Abstract
Canine prostate cancer (cPCa) is an untreatable malignant neoplasm resulting in local tissue invasion and distant metastasis. MicroRNAs (miRs) are small non-coding RNAs that function as oncogenes or tumor suppressors. The purpose of this study was to characterize the expression of miRs that are altered in cPCa tissue. The expression levels of 277 mature miRs in prostatic tissue (n=5, respectively) were compared between the non-tumor and tumor groups using real-time PCR. Five miRs (miR-18a, 95, 221, 222 and 330) were up-regulated, but 14 miRs (miR-127, 148a, 205, 299, 329b, 335, 376a, 376c, 379, 380, 381, 411, 487b and 495) were down-regulated specifically in cPCa (P<0.05). These miRs have potential use as early diagnosis markers for cPCa and in miR-based therapy.
Keywords: canine, expression profiling, microRNA, prostate cancer, real-time PCR
Canine prostate cancer (cPCa) is a malignant neoplasm that causes local invasion of surrounding tissue and early metastasis to the lymph node, lung and bone [4, 18, 29]. The incidence of cPCa is 0.2−0.6%, which is lower than found in humans [4, 22]. Most cases of cPCa are characterized histopathologically as prostatic adenocarcinoma or transitional cell tumor [17]. Androgens contribute to the development of human PCa (hPCa); however, it has been reported that the etiology of cPCa may not be related to androgens [22], which is supported by an immunohistochemical study showing that most cPCa originates from the ductal or urothelial epithelium in an androgen-independent process [26]. In fact, the risk of cPCa in castrated dogs was increased compared with that in intact dogs [16]. Currently, there is no effective therapy for cPCa.
Mature microRNAs (miRs) are small, single-stranded, noncoding RNAs approximately 18−25 nucleotides in length that bind to the 3′-untranslated region of target messenger RNAs (mRNAs) and regulate protein expression via mRNA degradation or translational repression [1, 7, 27]. MiRs play an important role in biological phenomena, such as development, differentiation, proliferation, cell migration and apoptosis [2, 31]. The deregulation of miR expression is involved in tumorigenesis, and some miRs are known to act as oncogenes or tumor-suppressor genes [2]. Several miRs are up- or down-regulated in hPCa tissue during pathogenesis, and miRs have great potential as diagnostic or prognostic biomarkers and novel therapeutic targets in PCa [24]. However, the change of miR expression in cPCa tissue has not been elucidated.
In this study, quantitative real-time PCR was employed to investigate the expression levels of 277 miRs in canine non-tumor prostatic tissue and prostatic adenocarcinoma tissue to identify miRs associated with the pathological process in this type of cancer.
Tissue from two intact healthy male dogs, two intact male dogs with benign prostatic hyperplasia (BPH) that showed mild dysuria and dyschezia, and one castrated dog with moderate chronic prostatitis was used for the non-tumor group, and tissue from five castrated male dogs with pathologically diagnosed prostatic adenocarcinoma was used for the tumor group (Table 1). All prostate biopsies were performed under general anesthesia. The animal study was approved by the Animal Care and Use Committee of Nippon Veterinary and Life Science University (approval no. 26S-9).
Table 1. Clinical profile of the 10 male dogs used in this study.
| Case number | Breed | Sterilization | Age (years) | Diagnosis | Metastasis |
|---|---|---|---|---|---|
| Non-tumor group | |||||
| 1 | Beagle | I | 1 | Normal | − |
| 2 | Beagle | I | 1 | Normal | − |
| 3 | Beagle | I | 9 | BPH | − |
| 4 | Mixed | I | 9 | BPH | − |
| 5 | Border collie | N | 10 | Prostatitis | − |
| Tumor group | |||||
| 6 | Miniature dachshund | N | 12 | PCa | None |
| 7 | Russell terrier | N | 10 | PCa | Lymph node |
| 8 | Miniature dachshund | N | 9 | PCa | None |
| 9 | Welsh corgi | N | 9 | PCa | Lymph node |
| 10 | Shetland sheepdog | N | 12 | PCa | None |
I, intact; N, neutered; BPH, benign prostatic hyperplasia; PCa, prostate cancer (prostatic adenocarcinoma).
Tissue samples were homogenized in QIAzol Lysis Reagent (QIAGEN, Frederick, MD, U.S.A.) and total RNA was extracted, and reverse transcribed using the miRNeasy Mini Kit and miScript II RT Kit (QIAGEN). To profile mature miRs, real-time PCR was performed using a canine miRNome miScript miRNA PCR Array and miScript SYBR Green PCR Kit (QIAGEN). This array analyzes 277 mature miRs and six control RNAs (SNORD61, SNORD68, SNORD72, SNORD95, SNORD96A and RNU6-2). A thermal cycler (Applied Biosystems, Foster City, CA, U.S.A.) was used with the following real-time PCR run protocol: 95°C for 15 min for activation of the DNA polymerase and 40 amplification and quantification cycles (94°C for 15 sec, 55°C for 30 sec and 70°C for 34 sec) with fluorescence data collection. The threshold cycle number (Ct) was recorded automatically by setting baseline and threshold levels. The level of miR expression was calculated using the 2-ΔΔCt method. Data (CtmiR) were normalized by averaging the Ct value of six control RNAs (Ctcontrol) using the formula: ΔCt=CtmiR−Ctcontrol. To evaluate the relative change in miR expression between tumor and non-tumor tissue, the following formula was used: ΔΔCt=ΔCttumor−ΔCtnon-tumor; 2-ΔCt was calculated and used to plot miR expression. The unpaired Student’s t-test was used to compare miR expression, and a P-value <0.05 was considered statistically significant.
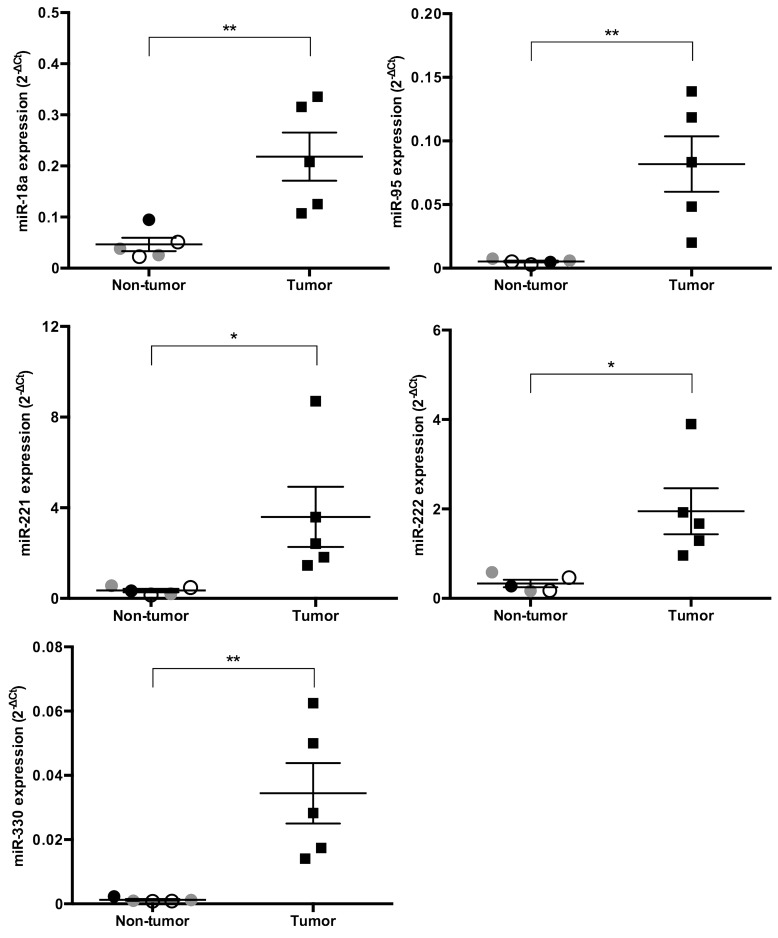
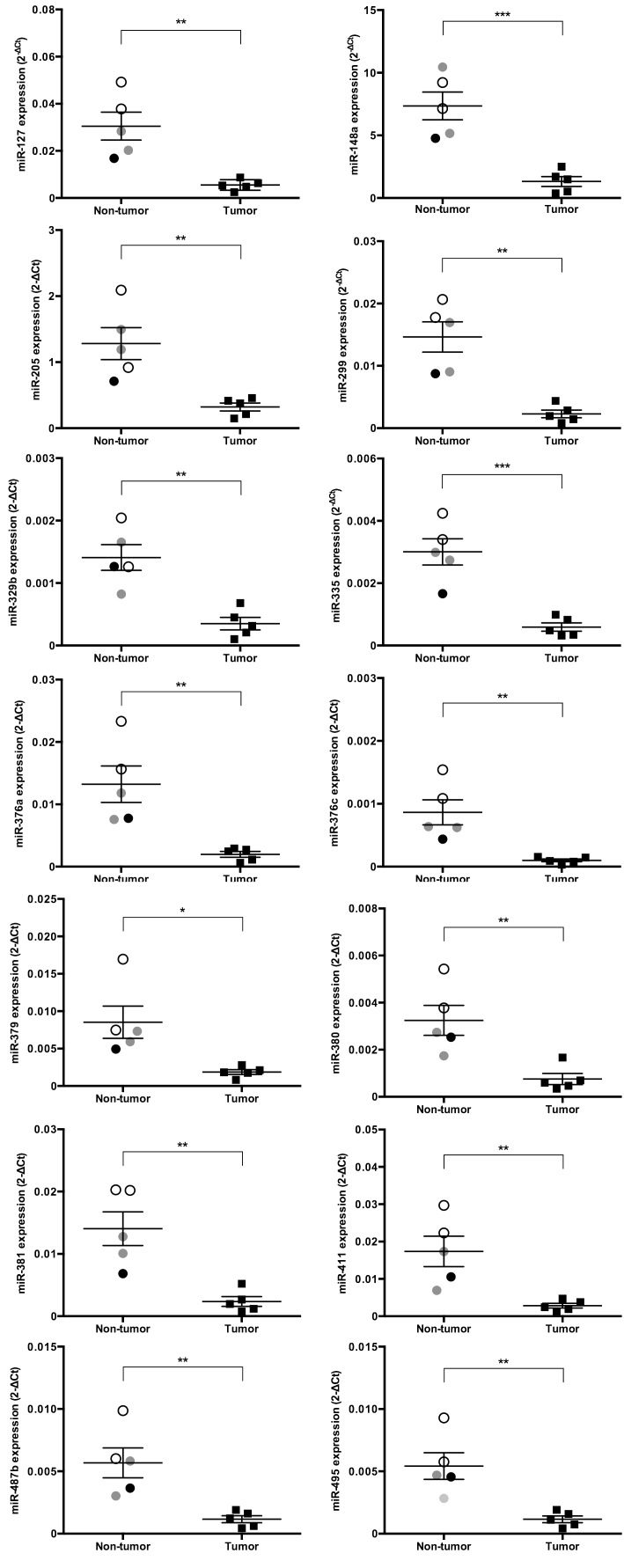
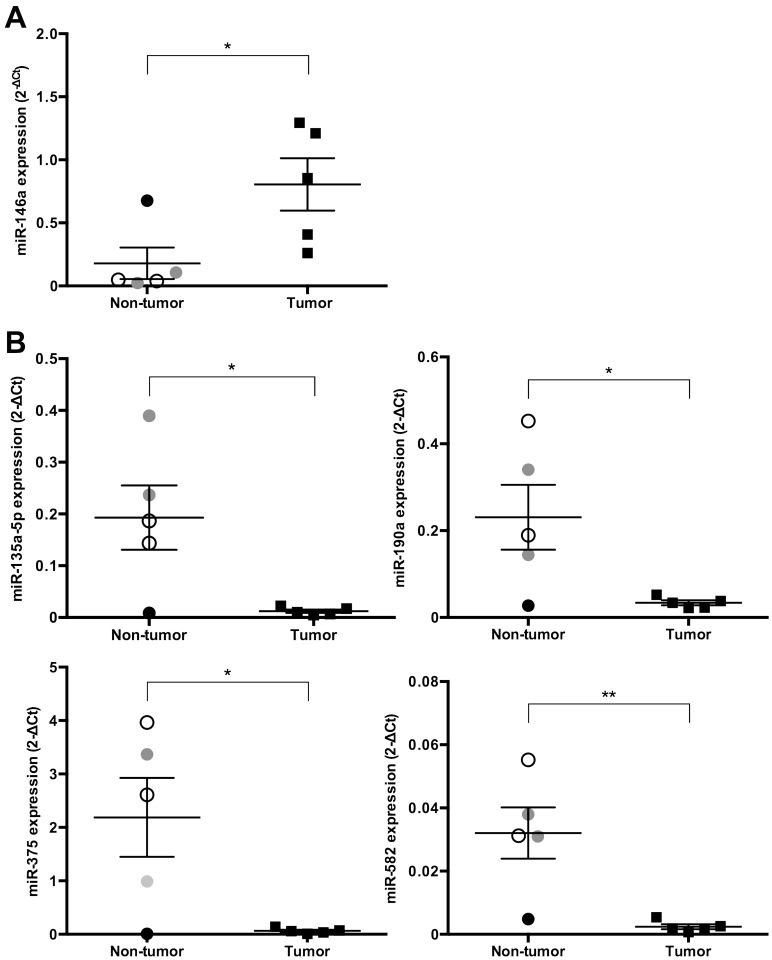
We identified the expression levels of miRs that significantly increased or decreased by more than 4-fold in cPCa tissue compared with their expression in non-tumor prostatic tissue (Figs. 1, 2, 3). Five miRs (miR-18a, 95, 221, 222 and 330) were significantly up-regulated in cPCa tissue in comparison with non-tumor prostatic tissue (13.3-fold, P=0.008; 4.9-fold, P=0.008; 9.3-fold, P=0.040; 5.9-fold, P=0.015 and 26.0-fold, P=0.008, respectively) (Fig. 1). The expression level of miR-146a was also higher in cPCa tissue than in non-tumor tissue (8.5-fold, P=0.032), but miR-146a expression in the dog with prostatitis was up-regulated to the same level as that in the tumor group (Fig. 3A). Conversely, 14 miRs (miR-127, 148a, 205, 299, 329b, 335, 376a, 376c, 379, 380, 381, 411, 487b and 495) were significantly down-regulated in cPCa tissue compared with non-tumor prostatic tissue (5.5-fold, P=0.003; 6.7-fold, P=0.001; 4.0-fold, P=0.005; 7.1-fold, P=0.001; 4.6-fold, P=0.002; 5.4-fold, P=0.001; 7.1-fold, P=0.005; 8.9-fold, P=0.005; 4.4-fold, P=0.016; 4.7-fold, P=0.006; 6.8-fold, P=0.003; 5.9-fold, P=0.008; 5.2-fold, P=0.006 and 5.0-fold, P=0.005, respectively) (Fig. 2). The expression levels of miR-135a-5p, 190a, 375 and 582 were also lower in cPCa tissue than in non-tumor tissue (11.2-fold, P=0.020; 5.1-fold, P=0.030; 16.6-fold; P=0.021 and 12.9-fold, P=0.007, respectively), but the expression levels of these miRs in prostatitis tissue were equivalent to those found in cPCa (Fig. 3B).
Fig. 1.
Scatter plots of up-regulated miRs in cPCa tissue (n=5) compared with non-tumor prostatic tissue (n=5). The expression levels of various miRs were measured using real-time PCR, and the 2-ΔCt levels of individual samples were normalized using an average Ct value of 6 control RNAs. The expression levels of five miRs were significantly increased (more than 4-fold) in prostatic tissue from cPCa compared with non-tumor dogs. Open circles, gray circles, closed circles and closed squares denote normal, BPH, prostatitis and cPCa tissue, respectively. Results are expressed as mean ± standard error (SE). *P<0.05, **P<0.01 (unpaired t-test).
Fig. 2.
Scatter plots of down-regulated miRs in cPCa tissue (n=5) compared with non-tumor prostatic tissue (n=5). The expression levels (2-ΔCt) of various miRs were calculated using the same method as detailed in Fig. 1, and those of 14 miRs of prostatic tissue were significantly decreased (more than 4-fold) in cPCa compared with non-tumor dogs. Open circles, gray circles, closed circles and closed squares denote normal, BPH, prostatitis and cPCa tissue, respectively. Results are expressed as mean ± SE. *P<0.05, **P<0.01, ***P<0.001 (unpaired t-test).
Fig. 3.
Scatter plots of miRs up-regulated or down-regulated at the same level between the dog with prostatitis and the tumor group. The expression levels (2-ΔCt) of various miRs were calculated using the same method as detailed in Figs. 1 and 2. (A) The expression level of one miR (miR-146a) was up-regulated (more than 4-fold) in prostatic tissue from cPCa compared with non-tumor dogs, but miR-146a was up-regulated to the same level in the dog with prostatitis and in the tumor group. (B) The expression levels of four miRs (miR-135a-5p, 190a, 375 and 582) were down-regulated (more than 4-fold) in prostatic tissue from cPCa compared with non-tumor dogs, but those of miR-135a-5p, 190a, 375 and 582 were down-regulated to the same level as in the tumor group. Open circles, gray circles, closed circles and closed squares indicate normal, BPH, prostatitis and cPCa tissue, respectively. Results are expressed as mean ± SE. *P<0.05, **P<0.01 (unpaired t-test).
MiRs function as oncogenes or tumor suppressors in many human cancers including PCa, and their level of expression influences the development and progression of cancer [3, 32]. The expression of miR-221 and 222 increased in cPCa tissue compared with non-tumor tissue. miR-221 and 222, known as onco-miRs, were also up-regulated in hPCa [10, 28], and these miRs target p27kit1, which regulates cell cycle progression and promotes cell proliferation [10]. In addition, the expression of miR-221 and 222 was up-regulated in human androgen-independent PCa (AIPCa) cells compared with androgen-dependent PCa (ADPCa) cells and were involved in the development or maintenance of an androgen-independent phenotype [28]. In the present study, all cPCa cases had been castrated previously. From these results, the up-regulation of miR-221 and 222 in cPCa may be associated with cell proliferation, as found in hPCa. The expression of miR-95 and 18a was higher in cPCa tissue than in non-tumor tissue. MiR-95 expression increased in hPCa specimens compared with normal tissue, and miR-95 overexpression resulted in tumor growth and the induction of radiation resistance [13]. Radiation therapy has been attempted in cPCa, but its effect was limited [18]. One of the reasons for the low radiation sensitivity of cPCa may involve the up-regulation of miR-95 expression. miR-18a was also highly expressed in hPCa tissues and cells and induced tumorigenesis [12]; therefore, as found with miR-221 and 222, the elevated expression of miR-95 and 18a in cPCa may result in cancer progression and malignant transformation.
The expression of miR-127, 148a, 205, 299, 335, 376a, 376c, 381, 487b and 495 significantly decreased in cPCa tissue compared with non-tumor tissue. miR-127 was also found to be down-regulated in hPCa tissue [25]. In addition, a previous report indicated that miR-127 was located within a CpG island and was induced by DNA demethylation and histone deacetylase inhibition, and a potential target of miR-127 was proto-oncogene B-cell lymphoma 6 [25]. The expression level of miR-148a was lower in AIPCa cells than in normal prostatic epithelial cells and ADPCa cells, and this miR inhibited cell growth, cell migration and cell invasion and increased sensitivity to the anti-cancer drug paclitaxel [9]. Overexpression of miR-299 resulted in the down-regulation of androgen receptor, and the increased expression of androgen receptor has been shown to lead to the development of AIPCa cells [6, 23]. Dogs develop PCa regardless of the presence or absence of androgens, and anti-androgenic therapies, such as castration or anti-androgen treatment, are not effective against cPCa. In addition, the expression level of miR-335 was decreased in various human cancers including PCa [30]. Furthermore, a recent study demonstrated that the expression of miR-205, 376a, 376c, 381, 487b and 495 was lower in hPCa metastatic cells than in normal prostatic epithelial cells, and lower miR levels correlated with higher metastatic incidence or prostate-specific antigen levels [8, 15]. In particular, miR-205 acts as a tumor suppressor in canine malignant melanoma and regulated epithelial-to-mesenchymal transition [11, 21]. Our results on cPCa and the previous reports on hPCa show that these miRs may act as candidate tumor suppressors in cPCa. Although the expression of miR-329b, 330, 379, 380 and 411 was altered in cPCa, there are few reports regarding their role in tumorigenesis; however, it is possible that their altered expression is specific to cPCa. In particular, recent studies have indicated that miR-330 expression is up-regulated in human non-small-cell lung cancer and miR-330 controls cell proliferation [19]. Furthermore, our results show that the expression levels of miR-146a, 135a-5p, 190a, 375 and 582 are higher or lower in cPCa and also prostatitis tissue than in normal and BPH tissue. Histological specimens of prostate cancer tissue frequently show inflammation and inflammation-associated lesions in association with the tumor [5]. In fact, it is reported that the change of miR-146a or 375 expression levels was a response to immunoinflammatory molecules, such as IL-1β or IL-13 [14, 20]; therefore, it is presumed that the alteration of the expression levels of miRs is a result of inflammation rather than tumor.
In conclusion, the present study profiled miR expression in cPCa for the first time. Five miRs (miR-18a, 95, 221, 222 and 330) were up-regulated, but 14 miRs (miR-127, 148a, 205, 299, 329b, 335, 376a, 376c, 379, 380, 381, 411, 487b and 495) were down-regulated in cPCa. Further studies are required to determine the specific role of these miRs in cPCa and to clarify the influence of prostatic disease, such as BPH and prostatitis, and aging on miR expression, but our data suggest that these miRs have diagnostic and miR-based therapeutic potential in cPCa.
REFERENCES
- 1.Ambs S., Prueitt R. L., Yi M., Hudson R. S., Howe T. M., Petrocca F., Wallace T. A., Liu C. G., Volinia S., Calin G. A., Yfantis H. G., Stephens R. M., Croce C. M.2008. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 68: 6162–6170. doi: 10.1158/0008-5472.CAN-08-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel D. P.2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 3.Bartel D. P.2009. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell F. W., Klausner J. S., Hayden D. W., Feeney D. A., Johnston S. D.1991. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970-1987). J. Am. Vet. Med. Assoc. 199: 1623–1630. [PubMed] [Google Scholar]
- 5.De Marzo A. M., Platz E. A., Sutcliffe S., Xu J., Grönberg H., Drake C. G., Nakai Y., Isaacs W. B., Nelson W. G.2007. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7: 256–269. doi: 10.1038/nrc2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman B. J., Feldman D.2001. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1: 34–45. doi: 10.1038/35094009 [DOI] [PubMed] [Google Scholar]
- 7.Filipowicz W., Bhattacharyya S. N., Sonenberg N.2008. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9: 102–114. doi: 10.1038/nrg2290 [DOI] [PubMed] [Google Scholar]
- 8.Formosa A., Markert E. K., Lena A. M., Italiano D., Finazzi-Agro’ E., Levine A. J., Bernardini S., Garabadgiu A. V., Melino G., Candi E.2014. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 33: 5173–5182. doi: 10.1038/onc.2013.451 [DOI] [PubMed] [Google Scholar]
- 9.Fujita Y., Kojima K., Ohhashi R., Hamada N., Nozawa Y., Kitamoto A., Sato A., Kondo S., Kojima T., Deguchi T., Ito M.2010. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J. Biol. Chem. 285: 19076–19084. doi: 10.1074/jbc.M109.079525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galardi S., Mercatelli N., Giorda E., Massalini S., Frajese G. V., Ciafrè S. A., Farace M. G.2007. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 282: 23716–23724. doi: 10.1074/jbc.M701805200 [DOI] [PubMed] [Google Scholar]
- 11.Gregory P. A., Bert A. G., Paterson E. L., Barry S. C., Tsykin A., Farshid G., Vadas M. A., Khew-Goodall Y., Goodall G. J.2008. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10: 593–601. doi: 10.1038/ncb1722 [DOI] [PubMed] [Google Scholar]
- 12.Hsu T. I., Hsu C. H., Lee K. H., Lin J. T., Chen C. S., Chang K. C., Su C. Y., Hsiao M., Lu P. J.2014. MicroRNA-18a is elevated in prostate cancer and promotes tumorigenesis through suppressing STK4 in vitro and in vivo. Oncogenesis 3: e99. doi: 10.1038/oncsis.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X., Taeb S., Jahangiri S., Emmenegger U., Tran E., Bruce J., Mesci A., Korpela E., Vesprini D., Wong C. S., Bristow R. G., Liu F. F., Liu S. K.2013. MiRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 73: 6972–6986. doi: 10.1158/0008-5472.CAN-13-1657 [DOI] [PubMed] [Google Scholar]
- 14.Iyer A., Zurolo E., Prabowo A., Fluiter K., Spliet W. G., van Rijen P. C., Gorter J. A., Aronica E.2012. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLOS ONE 7: e44789. doi: 10.1371/journal.pone.0044789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalogirou C., Spahn M., Krebs M., Joniau S., Lerut E., Burger M., Scholz C. J., Kneitz S., Riedmiller H., Kneitz B.2013. MiR-205 is progressively down-regulated in lymph node metastasis but fails as a prognostic biomarker in high-risk prostate cancer. Int. J. Mol. Sci. 14: 21414–21434. doi: 10.3390/ijms141121414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami E., Kobayashi M., Ikeda A., Hori T.2014. Occurrence of prostatic adenocarcinoma in castrated dogs and prostatic superoxide dismutase activity of healthy dogs before and after castration. Asian J. Anim. Vet. Adv. 9: 362–366. doi: 10.3923/ajava.2014.362.366 [DOI] [Google Scholar]
- 17.LeRoy B. E., Nadella M. V., Toribio R. E., Leav I., Rosol T. J.2004. Canine prostate carcinomas express markers of urothelial and prostatic differentiation. Vet. Pathol. 41: 131–140. doi: 10.1354/vp.41-2-131 [DOI] [PubMed] [Google Scholar]
- 18.Leroy B. E., Northrup N.2009. Prostate cancer in dogs: comparative and clinical aspects. Vet. J. 180: 149–162. doi: 10.1016/j.tvjl.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Shi H., Liu B., Li J., Liu Y., Yu B.2015. MiR-330-3p controls cell proliferation by targeting early growth response 2 in non-small-cell lung cancer. Acta Biochim. Biophys. Sin. (Shanghai) 47: 431–440. doi: 10.1093/abbs/gmv032 [DOI] [PubMed] [Google Scholar]
- 20.Lu T. X., Lim E. J., Wen T., Plassard A. J., Hogan S. P., Martin L. J., Aronow B. J., Rothenberg M. E.2012. MiR-375 is downregulated in epithelial cells after IL-13 stimulation and regulates an IL-13-induced epithelial transcriptome. Mucosal Immunol. 5: 388–396. doi: 10.1038/mi.2012.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi S., Mori T., Hoshino Y., Yamada N., Maruo K., Akao Y.2013. MicroRNAs as tumour suppressors in canine and human melanoma cells and as a prognostic factor in canine melanomas. Vet. Comp. Oncol. 11: 113–123. doi: 10.1111/j.1476-5829.2011.00306.x [DOI] [PubMed] [Google Scholar]
- 22.Obradovich J., Walshaw R., Goullaud E.1987. The influence of castration on the development of prostatic carcinoma in the dog. 43 cases (1978-1985). J. Vet. Intern. Med. 1: 183–187. doi: 10.1111/j.1939-1676.1987.tb02013.x [DOI] [PubMed] [Google Scholar]
- 23.Östling P., Leivonen S. K., Aakula A., Kohonen P., Mäkelä R., Hagman Z., Edsjö A., Kangaspeska S., Edgren H., Nicorici D., Bjartell A., Ceder Y., Perälä M., Kallioniemi O.2011. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 71: 1956–1967. doi: 10.1158/0008-5472.CAN-10-2421 [DOI] [PubMed] [Google Scholar]
- 24.Pang Y., Young C. Y., Yuan H.2010. MicroRNAs and prostate cancer. Acta Biochim. Biophys. Sin. (Shanghai) 42: 363–369. doi: 10.1093/abbs/gmq038 [DOI] [PubMed] [Google Scholar]
- 25.Saito Y., Liang G., Egger G., Friedman J. M., Chuang J. C., Coetzee G. A., Jones P. A.2006. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 9: 435–443. doi: 10.1016/j.ccr.2006.04.020 [DOI] [PubMed] [Google Scholar]
- 26.Sorenmo K. U., Goldschmidt M., Shofer F., Goldkamp C., Ferracone J.2003. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet. Comp. Oncol. 1: 48–56. doi: 10.1046/j.1476-5829.2003.00007.x [DOI] [PubMed] [Google Scholar]
- 27.Stefani G., Slack F. J.2008. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 9: 219–230. doi: 10.1038/nrm2347 [DOI] [PubMed] [Google Scholar]
- 28.Sun T., Wang Q., Balk S., Brown M., Lee G. S., Kantoff P.2009. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 69: 3356–3363. doi: 10.1158/0008-5472.CAN-08-4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver A. D.1981. Fifteen cases of prostatic carcinoma in the dog. Vet. Rec. 109: 71–75. doi: 10.1136/vr.109.4.71 [DOI] [PubMed] [Google Scholar]
- 30.Xiong S. W., Lin T. X., Xu K. W., Dong W., Ling X. H., Jiang F. N., Chen G., Zhong W. D., Huang J.2013. MicroRNA-335 acts as a candidate tumor suppressor in prostate cancer. Pathol. Oncol. Res. 19: 529–537. doi: 10.1007/s12253-013-9613-5 [DOI] [PubMed] [Google Scholar]
- 31.Yang X., Yang Y., Gan R., Zhao L., Li W., Zhou H., Wang X., Lu J., Meng Q. H.2014. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLOS ONE 9: e98833. doi: 10.1371/journal.pone.0098833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B., Pan X., Cobb G. P., Anderson T. A.2007. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 302: 1–12. doi: 10.1016/j.ydbio.2006.08.028 [DOI] [PubMed] [Google Scholar]