Abstract
Seabirds are marine top predators and accumulate high levels of metals and metalloids in their tissues. Contamination by metals in the highly productive offshore region has become a matter of public concern. It is home to 80% of the seabird population in the U.S.A., 95% of northern fur seals (Callorhinus ursinus), and major populations of Steller sea lions (Eumetopias jubatus), walruses (Odobenus rosmarus) and whales. Here, the concentrations of eight heavy metals (Hg, Cd, Cr, Co, Ni, Cu, Zn and Pb) and a metalloid (As) in the liver and kidneys of the northern fulmar (Fulmarus glacialis), thick-billed murre (Uria lomvia), short-tailed shearwater (Puffinus tenuirostris), tufted puffin (Fratercula cirrhata) and horned puffin (Fratercula corniculata) collected in the Bering Sea were measured. As proxies of trophic level and habitat, nitrogen (δ15N) and carbon (δ13C) stable isotope ratios of breast muscles were also measured. Hepatic Hg concentration was high in northern fulmar, whereas Cd level was high in tufted puffin and northern fulmar. The Hg concentration and δ15N value were positively correlated across individual birds, suggesting that Hg uptake was linked to the trophic status of consumed prey. Furthermore, Hg concentration in our study was higher than those of the same species of seabirds collected in 1990.
Keywords: Bering Sea, cadmium, mercury, seabird
The Bering Sea is an important habitat for seabirds and marine animals, because of its large volume of plankton and the numerous fish species [30, 41]. It is home to 80% of the seabird population in the U.S.A., 95% of northern fur seals (Callorhinus ursinus), and major populations of Steller sea lions (Eumetopias jubatus), walruses (Odobenus rosmarus) and whales [30].
It has been reported that marine mammals accumulate high concentrations of heavy metals, such as mercury (Hg) and cadmium (Cd) as well as metalloids [19]. Aquatic ecosystems appear to be the most susceptible to monomethylmercury contamination, as they are major repositories of natural and pollution-derived Hg and host active populations of Hg methylating bacteria [12]. These findings suggest that wildlife species that have a primarily marine-based diet are at a higher risk of metal exposure in their feeding habitats. Methylmercury has greater toxicity and bioaccumulation than Hg itself. Inorganic Hg exerts its greatest effect on the kidney, whereas MeHg readily penetrates the blood–brain barrier in birds and mammals, producing brain lesions, spinal cord degeneration and central nervous system dysfunction [50]. Seabirds are marine top predators and also accumulate high levels of heavy metals in their tissues, and sometimes suffer lower reproductive success and survival [6, 11, 22, 43, 48, 50]. The ocean receives about 90% of its Hg through wet and dry atmospheric deposition [23], and anthropogenic interferences in the global Hg cycle are significant [12]. The major contributions are fossil-fuel fired power plants, artisanal small scale gold mining and non-ferrous metals manufacturing [34]. At present, the Hg concentrations in arctic marine animals are generally about 10−12 times higher than those in pre-industrial times [46], indicating that Hg accumulation is due to recent anthropogenic sources.
High concentrations of heavy metals have been reported in tissues of marine mammals and seabirds in the coastal region and the sea around the Aleutian Islands [8, 9, 36]. The level of Cd that causes renal tubular necrosis in birds [49] is also high in several seabirds. However, information of metal accumulation on those living in the offshore is scarce [13].
To clarify the metal contamination in seabirds in the central Bering Sea, the present study investigated the accumulation patterns of eight heavy metals (Hg, Cd, Cr, Co, Ni, Cu, Zn and Pb) and one metalloid (As) in the liver and kidneys of five species of seabirds, i.e., northern fulmar (Fulmarus glacialis), thick-billed murre (Uria lomvia), short-tailed shearwater (Puffinus tenuirostris), tufted puffin (Fratercula cirrhata) and horned puffin (Fratercula corniculata), collected in the central Bering Sea.
As some heavy metals are known to be biomagnified through the food chain [2] and also vary across habitats [21], nitrogen (δ15N) and carbon (δ13C) stable isotope ratios in muscle were also measured as proxies of trophic level and habitat.
In the present study, differences in metal concentrations among species and the sources of metals were studied to determine the accumulation pattern of metals in each seabird species. Furthermore, the accumulation levels of metals and arsenic between sexes and ages in short-tailed shearwater that number was enough for the statistical analysis were analyzed. Hg pollution is especially serious all over the world. Therefore, we compared the Hg concentrations among seabirds in the Bering Sea with previous reports to clarify its influence in seabirds.
MATERIALS AND METHODS
Sampling
Specimens were sampled from seabirds entangled in gill nets during the Japan–US joint research on salmon in June or July from 2008 to 2010 in the central Bering Sea (44°00′N–57°30′N, 178°00′E–177°00′W) (Fig. 1, Table S1). Birds were kept in a freezer (−20°C), thawed at room temperature and dissected in the laboratory. The liver and kidneys were collected from northern fulmar (Fulmarus glacialis) (n=4), thick-billed murre (Uria lomvia) (n=4), short-tailed shearwater (Puffinus tenuirostris) (n=24), tufted puffin (Fratercula cirrhata) (n=5) and horned puffin (Fratercula corniculata) (n=3). Data regarding sex and age are shown in Table S1. Breast muscles of northern fulmar (n=4), thick-billed murre (n=3), short-tailed shearwater (n=23), tufted puffin (n=3) and horned puffin (n=2) were also collected. All samples were kept at −20°C in a freezer until metal analysis.
Fig. 1.
Map of the Bering Sea. The dotted line indicates the sampling area.
Extraction and analysis of heavy metals and a metalloid
Analyses were performed according to the method of Yabe et al. [51]. For metal analysis, 0.3 g of liver and kidney samples were dried for 15 hr at 50°C and then digested with 6 ml of 60% nitric acid (Kanto Chemical Corporation, Tokyo, Japan) and 1 ml of 30% hydrogen peroxide (Kanto Chemical Corporation) in a microwave digestion system (Speedwave Two; Berghof, Eningen, Germany). Digestion was performed under the following conditions: 180°C for 15 min, 200°C for 20 min and 100°C for 20 min. After the samples were cooled, they were transferred to plastic tubes into which was added 0.1 ml of lanthanum chloride (Wako Pure Chemical Industries, Osaka, Japan). The volume was then brought to 10 ml with 2% nitric acid. The concentrations of heavy metals and a metalloid (Cd, Cr, Co, Ni, Cu, Zn, Pb and As) were measured using an atomic absorption spectrophotometer (AAS) (Z-2010; Hitachi High-Technologies Corporation, Tokyo, Japan) with acetylene flame or argon non-flame method. The instrument was calibrated using standard solutions of the respective heavy metals to establish standard curves before analysis. Concentrations of Cu and Zn were determined through the flame method with acetylene gas, whereas concentrations of Cd, Cr, Ni, Pb and As were determined using a graphite furnace with argon gas. All chemicals and standard stock solutions were of analytical reagent grade (Wako Pure Chemicals Industries). Water was distilled and deionized (Milli-Q; Merck Millipore, Billerica, MA, U.S.A.). Analytical quality control was performed using DOLT-4 (dogfish liver) and DORM-3 (fish protein) certified reference materials (both from the National Research Council of Canada). Recovery rates (%) of all elements were acceptable: Cd (91−108), Cr (91−108), Co (96−111), Ni (98−111), Cu (88−90), Zn (78−83) and Pb (89−98). Arsenic had lower recovery rates (50−67%). Detection limits (µg/kg) for Cd, Cr, Co, Ni, Cu, Zn, Pb and As were 0.2, 0.5, 0.5, 0.5, 1.0, 0.1, 1.0 and 2.0, respectively.
Analysis of total mercury (Hg)
The concentrations of total Hg in the liver and kidneys were measured directly without any pre-treatment by thermal decomposition, gold amalgamation and atomic absorption spectrophotometry using a mercury analyzer (MA-3000; Nippon Instruments Corporation, Tokyo, Japan), after preparation of the calibration standard. The recovery rates of Hg for the certified reference material, DOLT-4, ranged from 92 to 103%. The concentration of Hg was converted from mg/kg wet weight to mg/kg dry weight using the calculated water content of specimens before and after drying.
Stable isotope ratio analysis
Stable isotope ratio analysis was performed according to the method of Nakayama et al. [25]. Muscle tissue has longer turnover time than liver and is the most common samples used for stable isotope analysis [35]. Muscles were washed with distilled water to remove blood and then dried at 45°C for 48 hr. Samples were then ground into a homogeneous powder and treated with a 2:1 chloroform-methanol solution (Kanto Chemical Corporation) to remove lipids, and the residue was dried. Each sample was weighed (0.5−1.0 mg) into a tin capsule (Säntis Analytical AG, Teufen, Switzerland), and stable isotope ratios were determined by the flow injection method using a Finnigan MAT-252 mass spectrometer (Finnigan MAT GmbH, Bremen, Germany) connected to a Fisons NA1500 elemental analyzer (Fisons Instruments SpA, Strada Rivoltana, Italy). Stable isotope ratios were expressed in δ notation (as deviation from standards in parts per thousand (‰)) according to the following formula: δX=[(Rsample/Rstandard−1)] ×1,000, where X is 13C or 15N and R is the corresponding ratio 13C/12C or 15N/14N [24]. Data are presented as values based on the international standard of v-PDB (Vienna Peedee Belemnite; fossilized shells from the PeeDee Formation in South Carolina) and as atmospheric N2 for C and N, respectively [22]. Replicate errors were within 0.2‰ for both δ13C and δ15N analyses.
Statistics
Differences in metal and metalloid concentrations and isotope ratio values among five species were examined by Tukey’s HSD test (P<0.05). We also analyzed the levels of metal and metalloid accumulation between sexes and ages in short-tailed shearwater by Kruskal-Wallis Test (P<0.05), because their number was sufficient for analysis. Analyses were performed in JMP Pro 10.0.2 (SAS Institute, Cary, NC, U.S.A.). Pearson product-moment correlation (r) was used to analyze relationships between pollutant concentrations, body weight and stable isotope ratio (n=40). Principal component analysis (PCA) was also performed with each metal and metalloid concentration, body weight and stable isotope ratio.
RESULTS
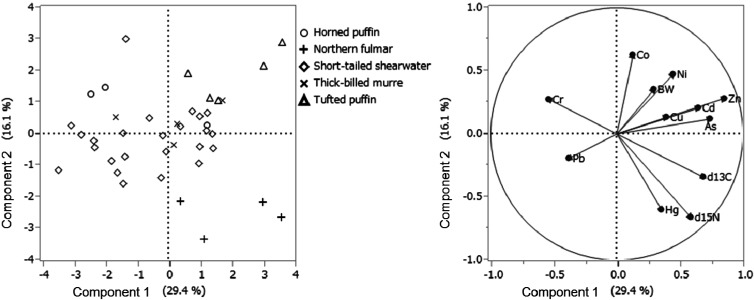
The concentrations of heavy metals and arsenic in the liver and kidney tissues of seabirds are shown in Table 1. Concentrations of Hg and Cd were high compared to those of terrestrial bird species that are herbivorous or insectivorous [1]. It was suggested that seabirds accumulate high levels of Hg and Cd. Northern fulmar accumulated significantly higher liver Hg levels ranged from 6.82−40.3 mg/kg dry weight (2.05−13.5 mg/kg wet weight) than the other species (Table 1). Tufted puffin and northern fulmar accumulated high Cd levels (114.4 ± 50.1 and 102.7 ± 31.7 mg/kg dry weight of kidney, respectively). Accumulation patterns of metals were different for each species. For example, northern fulmar accumulated high concentrations of Hg and Cd, whereas tufted puffin had high levels of Cd. In contrast, Co, Ni and As concentrations in the liver and Cr, Cu and Pb concentrations in the liver and kidney did not differ among species. PCA results indicate species-specific accumulation characteristics. Northern fulmar had a high correlation of Hg and δ15N, whereas tufted puffin accumulated more Cd, Ni and Zn (Fig. 2).
Table 1. Sample size (N), body weight (BW) and stable isotope ratio in muscle (δ13C and δ15N, ‰) and metal and metalloid concentrations in liver and kidney (mg/kg dry weight).
| Species | No. | Age | Sex | BW (g) | δ15N | δ13C | Hg | Cd | Cr | Co | Ni | Cu | Zn | As | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Northern fulmarF. glacialis | 4 | 4Ad | 1M & 2F & 1U | 616.3 ± 56.9c) | 13.0 ± 0.54a) | −21.0 ± 0.64 | liver | 24.5 ± 12.6a) | 26.7 ± 11.3a) | 0.15 ± 0.09 | 0.05 ± 0.01 | 0.06 ± 0.07 | 16.4 ± 3.60 | 135.2 ± 25.1a,b) | 1.50 ± 1.76 | 0.06 ± 0.04 |
| kidney | 7.69 ± 4.12a) | 102.7 ± 31.7a,b) | 0.32 ± 0.05 | 0.16 ± 0.04b) | 0.80 ± 1.25b) | 15.3 ± 1.76 | 148.6 ± 9.49a,b) | 1.22 ± 1.06a,b) | 0.38 ± 0.27 | |||||||
| Thick-billed murreU. lomvia | 4 | 1Ad & 3Ju | 1M & 2F & 1U | 1,066.0 ± 37.1a) | 11.2 ± 0.98a,b) | −21.5 ± 1.86 | liver | 1.89 ± 1.75b) | 12.7 ± 4.85b) | 0.47 ± 0.24 | 0.09 ± 0.02 | 0.84 ± 0.76 | 18.0 ± 2.01 | 99.9 ± 21.0a,b) | 0.34 ± 0.45 | 0.02 ± 0.01 |
| kidney | 1.21 ± 0.88b) | 27.4 ± 13.4a,b) | 0.45 ± 0.21 | 0.12 ± 0.03b) | 2.42 ± 3.53b) | 17.1 ± 1.52 | 103.4 ± 9.46b,c) | 0.33 ± 0.38b) | 0.11 ± 0.06 | |||||||
| Short-tailed shearwaterP. tenuirostris | 24 | 14Ad & 8Ju & 2U | 9M & 7F & 8U | 510.2 ± 61.3d) | 10.3 ± 0.74b,c) | −22.0 ± 1.27 | liver | 1.33 ± 0.66b) | 6.06 ± 4.06b) | 0.28 ± 0.32 | 0.11 ± 0.08 | 0.53 ± 0.74 | 21.5 ± 10.7 | 105.2 ± 32.8b) | 0.69 ± 0.58 | 0.08 ± 0.08 |
| kidney | 0.83 ± 0.40b) | 43.4 ± 49.4b) | 0.39 ± 0.21 | 0.18 ± 0.11b) | 2.40 ± 3.79b) | 22.9 ± 13.8 | 102.7 ± 20.5c) | 0.60 ± 0.45b) | 0.26 ± 0.35 | |||||||
| Tufted puffinF. cirrhata | 5 | 1Ad & 3Ju & 1 U | 4M & 1F | 840.8 ± 72.4b) | 9.27 ± 1.01c,d) | −22.7 ± 0.36 | liver | 1.44 ± 0.33b) | 36.0 ± 9.31a) | 0.10 ± 0.03 | 0.13 ± 0.04 | 1.48 ± 1.03 | 24.4 ± 4.71 | 156.1 ± 21.1a) | 1.80 ± 0.88 | 0.03 ± 0.01 |
| kidney | 1.63 ± 0.41b) | 114.4 ± 50.1a) | 0.42 ± 0.12 | 0.53 ± 0.37a) | 15.5 ± 6.40a) | 22.9 ± 10.8 | 153.2 ± 40.9a) | 1.53 ± 0.35a) | 0.04 ± 0.03 | |||||||
| Horned puffin F. corniculata | 3 | 3Ad | 2M & 1F | 631.7 ± 78.2c) | 7.02 ± 0.20d) | −23.4 ± 0.04 | liver | 1.63 ± 0.22b) | 10.5 ± 5.30b) | 0.45 ± 0.27 | 0.10 ± 0.02 | 0.79 ± 0.54 | 18.0 ± 2.62 | 103.6 ± 16.6a,b) | 0.68 ± 0.85 | 0.03 ± 0.02 |
| kidney | 2.50 ± 0.16b) | 52.8 ± 36.4a,b) | 0.31 ± 0.10 | 0.28 ± 0.13a,b) | 3.76 ± 5.27b) | 18.8 ± 4.09 | 120.9 ± 20.7a–c) | 0.54 ± 0.55a,b) | 0.17 ± 0.17 |
Different letters indicate significant differences among species (Tukey test, P<0.05). Ad: adult, Ju: juvenile, M: male, F: female, U: unidentified.
Fig. 2.
Principal component analysis (PCA) of body weight (BW), metal and metalloid concentrations (mg/kg dry weight) in the liver, and stable isotope ratio (δ15N, δ13C) in muscle. The value of component 1 plus component 2 was low (45.5%).
Level of δ15N was the highest in northern fulmar, followed by thick-billed murre, short-tailed shearwater, tufted puffin and horned puffin (Table 1). Hg concentration in liver and δ15N value in muscle were positively correlated (r=0.54, P=0.0008) (Table 3). There were significant differences of Hg concentration between northern fulmar and other species (Table 1). Cd and Zn showed a strong positive correlation (Table 2). There was also a positive correlation between the concentrations of As and Zn (r=0.66, P<0.0001), as well as between the concentrations of Cd and As (r=0.45, P=0.04), and body weight-Cd (r=0.50, P=0.001). δ13C showed a weak but significant correlation with Zn (r=0.45, P=0.006).
Table 3. Prey (crustacean, fish and squid) composition (% mass or volume) in the stomach contents in seabirds collected in the Bering Sea and Aleutians.
| Species | Area | Fish | Squids | Crustaceans | Refs |
|---|---|---|---|---|---|
| Northern fulmar | Bering Sea | 73 | 21 | 6 | [15] |
| F. glacialis | Gulf of Alaska | 3 | 96 | 1 | [10, 16] |
| Thick-billed murre | Bering Sea | 95 | 1 | 4 | [15] |
| U. lomvia | Bering Sea | 6 | <1 | 82 | [28] |
| Okhotsk | 82 | <1 | 11 | [27] | |
| Gulf of Alaska | 16 | 74 | 10 | [10, 16] | |
| Chukchi Sea | 100 | 0 | 0 | [33] | |
| Short-tailed shearwater | Bering Sea | 0–67 | 0 | 33–100 | [14] |
| P. tenuirostris | Bering Sea | 8–40 | 0–30 | 36–82 | [45] |
| Northern North Pacific | 12 | 1 | 87 | [29] | |
| Gulf of Alaska | 24 | 2 | 73 | [10, 16] | |
| Tufted puffin | Bering Sea | 81 | 2 | 3 | [15] |
| F. cirrhata | Bering Sea | 33.6 | 60.5 | 0 | [44] |
| Pribilofs | 99.3 | 0 | 0.7 | [18] | |
| Aleutians | 41.2 | 41.5 | 17 | [44] | |
| Gulf of Alaska | 81 | 8 | 11 | [10, 16] | |
| Horned puffin | Bering Sea | 80 | 1 | 11 | [15] |
| F. corniculata | Pribilofs | 52.1 | 47.5 | 0.4 | [18] |
| Aleutians | 62.4 | 33.4 | 0.8 | [31, 41] | |
| Gulf of Alaska | 98 | 1 | 1 | [10, 16] |
Table 2. Pairwise correlation coefficients r for body weight, each metal and metalloid in the liver and stable isotope ratio in muscle of various species.
| r | P-value | |
|---|---|---|
| Hg-δ15N | 0.54 | 0.0009 |
| Hg-Cd | 0.41 | 0.009 |
| Cd-BW | 0.50 | 0.001 |
| Cd-Zn | 0.60 | <0.0001 |
| Cd-As | 0.45 | 0.004 |
| Co-Zn | 0.41 | 0.008 |
| Ni-Zn | 0.41 | 0.008 |
| As-Zn | 0.66 | <0.0001 |
| δ13C-Zn | 0.45 | 0.006 |
| δ15N- δ13C | 0.70 | <0.0001 |
The accumulation levels of metals and arsenic between sexes and ages in liver of short-tailed shearwater were analyzed, because only this species had enough specimens for analysis. Between males and females, Hg concentration was significantly higher in males, whereas females accumulated higher levels of Cd and Pb. In the case of ages, adults had higher levels of Cr, whereas juvenile had higher levels of Co, Cu, δ13C and δ15N (Table 4).
Table 4. Gender and age diferrences in body weight (g), metal concentration (mg/kg, dry weight in liver) and stable isotope ratio (δ13C and δ15N, ‰) in short-tailed shearwater.
| Male (n=9) |
Female (n=7) |
Adult (n=14) |
Juvenil e(n=8) |
Kruskal-Wallis Χ2 (P) |
|
|---|---|---|---|---|---|
| Body weight | 476 ± 62 | 541 ± 28 | - | - | 3.85 (0.05) |
| Hg | 1.71 ± 0.74 | 0.88 ± 0.36 | - | - | 5.18 (0.02) |
| Cd | 3.06 ± 1.97 | 9.55 ± 4.14 | - | - | 8.47 (0.004) |
| Pb | 0.05 ± 0.02 | 0.09 ± 0.03 | - | - | 4.29 (0.04) |
| Body weight | - | - | 497 ± 55 | 505 ± 47 | 0.09 (0.76) |
| Cr | - | - | 0.37 ± 0.38 | 0.15 ± 0.15 | 8.44 (0.004) |
| Co | - | - | 0.11 ± 0.10 | 0.13 ± 0.04 | 4.11 (0.04) |
| Cu | - | - | 17.7 ± 5.53 | 29.4 ± 14.1 | 9.43 (0.002) |
| δ15N | - | - | 10.1 ± 0.66 | 10.8 ± 0.60 | 6.43 (0.01) |
| δ13C | - | - | –22.4 ± 1.18 | –21.0 ± 0.92 | 5.36 (0.02) |
Given are arithmetic means ± SD.
DISCUSSION
Correlations of metal concentrations and stable isotope ratio
The δ15N level indicates the trophic positions of individual species; thus, northern fulmar is at a trophic level higher than the other species. Northern fulmar eats various fish and squid species (Table 3), whereas horned puffin mainly eats small fish [12]. Trophic levels would have reflected these feeding habitats.
The significant differences of Hg concentration between northern fulmar and other species suggest that Hg uptake is linked to the trophic status of the prey consumed. It is generally accepted that Hg is a ubiquitous environmental contaminant that is transferred typically through aquatic and terrestrial food webs [3, 4]. Northern fulmar had high δ15N, indicating their position as a top predator of the food chain and their higher risk of toxicity due to Hg and other persistent pollutants compared with other birds. Furthermore, they have a long life span and accumulate Hg over long periods [36].
Cd and Zn showed a strong positive correlation, which was consistent with previous reports [13, 38]. These heavy metals are expected to interact with a detoxification system, such as metallothionein (MT), a low molecular weight cysteine-rich protein involved in the homeostasis of essential metals, such as Zn. The synthesis of MT can be induced by several heavy metals, such as Cd, Zn, Cu and inorganic Hg. Zn is essential for the synthesis of MT. When Cd is taken up, Zn in MT is replaced with Cd to achieve detoxification. It was reported that Cd and MT concentrations are positively correlated in many seabirds [11, 42]. High accumulation of Cd induces the synthesis of MT and increased Zn uptake for synthesis. MT can be synthesized or degraded rapidly in response to changes in metal levels (seasonal or through molting) and likely accounts for the range of Cd–Zn relationship [42]. Cd exposure increased Zn concentration in the kidney and livers of rats and increased the concentration of MT mRNA [52].
There was also a positive correlation between As–Zn and Cd–As. Squids accumulating high levels of Cd [42, 43] are known to also accumulate high levels of As [47]. Furthermore, Cd and Zn concentrations in the current study had high correlations. The correlation between As–Zn and Cd–As perhaps reflected these situations.
Body weight-Cd correlation was also high. There are several possibilities that chicks mainly eat small fish and later change their diet to squids or other organisms containing high Cd level, or they accumulate continuously this metal.
The weak correlation between δ13C and Zn suggested that the source of Zn was the food. However, δ13C did not explain the concentrations of any other metal examined (Table S2).
Gender and age differences in metal and arsenic concentrations and stable isotope ratio in short-tailed shearwater
Gender differences might be expected, if males and females eat 1) different foods, 2) different-sized foods or 3) different proportions of different foods [5]. Females can also reduce Hg transferred into the egg [37].
In short-tailed shearwater, females had significantly higher concentrations of Cd and Pb. It is reported that female black skimmers (Rynchops niger) accumulated higher levels of Cd and Pb than males in their breast feathers, and it might be due to a difference in species composition of the diet [7]. The current study showed that the range of δ13C in males was larger than that in females, although there is no significantly difference. There is a possibility that the foods are different between males and females, and it causes the differences of metal accumulation. Hg concentration was higher in males than that in females (Table 2). They breed in south-eastern Australia and lay one egg around the end of November [40]. Short-tailed shearwaters in our study were dead in summer, and almost one year has passed from the breeding. They would accumulate Hg continuously, and females would excrete some of them when they lay the egg.
Juveniles had higher levels of δ13C and δ15N than adults, indicating that the foods are different between adults and juveniles. Therefore, metal concentrations in them would reflect the differences of their food and continued accumulation with age.
Sources of Cd accumulation
Tufted puffin and northern fulmar accumulated high Cd levels. Mallard ducks with Cd concentration in the kidney >300 mg/kg dry weight show renal tubular necrosis [49], although the benchmark values in seabirds are unclear [6, 42]. Seabirds have been shown to contain high concentrations of Cd [17], and some seabird colonies featured the occurrence of kidney diseases caused by Cd [26]. If they appear to be healthy, perhaps they would have some influence because of their high accumulation.
Squids accumulate high levels of Cd [42, 43], and in the North Pacific, northern fulmar and tufted puffin often feed on squid, and the horned puffin sometimes eats squid, while thick-billed murre and short-tailed shearwater feed mainly on krill and fish (Table 2). Many seabirds change their feed depending on the place (Table 3) and season [31, 32]. In the case of oceanic habitats, squid makes up a very large population of the adult tufted puffin diet [35]. The high Cd concentration found in some species may be related to their squid intake.
Toxicological effect and historical trend of Hg
Hepatic concentration of Hg was higher than those in the kidneys of northern fulmar (Table 1), short-tailed shearwater and thick-billed murre, whereas renal levels were higher than those in the liver of tufted puffin and horned puffin. It is reported that some species that accumulated high levels of Hg had higher concentrations in the liver, whereas other species that accumulated low levels of Hg had almost the same levels in the liver and kidney [13], as was the case in the present study. It has been reported that the mean ratios of MeHg in total were 35, 36 and 66% in the liver, kidney and muscle, respectively in seabirds [20]. These authors also found a decrease in the mean proportion of MeHg with an increase in mean total Hg concentration in these tissues. These observations indicated that some seabirds are capable of demethylating MeHg in tissue and storing it in an inert inorganic form in the liver. Differences in accumulation ratio among tissues therefore depend on total Hg level and species-specific demethylation capacity. Higher levels of Hg in liver than the kidney indicate that MeHg is demethylated for the inorganic form in the liver [20]. Intestinal absorption of inorganic Hg is limited to a few percent, whereas MeHg can be absorbed nearly completely [50]. Absorption in the first pharmacokinetic process was also different among species. Differences in inorganic Hg absorption among bird species have been reported [39]; it follows that the capacities of MeHg absorption also vary.
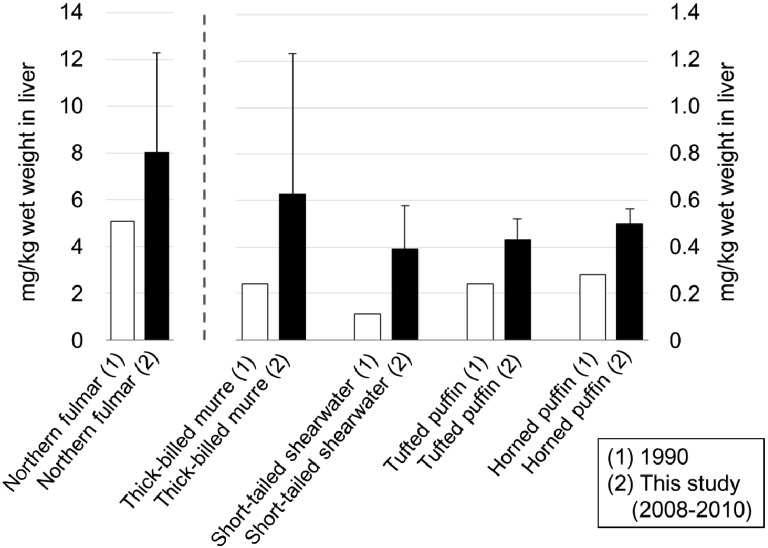
The present study showed that Hg concentrations in the liver and kidneys of seabirds collected in the central Bering Sea were almost double compared with those measured in each species of seabirds collected in 1990 [13] (Fig. 3). Other heavy metals (Cd, Cu and Zn) were not increased, suggesting that Hg concentration changed definitely and there is the possibility that Hg may cause poisoning in various animals in the future. However, Hg concentrations of northern fulmar and tufted puffins in Aleutian Island in 2000−2001 were 5.44−32.7 and 2.1−4.4 (mg/kg dry weight of liver), respectively [36]. The concentration in northern fulmar was lower than that in this study (6.82−40.3 mg/kg dry weight), whereas that of tufted puffin was higher than in this study (1.02−1.99 mg/kg dry weight). There is a possibility that Hg concentration is not increasing depending on species. However, in the case of seabirds in which it is easy for Hg to accumulate, such as northern fulmar, Hg concentration may have some influence.
Fig. 3.
Comparison of Hg levels in the livers of seabirds in the Bering Sea during the periods (1) 1982−1985 (Honda et al. 1990) and (2) 2008−2010 (this study) (mean, mg/kg wet weight).
In conclusion, heavy metal and metalloid concentrations in the liver and kidneys of seabird species collected in the central Bering Sea showed that 1) seabird species of high trophic level, such as northern fulmar, accumulated more Hg, 2) those feeding largely on squid, such as tufted puffin and northern fulmar, readily accumulated Cd, 3) accumulation pattern of several metals is different depending on gender and age, and 4) Hg concentration in the central Bering Sea would have increased over these two decades. High accumulation of heavy metals would adversely affect seabirds, and the risk is increasing.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan awarded to M. Ishizuka (No. 16H0177906), Y. Ikenaka (No. 26304043, 15H0282505 and 15K1221305) and S. Nakayama (16K16197), and the foundations of Sumitomo and JSPS Core to Core Program (AA Science Platforms) and Bilateral Joint Research Project (PG36150002 and PG36150003). We also acknowledge the financial support of The Mitsui & Co., Ltd., Environment Fund, the Soroptimist Japan Foundation, the Nakajima Foundation and the Inui Memorial Trust for Research on Animal Science. One of the authors (C. Ishii) is a Research Fellow of the Japan Society for the Promotion of Science (No. 15J01937). We are grateful to Mr. Takahiro Ichise (Laboratory of Toxicology, Graduate School of Veterinary Medicine, Hokkaido University) for technical support.
REFERENCES
- 1.Alleva E., Francia N., Pandolfi M., De Marinis A. M., Chiarotti F., Santucci D.2006. Organochlorine and heavy-metal contaminants in wild mammals and birds of Urbino-Pesaro Province, Italy: an analytic overview for potential bioindicators. Arch. Environ. Contam. Toxicol. 51: 123–134. doi: 10.1007/s00244-005-0218-1 [DOI] [PubMed] [Google Scholar]
- 2.Atwell L., Hobson K. A., Welch H. E.1998. Biomagnification and bioaccumulation of mercury in an arctic marine food web: insights from stable nitrogen isotope analysis. Can. J. Fish. Aquat. Sci. 55: 1114–1121. doi: 10.1139/f98-001 [DOI] [Google Scholar]
- 3.Blévin P., Carravieri A., Jaeger A., Chastel O., Bustamante P., Cherel Y.2013. Wide range of mercury contamination in chicks of southern ocean seabirds. PLoS ONE 8: e54508. doi: 10.1371/journal.pone.0054508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan A. L., Jr, Brant H. A., Jagoe C. H., Romanek C. S., Brisbin I. L., Jr.2012. Mercury concentrations in nestling wading birds relative to diet in the southeastern United States: a stable isotope analysis. Arch. Environ. Contam. Toxicol. 63: 144–152. doi: 10.1007/s00244-011-9745-0 [DOI] [PubMed] [Google Scholar]
- 5.Burger J.1995. Heavy metal and selenium levels in feathers of herring gulls (Larus argentatus): Differences due to year, gender, and age at Captree, Long Island. Environ. Monit. Assess. 38: 37–50. doi: 10.1007/BF00547125 [DOI] [PubMed] [Google Scholar]
- 6.Burger J.2008. Assessment and management of risk to wildlife from cadmium. Sci. Total Environ. 389: 37–45. doi: 10.1016/j.scitotenv.2007.08.037 [DOI] [PubMed] [Google Scholar]
- 7.Burger J., Gochfeld M.1992. Heavy metal and selenium concentrations in black skimmers (Rynchops niger): gender differences. Arch. Environ. Contam. Toxicol. 23: 431–434. doi: 10.1007/BF00203805 [DOI] [PubMed] [Google Scholar]
- 8.Castellini J. M., Rea L. D., Lieske C. L., Beckmen K. B., Fadely B. S., Maniscalco J. M., O’Hara T. M.2012. Mercury concentrations in hair from neonatal and juvenile Steller Sea Lions (Eumetopias jubatus): implications based on age and region in this northern Pacific marine sentinel piscivore. EcoHealth 9: 267–277. doi: 10.1007/s10393-012-0784-4 [DOI] [PubMed] [Google Scholar]
- 9.Day R. D., Roseneau D. G., Vander Pol S. S., Hobson K. A., Donard O. F., Pugh R. S., Moors A. J., Becker P. R.2012. Regional, temporal, and species patterns of mercury in Alaskan seabird eggs: mercury sources and cycling or food web effects? Environ. Pollut. 166: 226–232. doi: 10.1016/j.envpol.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 10.DeGange A. R., Sanger G. A.1986. Marine birds. pp. 479–524. In: Gulf Alaska Phys Environ Biol Resour (Hood, D.W. and Zimmerman, S.T. eds.), US Natl. Ocean. Atmos. Adm. Ocean. Assessments Div., Anchorage. [Google Scholar]
- 11.Elliott J. E., Scheuhammer A. M., Leighton F. A., Pearce P. A.1992. Heavy metal and metallothionein concentrations in Atlantic Canadian seabirds. Arch. Environ. Contam. Toxicol. 22: 63–73. doi: 10.1007/BF00213303 [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald W. F., Lamborg C. H., Hammerschmidt C. R.2007. Marine biogeochemical cycling of mercury. Chem. Rev. 107: 641–662. doi: 10.1021/cr050353m [DOI] [PubMed] [Google Scholar]
- 13.Honda K., Marcovecchio J. E., Kan S., Tatsukawa R., Ogi H.1990. Metal concentrations in pelagic seabirds from the North Pacific Ocean. Arch. Environ. Contam. Toxicol. 19: 704–711. doi: 10.1007/BF01183988 [DOI] [PubMed] [Google Scholar]
- 14.Hunt G. L., Baduini C., Jahncke J.2002. Diets of short-tailed shearwaters in the southeastern Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 49: 6147–6156. doi: 10.1016/S0967-0645(02)00338-7 [DOI] [Google Scholar]
- 15.Hunt G. L., Burgeson B., Sanger G. A.1981. Feeding ecology of seabirds of the eastern Bering Sea. East Bering Sea shelf. Oceanogr. Resour. 2: 629–648. [Google Scholar]
- 16.Hunt G. L., Drew G. S., Jahncke J., Piatt J. F.2005. Prey consumption and energy transfer by marine birds in the Gulf of Alaska. Deep Sea Res. Part II Top. Stud. Oceanogr. 52: 781–797. doi: 10.1016/j.dsr2.2004.12.024 [DOI] [Google Scholar]
- 17.Hutton M.1981. Accumulation of heavy metals and selenium in three seabird species from the United Kingdom. Environ. Pollut. A 26: 129–145. doi: 10.1016/0143-1471(81)90043-X [DOI] [Google Scholar]
- 18.Johnson S. R.1985. Population Estimation, Productivity, and Food Habits of Nesting Seabirds at Cape Peirce and the Pribilof Islands, Bering Sea, Alaska: Final Report. LGL Ecological Associates.
- 19.Kemper C., Gibbs P., Obendorf D., Marvanek S., Lenghaus C.1994. A review of heavy metal and organochlorine levels in marine mammals in Australia. Sci. Total Environ. 154: 129–139. doi: 10.1016/0048-9697(94)90083-3 [DOI] [PubMed] [Google Scholar]
- 20.Kim E. Y., Murakami T., Saeki K., Tatsukawa R.1996. Mercury levels and its chemical form in tissues and organs of seabirds. Arch. Environ. Contam. Toxicol. 30: 259–266. doi: 10.1007/BF00215806 [DOI] [Google Scholar]
- 21.Larison J. R., Likens G. E., Fitzpatrick J. W., Crock J. G.2000. Cadmium toxicity among wildlife in the Colorado Rocky Mountains. Nature 406: 181–183. doi: 10.1038/35018068 [DOI] [PubMed] [Google Scholar]
- 22.Lucia M., André J. M., Gontier K., Diot N., Veiga J., Davail S.2010. Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the southwest Atlantic coast of France. Arch. Environ. Contam. Toxicol. 58: 844–853. doi: 10.1007/s00244-009-9393-9 [DOI] [PubMed] [Google Scholar]
- 23.Mason R. P., Fitzgerald W. F., Morel F. M.1994. The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochim. Cosmochim. Acta 58: 3191–3198. doi: 10.1016/0016-7037(94)90046-9 [DOI] [Google Scholar]
- 24.Minagawa M., Wada E.1984. Stepwise enrichment of 15 N along food chains: further evidence and the relation between δ 15 N and animal age. Geochim. Cosmochim. Acta 48: 1135–1140. doi: 10.1016/0016-7037(84)90204-7 [DOI] [Google Scholar]
- 25.Nakayama S. M. M., Ikenaka Y., Muzandu K., Choongo K., Yabe J., Muroya T., Ijiri S., Minagawa M., Umemura T., Ishizuka M.2013. Geographic information system-based source estimation of copper pollution in Lake Itezhi-tezhi and metal-accumulation profiles in Oreochromis spp. from both field and laboratory studies. Arch. Environ. Contam. Toxicol. 64: 119–129. doi: 10.1007/s00244-012-9802-3 [DOI] [PubMed] [Google Scholar]
- 26.Norheim G.1987. Levels and interactions of heavy metals in sea birds from Svalbard and the Antarctic. Environ. Pollut. 47: 83–94. doi: 10.1016/0269-7491(87)90039-X [DOI] [PubMed] [Google Scholar]
- 27.Ogi H., Tsujita T.1978. Food and feeding habits of common murre and thick-billed murre in the Okhotsk Sea in summer, 1972 and 1973. Special Bulletin of Research Institute of North Pacific Fisheries Hokkaido University. [Google Scholar]
- 28.Ogi H., Hamanaka T.1982. The feeding ecology of Uria lomvia in the northwestern Bering Sea region. J. Yamashina Inst. Ornithol. 14: 270–280. doi: 10.3312/jyio1952.14.270 [DOI] [Google Scholar]
- 29.Ogi H., Kubodera T., Nakamura K.1980. The pelagic feeding ecology of the short-tailed shearwater Puffinus tenuirostris in the subarctic Pacific region. J. Yamashina Inst. Ornithol. 12: 157–182. doi: 10.3312/jyio1952.12.3_157 [DOI] [Google Scholar]
- 30.Overland J. E., Stabeno P. J.2004. Is the climate of the Bering Sea warming and affecting the ecosystem? Eos Trans. AGU 85: 309–312. doi: 10.1029/2004EO330001 [DOI] [Google Scholar]
- 31.Piatt J. F.2002a Horned puffin (Fratercula corniculata). Birds North Am. 611: 1–27. [Google Scholar]
- 32.Piatt J. F.2002b Tufted puffin (Fratercula cirrhata). Birds North Am. 708: 1–31. [Google Scholar]
- 33.Piatt J. F., Wells J. L., MacCharles A., Fadely B. S.1991. The distribution of seabirds and fish in relation to ocean currents in the southeastern Chukchi Sea. Studies of High-latitude Seabirds 1: 21–31. [Google Scholar]
- 34.Pirrone N., Cinnirella S., Feng X., Finkelman R. B., Friedli H. R., Leaner J., Mason R., Mukherjee A. B., Stracher G. B., Streets D. G., Telmer K.2010. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 10: 5951–5964. doi: 10.5194/acp-10-5951-2010 [DOI] [Google Scholar]
- 35.Post D. M., Layman C. A., Arrington D. A., Takimoto G., Quattrochi J., Montaña C. G.2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189. doi: 10.1007/s00442-006-0630-x [DOI] [PubMed] [Google Scholar]
- 36.Ricca M. A., Keith Miles A., Anthony R. G.2008. Sources of organochlorine contaminants and mercury in seabirds from the Aleutian archipelago of Alaska: inferences from spatial and trophic variation. Sci. Total Environ. 406: 308–323. doi: 10.1016/j.scitotenv.2008.06.030 [DOI] [PubMed] [Google Scholar]
- 37.Robinson S. A., Forbes M. R., Hebert C. E., Scheuhammer A. M.2011. Evidence for sex differences in mercury dynamics in double-crested cormorants. Environ. Sci. Technol. 45: 1213–1218. doi: 10.1021/es1021872 [DOI] [PubMed] [Google Scholar]
- 38.Scheuhammer A. M.1987. The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environ. Pollut. 46: 263–295. doi: 10.1016/0269-7491(87)90173-4 [DOI] [PubMed] [Google Scholar]
- 39.Serafin J. A.1984. Avian species differences in the intestinal absorption of xenobiotics (PCB, dieldrin, Hg2+). Comp. Biochem. Physiol. Part C Comp. Pharmacol 78: 491–496. [DOI] [PubMed] [Google Scholar]
- 40.Serventy D. L., Curry P. J.1984. Observations on colony size, breeding success, recruitment and inter-colony dispersal in a Tasmanian colony of Short-tailed Shearwaters Puffinus tenuirostris over a 30-year peroid. Emu 84: 71–79. doi: 10.1071/MU9840071 [DOI] [Google Scholar]
- 41.Springer A. M., Piatt J. F., Vliet G. V.1996. Sea birds as proxies of marine habitats and food webs in the western Aleutian Arc. Fish. Oceanogr. 5: 45–55. doi: 10.1111/j.1365-2419.1996.tb00016.x [DOI] [Google Scholar]
- 42.Stewart F. M., Furness R. W., Monteiro L. R.1996. Relationships between heavy metal and metallothionein concentrations in lesser black-backed gulls, Larus fuscus, and Cory’s shearwater, Calonectris diomedea. Arch. Environ. Contam. Toxicol. 30: 299–305. doi: 10.1007/BF00212287 [DOI] [PubMed] [Google Scholar]
- 43.Storelli M. M.2008. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 46: 2782–2788. doi: 10.1016/j.fct.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 44.Tanaka H.1989. Biology and bioaccumulation of PCBs in Tufted Puffins (Lunda cirrhata) of the northern North Pacific. J. Yamashina Inst. Ornithol. 21: 1–41. doi: 10.3312/jyio1952.21.1 [DOI] [Google Scholar]
- 45.Toge K., Yamashita R., Kazama K., Fukuwaka M., Yamamura O., Watanuki Y.2011. The relationship between pink salmon biomass and the body condition of short-tailed shearwaters in the Bering Sea: can fish compete with seabirds? Proc. R Soc. London B Biol. Sci. rspb20102345. [DOI] [PMC free article] [PubMed]
- 46.United Nations Environment Programme (UNEP) 2013. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. UNEP Chemicals Branch, Geneva. [Google Scholar]
- 47.Uneyama C., Toda M., Yamamoto M., Morikawa K.2007. Arsenic in various foods: cumulative data. Food Addit. Contam. 24: 447–534. doi: 10.1080/02652030601053121 [DOI] [PubMed] [Google Scholar]
- 48.Watanuki Y., Yamamoto T., Yamashita A., Ishii C., Ikenaka Y., Nakayama S. M., Ishizuka M., Suzuki Y., Niizuma Y., Meathrel C. E., Phillips R. A.2015. Mercury concentrations in primary feathers reflect pollutant exposure in discrete non-breeding grounds used by Short-tailed Shearwaters. J. Ornithol. 1: 4. [Google Scholar]
- 49.White D. H., Finley M. T.1978. Uptake and retention of dietary cadmium in mallard ducks. Environ. Res. 17: 53–59. doi: 10.1016/0013-9351(78)90060-9 [DOI] [PubMed] [Google Scholar]
- 50.Wolfe M. F., Schwarzbach S., Sulaiman R. A.1998. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. 17: 146–160. doi: 10.1002/etc.5620170203 [DOI] [Google Scholar]
- 51.Yabe J., Nakayama S. M. M., Ikenaka Y., Muzandu K., Choongo K., Mainda G., Kabeta M., Ishizuka M., Umemura T.2013. Metal distribution in tissues of free-range chickens near a lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 32: 189–192. doi: 10.1002/etc.2029 [DOI] [PubMed] [Google Scholar]
- 52.Zhang D., Gao J., Zhang K., Liu X., Li J.2012. Effects of chronic cadmium poisoning on Zn, Cu, Fe, Ca, and metallothionein in liver and kidney of rats. Biol. Trace Elem. Res. 149: 57–63. doi: 10.1007/s12011-012-9394-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.