Abstract
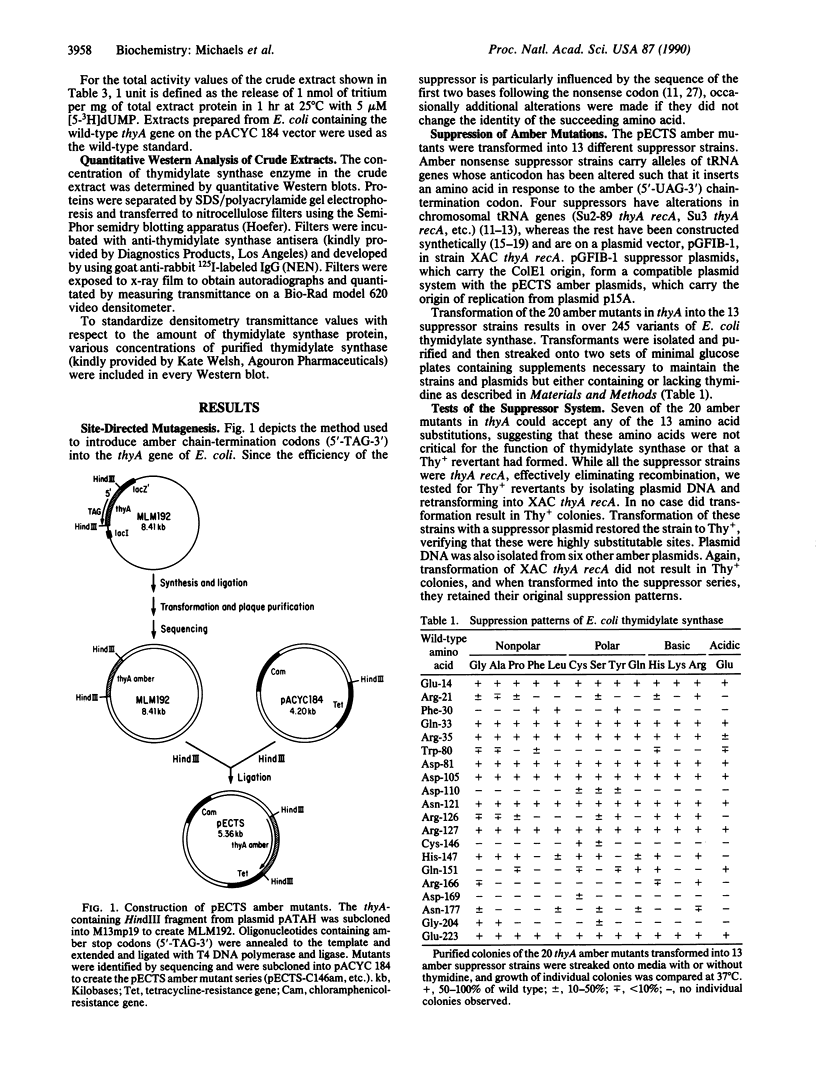
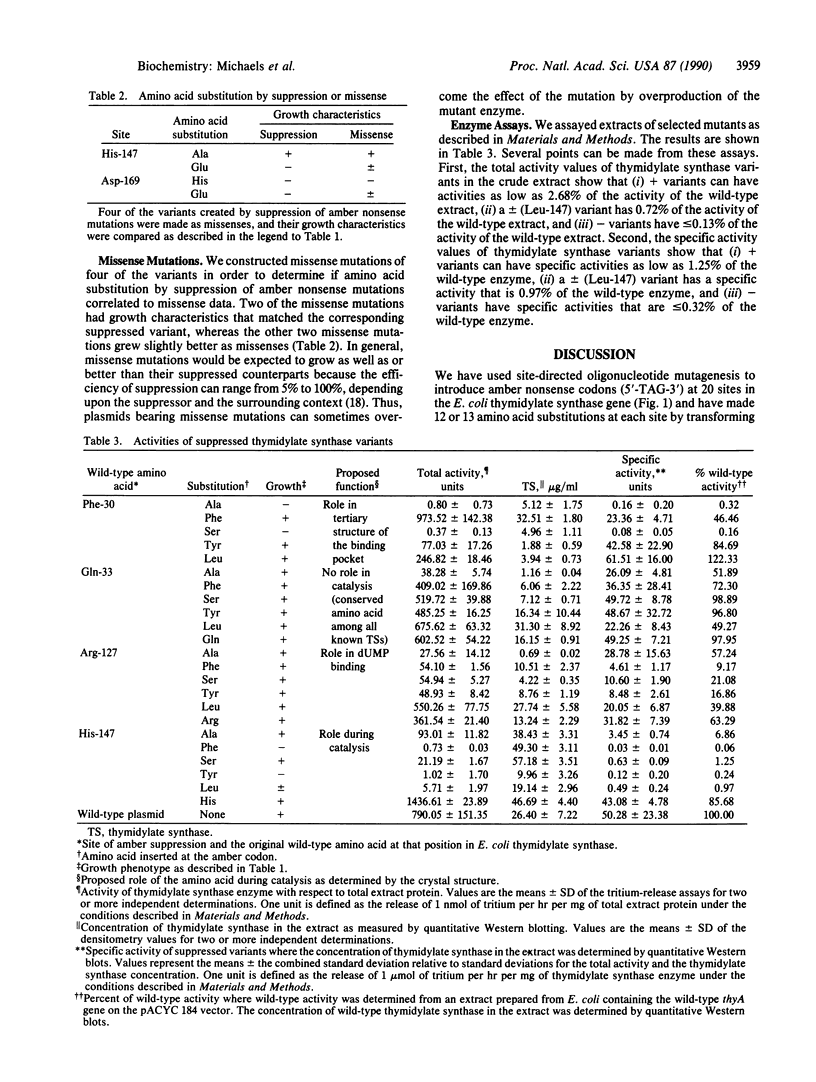
By using site-directed oligonucleotide mutagenesis, amber nonsense stop codons (5'-TAG-3') have been introduced at 20 sites in the Escherichia coli thymidylate synthase gene. By transforming the thyA mutant plasmids into 13 strains, each of which harbor different amber suppressor tRNAs, we were able to generate over 245 amino acid substitutions in E. coli thymidylate synthase (EC 2.1.1.45). Growth characteristics of these mutants have been studied, yielding a body of information that includes some surprising results in light of the recently published crystal structure of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Maley G., Pedersen-Lane J., Maley F. Primary structure of the Escherichia coli thyA gene and its thymidylate synthase product. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4914–4918. doi: 10.1073/pnas.80.16.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Pedersen-Lane J. Genetic system for analyzing Escherichia coli thymidylate synthase. J Bacteriol. 1984 Oct;160(1):371–378. doi: 10.1128/jb.160.1.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellisario R. L., Maley G. F., Galivan J. H., Maley F. Amino acid sequence at the FdUMP binding site of thymidylate synthetase. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1848–1852. doi: 10.1073/pnas.73.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi L. Context effects: translation of UAG codon by suppressor tRNA is affected by the sequence following UAG in the message. J Mol Biol. 1983 Feb 15;164(1):73–87. doi: 10.1016/0022-2836(83)90088-8. [DOI] [PubMed] [Google Scholar]
- Bradley D., Park J. V., Soll L. TRNA2Gln Su+2 mutants that increase amber suppression. J Bacteriol. 1981 Feb;145(2):704–712. doi: 10.1128/jb.145.2.704-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg P. V. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977 Dec 23;473(2):73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- Dev I. K., Yates B. B., Atashi J., Dallas W. S. Catalytic role of histidine 147 in Escherichia coli thymidylate synthase. J Biol Chem. 1989 Nov 15;264(32):19132–19137. [PubMed] [Google Scholar]
- Dev I. K., Yates B. B., Leong J., Dallas W. S. Functional role of cysteine-146 in Escherichia coli thymidylate synthase. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1472–1476. doi: 10.1073/pnas.85.5.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L. W., Finer-Moore J. S., Montfort W. R., Jones M. O., Santi D. V., Stroud R. M. Atomic structure of thymidylate synthase: target for rational drug design. Science. 1987 Jan 23;235(4787):448–455. doi: 10.1126/science.3099389. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Calvert A. H., Jackman A. L., Eakin M. A., Smithers M. J., Betteridge R. F., Newell D. R., Hayter A. J., Stocker A., Harland S. J. Quinazoline antifolates inhibiting thymidylate synthase: variation of the N10 substituent. J Med Chem. 1985 Oct;28(10):1468–1476. doi: 10.1021/jm00148a016. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Masson J. M., Miller J. H. Expression of synthetic suppressor tRNA genes under the control of a synthetic promoter. Gene. 1986;47(2-3):179–183. doi: 10.1016/0378-1119(86)90061-2. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Appelt K., Oatley S. J. Crystal structure of Escherichia coli thymidylate synthase with FdUMP and 10-propargyl-5,8-dideazafolate. Adv Enzyme Regul. 1989;29:47–60. doi: 10.1016/0065-2571(89)90093-9. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Appelt K., Oatley S. J. Stacked beta-bulges in thymidylate synthase account for a novel right-handed rotation between opposing beta-sheets. J Mol Biol. 1989 Jan 20;205(2):449–454. doi: 10.1016/0022-2836(89)90354-9. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Foss K. Changing the acceptor identity of a transfer RNA by altering nucleotides in a "variable pocket". Science. 1988 Sep 30;241(4874):1804–1807. doi: 10.1126/science.2459773. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Hofer M., Schmeissner U., Sommer H., Schmitz A., Lu P. Genetic studies of the lac repressor. IX. Generation of altered proteins by the suppression of nonsence mutations. J Mol Biol. 1979 Jun 25;131(2):191–222. doi: 10.1016/0022-2836(79)90073-1. [DOI] [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Ogden R. C., Horvath S. J., Abelson J. Changing the identity of a transfer RNA. Nature. 1986 May 15;321(6067):213–219. doi: 10.1038/321213a0. [DOI] [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- Rydén S. M., Isaksson L. A. A temperature-sensitive mutant of Escherichia coli that shows enhanced misreading of UAG/A and increased efficiency for some tRNA nonsense suppressors. Mol Gen Genet. 1984;193(1):38–45. doi: 10.1007/BF00327411. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]