Abstract
Autoantibodies to the Sm antigens are specifically found in 5 to 30% of patients with systemic lupus erythematosus (SLE) depending on the detection system and the patient group. Several immunoassays designed for research and diagnostic laboratory use have been developed. The autoantigens employed in these tests include purified native proteins, recombinant polypeptides, and synthetic peptides. In the present study, we compared the clinical accuracy of anti-Sm autoantibody assays from commercial suppliers including different conventional enzyme-linked immunosorbent assay (ELISA) systems based on purified Sm antigens, an addressable laser bead assay and a newly developed anti-Sm peptide assay. Although the clinical sensitivity of all assays under investigation was comparable, relatively poor correlations and significant differences in specificity were found with a patient cohort of 150 patients. The sensitivity and specificity were 10 and 94%, respectively, for the anti-Sm ELISA from Euroimmun, 10 and 90%, respectively, for the QuantaLite Sm (INOVA), 12 and 88%, respectively, for the Sm assay in the Varelisa ReCombi ANA profile (Pharmacia Diagnostics), 10 and 94%, respectively, for the QuantaPlex Sm (INOVA), and 12 and 100%, respectively, for the new SmD3 peptide-based ELISA (Varelisa Sm Antibodies). The majority of positive test results within the control groups were found in patients with mixed connective tissue disease. Based on the results, we conclude that the detection of anti-Sm antibodies strongly depends both on the nature of the antigen and on the detection system. Finally, we conclude that the recently identified SmD peptide containing a symmetrical dimethylarginine at position 112 of D3 represents a promising tool for the detection of a highly specific subpopulation of anti-Sm antibodies.
Systemic rheumatic diseases are characterized by the occurrence of circulating autoantibodies to defined intracellular targets (reviewed in reference 26). Among the earliest identified autoantibodies were those directed to components of U2-U6 small nuclear ribonucleoproteins (snRNPs), known as Sm, which are highly specific for systemic lupus erythematosus (SLE) (24). Thus, anti-Sm antibodies have been included as one of the SLE classification criteria of the American College of Rheumatology (25). Apart from autoantibodies targeting the Sm complex, anti-double-stranded DNA, anti-proliferating cell nuclear antigen (PCNA), anti-U1-RNP, antinucleosome, antihistone, anti-Ro/SS-A, anti-La/SS-B, anti-ribosomal P, and anti-phospholipid antibodies are found in patients with SLE (26). Recent studies suggest that SLE-associated antibodies are present before the clinical onset of the disease and thus have high prognostic value (2).
Anti-Sm reactivity is found in 5 to 30% of patients with SLE, and this frequency varies depending on the detection system and the ethnicity of the SLE population under investigation (1, 8, 12, 16, 17, 26). The Sm antigen is part of the spliceosomal complex that catalyzes the splicing of nuclear pre-mRNA and is composed of at least nine different polypeptides ranging from 9 to 29.5 kDa: B (B1, 28 kDa), B′ (B2, 29 kDa), N (B3, 29.5 kDa), D1 (16 kDa), D2 (16.5 kDa), D3 (18 kDa), E (12 kDa), F (11 kDa) and G (9 kDa) (8, 11). All of these core proteins, but most frequently the B and D polypeptides, are targets of the anti-Sm autoimmune response (3, 8). However, since SmBB′ and U1-specific RNPs share the cross-reactive epitope motif PPPGMRPP, SmD is regarded as the most SLE-specific Sm antigen (1, 8).
Within the SmD autoantigen family, reactivity with the SmD1/D3 pattern is at least four times more common than SmD1/D2/D3 recognition with a pronounced immunoreactivity to SmD1 (9). In epitope-mapping studies, several linear and conformational epitopes have been mapped on the SmB and D proteins (1, 7, 9, 12, 14, 17, 19). On SmD1 and BB′, the major reactivity was predominantly found in the C-terminal extensions (7, 8, 19). Recently, it has been shown that the polypeptides D1, D3, and BB′ contain symmetrical dimethylarginine (sDMA) constituting a major autoepitope within the C terminus of SmD1 (4). Several enzyme-linked immunosorbent assay (ELISA) systems designed for research studies as well as diagnostic laboratory use have been developed. The antigenic analytes employed in these tests included purified native proteins, recombinant polypeptides, and synthetic peptides (7, 8, 12, 15, 17). In a recent study, a highly specific Sm peptide containing a dimethylarginine residue at position 112 of SmD3 comprising the sequence 108AARG-sDMA-GRGMGRGNIF122 has been identified and used for the development of a reliable ELISA system for the detection of a subpopulation of anti-Sm antibodies (12).
In the present study, we compared the clinical accuracy of this new peptide-based immunoassay with three other commercial ELISA systems and a multiplex addressable laser bead assay, all of which used purified Sm proteins from native sources for the detection of anti-Sm antibodies.
MATERIALS AND METHODS
Serum samples.
Sera were collected from patients with systemic lupus erythematosus (SLE; n = 50) and various control diseases, including rheumatoid arthritis (n = 50), mixed connective tissue disease (MCTD, n = 17), scleroderma (n = 17), polymyositis/dermatomyositis (n = 11), and other autoimmune disorders (n = 15). All samples were taken from a previous study and classified according to published criteria for each disease (13). Sera were stored in aliquots at −80°C until use and shipped on dry ice. None of the samples had more than two freezing and thawing cycles.
Varelisa Sm antibodies.
The new Varelisa Sm (Pharmacia Diagnostics, Freiburg, Germany) assay is based on a recently identified peptide derived from the SmD3 sequence (12). The SmD3 peptide comprises 16 amino acids, amino acids 108 to 122 of SmD3 (108AARG-sDMA-GRGMGRGNIF122) with an additional cysteine at the C terminus and a symmetrical dimethylarginine (sDMA) residue at position 112.
Addressable laser bead assay.
Microspheres embedded with laser-reactive dyes (Luminex Corporation, Austin, Tex.) that were coupled with native Sm antigen were part of a commercial kit (QuantaPlex 9; INOVA Diagnostics Inc., San Diego, Calif.). This profile test allows the semiquantitative detection of autoantibodies to chromatin, DNA, Jo-1, Rib-P, RNP, Scl-70, Sm, SS-A (Ro), and SS-B (La). The assay was performed according to the manufacturer's instructions. Briefly, each test serum was diluted to 1:1,000, and 50 μl was added to a well of a microtiter plate, mixed with the antigen-coated beads that were preserved in the well, and incubated for 30 min. Then 50 μl of phycoerythrin-conjugated goat anti-human immunoglobulin G (Jackson ImmunoResearch, Inc., West Grove, Pa.) was added to each well and incubated for an additional 30 min. The reactivity of the antigen-coated beads was determined on a Luminex 100 dual-laser flow cytometer (Luminex Corp.). The cutoff for a positive test result was based on the reactivity of control samples. The control samples were titrated to provide high, medium, low, and negative values. For further information see http://www.inovadx.com/detailfiles/708910.pdf.
Euroimmun Sm.
Antibodies against Sm proteins (immunoglobulin G) is a quantitative or semiquantitative ELISA system based on purified Sm proteins including B, B′, D, E, F, and G from calf thymus (Euroimmun, Lübeck, Germany; code no. EA 1593-9601 G). A cutoff value of 20 relative units/ml (REU/ml) is recommended by the manufacturer. For further information see the manufacturer's website.
QuantaLite Sm.
QuantaLite Sm (INOVA, San Diego, Calif.; code no. 708560) is a semiquantitative test based on affinity-purified Sm proteins. According to the manufacturer's instructions, less then 20 units is considered negative, 20 to 39 units is ranked as weak positive, 40 to 80 units is considered moderate positive, and >80 units is considered strong positive. For the calculations in this study, a cutoff of 40 units was selected since, with the lower cutoff of 20 units, 16 control sera were positive for Sm antibodies. For further information, see http://www.inovadx.com/detailfiles/708560.pdf.
Varelisa ReCombi ANA Profile (Pharmacia Diagnostics, Freiburg, Germany).
The Varelisa ReCombi ANA Profile allows the semiquantitative detection of autoantibodies to double-stranded DNA (recombinant plasmid double-stranded DNA), U1-snRNP (human recombinant), Sm (B, B′, D) (complex purified from human HeLa cells), SS-A/Ro (human recombinant 52 and 60 kDa), SS-B/La (human recombinant antigen), Scl-70 (human recombinant topoisomerase 1), CENP (human recombinant CENP-B), and Jo-1 (human recombinant histidyl tRNA synthetase). For further information see http://www.diagnostics.nu/upload/AUTOIMMUNITY/ReCombi_ANA_Profil-PI.pdf and http://www.diagnostics.nu/upload/AUTOIMMUNITY/ReCombi_ANA_Profil-PF.pdf.
Statistical evaluation of the results.
The results obtained from the comparative study were evaluated with Analyze-it software (version 1.62; Analyze-it Software, Ltd., Leeds, United Kingdom). Receiver operating characteristic (ROC) curves (Fig. 1), positive and negative predictive values, and clinical efficiency were calculated for each anti-Sm antibody assay. Furthermore, the correlation coefficients between all immunoassays were calculated.
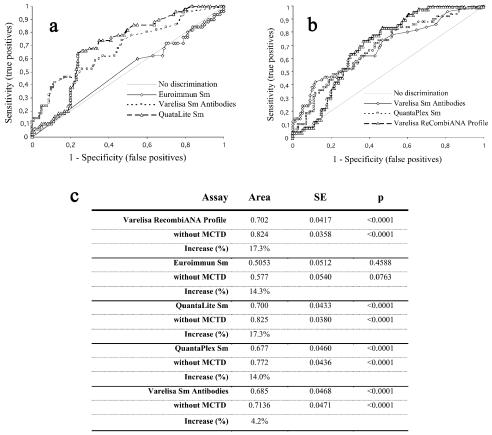
FIG. 1.
Receiver operating characteristic (ROC) analysis of different anti-Sm antibody assays. The results of this comparative study of different anti-Sm antibody assays were used to generate ROC curves. The discrimination between SLE patient samples and controls was significantly improved with the SmD3 peptide-based immunoassay compared to the anti-Sm antibody assays Euroimmun Sm, QuantaLite Sm (a), and Varelisa ReCombi ANA Profile, as well as the QuantaPlex Sm test (b). Results are summarized in panel c.
RESULTS
Sera from 50 unselected SLE patients and various control samples (n = 100) were tested in the anti-Sm ELISA systems from Euroimmun and INOVA. Furthermore, all sera were also tested in the Varelisa ReCombi ANA Profile (Pharmacia Diagnostics) and in the new QuantaPlex assay (INOVA) based on a multiplexed addressable laser bead immunoassay (ALBIA). Finally, the new Varelisa Sm antibodies assay (Pharmacia Diagnostics) was used to measure antibodies to the recently identified peptide of SmD3 (12).
We found that 5 of 50 (10%; INOVA QuantaLite Sm), 5 of 50 (10%; Euroimmun Sm), 6 of 50 (12% Varelisa ReCombi ANA Profile) and 6 of 50 (12% Varelisa Sm Antibodies) SLE patient sera were positive for anti-Sm antibodies (see Table 1). We found that 6 of 100 of the disease controls displayed anti-Sm reactivity under the respective conditions in the Euroimmun Sm ELISA, resulting in a specificity of 94%. Four of these patients were from the MCTD group, one had MCTD/SLE overlap syndrome, and one patient had systemic sclerosis. We found that 10 of 100 of the control patients tested positive in the QuantaLite Sm test from INOVA (one scleroderma, one MCTD/SLE overlap, and eight MCTD), resulting in a specificity of 90%. We found that 6 of 50 (12%), one patient with MCTD/SLE overlap syndrome, one scleroderma patient, and 10 patients suffering from MCTD tested false positive for anti-Sm antibodies in the Varelisa ReCombi ANA Profile. In contrast, none control sera were positive with the SmD3 peptide test.
TABLE 1.
Performance of different anti-Sm antibody assays with and without MCTD patients in the control groupa
| Parameter | Euroimmun Sm | QuantaLite Sm | QuantaPlex Sm | Varelisa RecombiANA Profile | Varelisa Sm antibodies |
|---|---|---|---|---|---|
| SLE patients | |||||
| No. (%) positive | 5 (10) | 5 (10) | 5 (10) | 5 (12) | 5 (12) |
| Mean value | 11.5 | 24.3 | 0.8 | 0.8 | 33.6 |
| SD | 37.9 | 42.5 | 2.1 | 1.4 | 175.5 |
| Mean + 3 SD | 125.1 | 151.9 | 7.2 | 5.0 | 560.0 |
| Max | 209.5 | 191.3 | 11.7 | 7.2 | 1232.0 |
| Min | 0.0 | 5.5 | 0.1 | 0.1 | 0.0 |
| Controls | |||||
| No. (%) positive | 6 (6)/1 (1.2) | 10 (10)/1 (1.2) | 6 (6)/2 (2.5) | 12 (12)/1 (1.2) | 0 (0)/0 (0) |
| Mean value | 6.4/1.1 | 16.2/7.8 | 0.4/0.2 | 0.58/0.22 | 1.4/1.1 |
| SD | 22.5/8.0 | 27.4/11.3 | 1.1/0.8 | 1.06/0.32 | 1.8/1.5 |
| Mean + 3 SD | 73.8/25.1 | 98.5/41.6 | 3.7/2.8 | 3.75/1.16 | 6.8/5.6 |
| Max | 136.0/72.2 | 153.7/105.3 | 7.9/7.6 | 5.36/2.8 | 9.9/8.3 |
| Min | 0.0/0.0 | 3.6/3.6 | 0.1/0.1 | 0.07/0.07 | 0.0/0.0 |
| MCTD no. (%) positive | 4 (23.5) | 8 (47.1) | 3 (17.7) | 10 (58.8) | 0 (0) |
| MCTD/SLE no. (%) positive | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 0 (0) |
| SSc no. (%) positive | 1 (5.9) | 1 (5.9) | 1 (5.9) | 1 (5.9) | 0 (0) |
| PM/DM no. (%) positive | 0 (0) | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) |
| Performance | |||||
| Sensitivity (%) | 10 | 10 | 10 | 12 | 12 |
| Specificity (%) | 94/98.8 | 90/98.8 | 94/97.5 | 88/98.8 | 100/100 |
| PPV (%) | 45.5/83.3 | 33.3/83.3 | 45.5/71.4 | 33.3/85.7 | 100/100 |
| NPV (%) | 67.6/63.7 | 66.7/63.7 | 67.6/63.4 | 66.7/64.2 | 69.4/63.7 |
| Efficiency (%) | 66/64.6 | 63.3/64.6 | 66/63.8 | 62.7/65.4 | 70.7/64.6 |
All values in the control and performance sections are calculated based on the control group with/without MCTD patients. SSc, scleroderma; PM/DM, polymyositis/dermatomyositis; PPV and NPV, positive and negative predictive value, respectively.
The positive and negative predictive values as well as the test efficiency were calculated at 45.5%, 67.6%, and 66%, respectively, for the Euroimmun Sm ELISA, at 33.3%, 66.7%, and 63.3%, respectively, for QuantaLite Sm, at 33.3%, 66.7%, and 62.7%, respectively, for Sm tested by Varelisa ReCombi ANA Profile, and at 100%, 69.4%, and 70.7%, respectively, for the SmD3 peptide-based ELISA (Varelisa Sm Antibodies). With a cutoff of 1 (ratio = value sample/value low positive) as suggested by the manufacturer of the new QuantaPlex Sm test, 5 of 50 patients with SLE tested positive for anti-Sm antibodies.
Six of the control sera (one scleroderma, one MCTD/SLE, one polymyositis/dermatomyositis, and three MCTD) had assay values above the suggested cutoff, resulting in a sensitivity of 10% and a specificity of 94% for lupus (Table 2). The positive and negative predictive values and the test efficiency were calculated at 45.5%, 67.6%, and 66%, respectively (Table 1). The results of all Sm assays were subjected to a comparative ROC analysis, which showed that the discrimination between positive sera and controls as expressed by the area under the curve varied from 0.505 (Euroimmun Sm) to 0.702 (Varelisa ReCombi ANA Profile). The results are summarized in Fig. 2. No significant improvement of the assay performance could be achieved by optimizing the cutoff values of the tests.
TABLE 2.
Selected results for anti-Sm-positive control samples
| Serum no. | ID no. | Diagnosisa | Euroimmun Sm (RE)b (cutoff = 20) | QuantaLite Sm (U) (cutoff = 40) | QuantaPlex Sm (ratio) (cutoff = 1) | Varelisa RecombiANA Profile (ratio) (cutoff = 1) | Varelisa Sm antibodies (U/ml) (cutoff = 13) |
|---|---|---|---|---|---|---|---|
| 99 | 25510 | RA | 1.2 | 25.5 | 0.1 | 0.5 | 0.3 |
| 102 | 25513 | PM/DM | 1.0 | 6.7 | 7.6 | 0.3 | 3.4 |
| 105 | 25516 | MCTD | 87.9 | 118.8 | 1.1 | 4.8 | 3.0 |
| 107 | 25518 | MCTD | 14.4 | 37.5 | 0.3 | 1.4 | 1.0 |
| 108 | 25519 | MCTD | 6.7 | 52.0 | 0.9 | 2.7 | 0.0 |
| 110 | 25521 | MCTD | 87.4 | 111.7 | 1.5 | 4.2 | 0.5 |
| 112 | 25523 | MCTD | 21.6 | 34.1 | 0.6 | 0.3 | 0.0 |
| 118 | 25529 | MCTD;SLE | 11.8 | 30.4 | 0.5 | 0.5 | 2.3 |
| 121 | 25532 | MCTD | 7.4 | 26.9 | 0.3 | 3.5 | 2.6 |
| 123 | 25534 | MCTD | 10.5 | 41.7 | 0.6 | 2.3 | 1.1 |
| 126 | 25537 | MCTD | 7.2 | 41.4 | 0.3 | 0.3 | 3.2 |
| 128 | 25539 | MCTD | 4.2 | 67.1 | 0.5 | 3.7 | 0.7 |
| 129 | 25540 | MCTD | 118.5 | 132.4 | 2.4 | 3.1 | 4.1 |
| 132 | 25543 | MCTD | 12.8 | 42.7 | 0.3 | 2.3 | 2.4 |
| 133 | 25544 | MCTD;SLE | 136.0 | 153.7 | 7.9 | 5.4 | 9.9 |
| 137 | 25448 | SSc | 72.2 | 105.3 | 1.2 | 2.8 | 2.2 |
| 145 | 25456 | MCTD | 8.4 | 30.9 | 0.3 | 1.5 | 1.1 |
| No. positive | 6 | 10 | 6 | 12 | 0 |
RA, rheumatoid arthritis. Also see Table 1, footnote a.
RE, relative units.
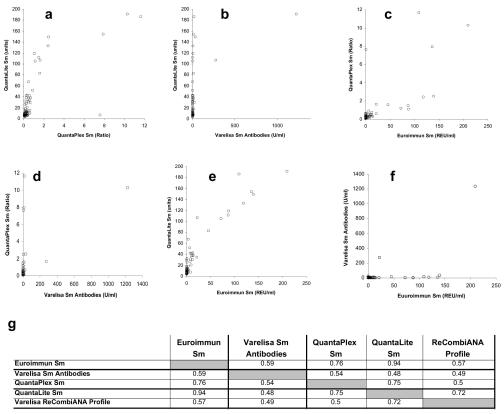
FIG. 2.
Correlation study of the anti-Sm antibody assays under investigation. The results obtained from the comparative study of different anti-Sm antibody tests were used for correlation plots showing significant differences in the degree of correlation. The correlation coefficient varied between 0.48 (QuantaLite Sm versus Varelisa Sm antibodies) and 0.94 (Euroimmun Sm versus QuantaLite Sm). All correlation coefficients are summarized in panel g.
The correlation between the anti-Sm antibody tests from different suppliers showed statistical R2 values ranging from 0.48 (QuantaLite Sm versus Varelisa Sm) to 0.94 (Euroimmun versus QuantaLite Sm). The correlation value between the QuantaPlex Sm results and the results from the QuantaLite test was unexpected low (R2 = 0.75), although both tests are based on the same native Sm antigen (Fig. 1).
The positive results in the control groups of the immunoassay with conventional Sm antigens (QuantaLite Sm, QuantaPlex Sm, Varelisa ReCombi ANA Profile, and Euroimmun Sm) were mostly found with samples from MCTD patients. When the MCTD samples were excluded from the control group, the assay performance of all assays with native antigens could be significantly increased. This can be expressed by the increased area under the curve and by the improved specificity and diagnostic efficiency of the assays (Table 1 and Fig. 1). The specificity increased to 98.8% (Euroimmun Sm), to 98.8% (QuantaLiteTM Sm), to 97.5% (QuantaPlexTM Sm), and to 98.8% (ReCombi ANA Profile).
DISCUSSION
Various techniques, in combination with a variety of different antigens, have been proposed for the detection of Sm antibodies: double immunodiffusion, immunoblotting, immunoprecipitation, ELISA, protein microarrays, and addressable laser bead immunoassay (ALBIA) with native antigens from different sources, purified or recombinant proteins, and synthetic peptides (7, 8, 9, 12, 15, 17, 19).
For several commercial immunoassays, recombinant SmBB′ from bacteria or insect cells has been used in kit development. Recombinant SmBB′ and purified Sm antigen containing SmBB′ both bear the disadvantage that they contain the cross-reactive epitope PPPGMRPP, which is present in SmBB′ and in the U1-specific RNPs (1). Since this epitope is frequently targeted by antibodies in sera from MCTD patients, common anti-Sm antibody assays with purified Sm or recombinant SmBB′ fail to differentiate between SLE and MCTD patients. As evidenced by classification criteria, MCTD tends to have different disease parameters than SLE (19, 25). Therefore, differentiation between these closely related autoimmune disorders can be improved by the use of the SmD3 peptide ELISA. Although no correlations between anti-SmD3 peptide reactivity and clinical symptoms were found in a previous study, extended multicenter evaluations are desirable to address this question and achieve statistical validity (12).
In this comparative study with kits from several suppliers, we have shown that the assays used in this study yielded sensitivities ranging from 10% to 12% and specificities varying from 88% to 100% for SLE. Furthermore, as revealed by the correlation data, it became evident that the detection of anti-Sm antibodies strongly depends on both the detection system and the nature of the Sm antigen. The correlation coefficients varied between 0.48 (QuantaLite Sm versus Varelisa Sm Antibodies) and 0.94 (Euroimmun versus QuantaLite Sm). The QuantaPlex Sm and the QuantaLite Sm test, both from the same supplier and with the same native Sm antigen, showed a correlation value R2 of 0.75.
Several factors such as the titer, affinity, isotype, and binding specificity of the antibodies affect the outcome of an autoantibody assay because of the high complexity of the human immune response. Therefore, differences in the washing and blocking conditions, stabilization and amount of antigen, epitope exposure, and the detection system or assay platform may lead to interlaboratory discrepancies.
Even lower correlation coefficients could be found between the Varelisa Sm antibodies and the conventional anti-Sm antibody assay. This observation is most likely explained by the nature of the Sm antigen. The conventional anti-Sm antibody tests including the ELISA systems from Euroimmun, INOVA, and Pharmacia (Varelisa ReCombi ANA Profile) as well as the novel multiplex assay (QuantaLite Sm) use Sm antigens purified from a native source containing all Sm polypeptides or even low concentrations of other proteins such as U1-specific RNPs. Thus, these assays detect a heterogenous mixture of different autoantibody populations. In contrast the Varelisa Sm antibodies test is based on a single peptide derived from the SmD3 sequence comprising only 16 amino acids (12). Consequently, when the peptide-based assay is used, only a subset of anti-Sm antibodies are detected. Other Sm autoantibody specificities such as the cross-reactive antibodies recognizing the epitope PPPGMRPP which is shared between SmBB′ and U1-specific RNPs are not detected by the SmD3 peptide-based assay, but by the assays with purified Sm antigens.
Based on the relatively poor correlation between conventional anti-Sm immunoassays with purified Sm antigen from native sources and the peptide assay, we conclude that antibodies targeting the SmD3 peptide represent only a minor subpopulation of anti-Sm antibodies. Nevertheless, based on the high sensitivity and specificity and on the observation that anti-Sm antibodies can be used to discriminate MCTD from SLE patients, we conclude that this subpopulation represents an important SLE-specific antibody (12). All these findings indicate that not only the specificity of anti-Sm antibodies but also the binding properties such as the affinity of those antibodies may play an important role for the test results. In the recent study some SLE patient sera reacted with the SmD3 in the peptide-based assay (4 of 101) or in the ELISA with native antigens (5 of 101) (12). Thus, the combined use of the SmD3 peptide and native Sm antigens leads to the detection of more anti-Sm-positive SLE patients.
By excluding MCTD sera from the group of control sera, the specificity and thus the clinical accuracy of the conventional immunoassays from Euroimmun, INOVA, and Pharmacia with purified Sm antigens significantly increased. The specificities of the Varelisa ReCombi ANA Profile, Euroimmun ELISA, QuantLite Sm, and QuantaPlex Sm tests were then found to be 98.8%, 98.8%, 98.8%, and 97.5%, respectively, resulting in clinical accuracies of 64.6%, 64.6%, 63.8%, and 65.4%, respectively. This observation underlines the assumption that the cross-reactive antibodies to the epitope motif PPPGMRPP of SmBB′ and U1-specific RNPs present in high titers in sera of patients with MCTD finally lead to positive test results in anti-Sm antibody assays (1).
Recently, it has been shown that the polypeptides D1, D3, and BB′ contain symmetrical dimethylarginine (sDMA) constituting a major autoepitope within the C terminus of SmD1 (4). In one of these studies, a synthetic peptide of SmD1 (amino acids 95 to 119) containing symmetrical dimethylarginine demonstrated significant increased immunoreactivity compared to the nonmodified peptide (4). The new peptide assay is also based on an Sm peptide containing dimethylarginine (12). Whether this modified amino acid plays a central role in the development of the SLE-specific B-cell immune response to the Sm particles remains a matter of speculation.
Synthetic peptides represent ideal antigenic targets for immunoassays because they can easily be produced in high quality and quantity. Furthermore, lower lot-to-lot variations will be observed since production is not dependent on the biological variation of native sources of antigens. In 1998, Schellekens et al. described the identification of a citrullinated cyclic peptide which has become an important and reliable marker for the diagnosis of rheumatoid arthritis (20). Today's sophisticated epitope-mapping methods will likely lead to the identification of additional peptides that can be used as specific targets in diagnostic and therapeutic approaches to patient management. This may lead to a new scientific research area with high impact for the development of diagnostic and therapeutic products, to the area of peptide engineering.
Advances in multiplex technologies and microarrays allows for the development of sophisticated profile assays containing multiple different antigens (6, 18, 23). This may improve the diagnosis of a variety of disorders, especially of autoimmune diseases since for most of those disorders no highly sensitive marker is available. For example, the diagnosis of SLE might be improved by providing an antigen array that includes different Sm antigens in combination with double-stranded DNA and ribosomal antigens.
International standardization of laboratory testing of Sm antibodies has yet to be fully realized, although Sm antibodies have been included in the American College of Rheumatology criteria for lupus and Sm-specific sera are part of the reference serum panels from the Centers for Disease Control and from the Association of Medical Laboratory Immunologists (10, 22).
Summary.
In the present study, we have compared anti-Sm antibody assays with conventional purified antigens with the new Varelisa Sm antibodies based on an SmD3-derived peptide. In summary, we have found that the detection of anti-Sm antibodies strongly depends on both the nature and quality of the antigen and the detection system. While no remarkable difference could be observed in the sensitivity of the assay, a significant difference could be observed in the specificity of the tests, mainly caused by different results of MCTD patient samples. Relatively poor correlation and significant differences in the clinical accuracy of the Sm tests with purified Sm antigens were found. Furthermore, we were able to show that the autoantibodies detected by the novel SmD3 peptide-based ELISA have high specificity for SLE and represent a promising marker for the differentiation of autoimmune disorders, especially the distinction of SLE from MCTD.
Acknowledgments
We acknowledge the technical assistance of Joan Miller and Mark Fritzler at the University of Calgary.
This project was supported in part by a grant (MOP-38034) from the Canadian Institutes of Health Research and by Pharmacia Diagnostics (Freiburg, Germany).
REFERENCES
- 1.Abuaf, N., C. Johanet, P. Chretien, B. I. Absalon, J. C. Homberg, and J. F. Buri. 1990. Detection of autoantibodies to Sm antigen in systemic lupus erythematosus by immunodiffusion, ELISA and immunoblotting: variability of incidence related to assays and ethnic origin of patients. Eur. J. Clin. Investig. 20:354-359. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle, M. R., M. T. McClain, M. V. Rubertone, R. H. Scofield, G. J. Dennis, J. A. James, and J. B. Harley. 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349:1526-1533. [DOI] [PubMed] [Google Scholar]
- 3.Brahms, H., V. A. Raker, W. J. van Venrooij, and R. Lührmann. 1997. A major, novel systemic lupus erythematosus autoantibody class recognizes the E, F, and G Sm snRNP proteins as an E-F-G complex but not in their denatured states. Arthritis Rheum. 40:672-682. [DOI] [PubMed] [Google Scholar]
- 4.Brahms, H., J. Raymackers, A. Union., F. de Keyser, L. Meheus, and R. Lührmann. 2000. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form amajor B-cell epitope for anti-Sm autoantibodies. J. Biol. Chem. 275:17122-17129. [DOI] [PubMed] [Google Scholar]
- 5.De Keyser, F., S. O. Hoch, M. Takei, H. Dang, H. De Keyser, L. A. Rokeach, and N. Talal. 1992. Cross-reactivity of the B/B′ subunit of the Sm ribonucleoprotein autoantigen with proline-rich polypeptides. Clin. Immunol. Immunopathol. 62:285-290. [DOI] [PubMed] [Google Scholar]
- 6.Fritzler, M. J. 2002. New technologies in the detection of autoantibodies, p. 50-63. In K. Conrad et al. (ed.), Autoantigens, autoantibodies, autoimmunity. Pabst Scientific Publishers, Lengerich, Germany.
- 7.Hines, J. J., W. Danho, and K. B. Elkon. 1991. Detection and quantification of human anti-Sm antibodies using synthetic peptide and recombinant SmB antigens. Arthritis Rheum. 34:572-579. [DOI] [PubMed] [Google Scholar]
- 8.Hoch, S. O. 1994. The Sm antigens, p. B2.4/1-B2.4/29. In R. N. Maini and W. J. van Venrooji (ed.), Manual of biological markers of disease. Kluywer Academic, Dordrecht, The Netherlands.
- 9.Hoch, S. O., R. A. Eisenberg, and G. C. Sharp. 1999. Diverse antibody recognition patterns of the multiple Sm-D antigen polypeptides. Clin. Immunol. 92:203-208. [DOI] [PubMed] [Google Scholar]
- 10.James, K., A. B. Carpenter., L. Cook, R. Marchand, and R. M. Nakamura. 2000. Development of the antinuclear and anticytoplasmic antibody consensus panel by the Association of Medical Laboratory Immunologists. Clin. Diagn. Lab. Immunol. 7:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner, M. R., J. A. Boyle, S. M. Mount, S. L. Wolin, and J. A. Steitz. 1980. Are snRNPs involved in splicing? Nature 283:220-224. [DOI] [PubMed] [Google Scholar]
- 12.Mahler, M., M. J. Fritzler, and M. Blüthner. 2004. Identification of a SmD3 epitope with a single symmetrical dimethylation of an arginine residue as specific target of a subpopulation of anti-Sm antibodies. Arthritis Res. Ther. 7:R19-R29. [DOI] [PMC free article] [PubMed]
- 13.Mahler, M., K. Kessenbrock, J. Raats, and M. J. Fritzler. 2004. Technical and clinical evaluation of anti-ribosomal P protein immunoassays. J. Clin. Lab. Anal. 18:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClain, M. T., P. A. Ramsland, K. M. Kaufman, and J. A. James. 2002. Anti-sm autoantibodies in systemic lupus target highly basic surface structures of complexed spliceosomal autoantigens. J. Immunol. 168:2054-2062. [DOI] [PubMed] [Google Scholar]
- 15.Ou, Y., S. Sun, G. C. Sharp, and S. O. Hoch. 1997. Screening of SLE sera using purified recombinant Sm-D1 protein from a baculovirus expression system. Clin. Immunol. Immunopathol. 83:310-317. [DOI] [PubMed] [Google Scholar]
- 16.Quintero-Del-Rio, A. I., D. Bacino, J. Kelly, T. Aberle, and J. B. Harley. 2001. Familial systemic lupus erythematosus: a comparison of clinical manifestations and antibody presentation in three ethnic groups. Cell. Mol. Biol. 47:1223-1227. [PubMed] [Google Scholar]
- 17.Riemekasten, G., J. Marell, G. Trebeljahr, R. Klein, G. Hausdorf, T. Haupl, J. Schneider-Mergener, G. R. Burmester, and F. Hiepe. 1998. A novel epitope on the C terminus of SmD1 is recognized by the majority of sera from patients with systemic lupus erythematosus. J. Clin. Investig. 102:754-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson, W. H., C. DiGennaro, W. Hueber, B. B. Haab, M. Kamachi, E. J. Dean, S. Fournel, D. Fong, M. C. Genovese, H. E. de Vegvar, K. Skriner, D. L. Hirschberg, R. I. Morris, S. Muller, G. J. Pruijn, W. J. van Venrooij, J. S. Smolen, P. O. Brown, L. Steinman, and P. J. Utz. 2002. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 8:295-301. [DOI] [PubMed] [Google Scholar]
- 19.Rokeach, L. A., M. Jannatipour, J. A. Haselby, and S. O. Hoch. 1992. Mapping of the immunoreactive domains of a small nuclear ribonucleoprotein-associated Sm-D autoantigen. Clin. Immunol. Immunopathol. 65:315-324. [DOI] [PubMed] [Google Scholar]
- 20.Schellekens, G. A., B. A. de Jong, F. H. van den Hoogen, L. B. van de Putte, and W. J. van Venrooij. 1999. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 101:273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp, G. C., W. S. Irvin, E. M. Tan, R. G. Gould, and H. R. Holman. 1972. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am. J. Med. 52:148-159. [DOI] [PubMed] [Google Scholar]
- 22.Smolen, J. S., B. Butcher, M. J. Fritzler, T. Gordon, J. Hardin, J. R. Kalden, R. Lahita, R. N. Maini, W. Reeves, M. Reichlin, N. Rothfield, Y. Takasaki, W. J. van Venrooij, and E. M. Tan. 1997. Reference sera for antinuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and western blotting. Arthritis Rheum. 40:413-418. [DOI] [PubMed] [Google Scholar]
- 23.Steinman, L., and S. Zamvil. 2003. Transcriptional analysis of targets in multiple sclerosis. Nat. Rev. Immunol. 6:483-492. [DOI] [PubMed] [Google Scholar]
- 24.Tan, E. M., and H. G. Kunkel. 1966. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J. Immunol. 96:464-471. [PubMed] [Google Scholar]
- 25.Tan, E. M., A. S. Cohen, J. F. Fries, A. T. Masi, D. J. McShane, N. F. Rothfield, J. G. Schaller, N. Talal, and R. J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271-1277. [DOI] [PubMed] [Google Scholar]
- 26.von Muhlen, C. A., and E. M. Tan. 1995. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthritis Rheum. 24:323-358. [DOI] [PubMed] [Google Scholar]