Abstract.
We performed this study to evaluate the associations of hypothyroidism with clinical severity and the occurrence of diabetic ketoacidosis (DKA) at initial diagnosis among pediatric patients with type 1 diabetes mellitus (T1DM). 330 children with T1DM who referred to Diabetes Clinic were enrolled. The medical records were e valuated and a blood sample was drawn from patients for measuring thyroid function and antibodies, blood glucose, and glycated hemoglobin (HbA1C) levels. Hypothyroidism was detected in 9.6% of children with T1DM and was associated with higher rates of DKA (OR = 3.15, 95%CI = 1.48–6.71) and younger age at initial diagnosis (7.3 ± 3.2 vs. 10.1 ± 2.5, p = 0.04), higher levels of HbA1C upon enrolment (9.8 ± 2.2 vs. 8.8 ± 1.9, p = 0.02) and the requirement for higher insulin doses to control the disease (0.9 ± 0.42 vs. 0.81 ± 0.2, p = 0.03) compared to children with T1DM and normal thyroid function. Additionally children with T1DM and hypothyroidism had significantly higher rates of anti-TPO antibodies (p < 0.001), consanguinity in their parents (p =0.01), and family history of diabetes mellitus (p = 0.02) in their first degree relatives. In conclusion autoimmune hypothyroidism is prevalent among children with T1DM and is associated with a more aggressive disease at initial presentation, poorly controlled T1DM, and requirement for higher Insulin doses for controlling the disease.
Keywords: autoimmune, DKA, insulin, ketoacidosis, thyroid
Introduction
Type 1 Diabetes Mellitus (T1DM) is one of the most prevalent endocrine disorders among children worldwide; annually an estimated of 65,000 children develop the disease and its incidence is increasing 3% each year (1). Between 10 to 70% of these children present in Diabetic Ketoacidosis (DKA) as their first presentation of the disease (1). In addition to its life threatening aspects, DKA is associated with a heavy financial burden; it accounts for more than 500,000 hospital days per year with an estimated annual cost of 2.4 billion USD (2).
Some studies have shown a higher prevalence of autoimmune disorders among patients with diabetes mellitus which might increase their chance of developing DKA as the first manifestation of their disease (3).
Hypothyroidism is prevalent among pediatric patients with T1DM; while the reported prevalence of hypothyroidism among general pediatric population is 0.1 to 2% (4, 5), the prevalence of hypothyroidism in patients with T1DM is much higher ranging from 3 to 30% (6, 7). This could be due to the shared autoimmune disposition for both T1DM and hypothyroidism; recent studies have identified some shared genes involved in the susceptibility for both conditions (7, 8). Additionally hypothyroidism and metabolic derangement in patients with T1DM might form a vicious cycle aggravating disease severity; hypothyroidism have been shown to increase insulin resistance and impair glucose metabolism (8, 9). while metabolic derangement have been shown to depress pituitary–thyroid axis impairing thyroid function (9).
Given the high prevalence of hypothyroidism among pediatric patients with T1DM and the increased insulin resistance caused by hypothyroidism state and the increased risk of developing DKA as the first manifestation of diabetes in individuals who have other autoimmune disorders (3, 6), we postulate that children with T1DM who have simultaneous hypothyroidism might have a more aggressive form of the disease requiring tighter control and also might have higher chance of developing DKA at initial diagnosis.
Searching the literature we found a considerable gap in this area of research; we could not find enough studies searching for the relationship between hypothyroidism and the severity of T1DM, the Hemoglobin A1C (HbA1C) levels, and the required Insulin doses to control the disease. Whether pediatric patients with both T1DM and hypothyroidism have more aggressive onset of the disease (DKA) is not clearly identified. Furthermore it needs to be identified whether the family history of diabetes or hypothyroidism could affect the presence of both conditions in their affected children. This is especially important in countries with high rates of consanguineous marriages.
We therefore conducted this study to assess the prevalence of hypothyroidism among pediatric patients with T1DM in a developing country where the rate of consanguineous marriage is very high and to evaluate whether the presence of hypothyroidism has any associations with the severity of T1DM as reflected in the required insulin dose, age at initial diagnosis, HbA1C levels, and the occurrence of DKA as the first manifestations of the disease.
Materials and Methods
Study population and design
We studied the pediatric patients with T1DM who were referred to the Diabetes Clinic of our institute, from January 2013 through January 2015 in a hospital based retrospective cohort study. The patients were considered eligible if they had less than 18 yr of age, had a documented T1DM diagnosis based on the American Diabetes Association (ADA) criteria and had not been on any thyroid medication. A total of 357 patients were eligible to participate in the study, and 27 were excluded due to the following criteria: age more than 18 yr, missing the medical records including the records on the first presentation of the disease and missing an informed consent. A total of 330 children aged from 2 to 18 yr completed this study which was approved by the Research Deputy and the Ethics Committee of our institute.
Data and specimen collection
After explaining the whole process an informed written consent was obtained from all the parents and when applicable from the patients who accepted to participate in the study. The patients were visited in a separate appointment and a questionnaire was completed for them through medical interviews and investigating their medical records. Then a blood sample was obtained from patients to measure their Thyroid Stimulating Hormone (TSH), free Thyroxin (T4), Triiodothyronine (T3), Anti-thyroid peroxidase (anti-TPO) antibodies, fasting blood glucose (FBS), and glycated hemoglobin (HbA1C) levels. The following information was retrieved from the patients’ medical records: Age at diagnosis of T1DM, occurrence of DKA as the first clinical manifestation of the disease, the duration of T1DM, the required Insulin dose, and family history of autoimmune diseases in the first degree relatives.
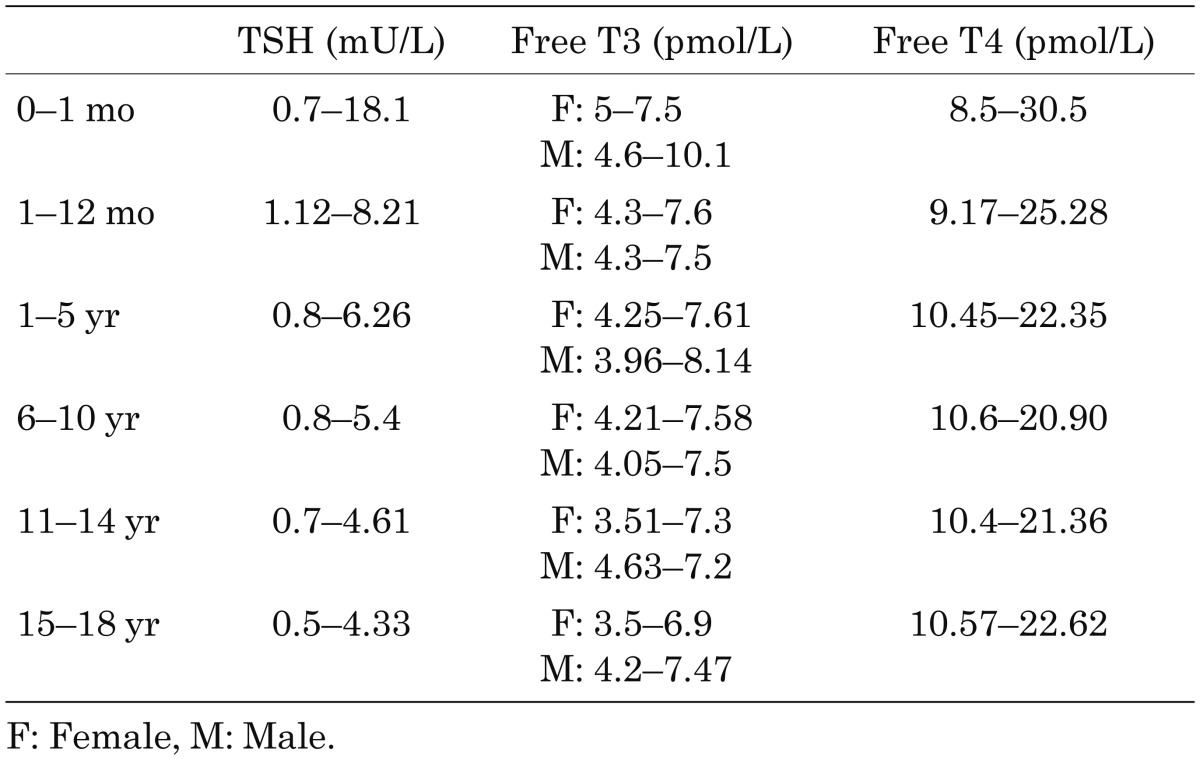
DKA was diagnosed based on the following criteria: hyperglycemia over 250 mg/dL, plasma pH less than 7.3, plasma HCO3 less than 15 mEq/L and the presence of ketonuria. Clinical hypothyroidism was diagnosed based on the increased TSH level and decreased free T4 and/or T3 levels according to the normal range in different pediatric age groups (Table1) (10).
Table 1. Reference range for the thyroid function tests in different pediatric age groups.
Patients were divided into two groups, based on thyroid function tests; patients with T1DM and hypothyroidism and patients with T1DM and normal thyroid function. The HbA1C levels, required Insulin dose, presence of thyroid antibodies, age at initial diagnosis of T1DM, the occurrence of DKA at initial diagnosis and demographics were compared between the two study groups.
Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 18.0.0: PASW, Chicago, IL). Chi-squared analysis, Fisher’s exact test, independent-samples T test and Multivariate logistic regression were used to for statistical analysis of the study. Sample size was calculated for a power of 80% and an alpha error of 0.05 where at least 28 persons would be required in each group to detect a difference in presenting with DKA at initial diagnosis of T1DM. Estimated odds ratios (ORs) with 95% confidence intervals (95% CIs) and P value were used to evaluate the statistical significant. A P value < 0.05 was considered statistically significant.
Results
This study evaluated 330 children with T1DM who were referred to our Diabetes Clinic between 2013 and 2015. The mean ± standard deviation (SD) for the patients’ age was 11.6 ± 2.4 yr; the duration of T1DM was 2.2 ± 1.2 ys; the age at diagnosing T1DM was 9.1 ± 2.3 yr; the required Insulin dose was 0.83 ± 0.23 international unit (IU)/kg/d; the HbA1C level was 8.8 ± 1.9. 193 patients (58.4%) were female with a male: female ratio of 1:1.4; 32 patients (9.6%) had hypothyroidism; 63 patient (19%) had positive anti-TPO antibodies; in 123 children (37.2%) DKA was the first clinical presentation of T1DM; parents’ consanguinity was observed in 86 patients (26%); 55 children (16.6%) had a family history of diabetes mellitus in their first degree relatives; 39 children (11.8%) had a family history of hypothyroidism in their first degree relatives.
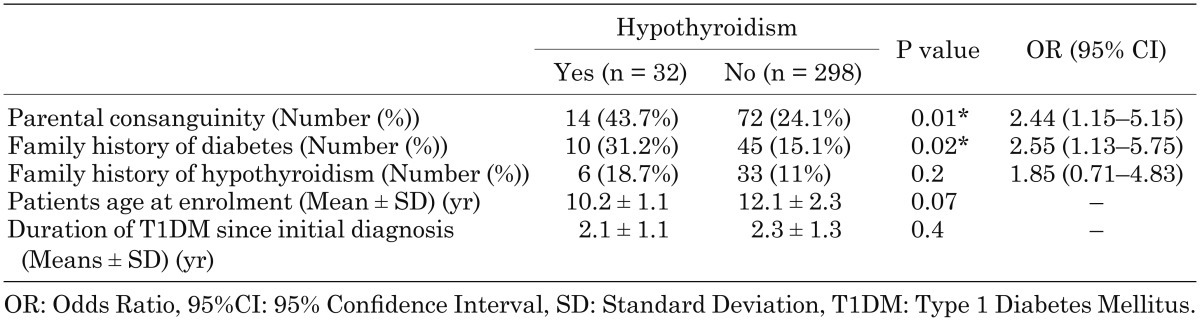
Children with T1DM and hypothyroidism tended to have a higher rate of consanguinity in their parents (43.7 vs. 24.1%, P = 0.01), and a higher rate of diabetes mellitus in their first degree relatives (31.2 vs. 15.1%, P = 0.02) compared to children with T1DM who had normal thyroid function, while the family history of hypothyroidism was not significantly different between the two groups (18.7 vs. 11%, P = 0.2) (Table 2).
Table 2. Comparison of the patients’ demographics between diabetic patients with and without hypothyroidism.
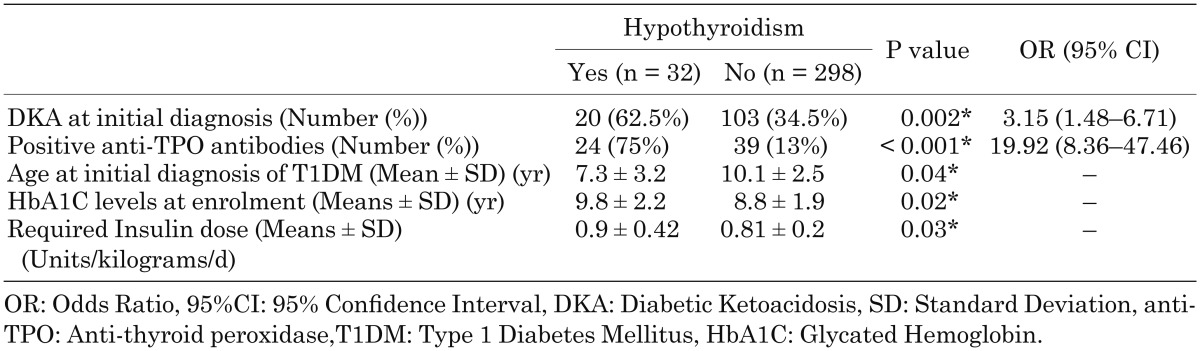
Patients with T1DM and hypothyroidism had significantly higher rates of DKA at initial diagnosis (62.5 vs. 34.5%, P = 0.002), younger age at initial diagnosis (P = 0.04), higher rates of positive anti-TPO antibodies (75 vs. 13%, P < 0.001), and higher levels of HbA1C upon enrolment in the study (P = 0.02) compared to patients with T1DM who had normal thyroid function. Patients with hypothyroidism also required higher doses of insulin to control their disease (P = 0.03) (Table 3).
Table 3. Comparison of the disease severity between diabetic patients with and without hypothyroidism.
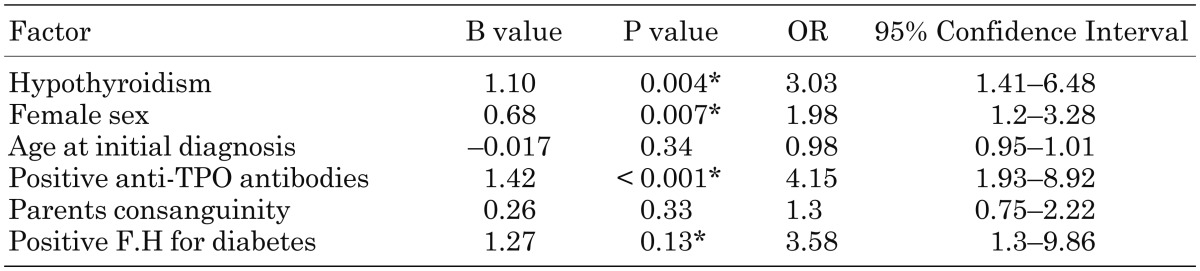
We used Logistic Regression model to adjust the results for patients’ sex, age at diagnosis, positive anti-TPO antibodies, parents consanguinity and family history of diabetes mellitus; after these adjustments hypothyroidism remained significantly associated with the occurrence of DKA as the initial manifestation of the disease in patients with T1DM (B = 1.1, P = 0.004, OR = 3.03, 95%CI = 1.41–6.48) (Table 4).
Table 4. Logistic regression analysis of the factors associated with the occurrence of DKA at the initial diagnosis of T1DM.
Discussion
In this study hypothyroidism was detected in 9.6% of children who suffer from T1DM and was associated with a more aggressive disease; in comparison to children with T1DM who had normal thyroid function, children who had both T1DM and hypothyroidism had significantly higher HbA1C levels at enrolment, required higher insulin doses to control their disease, their disease tended to present at younger ages and most of them experienced DKA as the first presentation of their disease. Additionally children with T1DM and hypothyroidism had significantly higher rates of anti-TPO antibodies, consanguinity among their parents and higher rates of diabetes in their first-degree relatives.
In the current study hypothyroidism was a prevalent problem among children with T1DM, complicating 9.6% of these children. This was in accordance with other studies; based on a 2010 review 3–8% of pediatric patients with T1DM have been reported to develop autoimmune hypothyroidism (6). While a recently published review and a meta-analysis reported a much higher rate of 7–30% for the prevalence of hypothyroidism in patients with T1DM (7, 11). The heterogeneity in the reported prevalence of hypothyroidism in these studies could be due to the variable population characteristics including age and ethnicity of patients, the differences in study design including the cut-off levels and classification of the disease with different definitions including autoimmune, subclinical, and clinical hypothyroidism (6, 7, 11).
In our study more than 70% of the patients with T1DM and hypothyroidism had positive anti-TPO antibodies, in addition parental consanguinity and the presence of diabetes mellitus in the first-degree relatives were significantly higher among patients with both T1DM and hypothyroidism, compared to patients with T1DM but without hypothyroidism. These results strongly support the role of autoimmunity and the shared genetic susceptibility in the pathogenesis of hypothyroidism in patients with T1DM. Recent studies have revealed some genes that might be responsible for the joint susceptibility to T1DM and autoimmune thyroid dysfunction (8); HLA class II loci, CTLA4, INS (12, 13, 15), PTPN22 (14, 15), and FOXP3 (15), are among the identified genes. These genes have been recognized as the key role players in the regulation of the immune response (15,16,17,18); these genes are involved in the differentiation, regulation, activation and function of regulatory T-cells (15,16,17,18), and their polymorphism have been linked to a number of autoimmune diseases including T1DM and autoimmune thyroid dysfunction (12,13,14,15,16,17,18).
In this study hypothyroidism was associated with a more aggressive disease in pediatric patients with T1DM; patients with T1DM and hypothyroidism had higher rates of DKA at presentation, were younger at the onset of the disease, had higher HbA1C levels and required higher insulin doses for the control of the disease. Balsamo et al. in their study documented the association of severe metabolic derangement and impaired thyroid function in patients with newly diagnosed T1DM (9). Lin et al. also showed a significantly lower levels of T3, T4 and free T4 among children newly diagnosed T1DM who presented with DKA compared to patients without DKA at initial diagnosis (19).
The association of thyroid dysfunction with the disease severity in T1DM patients could be explained through several mechanisms; our results supported by the aforementioned studies (12,13,14,15,16,17,18), document that the occurrence of both T1DM and hypothyroidism might show the presence of polymorphism in some of the immune response regulatory genes (12,13,14,15,16,17,18), causing a more severe disease with stronger features of autoimmunity (15,16,17,18). Another mechanism might be through the effect of hypothyroidism on increasing insulin resistance and glucose metabolism(8, 19,20,21,22); thyroid hormones stimulate glucose uptake into peripheral tissues and enhance an additive effect on insulin action on glucose transport into cells (19,20,21,22). Therefore in hypothyroid patients insulin-dependent glucose clearance could get defected (19,20,21,22), putting diabetic patients with hypothyroidism at significantly higher risk of experiencing acute and possibly chronic complications of diabetes (8). Furthermore metabolic derangement and poor diabetic control for a long time might cause complete depression of hypothalamus-pituitary-thyroid axis causing clinical hypothyroidism (9), which in turn aggravates insulin resistance, thus might form a vicious cycle resulting in a more aggressive disease.
This study is among the rare studies that evaluate the clinical severity, insulin requirement and HbA1C levels in pediatric patients with T1DM who have hypothyroidism. However our study has some limitations that should be considered when interpreting the results; we do not have information regarding thyroid function at the onset of T1DM therefore it is not clear whether thyroid dysfunction developed though the course of the disease or it was present at the initial presentation of T1DM. Another limitation was that our patients were not followed to evaluate whether treating hypothyroidism could improve the severity of diabetes and lower the required insulin dose. Additionally in our study thyroid scan and anti-thyroglobulin antibodies were not evaluated, this might have caused an underestimation of the presence of autoimmune thyroiditis among the patients with T1DM. Prospective and interventional studies are required to evaluate whether treating hypothyroidism could alleviate the severity of diabetes and lower the required insulin dose in patients with concurrent T1DM and hypothyroidism, and to answer whether controlling diabetes could result in improvement of thyroid function in these patients.
Conclusions
Hypothyroidism is prevalent among pediatric patients with T1DM and is associated with a more aggressive form of the disease. Patients with T1DM and hypothyroidism have higher rates of DKA, develop the disease at younger ages, and require higher insulin doses. Therefore all patients with T1DM especially those with poorly controlled disease and those with positive family history for diabetes should be screened for thyroid dysfunction. Interventional studies are required to evaluate the efficacy of treating thyroid dysfunction in these patients and to assess if hormone therapy in these patients could help in a better management of diabetes and reduce its complications.
Acknowledgement
This study was funded by the Research Deputy of the Tehran University of Medical Sciences.
References
- 1.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ 2011;343: d4092. doi: 10.1136/bmj.d4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32: 1335–43. doi: 10.2337/dc09-9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valerio G, Maiuri L, Troncone R, Buono P, Lombardi F, Palmieri R, et al. Severe clinical onset of diabetes and increased prevalence of other autoimmune diseases in children with coeliac disease diagnosed before diabetes mellitus. Diabetologia 2002;45: 1719–22. doi: 10.1007/s00125-002-0923-5 [DOI] [PubMed] [Google Scholar]
- 4.Hunter I, Greene SA, MacDonald TM, Morris AD. Prevalence and aetiology of hypothyroidism in the young. Arch Dis Child 2000;83: 207–10. doi: 10.1136/adc.83.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shriraam M, Sridhar M. Subclinical hypothyroidism in children. Indian Pediatr 2014;51: 889–95. doi: 10.1007/s13312-014-0522-9 [DOI] [PubMed] [Google Scholar]
- 6.Brenta G. Diabetes and thyroid disorders. Br J Diabetes Vasc Dis 2010;10: 172–7. doi: 10.1177/1474651410371321 [DOI] [Google Scholar]
- 7.Joffe BI, Distiller LA. Diabetes mellitus and hypothyroidism: Strange bedfellows or mutual companions? World J Diabetes 2014;5: 901–4. doi: 10.4239/wjd.v5.i6.901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011;439463. doi: 10.4061/2011/439463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balsamo C, Zucchini S, Maltoni G, Rollo A, Martini AL, Mazzanti L, et al. Relationships between thyroid function and autoimmunity with metabolic derangement at the onset of type 1 diabetes: a cross-sectional and longitudinal study. J Endocrinol Invest 2015;38: 701–7. doi: 10.1007/s40618-015-0248-0 [DOI] [PubMed] [Google Scholar]
- 10.Kapelari K, Kirchlechner C, Högler W, Schweitzer K, Virgolini I, Moncayo R. Pediatric reference intervals for thyroid hormone levels from birth to adulthood: a retrospective study. BMC Endocr Disord 2008;8: 15. doi: 10.1186/1472-6823-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med 2014;31: 126–35. doi: 10.1111/dme.12318 [DOI] [PubMed] [Google Scholar]
- 12.Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab 2005;90: 4904–11. doi: 10.1210/jc.2004-2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikegami H, Awata T, Kawasaki E, Kobayashi T, Maruyama T, Nakanishi K, et al. The association of CTLA4 polymorphism with type 1 diabetes is concentrated in patients complicated with autoimmune thyroid disease: a multicenter collaborative study in Japan. J Clin Endocrinol Metab 2006;91: 1087–92. doi: 10.1210/jc.2005-1407 [DOI] [PubMed] [Google Scholar]
- 14.Dultz G, Matheis N, Dittmar M, Röhrig B, Bender K, Kahaly GJ. The protein tyrosine phosphatase non-receptor type 22 C1858T polymorphism is a joint susceptibility locus for immunthyroiditis and autoimmune diabetes. Thyroid 2009;19: 143–8. doi: 10.1089/thy.2008.0301 [DOI] [PubMed] [Google Scholar]
- 15.Villano MJ, Huber AK, Greenberg DA, Golden BK, Concepcion E, Tomer Y. Autoimmune thyroiditis and diabetes: dissecting the joint genetic susceptibility in a large cohort of multiplex families. J Clin Endocrinol Metab 2009;94: 1458–66. doi: 10.1210/jc.2008-2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue N, Watanabe M, Morita M, Tomizawa R, Akamizu T, Tatsumi K, et al. Association of functional polymorphisms related to the transcriptional level of FOXP3 with prognosis of autoimmune thyroid diseases. Clin Exp Immunol 2010;162: 402–6. doi: 10.1111/j.1365-2249.2010.04229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP. Why is PTPN22 a good candidate susceptibility gene for autoimmune disease? FEBS Lett 2011;585: 3689–98. doi: 10.1016/j.febslet.2011.04.032 [DOI] [PubMed] [Google Scholar]
- 18.Yeşilkaya E, Koç A, Bideci A, Camurdan O, Boyraz M, Erkal O, et al. CTLA4 gene polymorphisms in children and adolescents with autoimmune thyroid diseases. Genet Test 2008;12: 461–4. doi: 10.1089/gte.2008.0053 [DOI] [PubMed] [Google Scholar]
- 19.Lin CH, Lee YJ, Huang CY, Kao HA, Shih BF, Wang AM, et al. Thyroid function in children with newly diagnosed type 1 diabetes mellitus. Acta Paediatr Taiwan 2003;44: 145–9. [PubMed] [Google Scholar]
- 20.Teixeira SS, Tamrakar AK, Goulart-Silva F, Serrano-Nascimento C, Klip A, Nunes MT. Triiodothyronine acutely stimulates glucose transport into L6 muscle cells without increasing surface GLUT4, GLUT1, or GLUT3. Thyroid 2012;22: 747–54. doi: 10.1089/thy.2011.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, et al. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab 2006;91: 4930–7. doi: 10.1210/jc.2006-0478 [DOI] [PubMed] [Google Scholar]
- 22.Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol 2009;160: 785–90. doi: 10.1530/EJE-08-0797 [DOI] [PubMed] [Google Scholar]