Abstract
A 9-year-old, spayed female Golden Retriever dog was referred to us for lymphocytosis and lymphadenopathy, secondary to suspected chronic lymphocytic leukemia (CLL). The dog had a clinical history of anorexia, vomiting and melena lasting two days. The popliteal lymph node contained small-to-intermediate lymphocytes, which led us to suspect low-grade lymphoma. Thickened lesions in the stomach and small intestine were detected by ultrasonography. Histopathology of the popliteal lymph node and small intestine revealed a simultaneous presence of T-zone lymphoma (TZL) and high-grade gastrointestinal (GI) cytotoxic T-cell lymphoma. Large granular lymphocytes (LGLs) were seen on cytological examination. Polymerase chain reaction (PCR) revealed that both lymphomas originated in the T-cells. The dog died 15 days after diagnosis, despite chemotherapy.
Keywords: canine, gastrointestinal lymphoma, large granular lymphocytic lymphoma, PARR, T-zone lymphoma
Malignant lymphoma is the most common hematopoietic malignancy in dogs. It occurs in multicentric, alimentary, mediastinal and extranodal forms. There are infrequent reports of two lymphoid tumors in a single case, such as the transformation of an indolent lymphoma to an aggressive lymphoma [3] or in Richter syndrome (RS), which involves CLL transforming into aggressive lymphoma [15].
T-zone lymphoma (TZL) is not rare in occurrence and is characterized by long survival times and indolent progression. In a recent study, approximately 10% of all dogs diagnosed with TZL developed a second malignancy [11]. Our report describes a unique case presenting with two different types of lymphoma, TZL and high-grade gastrointestinal (GI) lymphoma. We were unable to determine, if this was a case of progression similar to RS or an unknown characteristic of TZL.
A 9-year-old, 29.1-kg, spayed female Golden Retriever dog with lymphocytosis and lymphadenopathy and suspected underlying CLL was referred to our animal hospital. The dog began displaying signs of anorexia, vomiting and melena two days prior. Physical examination revealed generalized lymphadenopathy with the left superficial cervical lymph node being the largest at 25 × 25 × 20 mm. The complete blood count (CBC) revealed mild lymphocytosis (11,470/µl, reference interval 1,000–4,800/µl) with small lymphocytes (Fig. 1A), while the chemistry panel was normal. A cytological examination of a fine-needle aspirate from the right popliteal lymph node revealed that almost all the lymphocytes were small-to-intermediate and mature. Some lymphocytes showed the same “hand mirror” type of cytoplasmic extension that is seen in peripheral blood. Using the updated Kiel classification [6], these findings coincided with the clear cell subtype of the small cell category under low-grade T-cell lymphoma. These abnormal lymphocytes are found in the lymph node with TZL, as previously described [19] (Fig. 1B). Thus, the dog was suspected to have a low-grade lymphoma, such as TZL. Abdominal ultrasound revealed partial hypoechogenic thickening of the gastric and small intestine walls (approximately 9.0 mm) (Fig. 2A) and enlargement of the abdominal lymph nodes. Ultrasound-guided fine-needle aspiration of these intestinal lesions was performed, but we did not obtain enough samples for testing.
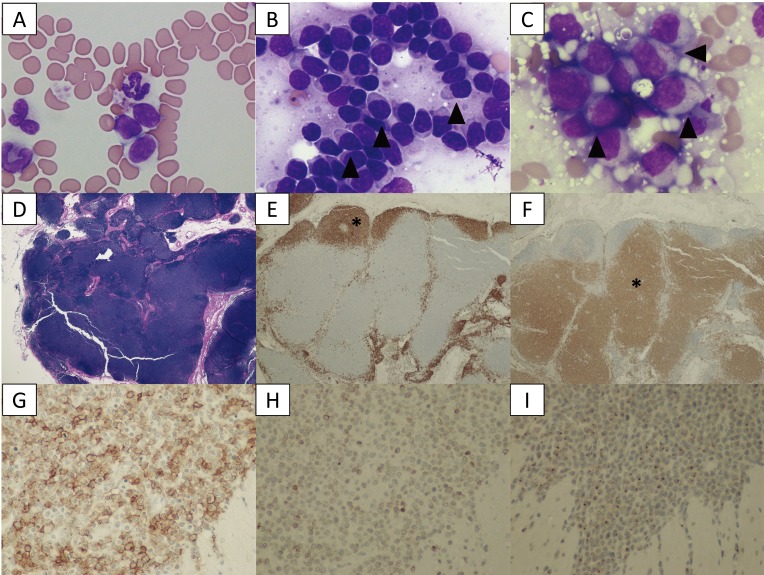
Fig. 1.
A–C: Cytological images on day 1. (A) Peripheral blood smear. Increased numbers of small lymphocytes. (B) Cytology of the popliteal lymph node biopsy. Most lymphocytes are small-to-intermediate, mature lymphocytes. Some lymphocytes show a “hand mirror” type of cytoplasmic extension (arrowhead) (Wright-Giemsa stain, × 400). (C) Slide preparation of tissue from the small intestine. The lymphocytes are intermediate-to-large, immature cells, and some display azurophilic granules in the cytoplasm (LGLs, arrowhead). D–F: Histological images of popliteal lymph node tissue. (D) Hematoxylin and eosin (H&E) staining. (E) The lymphocytes with fading follicular structures are CD20 positive (asterisk). Immunolabeling with anti-CD20, a hematoxylin counterstain. (F) The nodal capsule (CD3 positive) is thinned without the involvement of the perinodal tissue (asterisk). Immunolabeling with anti-CD3, hematoxylin counterstain. G–I: Histological images of the intestinal tissue. All lymphocytes are positive for CD20 (G), CD3 (H) and granzyme B (I).
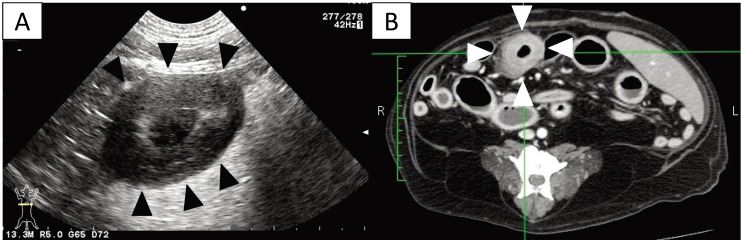
Fig. 2.
(A) Transverse ultrasound image on day 1 showing a thickened intestinal wall (approximately 9.0 mm, arrowhead). (B) Post-contrast transverse CT image on day 2 also showing a thickened intestinal wall (arrowheads). The intrathoracic and abdominal lymph nodes are enlarged.
On the following day, bone marrow aspiration, computed tomography (CT) and endoscopic examinations were performed under anesthesia. The bone marrow aspiration, endoscopy and biopsy of the stomach and duodenum were normal. The CT revealed thickened gastric and duodenal walls (Fig. 2B) and enlargement of the intrathoracic and abdominal lymph nodes. The right popliteal lymph node and the section of the duodenum containing thickened lesions were surgically resected. Microscopic examination of the slides prepared from the duodenal tissue revealed intermediate-to-large immature lymphocytes, some of which, called large granular lymphocytes (LGLs), displayed azurophilic granules in the cytoplasm. They were not linked with other lymphocytes seen in the peripheral blood and the popliteal lymph node (Fig. 1C). A polymerase chain reaction for antigen receptor rearrangement (PARR) analysis (Kahotechno, Fukuoka, Japan) of the peripheral blood and intestinal tissue sample to detect the clonal expansion of the lymphocytes was also performed (Fig. 3A and 3B). The analysis demonstrated clonal rearrangement of TCRγ genes in both samples. Three primer sets were used for the amplification of TCRγ (TCR1-3, Fig. 3 for example, TCR1 contained two primer pairs: TCRγ1 (5′-ACCCTGAGAATTGTGCCAGG-3′) and TCRγ3 (5′-TCTGGGA/GTGTAC/TTACTGTGCTGTCTGG), and TCRγ2 (5′-GTTACTATAAACCTGGTAAC-3′) and TCRγ3 [1]. Primers for TCR1 and TCR2 were used to detect the clonal expansion in the peripheral blood, and TCR3 primer was used for the intestinal tissue. Based on the histopathological and immunohistochemistrical analyses, the popliteal lymph node was diagnosed with TZL (Fig. 1D–1F). In the intestinal tissue, immunohistochemical analysis showed that most lymphocytes were positive for CD20, CD3 and granzyme B (Fig. 1G–1I). The cytoplasms of the neoplastic lymphoid cells displayed a strong, punctate staining pattern for granzyme B. Based on these results, the intestinal tissue was diagnosed with GI cytotoxic T-cell lymphoma.
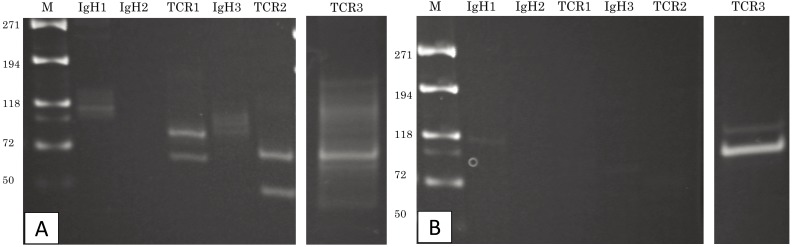
Fig. 3.
PARR analysis. (A) The peripheral blood sample shows TCRγ gene rearrangement. (B) The intestinal tissue sample also shows TCRγ gene rearrangement. The two tumors demonstrate clonal expansions from different primers.
Based on the diagnosis, the dog was treated with chemotherapy using L-asparaginase on day nine after diagnosis and with vincristine on day 10. Five days after the first administration of vincristine, the dog went into shock, displaying neutropenia and thrombocytopenia (band neutrophils, 2,541/µl; segmented neutrophils, 343/µl; and PLT, 21 × 103/µl) and coagulation abnormalities (prothrombin time, 8.4 sec, reference interval 7.4–8.8 sec; activated partial thromboplastin time, 30.8 sec, reference interval 12.0–28.0 sec; fibrin/fibrinogen degradation products, 15.4 µg/ml, reference interval <5 µg/ml; antithrombin activity, 49%, reference interval 80–130%; and D-dimer, 7.11 µg/ml, reference interval <1.0 µg/ml). Based on these results, the causes of the neutropenia and thrombocytopenia were likely multifactorial and may have included disseminated intravascular coagulation (DIC), septic shock or endotoxin shock secondary to perforation of the intestinal tract. The dog died on the same day, and the owner did not permit a necropsy.
This case was complicated by two different types of lymphomas, TZL and high-grade GI lymphoma. Although both immunophenotypes originated in the T cells, PARR analysis showed that clonal expansions were detected by different primer sets in the peripheral blood and the intestinal tissue. Therefore, these lymphomas were reasoned to be derived from different genes.
In humans and dogs, an indolent lymphoma/leukemia is associated with an increased risk of developing a second malignant disorder, such as solid tumors or other lymphoid malignancies. For example, CLL may transform into an aggressive lymphoma (for e.g., RS), and follicular lymphoma may transform into one of the more aggressive lymphoma subtypes [2, 3, 9, 10, 16]. There has been only one previous case report of canine CLL diagnosed concurrently with large granular GI lymphoma [17]. In another study, a group of 16 dogs that had been diagnosed with TZL were observed; only one dog developed DLBCL during the disease progression [13].
In this case, we believe that the TZL occurred before the GI lymphoma based on the unique features of each disease. We could not determine, if the TZL transformed or whether the lymphomas originated concurrently (a possibility because the lymphomas did derive from different genes). The underlying causes in the development of secondary malignancies remain unclear, but possible mechanisms include a common genetic predisposition and impaired immunological surveillance [10, 11].
In the popliteal lymph node, histopathological analysis revealed TZL. There are two different phenotypes; some patients live for years without therapy, and others eventually reach a stage at which therapy is indicated [19]. In contrast, the intestinal tissue was diagnosed with GI cytotoxic T-cell lymphoma, which requires aggressive chemotherapy. Only few cases of canine GI lymphoma have been reported previously [4, 5]. Rassnick et al. observed that the median overall survival times for 13 dogs with primary GI lymphoma and five dogs with GI lymphoma as a manifestation of multicentric disease were 77 days and 100 days, respectively [14]. The dog observed in our study showed LGLs characterized by abundant pale blue cytoplasm containing prominent azurophilic granules [18]. In humans and dogs, LGL tumors lead to poor prognoses, because they are typically highly aggressive and they are presumed to arise from extranodal sites [7, 12]. Immunohistochemistry of the small intestine demonstrated that most of the lymphocytes were positive for CD20, CD3 and granzyme B. The CD20-positive primary gastric T-cell lymphoma has been previously reported in humans [8]. The CD20 antigen, a transmembrane protein that functions as a calcium channel, has long been designated a pan-B antigen that is present on a wide range of normal and neoplastic B lymphocytes. However, CD20 has been identified in a small subset of normal and neoplastic T lymphocytes using flow cytometry [8]. Although the diagnosis is not well-defined for dogs, we regarded this GI lymphoma as T-cell lymphoma based on the result of positive granzyme B staining, which was expressed exclusively by cytotoxic T lymphocytes. Further, PARR analysis was positive for CD3.
In conclusion, this case report describes a low-grade lymphoma seen concurrently with high-grade lymphoma. Close follow-up with or without chemotherapy may be necessary in cases with TZL in which genetic predisposition and impaired immune function play a role.
Acknowledgments
We would like to thank Dr. Mika Tanabe, Dr. Keitaro Morishita and Dr. Hiroshi Shimoda for their advice.
REFERENCES
- 1.Burnett R. C., Vernau W., Modiano J. F., Olver C. S., Moore P. F., Avery A. C.2003. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet. Pathol. 40: 32–41. doi: 10.1354/vp.40-1-32 [DOI] [PubMed] [Google Scholar]
- 2.Comazzi S., Gelain M. E., Martini V., Riondato F., Miniscalco B., Marconato L., Stefanello D., Mortarino M.2011. Immunophenotype predicts survival time in dogs with chronic lymphocytic leukemia. J. Vet. Intern. Med. 25: 100–106. doi: 10.1111/j.1939-1676.2010.0640.x [DOI] [PubMed] [Google Scholar]
- 3.Comazzi S., Martini V., Riondato F., Poggi A., Stefanello D., Marconato L., Albonico F., Gelain M. E.2015. Chronic lymphocytic leukemia transformation into high-grade lymphoma: a description of Richter’s syndrome in eight dogs. Vet. Comp. Oncol. doi: 10.111/vco.12172 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Couto C. G., Rutgers H. C., Sherding R. G., Rojko J.1989. Gastrointestinal lymphoma in 20 dogs. A retrospective study. J. Vet. Intern. Med. 3: 73–78. doi: 10.1111/j.1939-1676.1989.tb03082.x [DOI] [PubMed] [Google Scholar]
- 5.Coyle K. A., Steinberg H.2004. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet. Pathol. 41: 141–146. doi: 10.1354/vp.41-2-141 [DOI] [PubMed] [Google Scholar]
- 6.Fournel-Fleury C., Magnol J. P., Bricaire P., Marchal T., Chabanne L., Delverdier A., Bryon P. A., Felman P.1997. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas. J. Comp. Pathol. 117: 35–59. doi: 10.1016/S0021-9975(97)80065-5 [DOI] [PubMed] [Google Scholar]
- 7.Greer J. P., Kinney M. C., Loughran T. P., Jr.2001. T cell and NK cell lymphoproliferative disorders. Hematology (Am Soc Hematol Educ Program) 2: 259–281. [DOI] [PubMed] [Google Scholar]
- 8.Kakinoki Y., Hashiguchi J., Ishio T., Chiba K., Niino D., Ohshima K.2015. CD20-positive primary gastric T-cell lymphoma poorly responding to initial treatment with rituximab plus CHOP, and a literature review. Int. J. Hematol. 102: 702–708. doi: 10.1007/s12185-015-1841-x [DOI] [PubMed] [Google Scholar]
- 9.Leifer C. E., Matus R. E.1986. Chronic lymphocytic leukemia in the dog: 22 cases (1974-1984). J. Am. Vet. Med. Assoc. 189: 214–217. [PubMed] [Google Scholar]
- 10.Maddocks-Christianson K., Slager S. L., Zent C. S., Reinalda M., Call T. G., Habermann T. M., Bowen D. A., Hoyer J. D., Schwager S., Jelinek D. F., Kay N. E., Shanafelt T. D.2007. Risk factors for development of a second lymphoid malignancy in patients with chronic lymphocytic leukaemia. Br. J. Haematol. 139: 398–404. doi: 10.1111/j.1365-2141.2007.06801.x [DOI] [PubMed] [Google Scholar]
- 11.Martini V., Marconato L., Poggi A., Riondato F., Aresu L., Cozzi M., Comazzi S.2016. Canine small clear cell/T-zone lymphoma: clinical presentation and outcome in a retrospective case series. Vet. Comp. Oncol. 14Suppl 1: 117–126. doi: 10.1111/vco.12155 [DOI] [PubMed] [Google Scholar]
- 12.McDonough S. P., Moore P. F.2000. Clinical, hematologic, and immunophenotypic characterization of canine large granular lymphocytosis. Vet. Pathol. 37: 637–646. doi: 10.1354/vp.37-6-637 [DOI] [PubMed] [Google Scholar]
- 13.Mizutani N., Goto-Koshino Y., Takahashi M., Uchida K., Tsujimoto H.2016. Clinical and histopathological evaluation of 16 dogs with T-zone lymphoma. J. Vet. Med. Sci. 78: 1237–1244. doi: 10.1292/jvms.15-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rassnick K. M., Moore A. S., Collister K. E., Northrup N. C., Kristal O., Chretin J. D., Bailey D. B.2009. Efficacy of combination chemotherapy for treatment of gastrointestinal lymphoma in dogs. J. Vet. Intern. Med. 23: 317–322. doi: 10.1111/j.1939-1676.2008.0270.x [DOI] [PubMed] [Google Scholar]
- 15.Rossi D.2016. Richter’s syndrome: Novel and promising therapeutic alternatives. Best Pract. Res. Clin. Haematol. 29: 30–39. doi: 10.1016/j.beha.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Schöllkopf C., Rosendahl D., Rostgaard K., Pipper C., Hjalgrim H.2007. Risk of second cancer after chronic lymphocytic leukemia. Int. J. Cancer 121: 151–156. doi: 10.1002/ijc.22672 [DOI] [PubMed] [Google Scholar]
- 17.Snead E. C.2007. Large granular intestinal lymphosarcoma and leukemia in a dog. Can. Vet. J. 48: 848–851. [PMC free article] [PubMed] [Google Scholar]
- 18.Timonen T., Ortaldo J. R., Herberman R. B.1981. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J. Exp. Med. 153: 569–582. doi: 10.1084/jem.153.3.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valli V. E., Kass P. H., San Myint M., Scott F.2013. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 50: 738–748. doi: 10.1177/0300985813478210 [DOI] [PubMed] [Google Scholar]