Abstract
Multibacillary and paucibacillary paratuberculosis are both caused by Mycobacterium avium subspecies paratuberculosis. Multibacillary lesions are composed largely of infected epithelioid macrophages and paucibacillary lesions contain T cells but few bacteria. Multibacillary disease is similar to human lepromatous leprosy, with variable/high levels of antibody and a dysfunctional immune response. Animals with paucibacillary disease have high cell-mediated immunity and variable levels of antibody. This study aims to characterize the immunological dysfunction using TruSeq analysis of the ileocaecal lymph node that drains disease lesions. Immune dysfunction is highlighted by repression of TCR/CD3 genes, T cell co-receptors/co-stimulators, T cell activation and signal-transduction genes. Inflammation was an acute phase response and chronic inflammation, with little evidence of acute inflammation. The high levels of immunoglobulin and plasma cell transcripts is consistent with the anti-MAP antibody responses in paratuberculosis sheep. Also notable was the overwhelming reduction in mast cell transcripts, potentially affecting DC activation of the immune response. This study also shows that there were no fundamental differences in the gene expression patterns in multibacillary and paucibacillary disease, no shift in T cell genes from Th1 to Th2 pattern but rather an incremental decline into immune dysfunction leading to multibacillary pathology.
Mycobacterium avium subspecies paratuberculosis (MAP) is the causative agent of paratuberculosis or Johne’s disease1. This is an enteric disease of ruminants and an endemic problem for livestock worldwide2,3,4,5. MAP is an intracellular bacterium, typically infecting macrophages of the small intestine, with severe lesions often occurring in the terminal ileum6,7,8. As with human tuberculosis and leprosy, only a minority of infected individuals develop clinical pathology, and infection results in a spectrum of disease forms6,9 graded by a range of pathological characteristics. Similarities in the pathology of paratuberculosis with leprosy have led to the broad classification of these disease forms in sheep as paucibacillary (tuberculoid) and multibacillary (lepromatous)6,7. Paucibacillary (P) lesions consist largely of T cells and Langhan’s giant cells but few bacteria; in contrast multibacillary (M) lesions are characterized by heavily-infected epithelioid macrophages10,11. It is this form of the disease that is most problematic in relation to the dissemination of infection because of the high numbers of mycobacteria in faeces12 and milk11,13.
The different cell infiltrates of P and M lesions are likely to be linked to the contrasting immune responses associated with the two disease forms. This is manifest by variable levels of anti-MAP antibody with high cell mediated immunity (CMI) in P pathology and often high levels of antibody and low CMI in M disease14,15. Studies on lesion tissue in sheep with advanced disease have linked P pathology with a Th1 response and high levels of IFNγ that controls bacterial growth; and M disease with Th2 activation, low IFNγ levels and consequently uncontrolled bacterial replication10,15,16. A similar dichotomy of polarized responses is seen with the progression from P to M pathology in cattle17,18 and in human tuberculosis and leprosy19,20. However, recent studies have cast doubt on the hypothesis that discrimination of P and M disease is simply a matter of a Th1 to Th2 switch21. Macrophages are the target cells for MAP1,22 with infection influencing their metabolism and transcriptome23,24. They also have a central role in the activation and polarization of T cells. MAP infection-associated changes to antigen processing pathways23, cytokine and innate receptor expression25,26,27 are also likely to play a role in the development of the two disease forms.
Experimental infection studies in both cattle28,29,30 and sheep21 have linked progression to M disease with T cell dysfunction that results in the loss of CMI, T cell unresponsiveness and exhaustion. Studies on the molecular nature of the reduced T cell function in bovine paratuberculosis have identified increased expression of immunoinhibitory molecules in the suppression of anti-MAP T cell responses in vitro30, and shown a reduction in expression of the T cell receptor second messenger ZAP7031.
Previous transcriptome studies on paratuberculosis had the broad aim of describing the effects of MAP infection on host gene expression. Studies in cattle and red deer examined in vitro infected macrophages24,32,33, blood leukocytes34,35 and the ileocaecal valve36 from infected animals. A recent project focussed on lymph node from experimentally-infected red deer with no defined pathology37. Investigations of the disease in sheep have largely focussed on lesions38,39 with the consequent difficulties of differentiating cause and effect, although lymph node and blood analyses have also been performed39. These studies showed that immune-mediated genes and pathways were associated with paratuberculosis pathologies. The immunological events that lead to the development of the different pathological forms of paratuberculosis are unlikely to occur within the gastrointestinal mucosa, but within the lymph nodes that drain that tissue40. Macrophages and dendritic cells (DC) may be the first cells to respond to intracellular infection but the host immune response will primarily be under T cell regulation. This current study used Illumina TruSeq to analyse the transcriptome of the Ileocaecal lymph node (ICLN), the major immune-inductive site for the terminal ileum; to investigate the immune and inflammatory responses associated with P and M disease, focussing on the nature of the immune dysfunction in M paratuberculosis.
Results
Diagnosis of multibacillary and paucibacillary paratuberculosis
Animals with M and P lesions were identified on the basis of: clinical signs, bacteriology and gross pathology, haematoxylin and eosin (H&E) and Ziehl-Neelsen (ZN) histopathology of terminal ileum and mesenteric lymph node (MLN) (see Supplementary Table S1). M lesions were composed largely of heavily-infected epithelioid macrophages; P lesions were composed largely of lymphocytes, with few ZN+ bacteria. Infected animals were culture and insertion sequence 900 (IS900) positive; amplicon sequences were identical to MAP IS900 (accession no. S74401.1). Uninfected controls (C) were all were IS900 negative.
Analysis of the ICLN transcriptome
TruSeq analysis of the ICLN of sheep with M and P paratuberculosis and uninfected C (n = 5 for each group) obtained an average of 56,080,477 paired-end reads (101 bp) per sample library and mapping efficiencies of 74.27% to 87.85% (see Supplementary Table S2). Reads were mapped to the Ovis aries genome assembly OAR3.1 and the relationships in expression profiles between samples were explored using multidimensional scaling (MDS) plot (see Supplementary Figure S1). The plot showed that the paratuberculosis samples were more heterogeneous than C; however there was clear separation along dimension 1 of infected and C samples. Differential expression analysis between groups using linear models fitted to the datasets revealed 4,852 differentially-expressed genes (DEG) in M vs. C, 2,761 in P vs. C and 62 in M vs. P (adjusted p-value ≤ 0.05) comparisons. The volcano plots of the M vs. C and P vs. C comparisons show a symmetrical distribution of up-regulated and down-regulated genes, although the absolute fold changes were generally higher for up-regulated genes (see Supplementary Figure S1).
The twenty most up-regulated and down-regulated genes in the M vs. C and P vs. C comparisons are listed in Tables 1 and 2. The most up-regulated transcript in M sheep was pentraxin PTX3 (+280.38 fold); SAA1 (+48.93 fold) and FGF23 (+36.69 fold) were also highly increased. The most up-regulated transcript in P sheep was PROC (+67.62 fold); others included seven immunoglobulin genes comprising IGHG1 (+15.62 fold) and IGHE (+13.25 fold) and five light chain genes (average + 11.64 fold). The most down-regulated transcripts in the M and P datasets were HDC (M, −54.25 and P, −10.6 fold) and GPR143 (M, −35.25 and P, −33.27 fold) respectively. A spreadsheet of all the DEG can be found as Supplementary Table S3.
Table 1. Genes up-regulated and down-regulated in the ICLN of multibacillary sheep.
| Ensembl id | Gene | P-value | FC | P-value | FC |
|---|---|---|---|---|---|
| Multi vs control | Pauci vs control | ||||
| ENSOARG00000002754 | PTX3 | 0.0031 | 280.38 | 0.0628 | 35.25 |
| ENSOARG00000000450 | S100A8 | 0.0025 | 179.69 | 0.0253 | 49.01 |
| ENSOARG00000004127 | FOLR1 | 0.0016 | 173.01 | 0.3852 | 4.51 |
| ENSOARG00000000432 | S100A12 | 0.0012 | 99.72 | 0.0188 | 27.02 |
| ENSOARG00000010953 | XDH | 0.0007 | 84.49 | 0.0355 | 14.07 |
| ENSOARG00000004536 | RPL17 | 0.0001 | 56.06 | 0.0072 | 10.26 |
| ENSOARG00000009847 | SAA1 | 0.0001 | 48.93 | 0.0136 | 10.09 |
| ENSOARG00000014440 | VNN1 | 0.0002 | 40.23 | 0.3848 | 2.27 |
| ENSOARG00000010198 | FGF23 | 0.0053 | 36.69 | 0.3494 | 3.89 |
| ENSOARG00000016105 | PROC | 0.0010 | 35.20 | 0.0010 | 67.62 |
| ENSOARG00000002862 | CD163 | 0.0002 | 33.82 | 0.0303 | 4.56 |
| ENSOARG00000009608 | RNF186 | 0.0056 | 33.48 | 0.0328 | 18.78 |
| ENSOARG00000003744 | HP | 0.0030 | 32.45 | 0.4915 | 2.36 |
| ENSOARG00000008959 | SLIT1 | 0.0067 | 26.06 | 0.0592 | 10.58 |
| ENSOARG00000013805 | COLCA2 | 0.0004 | 24.97 | 0.0036 | 14.46 |
| ENSOARG00000001869 | TREM1 | 0.0002 | 23.87 | 0.0099 | 8.25 |
| ENSOARG00000009963 | SAA3 | 0.0002 | 23.74 | 0.0021 | 11.79 |
| ENSOARG00000009344 | FABP4 | 0.0002 | 23.05 | 0.0131 | 6.04 |
| ENSOARG00000007086 | FCRL2 | 0.0337 | 21.38 | 0.3128 | 5.10 |
| ENSOARG00000010231 | MX2 | 0.0310 | 20.40 | 0.0520 | 17.18 |
| ENSOARG00000007488 | SPTA1 | 0.0102 | −9.94 | 0.0259 | −4.37 |
| ENSOARG00000013263 | RNASE6 | 0.0314 | −10.83 | 0.0210 | −12.29 |
| ENSOARG00000003612 | ABCC8 | 0.0005 | −10.95 | 0.0075 | −3.09 |
| ENSOARG00000015144 | SERPINA5 | 0.0020 | −11.12 | 0.0024 | −12.31 |
| ENSOARG00000004611 | CCL7 | 0.0063 | −11.18 | 0.0087 | −6.05 |
| ENSOARG00000001188 | SBK3 | 0.0003 | −11.33 | 0.0011 | −8.79 |
| ENSOARG00000016974 | KRT5 | 0.0106 | −11.65 | 0.0235 | −6.09 |
| ENSOARG00000010570 | TMEM213 | 0.0208 | −12.57 | 0.0871 | −3.01 |
| ENSOARG00000003570 | LGI1 | 0.0010 | −13.27 | 0.0035 | −5.66 |
| ENSOARG00000008224 | DAB1 | 0.0026 | −13.78 | 0.0036 | −11.53 |
| ENSOARG00000020292 | MUC4 | 0.0073 | −14.85 | 0.0360 | −5.45 |
| ENSOARG00000018133 | CFTR | 0.0054 | −15.95 | 0.0270 | −4.72 |
| ENSOARG00000007787 | FCER1A | 0.0033 | −21.36 | 0.0493 | −3.72 |
| ENSOARG00000014689 | TPSAB1 | 0.0009 | −22.24 | 0.0032 | −5.21 |
| ENSOARG00000003456 | TH | 0.0002 | −24.73 | 0.0031 | −5.19 |
| ENSOARG00000015746 | RIMBP2 | 0.0004 | −26.13 | 0.0028 | −7.02 |
| ENSOARG00000008753 | GUCY1B2 | 0.0356 | −29.89 | 0.2217 | −5.10 |
| ENSOARG00000010016 | GPR143 | 5.1E-05 | −35.25 | 0.0001 | −33.27 |
| ENSOARG00000005274 | CPA3 | 0.0002 | −41.73 | 0.0054 | −6.63 |
| ENSOARG00000020992 | HDC | 8.9E-05 | −54.25 | 0.0018 | −10.60 |
The twenty most significantly (adjusted p-value ≤ 0.05) increased and decreased genes, ranked by fold change (FC).
Table 2. Genes up-regulated and down-regulated in the ICLN of paucibacillary sheep.
| Ensembl id | Gene | P-value | FC | P-value | FC |
|---|---|---|---|---|---|
| Pauci vs control | Multi vs control | ||||
| ENSOARG00000016105 | PROC | 0.0010 | 67.62 | 0.0010 | 35.20 |
| ENSOARG00000000450 | S100A8 | 0.0253 | 49.01 | 0.0025 | 179.69 |
| ENSOARG00000000432 | S100A12 | 0.0188 | 27.02 | 0.0012 | 99.72 |
| ENSOARG00000012259 | FMO3 | 0.0085 | 19.77 | 0.0184 | 11.55 |
| ENSOARG00000016677 | MGAT3 | 0.0068 | 18.48 | 0.0031 | 19.35 |
| ENSOARG00000009608 | RNF186 | 0.0328 | 16.75 | 0.0056 | 33.48 |
| ENSOARG00000009143 | IGHG1 | 0.0014 | 15.62 | 0.0006 | 16.65 |
| ENSOARG00000013805 | COLCA2 | 0.0036 | 14.46 | 0.0004 | 24.97 |
| ENSOARG00000012509 | IGLV9–49 | 0.0117 | 14.43 | 0.0463 | 6.79 |
| ENSOARG00000010953 | XDH | 0.0355 | 14.07 | 0.0007 | 84.49 |
| ENSOARG00000008994 | IGHE | 0.0097 | 13.25 | 0.0109 | 11.89 |
| ENSOARG00000012585 | IGLC1 | 0.0006 | 12.24 | 0.0002 | 14.84 |
| ENSOARG00000001593 | CFAP74 | 0.0029 | 11.86 | 0.0020 | 3.74 |
| ENSOARG00000009963 | SAA3 | 0.0021 | 11.79 | 0.0002 | 23.74 |
| ENSOARG00000013171 | C21orf58 | 0.0053 | 11.08 | 0.9875 | 1.02 |
| ENSOARG00000012537 | IGLV1-51 | 0.0030 | 11.07 | 0.0016 | 12.66 |
| ENSOARG00000003300 | DPEP2 | 0.0012 | 10.67 | 0.0002 | 13.08 |
| ENSOARG00000004536 | RPL17 | 0.0072 | 10.26 | 0.0001 | 56.06 |
| ENSOARG00000012457 | IGLV1-50 | 0.0006 | 10.25 | 0.0001 | 12.92 |
| ENSOARG00000012343 | IGLV8-61 | 0.0044 | 10.22 | 0.0016 | 11.54 |
| ENSOARG00000004611 | CCL7 | 0.0087 | −6.05 | 0.0063 | −11.18 |
| ENSOARG00000016974 | KRT5 | 0.0235 | −6.09 | 0.0106 | −11.65 |
| ENSOARG00000000778 | MET | 0.0024 | −6.38 | 0.0036 | −4.86 |
| ENSOARG00000018446 | PTPRZ1 | 0.0120 | −6.54 | 0.0147 | −6.62 |
| ENSOARG00000005274 | CPA3 | 0.0054 | −6.63 | 0.0002 | −41.73 |
| ENSOARG00000011529 | ZFR2 | 0.0002 | −6.70 | 0.0001 | −9.79 |
| ENSOARG00000015746 | RIMBP2 | 0.0028 | −7.02 | 0.0004 | −26.13 |
| ENSOARG00000009731 | RGS13 | 0.0174 | −7.12 | 0.0206 | −7.34 |
| ENSOARG00000015393 | RASSF9 | 0.0020 | −7.18 | 0.0113 | −3.27 |
| ENSOARG00000017420 | GLI3 | 0.0007 | −7.21 | 0.0007 | −5.62 |
| ENSOARG00000000744 | LAMC2 | 0.0095 | −7.97 | 0.0229 | −5.07 |
| ENSOARG00000014271 | MOXD1 | 0.0004 | −8.05 | 0.0043 | −2.65 |
| ENSOARG00000001188 | SBK3 | 0.0011 | −8.79 | 0.0003 | −11.33 |
| ENSOARG00000006864 | FCAMR | 0.0128 | −8.85 | 0.0160 | −8.79 |
| ENSOARG00000020992 | HDC | 0.0018 | −10.60 | 8.9E-05 | −54.25 |
| ENSOARG00000008224 | DAB1 | 0.0036 | −11.53 | 0.0026 | −13.78 |
| ENSOARG00000013263 | RNASE6 | 0.0210 | −12.29 | 0.0314 | −10.83 |
| ENSOARG00000015144 | SERPINA5 | 0.0024 | −12.31 | 0.0020 | −11.12 |
| ENSOARG00000018455 | OLFM3 | 0.0014 | −15.18 | 0.0117 | −4.26 |
| ENSOARG00000010016 | GPR143 | 0.0001 | −33.27 | 5.1E-05 | −35.25 |
The twenty most significantly (adjusted p-value ≤ 0.05) increased and decreased genes, ranked by fold change (FC).
Comparison of multibacillary and paucibacillary disease
Of the forty most DEG in M sheep (Table 1) and P sheep (Table 2), eighteen were common to both. Common genes included XDH (M, +84.49 and P, +14.07 fold), and the calcium binding proteins S100A8 (M, +179.69 and P, +49.01 fold) and S100A12 (M, +99.72 fold and P, +27.02 fold). S100A2 (+10.19 fold) and S100A9 (+5.50 fold) were also significantly raised in M, but not in P. The mast cell specific transcripts HDC and CPA3 (M, −41.73 and P, −6.63 fold) were highly repressed genes common to both disease pathologies. Additional mast cell transcripts down-regulated in both datasets were TPSAB1 (M, −22.24 and P, −5.21 fold), FCER1A (M, −21.36 and P, −3.72 fold), KIT (M, −2.69 and P, −2.51 fold) and KITLG (M, −2.44 and P, −2.47 fold) (see Supplementary Table S3). Preliminary observations of toluidine blue histology shows many more mast cells in the ICLN of uninfected C sheep than in M animals (see Supplementary Figure S2); the number of mast cells are similar in M and P.
Spearman’s correlation analysis of fold changes of the top genes (Tables 1 and 2) identified a significant correlation (Spearman’s rs 0.79, p < 0.0001) between M and P sheep. This high level of similarity reflects the fact that there were only 62 significant DEG in the comparison of the M and P datasets (see Supplementary Table S3); none was affected in the opposite direction in the two disease forms. The two most up-regulated genes in the M vs. P comparison were CYP4F11 (+29.5 fold) and VNN1 (+17.69 fold), and the most down-regulated (annotated) gene was CD40LG (−2.7 fold).
Ingenuity pathway analysis
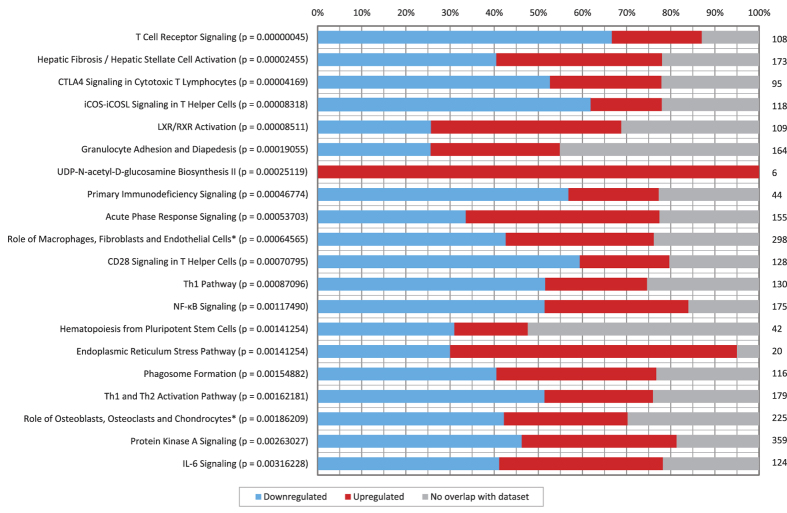
The functional classification of 1,097 annotated DEG (fold change ≥ 2; adjusted p-value ≤ 0.05) in the M dataset, by Ingenuity pathway analysis (IPA), identified a significant enrichment of genes in canonical pathways mostly associated with T cell immunity/activation, and inflammation and tissue repair (Fig. 1). Similar analysis with the 481 annotated DEG in the P dataset also identified pathways associated with inflammation and tissue repair, but did not identify a significant enrichment of genes linked with the adaptive immune response (see Supplementary Figure S3).
Figure 1. Pathway Analysis of Differentially Expressed Genes of the ICLN in Multibacillary Paratuberculosis.
The top twenty canonical pathways enriched for differentially expressed genes identified in the M versus C dataset are displayed along the y-axis, the significance values (shown in brackets) were calculated by Fisher’s exact test right-tailed in IPA. The percentage of differentially expressed genes down-regulated and up-regulated within each canonical pathway are shown on the x-axis. The total number of genes in a pathway is displayed to the right of each of the stacked bars. (* in Rheumatoid Arthritis).
Disease-associated changes to the T cell transcriptome
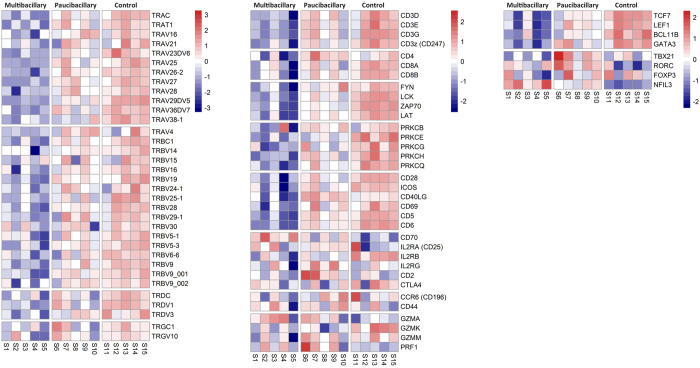
Of the top twenty canonical pathways identified in the M dataset seven were associated with adaptive immunity. The majority of identified genes within these pathways were repressed. All thirty-four identified TCR genes in the ‘T cell receptor signalling’ pathway were repressed including transcripts of the alpha/beta (average −2.92 fold) and gamma/delta chains (average −2.40 fold). Transcripts of all four identified CD3 genes (average −2.07 fold) and the TCR/CD3 signalling pathway members LCK (−2.99 fold), FYN (−1.48 fold), ZAP70 (−2.66 fold) and LAT (−2.68 fold) were significantly reduced (Fig. 2). Within the other T cell pathways, repressed genes included; TCR co-receptors CD4 (−1.57 fold); CD8A (−2.33 fold) and CD8B (−2.28 fold), T cell co-stimulators CD28 (−2.62 fold), ICOS (−2.35 fold) and CD40LG (−1.94 fold); and the T cell activation marker CD69 (−2.01 fold). The lymphocyte surface markers CD5 (−2.48 fold) and CD6 (−2.92 fold) were also repressed as were the protein kinase C molecules, PRKCB, PRKCE, PRKCG, PRKCH and PRKCQ (average −2.24 fold). Not all T cell genes were down-regulated; CD70 was increased (+4.30 fold) and the IL2 receptor genes, IL2RA, IL2RB and IL2RG were largely unaffected, as were CD2 and CTLA4 and the memory T cell markers CCR6 and CD44. The expression of molecules associated with cytotoxic T cells and NK cells was variable; the granzymes GZMK (−3.23 fold) and GZMM (−1.65 fold) were significantly down-regulated, GZMA was increased (+2.23 fold) and the cytolytic protein PRF1 was unchanged (Fig. 2).
Figure 2. Heatmap of paratuberculosis associated changes to the T cell transcriptome.
Heatmap profile of identified TCR/CD3 and other T cell associated genes expressed in the ICLN of diseased and control sheep. The colour key indicates the direction of changes, with red depicting genes up-regulated and blue showing genes down-regulated. The colour represents log2 counts per million for each sample, centred and scaled for each row.
The expression of T cell associated genes in P sheep was either unchanged or slightly repressed, with fold changes less than in M sheep (Fig. 2). One notable difference between the two datasets was IFNG, not significantly affected in M but increased in P (+2.72 fold).
Disease-associated changes to the B cell and macrophage transcriptomes
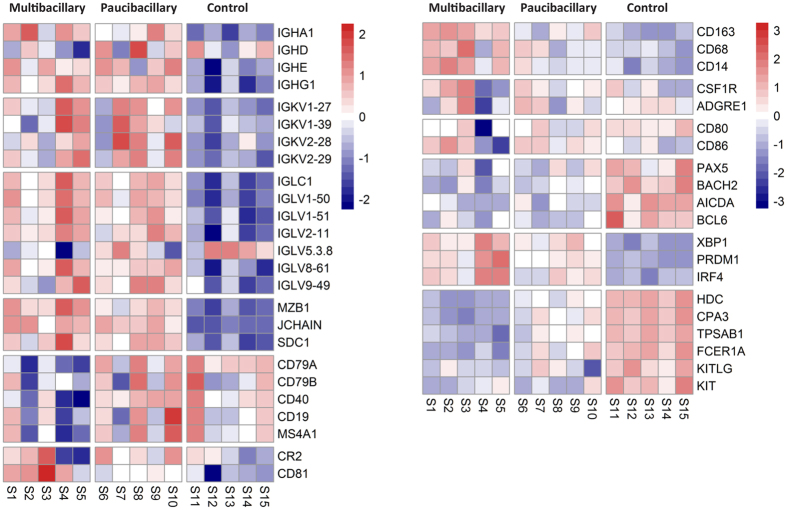
The immunoglobulin heavy chains IGHG1 (M, +16.65 and P, +15.62 fold), IGHE (M, +11.89 and P, +13.25 fold) and IGHA1 (M, +6.42 and P, +3.79 fold) were significantly up-regulated in both M and P sheep but IGHD was unaffected in both (Fig. 3). We have no information on IGHM, it had been assigned to an ‘unplaced scaffold’ (Scaffold JH921924.1: 50,045–53,838) in OAR3.1 so transcripts were not mapped. The IGK and IGL light chains in both datasets were also increased (average M, +7.47 and P, +8.09 fold) as was JCHAIN (M, +5.41 and P, +4.35 fold) and MZB1 (M, +12.46 and P, +6.34 fold). Other activated B cell and plasma cell markers were also raised in both infected groups, including SDC1 (M, +4.52 and P, +5.93 fold). The expression levels of non-activated and memory B cell molecules were marginally-repressed or unaffected by MAP infection including the CD79A and CD79B components of the B cell antigen receptor, CD19 and MS4A1 (average M, −1.56 and P, +1.10 fold). Expression level of the costimulatory protein CD40, constitutively expressed by antigen presenting cells, was marginally repressed in M sheep (−1.65 fold) but unchanged in P sheep.
Figure 3. Heatmap of paratuberculosis associated changes to professional antigen presenting cell and mast cell transcriptome.
Heatmap profile of identified TCR/CD3 and other T cell associated genes expressed in the ICLN of diseased and control sheep. The colour key indicates the direction of changes, with red depicting genes up-regulated and blue showing genes down-regulated. The colour represents log2 counts per million for each sample, centred and scaled for each row.
Expression levels of macrophage-associated transcripts was variable in both disease forms, illustrated by two scavenger receptors; CD163 (M, +33.82 and P, +4.56 fold) and CD68 (M, +1.88 and P, +1.32 fold). However, other common macrophage markers were unchanged including IL10, CSF1R and ADGRE1. Variation also occurred with Fc receptors, FCAMR (M, −8.79 and P, −8.85 fold) was repressed and FCGR1A (M, +6.29 and P, +3.1 fold) was increased, in both infected groups. There were twenty MHC class II genes identified in both datasets with Ovar-DYA significantly repressed (M, −5.62 and P, −3.42 fold) in both although the other MHC genes were largely unaffected (see Supplementary Table S3).
Inflammation-associated canonical pathways linked with paratuberculosis pathologies
At least nine of the top twenty canonical pathways in M sheep were associated with the inflammation and tissue repair (Fig. 1); and eleven in P sheep (see Supplementary Figure S3). Approximately equal numbers of genes in these pathways were increased and repressed in both datasets. ‘Hepatic Fibrosis/Hepatic Stellate Cell Activation’ was the most significant inflammation/tissue repair pathway identified in both datasets. There were twelve matrix metalloproteinases in each dataset, most were unaffected but MMP9 (−4.95 and −5.37 fold) was repressed in both M and P. The inflammation pathways ‘LXR/RXR Activation’, ‘Granulocyte Adhesion and Diapedesis’, ‘Acute Phase Response’ and ‘Role of Osteoblasts, Osteoclasts and Chondrocytes in Rheumatoid Arthritis’ were also common to the two datasets.
The common mediators of acute inflammation IL1B, TNF and LTA were unchanged or slightly repressed in both pathologies (average M, −1.90 and P, −1.13 fold), while IL1A was not significantly changed in M but was up-regulated in P (+2.52 fold). In contrast, mediators or indicators of chronic inflammation were significantly raised in both M and P, including IL6 (M, +10.03 and P, +2.07 fold), CSF3R (M, +20.23 and P, +5.43 fold), DPEP2 (M, +13.08 and P, +10.67 fold) and DPEP3 (M, +11.86 and P, +8.22 fold). Anti-inflammatory genes like PROC (+35.2 and + 67.62 fold) and IL1R2 (+8.46 and + 3.83 fold) were also significantly increased in both pathologies.
Cell communication/adhesion pathways linked with paratuberculosis
Other significant canonical pathways identified in the M dataset were ‘Communication between Innate and Adaptive Immune Cells’ and ‘Granulocyte Adhesion and Diapedesis’, which was also identified in the P dataset, with an almost equal split between up-regulated and down-regulated genes. Sixteen chemokines and chemokine receptors were significantly differentially expressed in M and nine in P sheep, but with no obvious expression pattern. CCL15 (M, +20.39 and P, +5.9 fold), CCL3 (M, +4.29 and P, +3.54 fold) and CCR10 (M, +8.83 and P, +4.74 fold) were up-regulated, but CCL7 (M, −11.18 and P, −6.05 fold) was down-regulated in both pathologies. CXCR1 (+6.55 fold) was raised, and CCR9 (−4.5 fold) and CCR7 (−3.59 fold) was repressed in M but unaffected in P. CXCL11 was significantly increased (+5.52 fold) in P but unaffected in M.
Disease-associated changes to the transcription regulators
Many transcription regulators that control lymphocyte maturation and differentiation were significantly affected in both disease forms. The core regulators of T cell and innate lymphoid cell (ILC) maturation, TCF7 (M, −5.21 and P, −2.30 fold), LEF1 (M, −4.62 and P, −1.8 fold) and BCL11B (M, −5.46 and P, 2.78 fold), were repressed in M and P with higher fold changes in M (Fig. 2). GATA3, a master regulator of Th2 and ILC2 development, was significantly down-regulated in M (−2.35 fold) but unchanged in P. The master regulators of Th1 and Treg cells, TBX21 and FOXP3, were unaffected in both; and the master regulator of Th17, RORC2, was unchanged in M but increased in P (+2.25 fold).
The core upstream regulators of B cell maturation, activation and memory development PAX5 (M, −6.60 and P −4.35 fold), BACH2 (M, −6.92 and P, −4.01 fold), AICDA (M, −6.59 and P, −5.98 fold) and BCL6 (M, −1.43 and −1.5 fold) were all significantly repressed in both pathologies (Fig. 3). In contrast, three transcription regulators of plasma cell differentiation were significantly raised, XBP1 (M + 4.94 and P, +3.25 fold), PRDM1 (M, +3.06 and + 2.21 fold) and IRF4 (M, +2.21 and P, +1.57 fold).
Validation of differential expression by quantitative real-time RT-PCR
Validation of the TruSeq data was by RT-qPCR with the Inflammatory Cytokine and Receptors RT2 Profiler PCR array (see Supplementary Table S4). There was a significant correlation with diseased cases compared to C (Spearman’s rs 0.49, p (two-tailed) <0.0001).
Discussion
The primary aim of this study was to characterize the immune and inflammatory responses of M and P paratuberculosis in sheep, focussing on the nature of the immune dysfunction21,28,29,30,31 associated with the M disease. TruSeq analysis was used to quantify transcripts expressed in the ICLN of diseased (M and P) and uninfected C animals. The ICLN was chosen as it is the organ of induction of the immune response for the terminal ileum, a major location of paratuberculosis lesions. The disease-associated response within the ICLN is likely to play a major role in the development of disease pathology. Analysis of the transcriptome at this strategic immunological site allowed an understanding of the immunological response to infection. This enabled the identification of physiological pathways and genes associated with the development and maintenance of the paratuberculosis phenotypes.
In M sheep, nine of the most up-regulated genes (Table 1) were associated with chronic rather than acute inflammation. PTX3 is an acute phase protein of the lectin pathway of complement and a regulator of inflammation induced by mycobacterial lipoarabinomannan41; polymorphisms of PTX3 are linked with pulmonary tuberculosis42. S100A8 and S100A12 are major pro-inflammation mediators43, that play significant roles in chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease (IBD)44. S100A8 is also prominent in lepromatous leprosy45 but with low anti-microbial activity. However, S100A12 can kill Mycobacterium leprae and Mycobacterium tuberculosis, although it is more highly expressed in tuberculoid leprosy46. XDH plays a major role in phagocytic killing47 and high expression levels are indicative of activated and infected macrophages in M paratuberculosis and is characteristic of oxidative stress. VNN1 is involved in cysteamine production, a major promotor of inflammation48 and polymorphisms of human VNN1 are linked to susceptibility to IBD49. FGF23 is expressed by activated macrophages, and elevated levels impair neutrophil recruitment50. The high levels of FGF23 expression and significant repression of its neutrophil receptor FGFR2 in M and P animals (M, −5.18 and P, −2.97 fold) is consistent with the low numbers of neutrophils seen in M pathology51. The presence of activated and infected macrophages in the ICLN of M animals is also indicated by high expression levels of CD163, which is also up-regulated in a range of chronic inflammatory diseases52. It is a scavenger receptor for the haemoglobin/haptoglobin complex and a major effect of receptor/ligand interaction is the stimulation of IL-6 production, the major cytokine mediator of the acute phase response53. The link between RNF186 and chronic inflammation originates from genome-wide association studies of IBD; overexpression of RNF186 is indicative of endoplasmic reticulum stress and a truncated variant of RNF186 confers protection against ulcerative colitis54,55. These genes were also up-regulated in P sheep, but with lower fold changes. Of these genes only VNN1 (M, +40.23 and P, +2.27 fold) is significantly different between M and P.
The most repressed transcripts in M sheep include four genes associated with mast cell function and may indicate a marked reduction in mast cells numbers in infected ICLN. HDC is the most down-regulated gene in the M dataset and encodes an enzyme that synthesizes histamine, a major component of mast cell granules56. Its importance in mycobacterial infection is highlighted by the greatly increased growth of Mycobacterium bovis BCG in macrophages from HDC −/− knock-out mice; addition of histamine to HDC−/− macrophages decreased the numbers of intracellular bacilli57. CPA3 and TPSAB1 encode proteases that are major constituents of mast cell granules and play important roles in the control of chronic inflammation and in regulating sepsis58. FCER1A encodes the high affinity IgE receptor alpha chain that is essential for mast cell interactions with antigen56. In addition, transcripts for mast cell growth factor KITLG and its receptor KIT are also repressed in infected animals. Mast cells play a significant role in the homeostasis of immune responses59,60 and the marked reduction of mast cell numbers/function in the ICLN may negatively influence myeloid DC activation and function, evidenced by the repression of the uniquely ruminant MHC class II transcript Ovar-DYA, which is exclusively expressed by DC61. These results, and the preliminary histological observations, highlight the need to more accurately characterize mast cells in the ICLN and investigate their role in paratuberculosis pathology.
The ICLN drains the M lesions in the terminal ileum, which contain large numbers of infected macrophages10,11. Lymph nodes that drain sites of infection are usually hypertrophic and their constituent lymphocytes are highly activated62. This activation is reflected here by the significant increase in the activation marker CD70; this may reflect activated B cells as most of the genes associated with T cell activation and signal transduction were significantly repressed. This down-regulation includes the TCR and CD3 clusters, most co-receptor and co-stimulatory molecules as well as members of signal transduction pathways. Antigen activation in sheep leads to a increases in MHC class II63 by T cells but in M sheep MHC class II expression levels (except Ovar-DY) were largely unaffected. In addition, MHC class II expression by macrophages was not significantly affected; which may be linked to the fact that IFNG levels were not significantly raised in M animals. Sheep with M disease express reduced levels of IFNG in MLN compared to subclinically infected animals64, which possibly results in uncontrolled MAP growth. IFNG levels are raised in P animals, which have few mycobacteria. The repression of T cell transcripts in M animals may be due to a reduction in T cell numbers, although the expression levels of genes that encode common T cell markers (e.g. CD2) is unchanged. One possible explanation for the depressed T cell response in M sheep could be that chronic bacterial infections, including Mycobacterium tuberculosis, trigger a programmed down-regulation of high avidity TCR65. This results in the reduction of TCR expression and T cell activation, but is hypothesized to preserve the polyclonality of the immune response and protect against immune-mediated inflammatory damage65.
After encountering antigen, B cells differentiate into plasma cells through a tightly organised gene-expression programme controlled by specific transcriptional regulators66. The up-regulation of immunoglobulin transcripts in M and P sheep is consistent with B cell activation, class switching and terminal differentiation into plasma cells (Fig. 3). Also required for Ig heavy chain biosynthesis is MZB167, which is up-regulated in infected animals. However, molecules associated with resting or memory B cells were reduced or unchanged including the B cell co-receptor complex (CR2, CD19 and CD81), BCR signal transduction heterodimer (CD79A and CD79B) and MS4A1 (CD20). The plasma cell transcriptional regulators, IRF4, PRDM1 and XBP1 were raised while those required for mature B cell maintenance, PAX5, BACH2, AICDA and BCL666 were repressed or unchanged in M and P sheep. High levels of PRDM1 (Blimp1) repress the key regulators of the B cell gene expression programme PAX5 and BCL6, thus a plasma cell-specific gene expression programme was maintained. Another key regulator of plasma cell differentiation and growth68 is IL-6, which is highly increased in M sheep, it up-regulates XBP1 and is itself regulated by XBP169. IL-6 is also a major driver of the acute phase response and chronic inflammation68. It is not significantly affected in P disease. High anti-MAP antibody levels is a common finding in M paratuberculosis11 and has also been described in human mycobacterial diseases, but their role in disease pathogenesis remains unclear70. As with inflammation and T cell genes, the expression patterns of these genes in P sheep is similar to M sheep, but usually with lower fold changes.
Communication between individual components of the innate and adaptive immune systems, highlighted by IPA analysis, is essential for the control and/or elimination of pathogens71. In M animals there is little consistency in chemokine/chemokine receptor expression. CCL15, CCL3 and CXCR1 influence T cell, monocyte and neutrophil migration and are significantly increased in M and P animals; as is CCR10, which plays a critical role in humoral immunity at mucosal sites71. However their respective ligands/receptors are either unaffected or not identified in the datasets. CCL7 also influences monocyte and lymphocyte mobilization but is repressed in both M and P; while CCR9 controls T cell homing to the gut, and is down-regulated only in M. As with the up-regulated chemokines, their respective ligands/receptors are also unaffected. The only receptor/ligand pair that shows consistent expression is CXCR3/CXCL10 in M animals, which control Th1 mediated T cell migration71.
The primary aim of this study was to begin to describe the basis of the immune dysfunction associated with M paratuberculosis. Lymph nodes draining the site of bacterial infections should show extensive evidence of T cell activation, acute inflammation and antibody synthesis. However, TruSeq analysis of the ICLN, the major immune inductive site of paratuberculosis lesions, showed that T cells were not activated, the T cell antigen-receptor complex genes (TCR and CD3) were all down-regulated or unaffected, as were most of the T cell co-receptor and co-stimulator transcripts, many T cell activation molecules and most of the signal-transduction cascade. There was clear evidence of inflammation in the ICLN draining the both M and P lesions, but this was clearly an acute phase response and chronic inflammation, with little evidence of acute inflammation. The high levels of immunoglobulin transcripts is consistent with the anti-MAP antibody responses frequently reported in M sheep, however similar high levels of immunoglobulin transcripts were also identified in P sheep, even though few bacteria can be detected and antibody levels are inconsistent. Also seen in M and P sheep was the repression of transcripts associated with resting and memory B cells. However, perhaps the most novel observation of these studies was the profound reduction in mast cell transcripts in both disease forms. This may negatively influence the activation state of myeloid DC, which are known to play a major role in the induction of the immune response. In conclusion, M and P diseases are two different pathologies, but this study shows that there were no fundamental differences in the gene expression patterns in M and P disease. There was no shift in T cell genes from a Th1 to Th2 pattern in P and M sheep, rather there was an incremental decline in the expression of T cell genes and an increase in the expression of chronic inflammation genes, eventually leading to the immune dysfunction associated with multibacillary pathology.
Materials and Methods
Animals and sample collection
Diseased sheep were out bred Scottish Blackface or Blackface X ewes with naturally-acquired MAP infection originating from local commercial flocks (see Supplementary Table S1). Uninfected control animals were all healthy Blackface sheep from a flock with no history of paratuberculosis, with no clinical disease or pathology. All animals were 2 to 4.5 years old, with no/low gastrointestinal nematode infection and none had been vaccinated against Johne’s disease. At post mortem the ICLN, distal MLN, ileocaecal valve and terminal ileum adjacent to the ileocaecal valve were collected and were fixed in 10% neutral buffered formalin for histopathology. Adjacent segments of terminal ileum (2 cm) were stored at −80 °C for subsequent detection of MAP growth. The ICLN was treated with RNAlater RNA Stabilization Solution (Ambion) and stored at −80 °C prior to RNA extraction (according to manufacturer’s instructions). No sheep were euthanized specifically for this work, uninfected sheep were euthanized for unrelated reasons and infected animals were culled for clinical reasons.
Pathological Diagnosis, Histopathology and Bacteriology
Formalin fixed tissues from MLN and terminal ileum were paraffin blocked and 5 μm sequential sections were stained with H&E, ZN or toluidine blue. All histopathology observations were described by a qualified veterinary pathologist (FC). The pathological diagnosis was evaluated and scored as described by Dennis et al. with modifications72. Frozen segments of terminal ileum were cultured for the subsequent detection of MAP growth on slopes of 7H1173. Colonies were confirmed as MAP using a standardized IS900 PCR (see Supplementary Table S1).
Nucleic acid isolation
Genomic DNA was extracted from ~20 mg (wet weight) ICLN for each sample using the Wizard® SV Genomic DNA Purification System (Promega) as described by the manufacturer and quantified using a NanoDrop ND-1000 spectrophotometer. For RNA isolation ICLN was disrupted and homogenized in a Lysing Matrix D tube (MP Biomedicals), containing ~40 mg of tissue and 1 ml of TRIzol reagent (Ambion), using a FastPrep FP120 Cell Disrupter (Thermo Electron). Total RNA was extracted using a TRIzol ® Plus RNA Purification Kit (Ambion) as described by the manufacturer, including DNase I treatment. RNA quality and integrity were assessed using an Agilent RNA ScreenTape on the Agilent 2200 TapeStation and quantified using a NanoDrop ND-1000 spectrophotometer. All samples had RIN values >7.5.
Detection of MAP by IS900 PCR
The presence of MAP in the ICLN was assessed by PCR using two previously validated primer sets: Eishi IS900 primer forward74; GTTCGGGGCCGTCGTTAGG; reverse; GCGGGCGGCCAATCTCCTT: and Bauerfeind MP3 primer75; CTGGCTACCAAACTCCCGA; MP4; GAACTCAGCGCCCAGGAT. All PCR reactions were performed with FastStart Taq DNA Polymerase (Roche Diagnostics) following manufacturer’s instructions, with 500 ng of gDNA and 0.2 μM of each primer. PCR reactions were performed using a Veriti® Thermal Cycler (Applied Biosystems); conditions were an initial denaturation step of 5 min at 95 °C, followed by 35 cycles of 15 sec at 95 °C, 15 sec at 55 °C, 58 °C, 60 °C or 62 °C, 30 sec at 72 °C, with a final elongation step of 10 min at 72 °C. PCR products were fractionated by 2% agarose gel electrophoresis, purified with MinElute Gel Extraction Kit (Qiagen), cloned using a TOPO TA cloning kit for sequencing (Invitrogen), and sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit and 3730 DNA Analyser (Applied Biosystems), according to the manufacturer’s instructions.
TruSeq library construction, sequencing and data processing
Template cDNA libraries for each sample were constructed using TruSeq™ RNA Sample Preparation Kit v2 according to the manufacturer’s instructions (Illumina). The cDNA libraries were sequenced from both ends (2 × 101-nucleotide paired-end reads) using an Illumina HiSeq 2500 sequencing system at Edinburgh Genomics (University of Edinburgh, UK) according to the manufacturer’s protocol. All sequence and metadata have been deposited in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5146.
Prior to alignment, read sequence quality and composition were checked using FastQC (v0.10.1) software then processed using Cutadapt v1.376 program for adapter and quality trimming. Using STAR (v2.3)77 software sequencing reads were aligned to the Ovis aries genome (Oar v3.1, GCA_000298735.1). The SAM files of the mapped reads were processed and organized using utilities from SAMtools78 prior to summarization of the read counts at the gene level using the script htseq-count(v0.6.1p1) of the Python package HTSeq79. Filtering of genes was performed to retain only those that had at least 1 count per million mapped reads, in at least five samples for further analysis.
Differential expression analysis of gene expression data
Filtered gene read counts were normalized using TMM scaling method80 before voom transformation81 with sample-specific quality weights applied before linear model analysis to assess differential gene expression with the limma package in R82. Gene names and symbols were retrieved for differentially expressed genes from Ensembl, annotation release 86, using the biomaRt package83. Manual annotation of unannotated genes of interest was performed using BLASTN analysis84.
Causal Network Analysis, Molecular Network and Pathway analysis
The differentially expressed genes datasets were analysed, by uploading the HUGO Gene Nomenclature Committee (HGNC) gene symbols for the sheep orthologues, fold change data and adjusted P-values, through the use of QIAGEN’s Ingenuity® Pathway Analysis (IPA®, QIAGEN Redwood City, www.qiagen.com/ingenuity). Overrepresented canonical pathways were identified, which, in addition to identifying enriched biological functions, pathway activity analysis allowed prediction of the activation status for each pathway using the activation z-score algorithm.
Inflammatory cytokines & receptors RT2 profiler™ PCR array
The Cow Inflammatory Cytokines & Receptors RT2 Profiler™ PCR Array (Qiagen) was used for the quantitative real-time RT-PCR (RT-qPCR) analysis of the ICLN of all sheep. Total RNA was extracted as described above and each sample of RNA was reverse transcribed using a RT2 First Strand Kit according to the manufacturer’s protocol (Qiagen). Real-time PCR was performed on each cDNA sample using the RT2 Profiler™ PCR Array with the RT2 SYBR Green/ROX FAST Mastermix in a Rotor-Gene Q cycler (Qiagen). The cycling profile was performed at 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Melting curve analysis of qPCR products confirmed the absence of secondary product. RT2 Profiler PCR Array Data Analysis version 3.5 software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php) was used for data analysis. Spearman rank-order correlation analysis was carried out using GraphPad Prism 6.07 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com).
Additional Information
How to cite this article: Gossner, A. et al. Pathways and Genes Associated with Immune Dysfunction in Sheep Paratuberculosis. Sci. Rep. 7, 46695; doi: 10.1038/srep46695 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant (BB/J004227/1). We acknowledge Joyce McLuckie (culture and PCR), Clare Underwood (Histopathology Labs) and Bioservices at the Moredun Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.H. designed the study. C.W. collected the tissue samples post mortem. F.C. provided pathological diagnosis and histopathological descriptions. A.G. conducted the experiments, analysed the data and wrote the manuscript with J.H. All authors read and approved the manuscript.
References
- Harris N. B. & Barletta R. G. Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin Microbiol Rev 14, 489–512, doi: 10.1128/CMR.14.3.489-512.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty T., Graham D. A., Mullowney P. & More S. J. A review of bovine Johne’s disease control activities in 6 endemically infected countries. Preventive Veterinary Medicine 116, 1–11, doi: 10.1016/j.prevetmed.2014.06.003 (2014). [DOI] [PubMed] [Google Scholar]
- Fernandez-Silva J. A., Correa-Valencia N. M. & Ramirez N. F. Systematic review of the prevalence of paratuberculosis in cattle, sheep, and goats in Latin America and the Caribbean. Trop Anim Health Prod 46, 1321–1340, doi: 10.1007/s11250-014-0656-8 (2014). [DOI] [PubMed] [Google Scholar]
- Garcia A. B. & Shalloo L. Invited review: The economic impact and control of paratuberculosis in cattle. J Dairy Sci 98, 5019–5039, doi: 10.3168/jds.2014-9241 (2015). [DOI] [PubMed] [Google Scholar]
- Nielsen S. S. & Toft N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev Vet Med 88, 1–14, doi: 10.1016/j.prevetmed.2008.07.003 (2009). [DOI] [PubMed] [Google Scholar]
- Perez V., Garcia Marin J. F. & Badiola J. J. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. Journal of comparative pathology 114, 107–122 (1996). [DOI] [PubMed] [Google Scholar]
- Clarke C. J. & Little D. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues. Journal of comparative pathology 114, 419–437 (1996). [DOI] [PubMed] [Google Scholar]
- Carrigan M. J. & Seaman J. T. The pathology of Johne’s disease in sheep. Australian Veterinary Journal 67, 47–50 (1990). [DOI] [PubMed] [Google Scholar]
- González J. et al. Histopathological classification of lesions associated with natural paratuberculosis infection in cattle. Journal of comparative pathology 133, 184–196 (2005). [DOI] [PubMed] [Google Scholar]
- Smeed J. A., Watkins C. A., Rhind S. M. & Hopkins J. Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis. BMC Vet Res 3, 18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C. The pathology and pathogenesis of paratuberculosis in ruminants and other species. Journal of comparative pathology 116, 217–261 (1997). [DOI] [PubMed] [Google Scholar]
- Reddacliff L., Eppleston J., Windsor P., Whittington R. & Jones S. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet Microbiol 115, 77–90, doi: 10.1016/j.vetmic.2005.12.021 (2006). [DOI] [PubMed] [Google Scholar]
- Grant I. R., Pope C. M., O’Riordan L. M., Ball H. J. & Rowe M. T. Improved detection of Mycobacterium avium subsp paratuberculosis in milk by immunomagnetic PCR. Veterinary Microbiology 77, 369–378 (2000). [DOI] [PubMed] [Google Scholar]
- Burrells C. et al. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne’s disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Veterinary immunology and immunopathology 66, 343–358 (1998). [DOI] [PubMed] [Google Scholar]
- Perez V., Tellechea J., Corpa J. M., Gutierrez M. & Garcia Marin J. F. Relation between pathologic findings and cellular immune responses in sheep with naturally acquired paratuberculosis. Am J Vet Res 60, 123–127 (1999). [PubMed] [Google Scholar]
- Stabel J. Transitions in immune responses to Mycobacterium paratuberculosis. Vet Microbiol 77, 465–473 (2000). [DOI] [PubMed] [Google Scholar]
- Coussens P. M. Mycobacterium paratuberculosis and the bovine immune system. Anim Health Res Rev 2, 141–161 (2001). [PubMed] [Google Scholar]
- Sweeney R. W. Pathogenesis of paratuberculosis. Vet Clin North Am Food Anim Pract 27, doi: 10.1016/j.cvfa.2011.07.001 (2011). [DOI] [PubMed] [Google Scholar]
- Yamamura M. et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254, 277–279 (1991). [DOI] [PubMed] [Google Scholar]
- Barnes P. F. et al. Cytokine production at the site of disease in human tuberculosis. Infection and Immunity 61, 3482–3489 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg D. J. et al. Does a Th1 over Th2 dominancy really exist in the early stages of Mycobacterium avium subspecies paratuberculosis infections? Immunobiology 216, 840–846, doi: 10.1016/j.imbio.2010.12.004 (2011). [DOI] [PubMed] [Google Scholar]
- Zurbrick B. G. & Czuprynski C. J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect Immun 55, 1588–1593 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. J., Evanson O. A., McClenahan D. J., Abrahamsen M. S. & Walcheck B. K. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infection and Immunity 69, 1002–1008 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. E. et al. Analysis of the Bovine Monocyte-Derived Macrophage Response to Mycobacterium avium Subspecies Paratuberculosis Infection Using RNA-seq. Front Immunol 6, 23, doi: 10.3389/fimmu.2015.00023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHugh D. E. et al. Pan-genomic analysis of bovine monocyte-derived macrophage gene expression in response to in vitro infection with Mycobacterium avium subspecies paratuberculosis. Veterinary Research 43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelaar M. F. et al. Cytokine gene expression profiles of bovine dendritic cells after interaction with Mycobacterium avium ssp. paratuberculosis (M.a.p.), Escherichia coli (E. coli) or recombinant M.a.p. heat shock protein 70. Veterinary immunology and immunopathology 107, 153–161 (2005). [DOI] [PubMed] [Google Scholar]
- Nalubamba K. et al. Differential expression of pattern recognition receptors in the three pathological forms of sheep paratuberculosis. Microbes Infect 10, 598–604 (2008). [DOI] [PubMed] [Google Scholar]
- Roussey J. A., Oliveira L. J., Langohr I. M., Sledge D. G. & Coussens P. M. Regulatory T cells and immune profiling in Johne’s disease lesions. Veterinary immunology and immunopathology, doi: 10.1016/j.vetimm.2016.03.008 (2016). [DOI] [PubMed] [Google Scholar]
- Roussey J. A., Steibel J. & Coussens P. M. Regulatory T cell activity and signs of T cell unresponsiveness in bovine paratuberculosis. Front Vet Sci 1, 20, doi: 10.3389/fvets.2014.00020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagawa T. et al. Bovine immunoinhibitory receptors contribute to suppression of Mycobacterium avium subsp. paratuberculosis-Specific T-cell responses. Infect Immun 84, 77–89, doi: 10.1128/iai.01014-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite F. L. et al. ZAP-70, CTLA-4 and proximal T cell receptor signaling in cows infected with Mycobacterium avium subsp. paratuberculosis. Veterinary immunology and immunopathology 167, 15–21, doi: 10.1016/j.vetimm.2015.06.017 (2015). [DOI] [PubMed] [Google Scholar]
- Kabara E. et al. A large-scale study of differential gene expression in monocyte-derived macrophages infected with several strains of Mycobacterium avium subspecies paratuberculosis. Brief Funct Genomics 9, 220–237 (2010). [DOI] [PubMed] [Google Scholar]
- Marfell B. J., O’Brien R. & Griffin J. F. Global gene expression profiling of monocyte-derived macrophages from red deer (Cervus elaphus) genotypically resistant or susceptible to Mycobacterium avium subspecies paratuberculosis infection. Developmental And Comparative Immunology 40, 210–217 (2013). [DOI] [PubMed] [Google Scholar]
- Purdie A., Plain K., Begg D., de Silva K. & Whittington R. Expression of genes associated with the antigen presentation and processing pathway are consistently regulated in early Mycobacterium avium subsp. paratuberculosis infection. Comp Immunol Microbiol Infect Dis 35, 151–162 (2012). [DOI] [PubMed] [Google Scholar]
- David J., Barkema H. W., Guan le L. & De Buck J. Gene-expression profiling of calves 6 and 9 months after inoculation with Mycobacterium avium subspecies paratuberculosis. Vet Res 45, 96, doi: 10.1186/s13567-014-0096-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel R. J., Bannantine J. P. & Stabel J. R. Transcriptional Profiling of Ileocecal Valve of Holstein Dairy Cows Infected with Mycobacterium avium subsp. Paratuberculosis. PLoS One 11, e0153932, doi: 10.1371/journal.pone.0153932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh C. G. et al. SOLiD SAGE sequencing shows differential gene expression in jejunal lymph node samples of resistant and susceptible red deer (Cervus elaphus) challenged with Mycobacterium avium subsp. paratuberculosis. Veterinary immunology and immunopathology 169, 102–110, doi: 10.1016/j.vetimm.2015.10.009 (2016). [DOI] [PubMed] [Google Scholar]
- Smeed J. A., Watkins C. A., Gossner A. G. & Hopkins J. Expression profiling reveals differences in immuno-inflammatory gene expression between the two disease forms of sheep paratuberculosis. Veterinary immunology and immunopathology 135, 218–225, doi: 10.1016/j.vetimm.2009.11.015 (2010). [DOI] [PubMed] [Google Scholar]
- Zhong L. et al. Identification of differentially expressed genes in ileum, intestinal lymph node and peripheral blood mononuclear cells of sheep infected with Mycobacterium avium subsp paratuberculosis using differential display polymerase chain reaction. Veterinary immunology and immunopathology 131, 177–189 (2009). [DOI] [PubMed] [Google Scholar]
- Coussens P. M., Verman N., Coussens M. A., Elftman M. D. & McNulty A. M. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis : Evidence for an inherent proinflammatory gene expression pattern. Infection and Immunity 72, 1409–1422 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouret-Craviari V. et al. Expression of a long pentraxin, PTX3, by monocytes exposed to the mycobacterial cell wall component lipoarabinomannan. Infect Immun 65, 1345–1350 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen R. et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun 8, 456–467, doi: 10.1038/sj.gene.6364410 (2007). [DOI] [PubMed] [Google Scholar]
- Donato R. et al. Functions of S100 proteins. Curr Mol Med 13, 24–57 (2013). [PMC free article] [PubMed] [Google Scholar]
- Ikhtaire S., Shajib M. S., Reinisch W. & Khan W. I. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol, doi: 10.1007/s00535-016-1182-4 (2016). [DOI] [PubMed] [Google Scholar]
- Sunderkotter C. H. et al. High expression of myeloid-related proteins 8 and 14 characterizes an inflammatorily active but ineffective response of macrophages during leprosy. Immunology 111, 472–480, doi: 10.1111/j.0019-2805.2004.01836.x (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realegeno S. et al. S100A12 Is Part of the Antimicrobial Network against Mycobacterium leprae in Human Macrophages. PLoS Pathog 12, e1005705, doi: 10.1371/journal.ppat.1005705 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. C. et al. Xanthine Oxidoreductase Function Contributes to Normal Wound Healing. Mol Med 21, 313–322, doi: 10.2119/molmed.2014.00191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitto T. & Onodera K. Linkage between coenzyme a metabolism and inflammation: roles of pantetheinase. J Pharmacol Sci 123, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- Gensollen T. et al. Functional polymorphisms in the regulatory regions of the VNN1 gene are associated with susceptibility to inflammatory bowel diseases. Inflamm Bowel Dis 19, 2315–2325, doi: 10.1097/MIB.0b013e3182a32b03 (2013). [DOI] [PubMed] [Google Scholar]
- Rossaint J. et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 126, 962–974, doi: 10.1172/JCI83470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Stabel J. & Kehrli M. E. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Veterinary immunology and immunopathology 82, 73–85 (2001). [DOI] [PubMed] [Google Scholar]
- Van den Heuvel M. M. et al. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol 66, 858–866 (1999). [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol 8, 253–278, doi: 10.1146/annurev.iy.08.040190.001345 (1990). [DOI] [PubMed] [Google Scholar]
- Fujimoto K. et al. Regulation of intestinal homeostasis by the ulcerative colitis-associated gene RNF186. Mucosal Immunol, doi: 10.1038/mi.2016.58 (2016). [DOI] [PubMed] [Google Scholar]
- Rivas M. A. et al. A protein-truncating R179X variant in RNF186 confers protection against ulcerative colitis. Nat Commun 7, 12342, doi: 10.1038/ncomms12342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson S. & Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 14, 478–494, doi: 10.1038/nri3690 (2014). [DOI] [PubMed] [Google Scholar]
- Megyeri K. et al. The role of histamine in the intracellular survival of Mycobacterium bovis BCG. Microbes Infect 8, 1035–1044, doi: 10.1016/j.micinf.2005.10.022 (2006). [DOI] [PubMed] [Google Scholar]
- Galli S. J., Grimbaldeston M. & Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 8, 478–486, doi: 10.1038/nri2327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed B. A., Christy A., Quirion M. R. & Brown M. A. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 26, 705–739, doi: 10.1146/annurev.immunol.26.021607.090320 (2008). [DOI] [PubMed] [Google Scholar]
- Galli S. J. et al. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. 749–786 (2005). [DOI] [PubMed]
- Ballingall K., MacHugh N., Taracha E., Mertens B. & McKeever D. Transcription of the unique ruminant class II major histocompatibility complex-DYA and DIB genes in dendritic cells. Eur J Immunol 31, 82–86, doi: (2001). [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway’s Immunobiology. 8th Edition edn, (Garland Science, 2011). [Google Scholar]
- Hopkins J., McConnell I., Dalziel R. G. & Dutia B. M. Patterns of major histocompatibility complex class II expression by T cell subsets in different immunological compartments. 2. Altered expression and cell function following activation in vivo. Eur J Immunol 23, 2889–2896 (1993). [DOI] [PubMed] [Google Scholar]
- Sweeney R. W., Jones D. E., Habecker P. & Scott P. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res 59, 842–847 (1998). [PubMed] [Google Scholar]
- Gallegos A. M. et al. Control of T cell antigen reactivity via programmed TCR downregulation. Nat Immunol 17, 379–386, doi: 10.1038/ni.3386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A. & Nutt S. L. Bach2: plasma-cell differentiation takes a break. Embo j 29, 3896–3897, doi: 10.1038/emboj.2010.282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M. et al. MZB1 is a GRP94 cochaperone that enables proper immunoglobulin heavy chain biosynthesis upon ER stress. Genes Dev 28, 1165–1178, doi: 10.1101/gad.240762.114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood 74, 1–10 (1989). [PubMed] [Google Scholar]
- Iwakoshi N. N., Lee A. H. & Glimcher L. H. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev 194, 29–38 (2003). [DOI] [PubMed] [Google Scholar]
- Lu L. L. et al. A Functional Role for Antibodies in Tuberculosis. Cell, doi: 10.1016/j.cell.2016.08.072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. W., Sokol C. L. & Luster A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32, 659 (2014). [DOI] [PubMed] [Google Scholar]
- Dennis M. M., Reddacliff L. A. & Whittington R. J. Longitudinal study of clinicopathological features of Johne’s disease in sheep naturally exposed to Mycobacterium avium subspecies paratuberculosis. Veterinary pathology 48, 565–575, doi: 10.1177/0300985810375049 (2011). [DOI] [PubMed] [Google Scholar]
- Hughes V., Smith S., Garcia-Sanchez A., Sales J. & Stevenson K. Proteomic comparison of Mycobacterium avium subspecies paratuberculosis grown in vitro and isolated from clinical cases of ovine paratuberculosis. Microbiology 153, 196–205, doi: 10.1099/mic.0.29129-0 (2007). [DOI] [PubMed] [Google Scholar]
- Eishi Y. et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. Journal of clinical microbiology 40, 198–204 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R. et al. Molecular characterization of Mycobacterium paratuberculosis isolates from sheep, goats, and cattle by hybridization with a DNA probe to insertion element IS900. Journal of clinical microbiology 34, 1617–1621 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12 (2011). [Google Scholar]
- Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079, doi: 10.1093/bioinformatics/btp352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T. & Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, doi: 10.1093/bioinformatics/btu638 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D. & Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome biology 11, R25, doi: 10.1186/gb-2010-11-3-r25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. W., Chen Y., Shi W. & Smyth G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome biology 15, R29, doi: 10.1186/gb-2014-15-2-r29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E. et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research 43, e47, doi: 10.1093/nar/gkv007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S., Spellman P. T., Birney E. & Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nature protocols 4, 1184–1191, doi: 10.1038/nprot.2009.97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic local alignment search tool. J. Mol Biol. 215, 403–410, doi: 10.1006/jmbi.1990.9999 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.