Abstract
Background:
The use of anticoagulants or antiplatelet medications has become increasingly common and is a well-established risk factor for worsening of hemorrhages in trauma patients. The current study addresses the need to investigate the efficacy of point-of-care tests (POC) as an adjunct to conventional coagulation testing in traumatic brain injury (TBI) patients.
Methods:
A retrospective review of 190 TBI patients >18 years of age who underwent both conventional and POC testing as part of their admission coagulopathy workup was conducted. Coagulation deficiency was defined as an international normalized ratio (INR) >1.4, a reaction time (r-value) on rapid thromboelastography >50 seconds, or a VerifyNow Aspirin (VN-ASA) level of < 550 Aspirin Reaction Units.
Results:
Among 190 patients, 91 (48%) disclosed a history of either warfarin or antiplatelet use or had documented INR >1.4. Of the 18 (9%) patients who reported warfarin use, 83% had elevated INR and 61% had elevated r-value. However, 41% of the patients without reported anticoagulant usage revealed significantly elevated r-value consistent with a post-traumatic hypocoagulable state. Of 64 (34%) patients who reported taking ASA, 51 (80%) demonstrated therapeutic VN-ASA. Interestingly, 31 of 126 (25%) patients not reporting ASA use were also noted to have therapeutic VN-ASA suggestive of platelet dysfunction.
Conclusions:
The coagulopathy POC panel consisting of r-TEG and VN-ASA successfully identified a subset of TBI patients with an occult coagulopathy that would have otherwise been missed. Standardization of these POC assays on admission in TBI may help guide patient resuscitation in the acute setting.
Keywords: Aspirin, coagulopathy, mild traumatic brain injury, thromboelastography, VerifyNow
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death and disability that affects approximately 1.7 million Americans annually.[22] Although most of these injuries are characterized as mild,[28] TBI accounts for an estimated 50,000 fatalities and 235,000 inpatient hospitalizations per year.[26] In these patients, the development of trauma-induced coagulopathy is recognized as a potential contributor to secondary brain injury and, consequently, to poorer clinical outcomes.[12,21,32] Similarly, the increasingly common use of anticoagulants or antiplatelet medications is a well-established risk factor for worsening of hemorrhages in trauma patients.[2,15] Thus, whether pharmacologically or traumatically induced, there is significant clinical benefit to rapidly identify and correct coagulation dysfunction in at risk patients.
Following TBI, evaluation of patients in the emergency department commonly involves the assessment of standard coagulation parameters, specifically the prothrombin time (PT), partial thromboplastin time (PTT), and INR.[30] In the past few years, however, increasing emphasis has been placed on the use of viscoelastic testing and various point-of-care (POC) laboratory tests to detect coagulation abnormalities in trauma patients. Compared with conventional studies, thromboelastography (TEG), in particular, has demonstrated utility in guiding resuscitation in trauma and surgical populations because it provides a more rapid, comprehensive assessment of a patient's coagulation status.[7,18,30,33] In fact, data from the rapid TEG (rTEG), a modification of TEG whereby tissue factor is used to accelerate clotting, may be available within 5 minutes and has been shown to correlate with conventional coagulation tests in general trauma patients.[7] In TBI, hypocoagulability as suggested by TEG or rotational thromboelastometry was associated with increased mortality, higher likelihood of neurologic decline at 24 hours, and higher rates of neurosurgical intervention.[20,29,34] Moreover, the TEG reaction time (r-value), a measure of the time to clot formation, was also associated with longer hospital and ICU admissions.[20]
A primary limitation of TEG, in its standard or rapid form, is its poor sensitivity to platelet inhibition induced by antiplatelet medications, including aspirin.[8,30] Given that antiplatelet drugs contribute to patient morbidity and mortality after TBI,[3] multiple POC platelet function tests have been developed to identify pharmacologically-induced coagulopathies. One of these, the VerifyNow Aspirin (VN-ASA) test (Accumetrics, San Diego, CA), has been well validated for use in patients with aspirin-induced platelet dysfunction and achieves a specificity of 96%.[4,25,27] In this rapid cartridge-based test, whole blood is used to measure platelet aggregation with fibrinogen-coated beads and a drug-specific agonist.[25] In a small cohort of 84 TBI patients, VN-ASA testing to objectively identify aspirin use revealed not only a population of aspirin-nonresponders but a population with platelet dysfunction who did not report aspirin use.[1] Using VN-ASA data to guide corrective platelet transfusions, the authors subsequently demonstrate a trend toward increased mortality in patients whose platelet function failed to normalize with transfusions.[1]
POC coagulation tests are increasingly used in the emergency department as an adjunct to standard coagulation testing. However, their role in the acute management of TBI and, specifically in identifying patients with trauma or pharmacologically-induced coagulopathy, requires further investigation. In this study, we evaluated the results of both conventional coagulation laboratory tests and a POC coagulopathy panel consisting of r-TEG and VerifyNow (VN-ASA) in TBI patients at the time of presentation to the emergency department. We hypothesized that these rapid POC tests would prove effective as a primary screening tool for coagulopathy in TBI patients on admission.
MATERIALS AND METHODS
Patient selection
The study was completed retrospectively at the University of Cincinnati Medical Center (UCMC), a primary teaching hospital and level 1 trauma center, and was correspondingly approved by the Institutional Review Board. Using the UCMC trauma registry, we first identified 259 adult patients (≥18 years of age) who sustained a TBI during a 2-year period from December 2012 through December 2014. TBI was defined as any intracranial hemorrhage associated temporally with a cranial traumatic force and identified on the initial noncontrast computed tomography (CT) scan performed at presentation. Subsequently, we included 190 patients who had undergone standard and POC coagulation testing, including platelet count, PT/INR, PTT, r-TEG, and VN-ASA. We excluded 69 patients who had incomplete laboratory coagulation profiles. Data obtained included relevant patient demographic characteristics (e.g., age, sex), mechanism of injury, presenting Glasgow Coma Scale (GCS) score, concurrent alcohol use, loss of consciousness at the time of injury, associated extracranial injuries, admission status, and active anticoagulant or antiplatelet medication use. Patients who reported the use of anticoagulants other than warfarin were excluded. In addition, the type and location of intracranial hemorrhage was recorded for each patient after review of the presenting noncontrast head CT.
Coagulation testing
Standard coagulation testing was performed in all 190 patients and included a platelet count, PT, PTT, and INR. POC testing was also universally performed in these patients as part of the primary trauma survey, and consisted of both r-TEG and VN-ASA. Coagulopathy was diagnosed if any of the following criteria were met: (1) platelet count ≤100,000, (2) INR ≥1.4, (3) r-value ≥50 seconds on r-TEG, (4) therapeutic VerifyNow Aspirin (VN-ASA) level of ≤550 ARU, or (5) maximum amplitude ≤52 mm on rTEG. The maximum amplitude reflects the maximal clot-shear elasticity, a measure of clot strength, and is primarily dependent of platelet function.[31] Of note, an INR cutoff of ≥ 1.4 was selected because this number is often cited as the target INR for reversal of anticoagulation.[9,14,24] Moreover, this INR cutoff represents the threshold value deemed safe to proceed with neurosurgical procedures at our institution.
Statistical analyses
SAS® version 9.3 (SAS Institute, Cary, NC) was used for analysis. Continuous variables, patient age, and GCS were reported as median and range; categorical variables as number and percentage. Kruskal–Wallis test was used to identify differences between the three TBI groups for age. Chi square or Fisher's exact test identified differences between the groups for the categorical variables. When the overall test was significant, differences between specific groups were tested, and the P- value was adjusted using a Bonferroni adjustment.
RESULTS
During the 2-year study period, 190 patients met entry criteria for inclusion in the study, having undergone both conventional coagulation laboratory testing and a rapid POC coagulopathy panel comprised r-TEG and VN-ASA. For these 118 men and 72 women, 64 (34%) reported aspirin use and 18 (9%) reported anticoagulant use. However, using POC panel testing, we identified evidence of coagulopathy in 128 (67%) post-TBI patients as defined by an elevated rTEG r-value or therapeutic VN-ASA. In contrast, the traditional combination of an abnormally elevated INR and a detailed medication history identified 90 (47%) patients at risk for coagulopathy.
Patient demographics and risk of intracranial hemorrhage progression
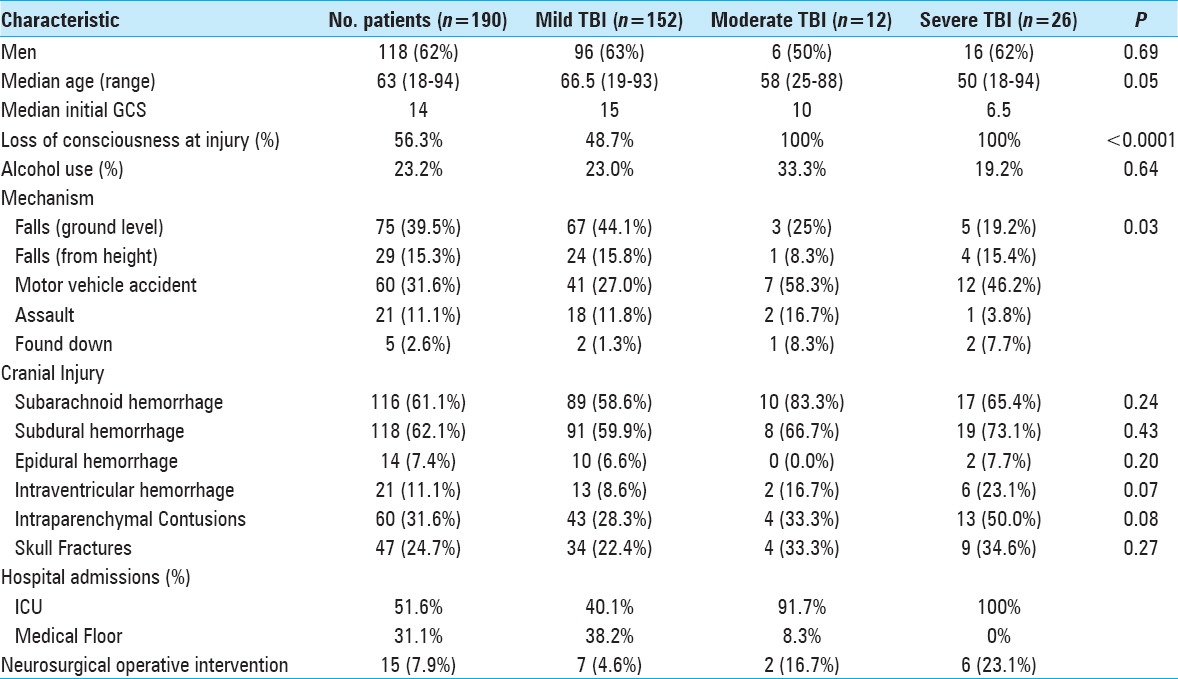
The 190 patients included in the study had a median (25th, 75th percentiles) age of 63 (45, 76) years [Table 1]. While the median GCS at presentation was 14, 38 (20%) patients suffered a moderate or severe TBI and 107 (56.3%) reported a loss of consciousness at the time of injury. Injuries resulted from a fall (55%), motor vehicle accident (32%), physical assault (11%), or were found down (3%). Severe TBI patients were more likely to be younger (P = 0.05) and experience a motor vehicle collision than patients with mild TBI (P < 0.05). Both moderate and severe TBI patients were more likely to have an associated loss of consciousness (P < 0.0001) than patients with mild TBI. Notably, there was no association between the mechanism of injury and the likelihood of subsequent coagulopathy as defined by an elevated INR (P = 0.38) or prolonged rTEG r-time (P = 0.70). However, when the mechanism was a fall from height, injured patients were significantly more likely to demonstrate radiographic progression of their intracranial hemorrhages (OR: 1.65; 95% CI: 1.01–2.71).
Table 1.
Patient demographics from retrospective review of 190 TBI patients >18 years of age who underwent both conventional and POC testing as part of their admission coagulopathy workup
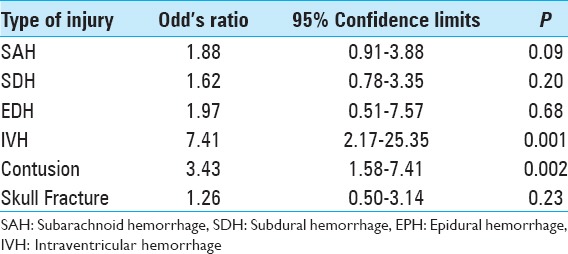
The most common intracranial findings identified on initial imaging of brain-injured patients were subdural hemorrhages (SDHs) and subarachnoid hemorrhages (SAHs). Regardless of the severity of TBI, these patterns of intracranial injuries seen in more than 60% of the 190 study patients did not predict elevated risk of intracranial hemorrhage progression. Conversely, patients with intraventricular hemorrhage (OR: 7.41, 95% CI: 2.16–25.35, P = 0.001) or intraparenchymal contusions (OR: 3.43, 95% CI: 1.58–7.40, P = 0.002) were significantly more likely to worsen radiographically on subsequent intracranial imaging [Table 2]. Interval progression of the intracranial hemorrhage was seen in 38.5% of patients who underwent a repeat head CT. Neurosurgical intervention, in the form of intracranial pressure monitoring or craniotomy, was indicated in 15 (7.9%) patients [Table 1]. Of these, 3 patients underwent craniotomy (1.6%); patients with mild TBI were significantly less likely to undergo surgical intervention (P = 0.004).
Table 2.
Pattern of intracranial hemorrhage and the risk of radiographic progression
Warfarin and aspirin use
Exposure to the oral anticoagulant warfarin before injury was documented in 9% of the study population. Of these, 83% of patients had an elevated INR above 1.4 [Figure 1], which was consistent with an expected pharmacologically-induced coagulopathy (sensitivity 0.83; specificity 0.90; NPV 0.98). Similarly, predicted prolongation of the rTEG R-time above 50 seconds was seen in 61% of patients reporting warfarin use (sensitivity 0.61; specificity 0.59; NPV 0.94). However, for patients without known anticoagulant use, rTEG R-value elevation occurred in 41% whereas increased INR was observed in only 10% (P < 0.0001). In this subset without pharmacologically-induced coagulopathy, a prolonged rTEG R-value was seen in 43.3% of the patients with documented progression of intracranial hemorrhage on follow-up imaging, while an elevated INR was observed in 8.9% (P = 0.001). Finally, neither an elevated INR (12.1%, P = 0.30) nor elevated rTEG R-value (11.0%, P = 0.19) significantly increased the risk of requiring a neurosurgical operative intervention.
Figure 1.
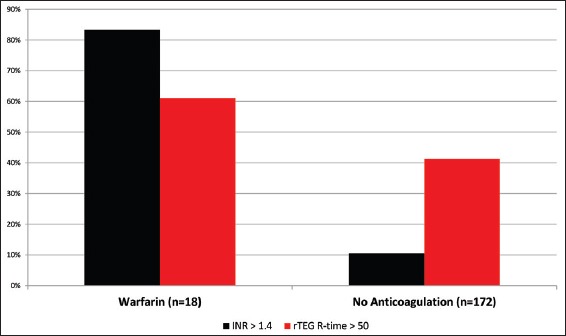
Percent accuracy of rapid TEG vs INR in the evaluation of mild TBI patients with and without warfarin therapy
In our 190 TBI patients, pharmacologically-induced coagulopathy in the form of aspirin-induced platelet dysfunction was evaluated. The 64 (34%) patients who reported active aspirin use were older (P < 0.0001), more likely to have fallen (P < 0.0001) as a mechanism of injury, and less often presented with alcohol intoxication (P = 0.001) [Table 3]. POC laboratory testing in aspirin-treated patients revealed therapeutic VN-ASA in 79.7% of patients, while only 7.9% were noted to have an abnormally reduced MA on rTEG suggestive of platelet dysfunction. Remarkably, VN-ASA was also noted to be therapeutic in nearly one-third of patients who reported no aspirin use [Figure 2].
Table 3.
Patient demographics by with (+) or without (−) warfarin or aspirin user
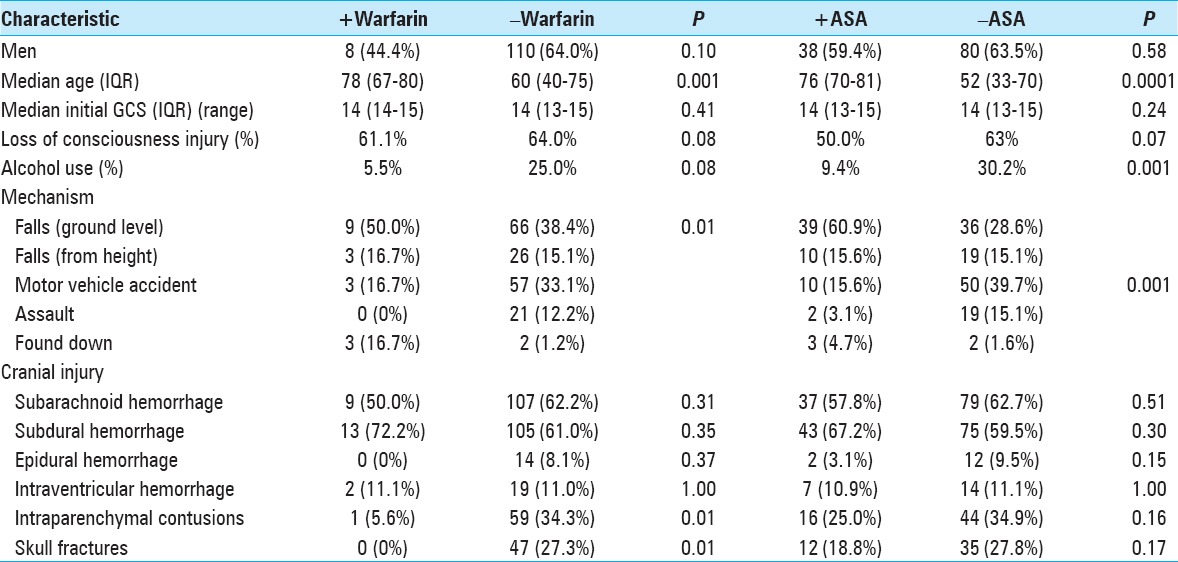
Figure 2.
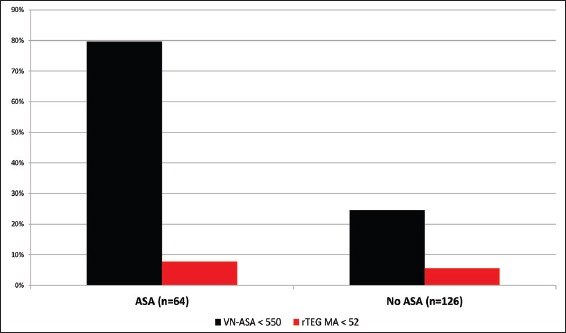
Percent accuracy of VN-ASA and rapid TEG in the identification of ASA-induced platelet dysfunction
DISCUSSION
Our study evaluating a panel of commercially available POC tests as adjuncts to conventional laboratory coagulation testing in 190 TBI patients highlights the importance of rapidly assessing for the presence of a coagulopathy. The information obtained via the r-TEG and VN-ASA may play a critical role in regulating the progression of intracranial hemorrhages, thus limiting the impact on patient morbidity and mortality. Given that 36% of American adults are prescribed aspirin to prevent cardiovascular disease[13] and additional millions are on chronic oral anticoagulation,[15] it is not surprising that 41% of our patients disclosed a history of either warfarin or aspirin use. By including results from conventional coagulation testing, we identified an additional 7% without history of anticoagulant use but in whom the INR was elevated above threshold. Notably, in POC panel testing, we found 67% of patients were coagulopathic based only on elevated rTEG R-value or therapeutic VN-ASA. The discrepancy in the rate of coagulopathy may reflect a portion of the estimated 25–45% of patients who experience trauma-induced dysfunction in coagulation[6,18,19] or partly derive from patients unable to disclose their use of coagulopathy-inducing medications.
Coagulation assessment and hemorrhage progression
INR has long been used in the acute evaluation of coagulopathy in trauma patients and for routine monitoring of patients receiving long-term antithrombotic therapy. Recently, several reports have suggested TEG-guided resuscitation may be superior to standard coagulation laboratory parameters in the trauma population, primarily because of the capacity for TEG to measure multiple aspects of clot formation rapidly in whole blood.[7,18,33] Although the rTEG R-value was prolonged in a majority of our patients who reported anticoagulant use, it remained inferior to the INR in both sensitivity and specificity in our study. In fact, our findings mirror the report by Dunham et al.[10] who suggested a 40.9% false negative rate for rTEG in detecting warfarin-induced coagulopathy. The authors proposed that the use of kaolin, an activator of the intrinsic coagulation cascade, in rTEG inconsistently measures extrinsic pathway dysfunction mitigated by vitamin K antagonists. Given the clinical implications of failing to identify a pharmacologically-induced hypocoagulable state in the context of TBI, we cannot recommend that rTEG replace INR in the acute evaluation of this at risk population. However, it is worth noting that, in patients without known warfarin use, the rTEG R-value was extremely sensitive in detecting coagulopathy, and ultimately correlated with increased risk of intracranial hemorrhage progression. The fact that this mild but clinically significant coagulopathy was largely undetected by INR supports the use of both conventional coagulation testing and the rTEG in the examination of post-traumatic coagulopathy.
Detection of aspirin-induced platelet dysfunction has historically depended on patient reporting because of the insensitivity of current conventional coagulation tests. In our study, we demonstrated the failure of the rTEG MA-value to detect aspirin-specific platelet inhibition. This phenomenon, which was previously reported,[16,17] spurred the development of novel independent assays with the capacity to quantify aspirin-associated effects. One such assay employed herein, the VN-ASA, successfully identified nearly 80% of patients who reported using aspirin therapy. These data are consistent with published reports that suggest the existence in approximately 25% of patients of aspirin-resistance, namely a state of hyporesponsiveness in which a functional proportion of platelet aggregation is maintained.[5,13] The VN-ASA test additionally identified a subset of patients (24.6%) with no defined history of aspirin use who were therapeutic on the laboratory test. Given the extremely high reported specificity of VN-ASA,[4,25,27] these patients likely reflect an at-risk population who either unknowingly ingested aspirin-containing medications or suffered an injury that prevented them from disclosing use. Nevertheless, the routine inclusion of VN-ASA in the acute evaluation of TBI patients would allow for correction of platelet dysfunction and prevent unnecessary platelet transfusions in aspirin nonresponders.
Study limitations
The retrospective study design and the inclusion of a wide array of intracranial injuries that likely differed in both pathophysiology and risk of traumatic coagulopathy represent our primary study limitations. Similarly, associated non-neurologic injuries may have played a role in promoting a hypocoagulable state. Future studies focused on the POC assessment of additional anticoagulant and antiplatelet agents must be undertaken to provide a more complete assessment of the coagulation state of TBI patients. Moreover, further investigation is needed to assess whether the additional expense incurred with POC testing can be offset by systemic cost savings,[23] including a reduction in the number of transfusions and savings derived from improved patient outcomes.[11]
CONCLUSIONS
This study evaluating conventional laboratory tests and POC coagulation tests by r-TEG and VerifyNow (VN-ASA) in the acute setting confirmed that rapid assessment can provide critical information to help regulate the progression of intracranial hemorrhages. As adjuncts to standard coagulation testing, rTEG and VN-ASA recognized occult coagulopathies in our TBI patients. With more than one-third of American adults prescribed aspirin and millions taking chronic oral anticoagulation, rapid coagulation testing in the acute setting may provide benefits for patients who are unresponsive or cannot provide information regarding such usage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was partially supported by a grant from the Mayfield Education and Research Foundation.
Footnotes
Contributor Information
Yair M. Gozal, Email: gozalyr@ucmail.uc.edu.
Christopher P. Carroll, Email: christopher.carroll@uc.edu;.
Bryan M. Krueger, Email: bryan.krueger@uc.edu.
Jane Khoury, Email: jane.khoury@cchmc.org.
Norberto O. Andaluz, Email: norberto.andaluz@uc.edu.
REFERENCES
- 1.Bachelani AM, Bautz JT, Sperry JL, Corcos A, Zenati M, Billiar TR, et al. Assessment of platelet transfusion for reversal of aspirin after traumatic brain injury. Surgery. 2011;150:836–43. doi: 10.1016/j.surg.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128:1300–5. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beynon C, Unterberg AW, Sakowitz OW. Point of care coagulation testing in neurosurgery. J Clin Neurosci. 2015;22:252–7. doi: 10.1016/j.jocn.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Blais N, Pharand C, Lordkipanidze M, Sia YK, Merhi Y, Diodati JG. Response to aspirin in healthy individuals. Cross-comparison of light transmission aggregometry, VerifyNow system, platelet count drop, thromboelastography (TEG) and urinary 11-dehydrothromboxane B(2) Thromb Haemost. 2009;102:404–11. doi: 10.1160/TH09-02-0126. [DOI] [PubMed] [Google Scholar]
- 5.Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Harmsze AM, Hackeng CM, et al. High on-treatment platelet reactivity to both aspirin and clopidogrel is associated with the highest risk of adverse events following percutaneous coronary intervention. Heart. 2011;97:983–90. doi: 10.1136/hrt.2010.220491. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra G, Sharma S, Subramanian A, Agrawal D, Sinha S, Mukhopadhyay AK. Coagulopathy as prognostic marker in acute traumatic brain injury. J Emerg Trauma Shock. 2013;6:180–5. doi: 10.4103/0974-2700.115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–14. doi: 10.1097/TA.0b013e31821e1bf0. [DOI] [PubMed] [Google Scholar]
- 8.Craft RM, Chavez JJ, Bresee SJ, Wortham DC, Cohen E, Carroll RC. A novel modification of the Thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J Lab Clin Med. 2004;143:301–9. doi: 10.1016/j.lab.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: A systematic review and proposed treatment algorithms. J Thromb Haemost. 2006;4:1853–63. doi: 10.1111/j.1538-7836.2006.01986.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunham CM, Rabel C, Hileman BM, Schiraldi J, Chance EA, Shima MT, et al. TEG(R) and RapidTEG(R) are unreliable for detecting warfarin-coagulopathy: A prospective cohort study. Thromb J. 2014;12:4. doi: 10.1186/1477-9560-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth. 2009;103(Suppl 1):i14–22. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 12.Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: A systematic review and meta-analysis. Injury. 2014;45:819–24. doi: 10.1016/j.injury.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Frelinger AL, Li Y, Linden MD, Tarnow I, Barnard MR, Fox ML, et al. Aspirin ‘resistance’: Role of pre-existent platelet reactivity and correlation between tests. J Thromb Haemost. 2008;6:2035–44. doi: 10.1111/j.1538-7836.2008.03184.x. [DOI] [PubMed] [Google Scholar]
- 14.Frontera JA, Lewin JJ, 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24:6–46. doi: 10.1007/s12028-015-0222-x. [DOI] [PubMed] [Google Scholar]
- 15.Grandhi R, Harrison G, Voronovich Z, Bauer J, Chen SH, Nicholas D, et al. Preinjury warfarin, but not antiplatelet medications, increases mortality in elderly traumatic brain injury patients. J Trauma Acute Care Surg. 2015;78:614–21. doi: 10.1097/TA.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 16.Hemlata, Verma A, Elhence P. Mapping the antiplatelet effects of clopidogrel and aspirin by modified thromboelastography. J Anaesthesiol Clin Pharmacol. 2013;29:421–3. doi: 10.4103/0970-9185.117094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: Current clinical applications and its potential role in interventional cardiology. Platelets. 2006;17:509–18. doi: 10.1080/09537100600935259. [DOI] [PubMed] [Google Scholar]
- 18.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: Experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–86. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 19.Kumar MA. Coagulopathy associated with traumatic brain injury. Curr Neurol Neurosci Rep. 2013;13:391. doi: 10.1007/s11910-013-0391-y. [DOI] [PubMed] [Google Scholar]
- 20.Kunio NR, Differding JA, Watson KM, Stucke RS, Schreiber MA. Thrombelastography-identified coagulopathy is associated with increased morbidity and mortality after traumatic brain injury. Am J Surg. 2012;203:584–8. doi: 10.1016/j.amjsurg.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Kuo JR, Chou TJ, Chio CC. Coagulopathy as a parameter to predict the outcome in head injury patients - Analysis of 61 cases. J Clin Neurosci. 2004;11:710–4. doi: 10.1016/j.jocn.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Laker SR. Epidemiology of concussion and mild traumatic brain injury. PM R. 2011;3(Suppl 2):S354–8. doi: 10.1016/j.pmrj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee-Lewandrowski E, Lewandrowski K. Perspectives on cost and outcomes for point-of-care testing. Clin Lab Med. 2009;29:479–89. doi: 10.1016/j.cll.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Loftus CM, editor. Anticoagulation and Hemostasis in Neurosurgery. Switzerland: Springer International Publishing; 2016. [Google Scholar]
- 25.Nielsen HL, Kristensen SD, Thygesen SS, Mortensen J, Pedersen SB, Grove EL, et al. Aspirin response evaluated by the VerifyNow Aspirin System and light transmission aggregometry. Thromb Res. 2008;123:267–73. doi: 10.1016/j.thromres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Niemeier JP, Grafton LM, Chilakamarri T. Treating persons with traumatic brain injury: History and updates. N C Med J. 2015;76:105–10. doi: 10.18043/ncm.76.2.105. [DOI] [PubMed] [Google Scholar]
- 27.Paniccia R, Antonucci E, Gori AM, Marcucci R, Poli S, Romano E, et al. Comparison of different methods to evaluate the effect of aspirin on platelet function in high-risk patients with ischemic heart disease receiving dual antiplatelet treatment. Am J Clin Pathol. 2007;128:143–9. doi: 10.1309/0G1PEJ00J8KP8357. [DOI] [PubMed] [Google Scholar]
- 28.Schaller B, Evangelopoulos DS, Muller C, Martinolli L, Pouljadoff MP, Zimmermann H, et al. Do we really need 24-h observation for patients with minimal brain injury and small intracranial bleeding? The Bernese Trauma Unit Protocol. Emerg Med J. 2010;27:537–9. doi: 10.1136/emj.2009.073031. [DOI] [PubMed] [Google Scholar]
- 29.Schochl H, Solomon C, Traintinger S, Nienaber U, Tacacs-Tolnai A, Windhofer C, et al. Thromboelastometric (ROTEM) findings in patients suffering from isolated severe traumatic brain injury. J Neurotrauma. 2011;28:2033–41. doi: 10.1089/neu.2010.1744. [DOI] [PubMed] [Google Scholar]
- 30.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol. 2012;8:513–20. [PMC free article] [PubMed] [Google Scholar]
- 32.Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: A prospective study. J Trauma. 2009;66:55–61. doi: 10.1097/TA.0b013e318190c3c0. [DOI] [PubMed] [Google Scholar]
- 33.Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74:378–85. doi: 10.1097/TA.0b013e31827e20e0. [DOI] [PubMed] [Google Scholar]
- 34.Windelov NA, Welling KL, Ostrowski SR, Johansson PI. The prognostic value of thrombelastography in identifying neurosurgical patients with worse prognosis. Blood Coagul Fibrinolysis. 2011;22:416–9. doi: 10.1097/MBC.0b013e3283464f53. [DOI] [PubMed] [Google Scholar]


