Abstract
A gender difference in stroke is observed throughout epidemiologic studies, pathophysiology, treatment and outcomes. We investigated the neuroprotective role of hemeoxygenase (HO) enzyme, which catabolizes free heme to bilirubin, carbon monoxide and biliverdin in the female brain after permanent ischemia. We have previously reported in male mice that genetic deletion of HO1 exacerbates the brain damage after permanent ischemia, and the mechanism of neuroprotection is dependent on the HO1/Wnt pathway; however, the role of HO1/Wnt mediated neuroprotection in the female brain is yet to be investigated. We subjected ovary intact female mice, HO1−/− intact, HO1 inhibitor tin mesoporphyrin (SnMP) treated intact and/or ovariectomized female mice to permanent ischemia (pMCAO), and the animals were sacrificed after 7 days. The SnMP treatment for 7 days significantly reduced the HO1 enzyme activity as compared to that of vehicle treated group. Infarct volume analysis showed significantly lower infarct in intact, HO1−/− intact, and SnMP treated group as compared to the OVX group, suggesting the role of estrogen in neuroprotection. However, there were no differences in infarct volume observed between the intact, HO1−/− and SnMP treated group, suggesting a sexually dimorphic role of HO1 neuroprotection. Western blot analysis on intact and SnMP-treated groups subjected to pMCAO suggested no significant differences in Wnt expression. Together, these results suggest that HO1 neuroprotection is sexually dimorphic and Wnt expression is independently regulated in the female brain following permanent ischemia.
Keywords: Stroke dimorphism, hemeoxygenase 1, Wnt signaling, ischemic stroke, Tin meso porphyrin
Introduction
Stroke is the fifth leading causes of death and permanent disability across the United States. The incidence of stroke is highly dependent on age and sex, and pre-menopausal woman experience a lower stroke risk as compared to men (Sudlow and Warlow, 1997), but after hitting menopause, the incidence and severity of stroke increase in women and become predominant between the ages of 55 and 75 (Mozaffarian et al., 2015). Therefore, it is the aging female population that bears the brunt of reduced stroke-related recovery and institutionalization (Lai et al., 2005). Epidemiological studies suggest that the ovarian hormone, estrogen, is responsible for early neuroprotection in females (Amantea et al., 2005). However, the comparative study of male versus female tissue injury and neurologic deficits at the time of stroke is understudied. A decade of research has shown that stroke is a sexually dimorphic disease, and worldwide databases have consistently shown significantly lower incidence of stroke in females (Gibson, 2013; Manwani and McCullough, 2011) than in males. Stroke kills 16% of post-menopausal females as compared to 8% of males (Mozaffarian et al., 2015). The difference in stroke outcomes between males and females has been found to be largely dependent on reproductive hormones (McCullough et al., 2016).
Experimental stroke studies in rodents have highlighted the loss of neuroprotection in females after removal of the ovaries which was restored upon exogenous estrogen treatment, evidence that estrogen is important for neuroprotection (Dubal et al., 1998). In female animals, tolerance against ischemic brain injury by estrogen is attributed to maintaining and restoring cerebral blood flow, having anti-inflammatory effects and protecting cells from apoptosis (Alkayed et al., 2001; Park et al., 2006; Yang et al., 2005). Experimental data on rodents pertaining to stroke gender differences suggests that cell death after the brain injury may follow different routes depending on sex steroid exposure. The evidence of sexual dimorphism has also been reported by studying inducible nitric oxide synthase (iNOS) expression in male and female mice brains. The reduction in ischemic injury was observed in iNOS-null male mice but not in iNOS-null female mice (Park et al., 2006).
Heme oxygenase (HO), also known as heat shock protein 32, is a microsomal enzyme that consists of three different isoforms: 1) inducible hemeoxygenase 1 (HO1), 2) constitutive HO2 and 3) HO3 (Li et al., 2014). The function of HO1 is controlled by number of stimuli, such as the presence of heme, heavy metals, hormones, oxidative stress (Platt and Nath, 1998) and traumatic brain injury (Okubo et al., 2013). The first report on the presence of HO enzymes in liver microsomes was provided by Tenhunen and co-workers (Tenhunen et al., 1968). HO catalyzes the first and rate-limiting step in the oxidative degradation of heme (Fe-protoporphyrin- IX) to carbon monoxide (CO), ferrous ion (Fe+2), and biliverdin-IX (Stocker and Perrella, 2006). HO is not a heme protein but uses heme as both its active center and substrate. CO activates cGMP to induce vasodilation and also acts as a potent anti-inflammatory mediator, whereas biliverdin is converted to bilirubin by bilirubin reductase (Li et al., 2014; Platt and Nath, 1998), also as an antioxidant, and contributes to the protective role of HO1 in ischemia (Sharp et al., 2013). Apart from its role in heme catabolism, HO1 plays important role in various pathological states associated with cellular stress and possesses antioxidant, anti-atherogenic and neuroprotective properties (Platt and Nath, 1998).
In the mammalian genome, the Wnt family is characterized by highly conserved cysteine-rich glycoproteins (Janda et al., 2012). Emerging evidence suggests that Wnt signaling has an essential role in biological processes, including embryonic development and maintenance of stem cells (Yang et al., 2016). Several studies indicate that the cell proliferation, differentiation, migration and central nervous system development is driven by canonical Wnt signaling pathway (Valvezan and Klein, 2012). Moreover, Wnt signaling also plays an important role in the development of neuronal progenitor and stem cells into neurons in male mice (Kuwabara et al., 2009). A recent study showed that HO1 acts upstream of the canonical Wnt signaling cascade (Vanella et al., 2013). In our previous study, we also demonstrated that Wnt expression is modulated by up-regulation of HO1 protein expression, which in turn enhances neurogenesis in male mice (Nada et al., 2013).
There were no differences observed in the infarct volume between WT and HO1 knockout (HO1−/−) male mice in the transient middle cerebral artery occlusion (tMCAO) model (1h occlusion, 23 h reperfusion) (Dore et al., 1999). However, studies conducted in our lab (Shah et al., 2011) on male mice showed the delayed ischemic effect (7 days post ischemia) of HO1 in permanent distal middle cerebral artery occlusion (pMCAO) ischemia. We have previously demonstrated that the neuroprotective mechanism of natural product, Ginkgo biloba in ovariectomized (OVX) female mice is not dependent on the HO1/Wnt canonical pathway (Tulsulkar et al., 2016) To our knowledge, the role of HO1 in the female brain is yet to be elucidated. Therefore, in this study we hypothesize that the role of HO1 neuroprotection is not beneficial in the female brain after stroke, and that HO1/Wnt pathways are independently regulated in the female brain after pMCAO.
Results
Tin mesoporphyrin (SnMP) administration significantly abrogated HO1 enzyme activity in the female mouse brain
To establish the inhibitory effect of SnMP on HO1 activity, which was determined ex vivo by monitoring the conversion of bilirubin in tissue lysates. Brain HO activity was significantly attenuated in SnMP treated female mice (233 ± 57 vs. 55 ± 31 pg/mg/h; p < 0.05), demonstrating a reduction of approximately 75% (Fig 1.). This data suggests that 7 days of continuous administration of SnMP had a significant inhibitory effect on HO activity in vivo.
Figure 1. SnMP treatment attenuates HO activity in female mice brain.
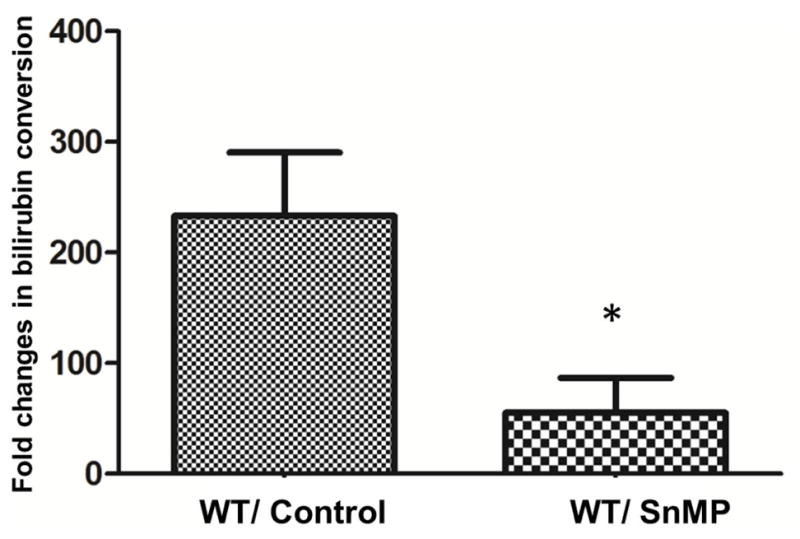
Vehicle animals were treated with Na3PO4, and the inhibitor group was treated with SnMP (5mg/Kg; i.p) prior to pMCAO and continued for 7 days and sacrificed on day 8. Data are expressed as mean ± SEM; * p<0.05, vs. WT/control n=3; SnMP intact n=3.
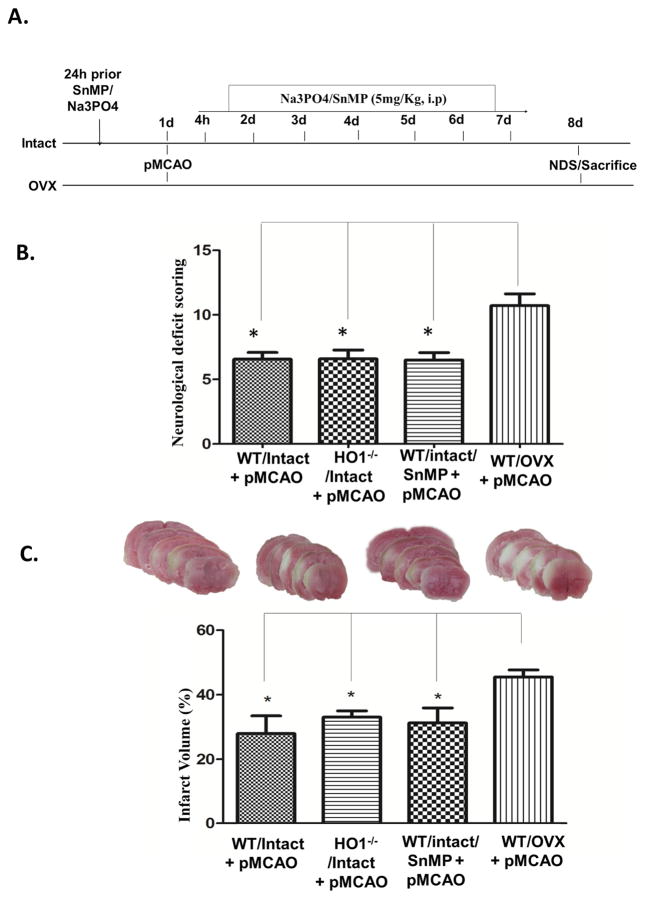
Inhibiting HO1 showed no significant differences in brain damage following pMCAO
Our lab has previously reported that HO1−/− male mice had increased infarct volume 7 days after pMCAO (Shah et al., 2011). Nevertheless, the role of HO1 in the female brain after ischemia is yet to be elucidated. Animals were treated with the HO activity inhibitor, SnMP, 24 hours prior to pMCAO and continued for 7 days and sacrificed on day 8 (Fig 2A). HO1−/−/Intact, WT/intact and WT/OVX animals also underwent pMCAO and were sacrificed on day 8. Neurological deficits were significantly lower in female animals with intact ovaries (HO1−/− and WT) as compared to those in the WT/OVX group. HO1−/− female animals with intact ovaries did not show any significant differences in neurological deficits as compared to WT/intact mice. To confirm these results, we further used SnMP, an HO activity inhibitor in female WT/intact mice, and then subjected them to pMCAO. SnMP treatment for 7 days did not show significant changes in neurological deficits between WT/intact and intact/HO1−/− females, but the WT/intact, HO1−/−/intact and WT/SnMP treated group showed significant reduction in neurological deficits when compared to WT/OVX animals (Fig 2B). Infarct volume analysis demonstrated that WT/intact, intact/HO1−/− and WT/SnMP group showed no significant differences after 7 days of pMCAO, but all groups were significantly lower than the WT/OVX group (Fig 2C). Together, these results suggest that HO1 neuroprotection is beneficial only in males and has no role in neuroprotection in the female brain after stroke.
Figure 2. Effect of HO1 inhibition in female brains after 7 days of pMCAO.
A) The schematic representation of the study design for inhibitor treatment. B) Neurological deficit scoring showed no significant differences between the groups except WT/OVX, which was significantly higher as compared to other groups after 7 days of ischemia. C) Infarct volume analysis at day 8 showed no differences between intact WT and HO1−/− and WT/SnMP-treated groups except in the WT/OVX group. Data are expressed as mean ± SEM; * p<0.05, vs. OVX/pMCAO; Intact n=6; OVX n=7, HO1−/− n= 6 and SnMP n=8.
HO1/Wnt pathway of neuroprotection is independently regulated in female brains after pMCAO
To further understand the effect of HO1 attenuation in the female brain after the 7 days of ischemia, brain tissues were isolated and Wnt protein expression levels were analyzed. The protein expression data shows that SnMP treatment does not affect Wnt expression in female brains after 7 days of pMCAO (Fig 3A). These results suggest that Wnt expression is independently regulated in the female brain.
Fig 3. Effect of SnMP treatment on Wnt expression after permanent ischemia.
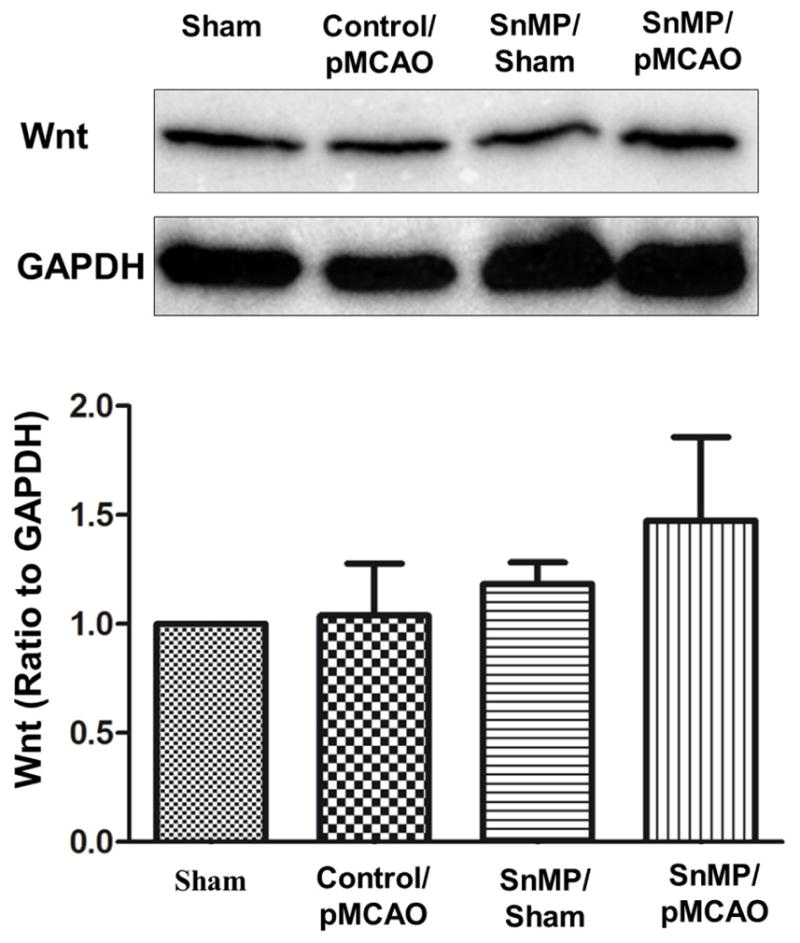
A) SnMp treatment for 7 days showed no significant differences between vehicle and SnMP-treated groups. B) The corresponding graph shows the densitometric analysis normalized to GAPDH. Data are expressed as mean ± SEM; Sham=3, vehicle/pMCAO n=3; SnMP sham n=4; and SnMP treated n=4
Discussion
In the present study, we demonstrate the dimorphic role of the HO1/Wnt pathway in the female brain after permanent ischemia, which may correspond to gender related differences during stroke. Treatment with the HO1 inhibitor, SnMP, significantly attenuated HO1 enzyme activity. Surprisingly, HO1−/− female mice and SnMP-treated groups showed no significant differences in neurological deficits when compared to the WT/Intact animals, but all of the groups had lower neurological deficits as compared to the OVX group. Furthermore, upon analyzing the brain infarct volume of the intact/HO1−/− and intact/SnMP treated groups, there was no significant difference in brain damage as compared to the WT/intact group, but the WT/OVX group showed significantly higher brain damage. Wnt expression also showed no differences between SnMP and WT/intact animals, suggesting that the expression of Wnt is independent of HO1 expression in the female brain after ischemia. Taken together, these results suggest that the HO1/Wnt pathway is independently regulated in the female mouse brain during stroke.
Degradation of the pro-oxidant free heme by HO results in iron, CO and biliverdin, which is further reduced to bilirubin. It has been previously shown that CO possesses a vasodilatory effect and that biliverdin and bilirubin act as antioxidants. It has also been reported that HO1−/− and WT male mice show no differences in brain infarct volume in the tMCAO reperfusion model (Dore et al., 1999) of 1 h occlusion and 23 hr reperfusion whereas in pMCAO model it was demonstrated that after 7 days of ischemia, HO1−/− male animals had significantly higher infarct volume as compared to WT male animals, suggesting a neuroprotective role of HO1(Shah et al., 2011). HO1 neuroprotective properties were also observed in NMDA-induced neurotoxicity model (Saleem et al., 2010) and after ischemic preconditioning, where male HO1−/− mice did not shown any protection and suffered from higher brain damage as compared to WT after 48 hrs of permanent ischemia (Zeynalov et al., 2009). We recently reported that HO1−/− male mice showed lower neurogenesis as compared to WT animals following permanent ischemia (Nada et al., 2014). On the other hand, HO1 exacerbates stroke damage in male mice after hemorrhagic stroke (Wang and Dore, 2007).
So far, HO1 has been studied in various models, and its role in ischemic and remote organ preconditioning (Lai et al., 2006) has already been established. Cell specificity, sex, origin and nature of the damage are the possible reasons for the variability in the HO1 function and signaling mechanisms. Interestingly, in a recent study, HO1 was shown to be protective in a model of myocardial infarction (Posa et al., 2013) in female rat hearts. Another important functional aspect of neuroprotective molecules in stroke is the biphasic role, which explicitly differentiates the roles of molecules in the injury and the recovery phases of stroke. In a recent study, it was noted that HO1 has a biphasic role after interacerebral hemorrhagic injury (Zhang et al., 2017). In ischemic brain injury, we have not seen the biphasic role of HO1, as most of our studies focused on 7-day survival after ischemia. In this study, we investigated the role of HO1 in the female brain after 7 days of permanent ischemia and, to our knowledge, this is the first study showing the dimorphic role of HO1 in stroke. The use of TTC stain for infarct volume staining and analysis after 7 days of ischemia is not considered the better approach. However, in all our previous studies involving 7 days survival, TTC staining has provided consistent results when supplemented with proper controls (Nada et al., 2012; Nada et al., 2013; Raghavan and Shah, 2014; Shah et al., 2011). In fact, TTC staining was found to be accurate for up to 7 days of ischemia when compared with Fluoro-Jade B and Neu N by another group (Liu et al., 2009). On treatment with SnMP, the conversion of bilirubin was significantly reduced as compared to the vehicle group, suggesting the complete inhibition of HO activity. Furthermore, intact/HO1−/− female animals showed no significant differences in brain damage as compared to the WT/intact female after 7 days of permanent ischemia. These results were confirmed by using SnMP, and infarct volume analysis demonstrated no significant differences between WT/Intact and HO1−/−/intact female mice. Taken together, our results suggest that HO1 is beneficial only in males and has no role in female neuroprotection following stroke. There are concerns about the off-target effects of the SnMP, which effect mostly iron metabolism, as it is the potent inhibitor of hepatic and microsomal HO activity and results in increased heme concentrations (Laftah et al., 2003). Increased heme concentrations are deleterious in hemorrhagic brain injury (Wang and Dore, 2007), but we did not observe these effects in ischemia.
As per our results, there is a clear association of estrogen and HO1, as the protective effect was not observed in OVX mice subjected to permanent ischemia. This was also in consonance with our previously published study, where OVX abrogated the HO1 expression in female mice brains, thus suggesting the association of HO1 and estrogen. Our finding can also draw credence from the evidence of increased HO1 expression and activity in the aorta and left ventricle of female rats (Posa et al., 2013) and elevated expression and activity of HO enzyme system observed to be induced by the circulating level of estradiol in the proestrus phase of the female rat estrous cycle. To understand the underlying mechanism of HO1 and estrogen association, further studies are required to validate this association.
Wnt proteins are extrinsic proteins that play an important role in the developed and matured nervous system. The proliferation of neural progenitor cells and differentiation of neurons in the sub ventricular zones (SVZ) is regulated by Wnt signaling (Inestrosa and Arenas, 2010). Likewise, the Wnt canonical signaling pathway is an important component of neural progenitor cell transformation into neurons. (Kuwabara et al., 2009). The role of HO1 acting upstream of the Wnt canonical pathway has been previously established by Vanella et al. in regard to adipocytes (Vanella et al., 2013). On the other hand, we demonstrated the connection of the HO1/Wnt canonical pathway in the brain after permanent ischemia (Nada et al., 2014) and overexpression of the HO1/Wnt pathway with natural product enhanced neuroprotection. However, of all these studies were performed in either a cell culture environment or in male rodents. Here, we have demonstrated that SnMP treatment for 7 days after permanent ischemia showed no significant changes in Wnt expression as compared to the control group, which suggests that HO1 and Wnt are independently regulated in female brains in neuropathological conditions like ischemia.
In conclusion, it was observed that the HO1/Wnt canonical pathway is beneficial only in males and has no role in female neuroprotection after ischemia. It was also observed that the upregulation of HO1 is estrogen dependent.
Materials and Methods
Animals and treatment
All animal protocols were approved by the University of Toledo Health Science Campus Institutional Animal Care and Utilization Committee, and the guidelines of the National Institutes of Health were followed throughout. Female C57BL/6 mice, 8–10 weeks old and 20–25 grams were procured from the in-house facility and were housed at 22 ± 1°C with a 12 h: 12 h light/dark cycles. pMCAO was carried out in four groups; group 1: WT/intact; group 2: HO1−/−/intact; group 3: WT/intact/SnMP and group 4:WT/OVX. All animals were randomized and distributed among different treatment groups.
HO1 Knockout Mice
Heterozygous HO1 (HO+/−) mice were obtained from Dr. Anupam Agarwal, University of Alabama (initially from the colony of Dr. Susumu Tonegawa, MIT). Knockout mice were produced by breeding male chimeras with C57BL/6 females followed by intercrossing of HO1+/− animals, which resulted in HO1−/− off springs. Mice genotypes were confirmed by PCR analysis of tail DNA (Chen et al., 2004) using primers for wild type (forward primer: 5′ GGTGACAGAAGAGGCTAAG 3′ and reverse primer: 5′ CTGTAACTCCACCTCCAAC 3′) and mutant (forward primer: 5′TCTTGACGA GTTCTTCTGAG 3′ and reverse primer: 5′ ACGAAGTGA CGCCATCTGT 3′) genotypes, which generated 450 and 350 BP, respectively.
Tin mesoporphyrin (SnMP) treatment
Animals were divided into control and SnMP-treated groups. The control group was treated with 0.4M Na3PO4 (pH 7.6) alone, whereas the SnMP group received 5 mg/Kg of SnMP dissolved in 0.4M Na3PO4 (pH 7.6) by intraperitoneal injections, 24 h before surgery and continued for 7 days.
Ovariectomy
One group of mice underwent bilateral OVX only. The animals were anesthetized initially under 5% isoflurane followed by 1.5% throughout the surgical procedure (Idris, 2012). A single incision of 5.5–10 mm was made on the right and left side, approximately 1/3 of the distance between the spinal cord and the ventral midline and muscle wall was exposed. The ovary and the oviduct were located through the muscle wall, and a hemostat was clamped around the uterine vasculature between the oviduct and the uterus. Each ovary and part of the oviduct was surgically removed with a single cut through the oviducts near the ovary. The hemostat was detached, and the remaining tissue was placed back into the peritoneal cavity. The ovary on the other side was removed in a similar fashion. All the animals were allowed to recover for 7 days following OVX before being subjected to pMCAO.
Permanent middle cerebral artery occlusion (pMCAO)
We used our previously optimized protocol for pMCAO model for this study (Nada et al., 2012; Nada et al., 2014). The animal surgeries were performed under absolute sterile conditions. Animals were anesthetized initially with 5% isoflurane and then continued at 1–2% for rest of the surgery. The core body temperature was maintained around 37° C ± 0.5 by using rectal thermometer connected to the heating blanket. Under the microscope, a vertical skin incision of approximately 1 cm was made between the right eye and ear, and the temporal muscle was detached to expose the underlying skull. A craniotomy was made with the use of a dental drill of about 2.0mm above the visible MCA region, and the dura was carefully removed from the distal part of the MCA. The part of the exposed MCA was carefully severed using a bipolar coagulator to establish the complete blockade of the artery. After the surgery, animals were moved to a temperature-controlled incubator and left to recover before shifting to their home cages.
Infarction volume analysis
Animals were euthanized after 7 days of pMCAO and brains were dissected out. The dissected brains were sliced into five 2-mm thick coronal sections and incubated in the solution containing 1% triphenyltetrazolium chloride (TTC, Sigma Co) and normal saline for 20 min at 37°C. The brain sections were fixed in 4% formaldehyde solution and used to perform infarct volume analysis using Image J software (NIH). The infarct volume was calculated from five coronal slices of each mouse brain. The calculations included measurement of infarct volume from rostral and caudal sides of each slice in combination with the thickness, and the final calculation was expressed as a percentage of the contralateral side. A person blinded to the treatment groups measured the infarct volume.
Neurological deficit scoring (NDS)
NDS was assessed by a 28-point score pattern (Nada et al., 2012) after 7 days of pMCAO by an investigator blinded to the experimental plan; the evaluation is a combination of sensory and motor deficit characteristics such as body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Deficits were rounded up to 28-point scale, with each being graded from 0 to 4 (4 indicating severe deficits and 0 indicating no deficits).
HO1 activity Assay
Determination of total HO activity in brain tissues was performed as described previously (Vera et al., 2007). The brain tissues were homogenized in radio-immunoprecipitation assay (RIPA) buffer, and 0.5mg of protein was mixed with 2mM glucose-6-phosphate, 0.2U of glucose-6-phosphatedehydrogenase, 0.8mM of nicotinamide-adenine dinucleotide phosphate, and 20.0μM hemin in a total reaction volume of 1200μl. The samples were incubated at 37 °C for 1 hour. The bilirubin produced in that time was chloroform extracted, and its concentration was determined by change in optical density at 464nm to 530nm with an extinction coefficient of 40mM/cm. Activity was expressed as picomoles of bilirubin per milligram of protein per hour. A total of three animals were tested from each group.
Western blotting
For Western blots, mice were sacrificed 7 days after ischemia and brain cortices of ischemic and non-ischemic mice were dissected out, weighed, and homogenized. The brain tissue was homogenized to extract cytoplasmic and nuclear proteins using a buffer containing 10mM HEPES-KOH pH 7.9, 1.5mM MgCl2, 10mM KCl and 1mM EDTA. Prior to extraction, freshly prepared 0.5 mM DTT and 0.2mM PMSF, along with phosphatase and protease inhibitors, was added to the buffer solution. The cytoplasmic protein was isolated by homogenizing the brain in buffer A and centrifuging at 5000 rpm for 10 mins. The supernatant was collected as the cytoplasmic fraction, and the pellet was treated with buffer B. The nuclear fraction was isolated by centrifuging the suspension again at 10,000 rpm for 10 mins. Both the fractions were stored at −20 °C for later use. Bradford reagent (Bio-Rad laboratories) was used to determine protein concentration, and samples were analyzed by loading equivalent amounts of cytoplasmic/nuclear proteins (20 μg) on 12% SDS polyacrylamide-gel. After separation, proteins were transferred from the gel to PVDF membrane and blocked by 5% dry nonfat milk for 1 h at RT followed by overnight incubation at 4 °C with primary antibodies: mouse anti-GAPDH (1:2000; Santacruz, TX); mouse anti-HO1 (1:1000; Santacruz) and anti rabbit Wnt (1:1000, Abcam, MA). The band pictures were captured and analyzed using the ChemiDoc molecular imager (Bio-Rad). The densitometric values were normalized with the values of loading control (GAPDH) to correct for any loading and transfer differences between samples.
Statistical Analysis
The behavioral parameters and infarct volumes between different groups were evaluated by one-way ANOVA followed by Newman–Keuls post hoc test. For the protein analysis, the differences between the groups were determined by Student’s t test, and a value of p< 0.05 was considered to be significant.
Female HO1 KO and WT mice showed no differences in the infarct volume.
Ovariectomized WT mice suffered from higher infarct volume compared to HO1 KO and WT.
HO1 neuroprotection is sexually dimorphic during ischemic injury.
Wnt expression is independently regulated in female brain following permanent ischemia.
Acknowledgments
Funding: This study was partly funded by the NIH-NCCAM grant R00AT004197 to ZAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21:7543–50. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–32. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Chen S, Sega M, Agarwal A. “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. Biotechniques. 2004;37:84–6. 88–9. doi: 10.2144/04371ST05. [DOI] [PubMed] [Google Scholar]
- Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med. 1999;5:656–63. [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Gibson CL. Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab. 2013;33:1355–61. doi: 10.1038/jcbfm.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AI. Ovariectomy/orchidectomy in rodents. Methods Mol Biol. 2012;816:545–51. doi: 10.1007/978-1-61779-415-5_34. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laftah AH, Raja K, Simpson RJ, Peters TJ. Effect of Tin-mesoporphyrin, an inhibitor of haem catabolism, on intestinal iron absorption. Br J Haematol. 2003;122:298–304. doi: 10.1046/j.1365-2141.2003.04376.x. [DOI] [PubMed] [Google Scholar]
- Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–7. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- Lai SM, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis. 2005;2:A13. [PMC free article] [PubMed] [Google Scholar]
- Li X, Song G, Jin Y, Liu H, Li C, Han C, Ren S. Higher level of heme oxygenase-1 in patients with stroke than TIA. J Thorac Dis. 2014;6:772–7. doi: 10.3978/j.issn.2072-1439.2014.06.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond) 2011;7:319–39. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 2016;8:1432–41. doi: 10.18632/aging.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nada SE, Tulsulkar J, Raghavan A, Hensley K, Shah ZA. A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem Int. 2012;61:1357–63. doi: 10.1016/j.neuint.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada SE, Tulsulkar J, Shah ZA. Heme Oxygenase 1-Mediated Neurogenesis Is Enhanced by Ginkgo biloba (EGb 761(R)) After Permanent Ischemic Stroke in Mice. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761(R)) after permanent ischemic stroke in mice. Mol Neurobiol. 2014;49:945–56. doi: 10.1007/s12035-013-8572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S, Xi G, Keep RF, Muraszko KM, Hua Y. Cerebral hemorrhage, brain edema, and heme oxygenase-1 expression after experimental traumatic brain injury. Acta Neurochir Suppl. 2013;118:83–7. doi: 10.1007/978-3-7091-1434-6_14. [DOI] [PubMed] [Google Scholar]
- Park EM, Cho S, Frys KA, Glickstein SB, Zhou P, Anrather J, Ross ME, Iadecola C. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26:392–401. doi: 10.1038/sj.jcbfm.9600194. [DOI] [PubMed] [Google Scholar]
- Platt JL, Nath KA. Heme oxygenase: protective gene or Trojan horse. Nat Med. 1998;4:1364–5. doi: 10.1038/3947. [DOI] [PubMed] [Google Scholar]
- Posa A, Kupai K, Menesi R, Szalai Z, Szabo R, Pinter Z, Palfi G, Gyongyosi M, Berko A, Pavo I, Varga C. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid Med Cell Longev. 2013;2013:521563. doi: 10.1155/2013/521563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan A, Shah ZA. Withania somnifera Improves Ischemic Stroke Outcomes by Attenuating PARP1-AIF-Mediated Caspase-Independent Apoptosis. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8907-2. [DOI] [PubMed] [Google Scholar]
- Saleem S, Shah ZA, Maruyama T, Narumiya S, Dore S. Neuroprotective properties of prostaglandin I2 IP receptor in focal cerebral ischemia. Neuroscience. 2010;170:317–23. doi: 10.1016/j.neuroscience.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–55. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4:685–92. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–89. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsulkar J, Glueck B, Hinds TD, Jr, Shah ZA. Ginkgo biloba Extract Prevents Female Mice from Ischemic Brain Damage and the Mechanism Is Independent of the HO1/Wnt Pathway. Transl Stroke Res. 2016;7:120–31. doi: 10.1007/s12975-015-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvezan AJ, Klein PS. GSK-3 and Wnt Signaling in Neurogenesis and Bipolar Disorder. Front Mol Neurosci. 2012;5:1. doi: 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther. 2013;4:28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1472–8. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Wang X, Zhang H, Wang Z, Nan G, Li Y, Zhang F, Mohammed MK, Haydon RC, Luu HH, Bi Y, He TC. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest. 2016;96:116–36. doi: 10.1038/labinvest.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wang X, Simpkins JW. Estrogens as protectants of the neurovascular unit against ischemic stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:169–77. doi: 10.2174/1568007053544174. [DOI] [PubMed] [Google Scholar]
- Zeynalov E, Shah ZA, Li RC, Dore S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis. 2009;35:264–9. doi: 10.1016/j.nbd.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhang Z, Li D, Zhu H, Liang R, Gu Y, Pang Y, Qi J, Wu H, Wang J. Distinct role of heme oxygenase-1 in early- and late-stage intracerebral hemorrhage in 12-month-old mice. J Cereb Blood Flow Metab. 2017;37:25–38. doi: 10.1177/0271678X16655814. [DOI] [PMC free article] [PubMed] [Google Scholar]