statistically significant.
After Bonferroni correction; P < 0.033 considered statistically significant.
Mann-Whitney U-test, P < 0.05 considered statistically significant.
FIG. 1.
IFN-γ production after in vitro whole-blood stimulation with tuberculin in early- and late-stage BUD patients, healthy control subjects, and active and treated TB patients.
FIG. 2.
IL-10 production after in vitro whole-blood stimulation with tuberculin in early- and late-stage BUD patients, healthy control subjects, and active and treated TB patients.
TB patients.
Active TB patients produced significantly higher PPD-responsive IL-10 levels than did treated TB patients (P = 0.0075, Fig. 2), but differences in IFN-γ production did not reach statistical significance. No differences were detected in cytokine production levels or ratios in PHA-stimulated cultures.
Patient and control variables and cytokine levels.
The BMI showed a low correlation with PHA-stimulated IFN-γ production (P = 0.048, ρ = 0.23) and tuberculin-stimulated IL-10 production (P = 0.03, ρ = 0.26). Age showed a correlation with PHA-stimulated IFN-γ concentrations (ρ = 0.44, P < 0.01), with IFN-γ divided by IL-4 (PHA stimulated) (ρ = 0.45, P < 0.01), with IFN-γ divided by IL-10 (ρ = 0.39, P < 0.01), and with IL-10 stimulated by tuberculin (ρ = −0.23, P < 0.05). In patients with extensive lesions, the median IFN-γ level stimulated with tuberculin was 1.5 (IQR, 0.3 to 3.4), whereas the median IFN-γ (tuberculin) level in patients with minimal lesions was 0.0 (IQR, 0.0 to 0.7). Therefore, BMI, age, and the extent of the lesion (if applicable) were included in the linear regression analysis. Both the (PHA-stimulated) IFN-γ levels and the (PHA-stimulated) level of IFN-γ divided by IL-4 were statistically significantly higher in the late-stage BUD patients compared with those of early-stage BUD patients. This difference was independent of age, BMI, and lesion extent (Table 2). Age was another independent determinant of the cytokine responses. The (PHA-stimulated) IFN-γ level was lower in the overall BUD group than in the controls. This difference was independent of age and BMI (Table 3). Levels of IFN-γ stimulated by tuberculin were higher in the overall BUD group than in the control group. This difference was independent of age and BMI (Table 3). Age was another independent determinant of the response of both of these cytokines, while BMI only influenced the IFN-γ response to tuberculin (Table 3).
TABLE 2.
Linear regression model for patients with early- and late-stage BUD
| Parameter | IFN-γ stimulated by PHA
|
IFN-γ/IL-4 stimulated by PHA
|
||
|---|---|---|---|---|
| Bb | SEc | Bb | SEc | |
| Early- vs late-stage BUD | 0.71a | 0.29 | 0.76a | 0.05 |
| Age (yr) | 0.05a | 0.02 | 0.06a | 0.02 |
| Extent of lesion | −0.25 | 0.31 | −0.19 | 0.63 |
| BMI (kg/m2) | −0.08 | 0.05 | −0.10 | 0.09 |
P < 0.05.
B, estimate of the change in the dependent variable that can be attributed to a change of 1 U in the independent variable.
SE, standard error.
TABLE 3.
Linear regression model for BUD patients versus controls
| Parameter | IFN-γ
|
|||
|---|---|---|---|---|
| Stimulated by PHA
|
Stimulated by tuberculin
|
|||
| Bb | SEc | Bb | SEc | |
| BUD patients vs controls | 0.45a | 0.20 | −1.80a | 0.82 |
| Age (yr) | 0.05a | 0.02 | 0.13a | 0.06 |
| BMI (kg/m2) | −0.03 | 0.04 | −0.40a | 0.18 |
P < 0.05.
B, estimate of the change in the dependent variable that can be attributed to a change of 1 U in the independent variable.
SE, standard error.
DISCUSSION
We were able to study cellular immune responses in a resource-poor environment where BUD is endemic with a simple but effective method to maintain pCO2 at around 5 kPa in the incubator jar with cell culture plates by letting a lit candle go out while sealing the top air tight.
Our results show differences in PHA-responsive IFN-γ production between the early and later stages of BUD. These results could reflect a restoration of previously depleted circulating IFN-γ-producing T cells in later stages. Overall, BUD patients produced higher IFN-γ levels than did matched community controls. However, when divided, only late-stage BUD patients produced higher IFN-γ levels than did controls. The increase in IFN-γ production that we found after nonspecific and mycobacterium-specific stimulation suggests an augmented Th-1 response in the late, healing stages of BUD. The fact that early-stage BUD patients and controls have comparable levels of PPD-induced IFN-γ production may reflect Th-1 downregulation in the early stages. Strangely, healthy community controls from areas of Ghana, where the disease is endemic, as opposed to Australian controls, do not appear responsive to common mycobacterial antigens (6). Moreover, the presence of a BCG scar was no independent predictor of PPD-induced IFN-γ production. This may reflect the fact that immune responses elicited by BCG are only short-lived; BCG vaccination in Ghana is typically carried out in the first week after birth. No evidence emerged for Th-2 preponderance in either early or late stages of BUD. This might, however, reflect technical difficulties in peripheral blood cultures, as detectable IL-4 levels were rarely produced. IL-10 levels were not elevated in BUD patients. This was not due to technical problems, as increased production was detected in active TB patients, consistent with other reports (9). Thus, the mechanism for Th-1 downregulation and change in IFN-γ response does not emerge from our data. These findings are all independent of the possible confounding factors listed in Materials and Methods. Whether the augmented IFN-γ production seen reflects protective immunity is unknown.
Recently, Prévot et al. evaluated cytokine production by peripheral whole-blood mononuclear cells in response to M. bovis BCG and M. ulcerans in nodular and ulcerative BUD patients and PPD-sensitized controls. Concomitantly, they compared intralesional cytokine mRNA levels (IFN-γ, IL-10, and IL-4, among others) between nodular and ulcerative lesions (15). These investigators too found marked Th-1 downregulation in BUD patients. However, in M. ulcerans-stimulated cultures, but not in M. bovis-stimulated cultures, IL-10 concentrations were significantly higher for BUD patients than for controls. Furthermore, patients with ulcerative rather than nodular lesions had significantly lower IFN-γ and higher IL-10 production in M. ulcerans-stimulated cultures only, mirrored by similar changes in intralesional mRNA levels. IL-4 mRNA was detected at very low levels, and IL-4 was undetectable in peripheral whole-blood mononuclear cell cultures, suggesting IL-10-mediated instead of Th-2-mediated Th-1 downregulation. Australian data substantiate these findings to some extent, as both IL-4 and IL-10 mRNAs in M. ulcerans-stimulated supernatants were detected nonquantitatively (6). Interestingly, Prévot et al. also found immune response differences depending on BUD stages. The fact that we did not find elevated IL-10 concentrations in active disease substantiates the M. ulcerans-specific nature of IL-10 production, as our data correspond to the M. bovis stimulation experiment results of Prévot et al. Similar divergent T-cell responses to different mycobacterial antigens have been reported in leprosy (10). Although it is not apparent from our data, it thus seems likely that Th-1 downregulation is induced by antigen-specific IL-10 production. The elevated IL-10 level seen could be either monocytic or T-cell derived (2, 12).
In conjunction with tuberculin PPD, we tried whole-blood cultures stimulated with burulin, the PPD of M. ulcerans (17). (courtesy of J. Stanford, University College London, London, United Kingdom). IFN-γ and IL-10 levels were detectable in very few cultures from the BUD, control, and TB groups. It is tempting to hypothesize a possible role for mycolactone, a polyketide toxin produced by M. ulcerans that has profound immunosuppressive activity (13). Gooding et al. reported a case of culture-confirmed M. ulcerans infection in one of the healthy household contacts included in their earlier study (7). Interestingly, 3 months after surgical excision, a marked decrease in M. ulcerans-responsive IFN-γ production and a change from a Th-1-type to a Th-2-type cytokine mRNA pattern were found, suggesting an M. ulcerans-induced immune shift. This calls for more research into the immunosuppressive activity of mycolactones in vivo. Future evaluation of immune response changes from early to later stages of BUD should have a prospective study design. A decrease in IL-10 in parallel with an increase in IFN-γ levels seems likely.
Our study has several limitations. Diagnosis of BUD was made by best clinical judgment. Although the diagnosis was based both on the clinical course and on the typical initial presentation, it is conceivable that some patients were erroneously diagnosed with BUD. However, existing diagnostic tools lack either sensitivity or specificity, thereby inducing bias (20). Furthermore, an arbitrary and heterogeneous division into early- and late-stage groups was partially based on suggested timing of the immune shift witnessed in TB. The number of days after surgery was no independent predictor of PPD-responsive IFN-γ production; nevertheless, a more rigid division into active and healing stages of BUD might yield more clearly different immune response patterns. Future research should reveal if augmented IFN-γ responses are transient or persistent. Furthermore, as mentioned above, a different immune response pattern could emerge with M. ulcerans-specific antigens. Nonetheless, our data provide the first evidence of augmented IFN-γ responses in the healing stages of BUD.
Acknowledgments
Y. Stienstra received a research grant from NWO-WOTRO (Netherlands Scientific Research Foundation). B. D. Westenbrink received a grant from the Junior Scientific Master Class (division of the GUIDE Research School), travel allowances from the Marco Polo Fund, and a laboratory bench fee from the J. K. de Cock Foundation.
REFERENCES
- 1.Al Attiyah, R., A. S. Mustafa, A. T. Abal, N. M. Madi, and P. Andersen. 2003. Restoration of mycobacterial antigen-induced proliferation and interferon-gamma responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol. Med. Microbiol. 38:249-256. [DOI] [PubMed] [Google Scholar]
- 2.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottrez, F., S. D. Hurst, R. L. Coffman, and H. Groux. 2000. T regulatory cells 1 inhibit a Th2-specific response in vivo. J. Immunol. 165:4848-4853. [DOI] [PubMed] [Google Scholar]
- 4.Dobos, K. M., E. A. Spotts, B. J. Marston, C. R. Horsburgh, Jr., and C. H. King. 2000. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg. Infect. Dis. 6:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooding, T. M., P. D. Johnson, D. E. Campbell, J. A. Hayman, E. L. Hartland, A. S. Kemp, and R. M. Robins-Browne. 2001. Immune response to infection with Mycobacterium ulcerans. Infect. Immun. 69:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooding, T. M., P. D. R. Johnson, M. Smith, A. S. Kemp, and R. M. Robins-Browne. 2002. Cytokine profiles of patients infected with Mycobacterium ulcerans and unaffected household contacts. Infect. Immun. 70:5562-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooding, T. M., A. S. Kemp, R. M. Robins-Browne, M. Smith, and P. D. R. Johnson. 2003. Acquired T-helper 1 lymphocyte anergy following infection with Mycobacterium ulcerans. Clin. Infect. Dis. 36:1076-1077. [DOI] [PubMed] [Google Scholar]
- 8.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch, C. S., Z. Toossi, C. Othieno, J. L. Johnson, S. K. Schwander, S. Robertson, R. S. Wallis, K. Edmonds, A. Okwera, R. Mugerwa, P. Peters, and J. J. Ellner. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J. Infect. Dis. 180:2069-2073. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J., K. Uyemura, M. K. Van Dyke, A. J. Legaspi, T. H. Rea, K. Shuai, and R. L. Modlin. 2001. A role for IL-12 receptor expression and signal transduction in host defense in leprosy. J. Immunol. 167:779-786. [DOI] [PubMed] [Google Scholar]
- 11.Marchant, A., A. Amedei, A. Azzurri, J. Vekemans, M. Benagiano, C. Tamburini, C. Lienhardt, T. Corrah, K. P. McAdam, S. Romagnani, M. M. D'Elios, and G. Del Prete. 2001. Polarization of PPD-specific T-cell response of patients with tuberculosis from Th0 to Th1 profile after successful antimycobacterial therapy or in vitro conditioning with interferon-alpha or interleukin-12. Am. J. Respir. Cell Mol. Biol. 24:187-194. [DOI] [PubMed] [Google Scholar]
- 12.Misra, N., M. Selvakumar, S. Singh, M. Bharadwaj, V. Ramesh, R. S. Misra, and I. Nath. 1995. Monocyte derived IL 10 and PGE2 are associated with the absence of Th 1 cells and in vitro T cell suppression in lepromatous leprosy. Immunol. Lett. 48:123-128. [DOI] [PubMed] [Google Scholar]
- 13.Pahlevan, A. A., D. J. Wright, C. Andrews, K. M. George, P. L. Small, and B. M. Foxwell. 1999. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-κB function. J. Immunol. 163:3928-3935. [PubMed] [Google Scholar]
- 14.Popa, E. R., C. F. Franssen, P. C. Limburg, M. G. Huitema, C. G. Kallenberg, and J. W. Cohen Tervaert. 2002. In vitro cytokine production and proliferation of T cells from patients with anti-proteinase 3- and antimyeloperoxidase-associated vasculitis, in response to proteinase 3 and myeloperoxidase. Arthritis Rheum. 46:1894-1904. [DOI] [PubMed] [Google Scholar]
- 15. Prévot, G., E. Bourreau, H. Pascalis, R. Pradinaud, A. Tanghe, K. Huygen, and P. Launois. 2004. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect. Immun. 72:958-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song, C. H., H. J. Kim, J. K. Park, J. H. Lim, U. O. Kim, J. S. Kim, T. H. Paik, K. J. Kim, J. W. Suhr, and E. K. Jo. 2000. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect. Immun. 68:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanford, J. L., W. D. L. Revill, W. J. Gunthorpe, and J. M. Grange. 1975. Production and preliminary investigation of burulin, a new skin-test reagent for Mycobacterium-ulcerans infection. J. Hyg. 74:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stienstra, Y., W. T. van der Graaf, G. J. te Meerman, T. H. The, L. F. de Leij, and T. S. van der Werf. 2001. Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors. Trop. Med. Int. Health 6:554-562. [DOI] [PubMed] [Google Scholar]
- 19.van den Biggelaar, A. H., R. van Ree, L. C. Rodrigues, B. Lell, A. M. Deelder, P. G. Kremsner, and M. Yazdanbakhsh. 2000. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356:1723-1727. [DOI] [PubMed] [Google Scholar]
- 20.van der Werf, T. S., T. Stinear, Y. Stienstra, W. T. A. van der Graaf, and P. L. Small. 2003. Mycolactones and Mycobacterium ulcerans disease. Lancet 362:1062-1064. [DOI] [PubMed] [Google Scholar]
- 21.van der Werf, T. S., W. T. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]