Abstract
Background:
Central nervous system infection with Cladophialophora bantiana (Black Mold) is rare. It carries a high mortality rate, that is more than 70%, despite multimodal therapy.
Case Description:
We present a rare case of “black mold” fungal brain abscess that was successfully treated with good patient outcome. The case is unusual because there were two fungal brain abscesses located bilaterally symmetrically in the mesial frontal lobes, and the response to different treatment strategies was well documented by over 25 magnetic resonance imaging (MRI) scans. Initial attempts to treat these lesions by repeated surgical excision and systemic amphotericin B was followed by continued growth rather than resolution. We realized that the application of treatment principles learned from bacterial brain abscess may not transpose intuitively to the treatment of fungal brain abscess. Therefore, a new treatment strategy was adopted that avoided further attempts at resection in favor of long-term oral voriconazole and repeated intracavitary aspiration and instillation of amphotericin B on an outpatient basis. Without further resection, the lesions stabilized and the aspirates eventually sterilized, however, the enhancing capsule never resolved on MRI scans. All treatment was stopped after 1 year. The apparently sterilized lesions have been followed for an additional 3 years without further growth, and the patient remains functionally, intellectually, and behaviorally normal.
Conclusion:
We conclude that, in the case of fungal abscess, it may be preferable to sterilize the lesion in situ rather than attempting to achieve resolution on imaging studies by repeated surgical resection of the capsule which can be counterproductive. This strategy accepts that the capsule may be important to the patient's immune defense against the fungus. Helping that defense barrier with intracapsular and systemic antifungal agents, rather than capsular removal, may be the better strategy for patients in whom early aggressive resection has failed. The basis for the apparent differences in the response of fungal versus bacterial brain abscess to surgical resection is discussed in the light of pathological findings from this and other cases.
Keywords: Abscess, antifungal, black mold, brain, excision
INTRODUCTION
Brain abscess caused by the fungus Cladophialophora bantiana (Black Mold) is notoriously difficult to treat with a mortality of over 70% reported in a large series.[18,38] This high mortality rate is presumed to stem only from the virulence and pathophysiology of the organism itself,[37] however, our experience with a recent case leads us to question the role that currently accepted surgical treatment strategy might have. Specifically, the application of treatment principles learned from decades of refinements in the surgical and pharmacological treatment of bacterial abscess may not transpose intuitively to the treatment of fungal brain abscess. Clearly, fungal brain abscess is different from bacterial brain abscess,[4,8,30] and the implications of these differences may not be fully appreciated currently for their implications in optimizing treatment for fungal brain abscess.
The case presented here is unusual in that there were two bilaterally symmetrical abscesses, with subtle differences that may account for their different response to treatment. In addition, there is extensive documentation by frequent MRI scans (25 in total) showing response of both lesions to various surgical and medical treatment attempts. In aggregate, the data appear to show that surgical extracapsular resection can be followed by enlargement of the lesion rather than aiding resolution. Early reformation and continued growth of a new enhancing capsule along the margins of the twice attempted resection was appreciated on one side and led to a change in treatment strategy away from further resection in favor of intracavitary catheter placement for repeated aspiration of pus and instillation of amphotericin B, in addition to the oral systemic therapy with voriconazole. This change in strategy was influenced by earlier experiences that repeated resection led to fatal outcome in two prior cases reported elsewhere.[20] The new in situ sterilization strategy adopted eventually led to stable lesions even after stopping all medications. While surgical resection appears to be a highly effective treatment for bacterial brain abscess, its role in treating fungal brain abscess may need to be reconsidered.
CASE PRESENTATION
Our patient is a 59-year-old right-handed male who presented to us after a new-onset seizure. He had a past medical history of well-controlled type II diabetes mellitus and mild chronic obstructive pulmonary disease. Neurological exam on admission was normal, however, initial imaging of the brain revealed bilateral mesial frontal ring-enhancing lesions most consistent with abscesses [Figure 1]. His initial labs were unremarkable with normal white blood cells, low erythrocyte sedimentation rate 8, and C-reactive protein 1.5. Further evaluation revealed a tooth infection, and his blood cultures came back suggestive of a bacillus. A presumptive diagnosis of bilateral bacterial brain abscesses was made and the patient was started on empiric antibiotics (ceftriaxone and metronidazole).
Figure 1.
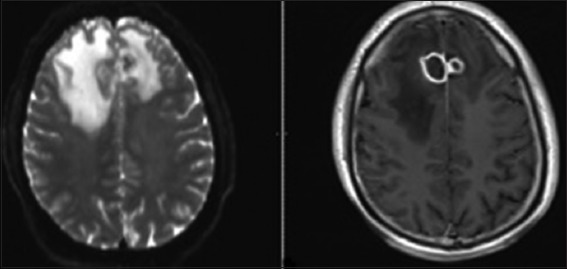
Magnetic resonance imaging demonstrated rim-enhancing 2 cm cystic lesions in the bilateral frontal lobes with associated cerebral edema
Magnetic resonance imaging (MRI) of the brain scan with contrast was repeated after 1 week of antibiotics, and the lesions appeared stable. The patient was then discharged home with a PICC line on intravenous Ertapenem and oral Levetiracetam. Four days after discharge, the patient presented with a recurrent seizure. The patient was readmitted to the hospital and placed back on ceftriaxone and metronidazole. Because of the unexpected worsening course, MRI brain was repeated approximately weekly from that point, showing convincing evidence of progression of both lesions at approximately 3 weeks despite antibiotic medication. He was then taken for a bifrontal burr hole craniotomy and stereotactic aspiration of both lesions. Pus was retrieved from both lesions which on gram stain did not reveal bacterial but instead showed septated hyphae typical of fungi. amphotericin B intravenously was then added for coverage [Figure 2]. One week after the stereotactic aspiration, MRI showed that the lesions had enlarged further. The patient was then taken back for bifrontal craniotomy and attempted aggressive total resection by extracapsular removal of both lesions and placement of bilateral intracavitary Ommaya reservoirs.
Figure 2.
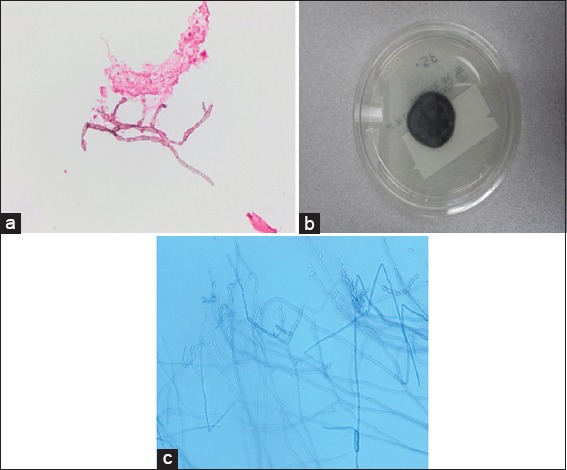
Fontana stain on brain aspirate revealed septated hyphae (a). Cultures on potato flake agar (day 6) grew black- gray velvety colony (b). Lactophenol cotton blue preparation revealed long, sparsely branched conidiophores producing long chains of terminal and lateral oval conidia (c)
Two days later, treatment with intracavitary amphotericin B 0.5 mg was started. Cultures later confirmed black mold (dark colored largely unbranched hyphae) C. bantiana species [Figure 3]. Oral voriconazole was started as well as intravenous (IV) amphotericin B. During this hospital stay, the patient developed several complications: Pancytopenia, pneumonia due to gram positive cocci infection, PICC line infection with gram positive cocci, acute kidney injury, and pleural effusion that required drainage with chest tube. IV amphotericin was then permanently stopped but intracavitary amphotericin B and oral voriconazole were continued.
Figure 3.
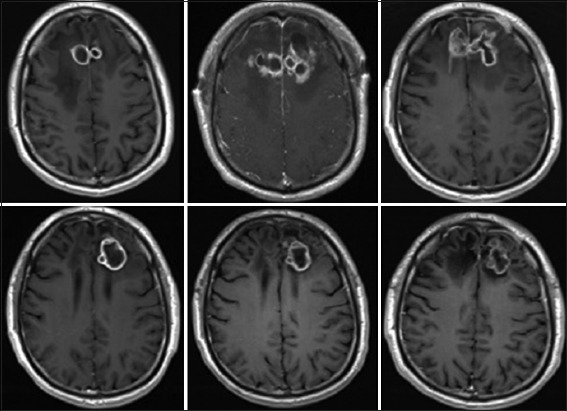
Serial MRI T1 sequence with contrast showing the disease course over time. (Top Left) after failure of antibacterial therapy. (Top Middle) After stereotactic aspiration. (Top Right) After surgical resection and placement of bilateral Ommaya reservoirs. (Bottom Left) Progression of lesion after stopping outpatient anti-fungal therapy. (Bottom Middle) Surveillance scan showing stability of lesion while patient on therapy. (Bottom Right) Last scan showing stability of lesion while the patient was off therapy for 3 years
Following surgery, the right lesion reformed an enhancing capsule, which then shrank and stabilized measuring approximately ~2.5 cm3. The left-sided lesion also reformed a capsule and initially continued to enlarge slowly. The left lesion measured 55 cm3 1 week after resection and progressed to a peak of 66 cm3 at approximately 1 month. During this time, the patient's neurological exam remained entirely normal, without focal deficits, signs, or symptoms of raised intracranial pressure or meningismus. After the initial post-resection enlargement, both abscesses started to decrease in size on the new regimen. After 2 months in the hospital, the patient had stable brain MRIs and was discharged on oral voriconazole and continued intracavitary aspiration with amphotericin B injections on an outpatient basis 3 times/week. This regimen was continued for a further 3 months at which point the lesions appeared stable. All medications were then stopped and the patient was followed with approximately monthly brain MRI scans. Two months after stopping treatment, brain MRI showed that the left frontal abscess was enlarging again and had not responded to treatment. At this point, the patient was re-admitted to the hospital and a bifrontal craniotomy was performed for internal debridement of the left-side brain abscess, with removal of the right-side Ommaya reservoir and replacement of only the left-sided Ommaya reservoir. At that time, analysis of intraoperative specimens showed septated hyphae on smear and C. Bantiana on cultures. The dose of intracavitary amphotericin treatment was then increased to 1 mg every other day. After 1 week in the hospital, he was discharged home on oral voriconazole and intracavitary aspiration and amphotericin B instillation 3 times/week in the clinic. Later, he was transitioned to weekly aspiration/injections that were continued for another 5 months, during which findings on periodic MRI remained stable [Figure 3].
From the last surgical debridement onward, analysis of aspirated material from the persistent left-side lesion continued to show septate hyphae on smear with no fungal growth on culture. Then, in September 2014 (13 months after beginning intracavitary therapy), analysis finally showed no fungal elements on smear and no growth on culture [Table 1]. From then, all anti-fungal treatments were stopped. At the time of writing this article, over 3 years from initial diagnosis, the patient remains off all antifungals and no longer receives intracavitary treatment [Figure 4]. He remains fully functional and socially appropriate with a normal neurologic exam and normal activity and interests. Although MRI scans continue to show ring enhancing lesions, they appear stable or slightly smaller on serial scans. We continue to monitor him with 6 monthly MRI scan.
Table 1.
Results of serial smear/culture of intracavitary aspirate
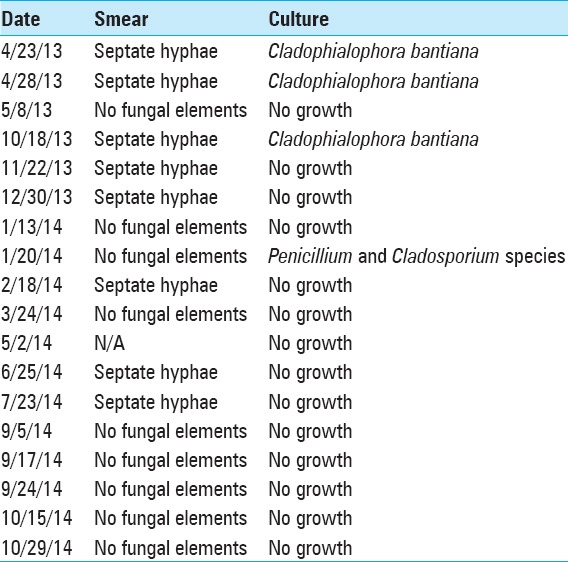
Figure 4.
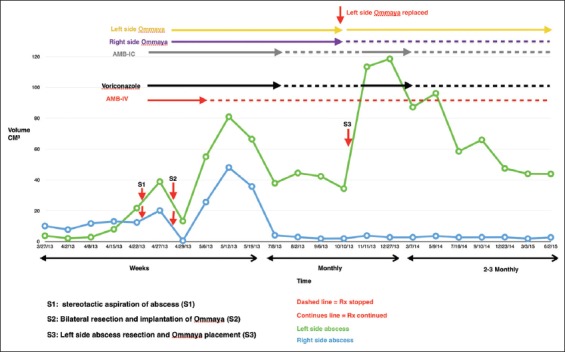
Change in the abscesses’ size over time based on serial brain MRIs; it also shows the timeline for medical treatment, as well as surgical interventions
DISCUSSION
Central nervous system infection with C. bantiana is a rare and often fatal infection.[6,14,21,33,34] The mortality rate of this condition is more than 70%, despite aggressive multimodal therapy.[37] Infection with this neurotropic fungus presents most commonly as brain abscess or rarely as meningitis.[5,32,39,41] Predisposing factors are not well established, and while some reports implicate steroids and immunosuppressant use after solid organ transplantation,[19,24,25,41] the majority of cases occur in immunocompetent patients.[7,11,17,28] The fungus is widely disseminated in the environment, and the mode of entry is only vaguely understood. Revankar et al.[38] suggested entry through inhalation of spores followed by hematogenous dissemination to the brain.
There are no clear guidelines regarding the treatment of this disease because of its rarity and lack of prospective trials. Treatments might include systemic antifungal therapy, burr-hole aspirations, open internal surgical debridement, wide extracapsular surgical resection, and rarely intrathecal/intracavitary therapy.[26]
Systemic antifungal monotherapy might be effective initially, but almost always leads to treatment failure from resistance and difficult antifungal penetration of the capsule of the abscess.[12,16,29] Amphotericin B is the most commonly used antifungal agent. However, studies have shown less than minimum inhibitory concentrations of amphotericin B in the cerebrospinal fluid and brain parenchyma after systemic administration of standard doses (70 mg/day).[3,23] Newer antifungals, such as voriconazole and posaconazole, appear to have high efficacy, improved bioavailability, and are well tolerated.[2,13,15,16,29,35] Alternatively, itraconazole was proposed if the patient does not respond to voriconazole.[38] Antifungal therapy can be guided by susceptibility testing and clinical efficacy. Animal studies have shown that in vivo antifungal efficacy against C. bantiana is consistent with in vitro antifungal susceptibility.[2,27,31] Despite these improved agents, failure with C. Bantiana continue to be reported.[21]
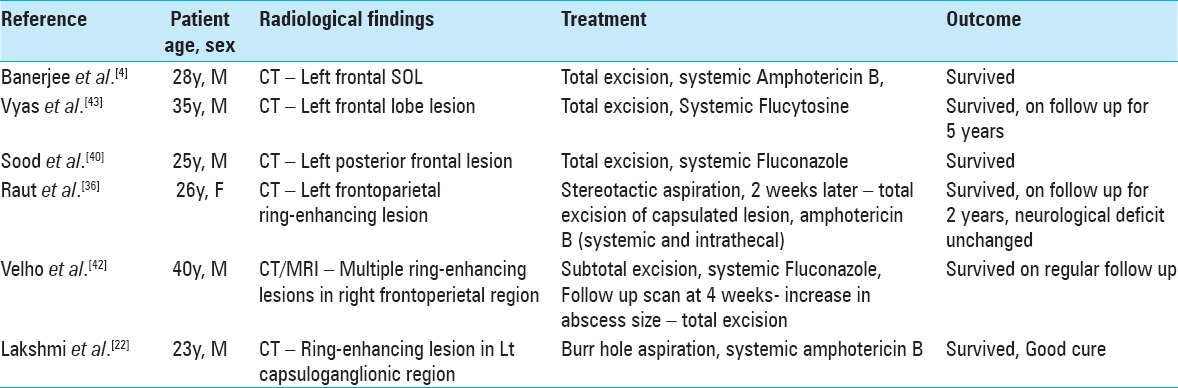
Surgical management of this disease may include initial biopsy with aspiration, possibly followed by more aggressive excision once hyphae are identified. For lesions that are amenable to early wide surgical resection, this may be an important factor in achieving a possible cure and improved outcome.[38] However, the role of surgical extracapsular resection is far from proven because many cases (9/24 reviewed here) that were similarly treated with excision did not survive,[41] while at least one case treated only with aspiration and systemic amphotericin B made a good recovery. Furthermore, because of size and location, a wide resection may not be desirable or possible. Table 2 summarizes several cases[4,22,36,40,42,43] in the literature that reported survival with C. bantiana brain abscess, and shows that each case was treated differently, adding to the existing uncertainty regarding the most appropriate treatment.
Table 2.
Summary of some of the reported cases of C. Bantiana that shows patient survival after different treatment strategies
Prolonged intracavitary treatment has seldom been proposed for intracranial fungal abscesses and appears not to have been published for the specific treatment of C. bantiana.[1,9,10] Intraventricular amphotericin treatment appears not to be well tolerated with significant side effects and potential for seeding the intraventricular space by passing a catheter.[1]
In the case documented here by serial brain MRIs, surgical resection was almost always followed by worsening of the abscess, despite continued systemic anti-fungal therapy. This observation raises several questions. First, how wide a margin is necessary to eradicate fungal brain abscess in comparison to bacterial brain abscess? In bacterial brain abscess, neuropathological studies have shown that in the stages of late cerebritis, early and late capsular formation, no bacteria could be identified in the collagen capsule or the surrounding reactive gliosis zone.[8,30] In contrast, fungal brain abscess showed diffuse infiltration of the brain parenchyma with fungal hyphae beyond the fibrous capsule. The adjacent brain parenchyma was reactive and hyperemic with granulomas containing fungi, necrotic debris, acute and chronic inflammatory cells, and multinucleated giant cells [Figure 5].[19]
Figure 5.
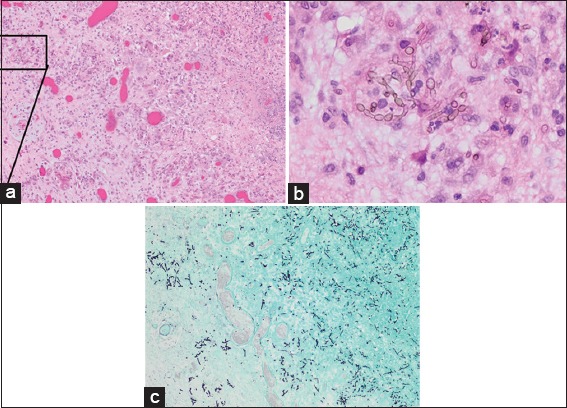
Resected cyst wall (hematoxylin and eosin; magnification, ×100) showing granulomatous inflammation with necrosis and reactive changes (a) and septated branching hyphae with oval conidia (b). Gomori methenamines Silver stain (GMS, ×100) of the tissue block in a showing the fungal elements (c)
Second, if an additional resection margin is needed, is that margin compatible with preservation of function in the affected location? Third, what are the effects of pus spillage and piecemeal removal? While with resection of bacterial brain abscess close margined extracapsular removal is typically effective, and some pus spillage is not ordinarily a major concern, the situation may be different for fungal brain abscess, for which anti-fungal agents may be relatively less effective than appropriate antibiotics for spilled bacteria.
Realizing that continued surgical resection attempts might be counterproductive in treating this patient's fungal brain abscess, we switched to a different strategy relying on a prolonged regimen of intracavitary aspirations and instillations of amphotericin B by an Ommaya reservoir already implanted at an earlier resection operation. Intracavitary amphotericin B injections have been documented to be safe with 5 μg daily increasing to 40 μg/day, and a dose as high as 0.5 mg/day was documented to be safe.[1,10] We used an initial intracavitary dose of 0.25 mg/day, and then escalated the dose to 0.5 mg/day after recurrence. Treatment failures have been noted with even 2 months of intracavitary treatment,[10] which we also experienced initially. Without further surgical resection, the patient's brain MRI scans showed stabilization of the rim enhancing lesions. Apart from the early seizures, the patient's clinical status remained stable throughout the course of treatment, and he never demonstrated any obvious symptoms or focal neurological deficits on exam even as all medications were eventually discontinued.
Although it is well accepted that surgical resection appears to be a highly effective treatment for bacterial brain abscess, its role in treating fungal brain abscess may be different and even sometimes counterproductive. Based on our experience with this case, patients with fungal brain abscess may benefit more from intracavitary amphotericin B in combination with systemic anti-fungal therapy. Further experience with future cases will be needed to verify these impressions.
Prolonged follow-up (>1 year) is essential because late relapses are reported to be possible.[37] Successful treatment courses have been as short as 3 months and lasted more than a year. Therapy should not be stopped until complete radiographic resolution occurs or there is evidence of halted progression on repeat MRIs spanning a prolonged period of time.[13,29,43] In our case, the length of treatment was guided by the gradually negative aspirate cultures and disappearance of fungal elements in the aspirate. Once these normalized, we did not attempt to resect the persistent enhancing capsule of the lesions because the patient was already asymptomatic and we feared reactivation of possible dormant residual elements by the surgery. At this writing, the patient has been followed for over 3 years without clinical or imaging evidence of reactivation. Remarkably, the patient remains neurologically normal and active.
Based on these observations, our current treatment paradigm for black mold fungal brain abscess is to first make a diagnosis by stereotactic aspiration, and if black mold is found, attempt as wide a surgical resection as possible, followed by treatment with maximal systemic medical therapy as tolerated with voriconazole and amphotericin. If the lesion shows early recurrence, rather than attempting further resection of the capsule, consider internal debridement leaving the capsule intact and place a catheter with Ommaya reservoir for intracavitary antifungal therapy. This strategy accepts that the capsule may be important to the patient's immune defense against the fungus and helping that defense barrier with intracapsular and systemic antifungal agents rather than capsular removal may be the better strategy for that patient. If this strategy is adopted, long-term regular treatment on an outpatient basis should be anticipated. Imaging showing persistence of the capsule should not be considered a treatment failure as long as further progression is not obvious. Aspiration and antifungal instillation should continue until the aspirate is cleared of fungal elements and the cultures turn persistently negative. Patience can pay off with eventual sterilization in situ of the lesion with good clinical outcome, despite imaging persistence of the lesion.
CONCLUSION
Optimal surgical treatment of fungal brain abscess may differ from that of bacterial brain abscess. In particular, the role of extracapsular surgical excision may not be as effective for fungal brain abscess as for bacterial abscess. While bacteria are confined by the fibrous capsule, fungal hyphae can extend beyond it. In addition, antibiotics for bacteria may be more effective than those for fungi. In cases where surgical resection of fungal brain abscess is followed by early enlargement or rapid recurrence, or the sensitive location does not allow initial wide resection, long-term repeated aspiration with intracavitary amphotericin B together with systemic oral voriconazole may still prove successful.
DISCLOSURE
We have read and understood SNI policy on declaration of interests and declare that we have no competing interests.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Zaid Aljuboori, Email: zaid.aljuboori@louisville.edu.
Rob Hruska, Email: rthruska@gmail.com.
Alae Yaseen, Email: yaseena@mymail.vcu.edu.
Forest Arnold, Email: fwarno01@louisville.edu.
Barbara Wojda, Email: btwojd01@louisville.edu.
Haring Nauta, Email: hjnauta@gmail.com.
REFERENCES
- 1.Adler DE, Milhorat TH, Miller JI. Treatment of rhinocerebral mucormycosis with intravenous interstitial, and cerebrospinal fluid administration of amphotericin B: Case report. Neurosurgery. 1998;42:644–8. doi: 10.1097/00006123-199803000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Al-Abdely HM, Alkhunaizi AM, Al-Tawfiq JA, Hassounah M, Rinaldi MG, Sutton DA. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med Mycol. 2005;43:91–5. doi: 10.1080/13693780400011104. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson AJ, Jr, Bennett JE. Amphotericin B pharmacokinetics in humans. Antimicrob Agents Chemother. 1978;13:271–6. doi: 10.1128/aac.13.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee U, Mohapatra AK, Sarkar C, Chaudhery R. Cladosporiosis (cerebral phaeohyphomycosis) of brain--a case report. Mycopathologia. 1989;105:163–6. doi: 10.1007/BF00437249. [DOI] [PubMed] [Google Scholar]
- 5.Biggs PJ, Allen RL, Powers JM, Holley HP., Jr Phaeohyphomycosis complicating compound skull fracture. Surg Neurol. 1986;25:393–6. doi: 10.1016/0090-3019(86)90216-8. [DOI] [PubMed] [Google Scholar]
- 6.Binford CH, Thompson RK, Gorham ME. Mycotic brain abscess due to Cladosporium trichoides, a new species; report of a case. Am J Clin Pathol. 1952;22:535–42. doi: 10.1093/ajcp/22.6.535. [DOI] [PubMed] [Google Scholar]
- 7.Borkar SA, Sharma MS, Rajpal G, Jain M, Xess I, Sharma BS. Brain abscess caused by Cladophialophora Bantiana in an immunocompetent host: Need for a novel cost-effective antifungal agent. Indian J Med Microbiol. 2008;26:271–4. doi: 10.4103/0255-0857.42050. [DOI] [PubMed] [Google Scholar]
- 8.Britt RH, Enzmann DR, Placone RC, Jr, Obana WG, Yeager AS. Experimental anaerobic brain abscess. Computerized tomographic and neuropathological correlations. J Neurosurg. 1984;60:1148–59. doi: 10.3171/jns.1984.60.6.1148. [DOI] [PubMed] [Google Scholar]
- 9.Broggi G, Franzini A, Peluchetti D, Servello D. Treatment of deep brain abscesses by stereotactic implantation of an intracavitary device for evacuation and local application of antibiotics. Acta Neurochir. 1985;76:94–8. doi: 10.1007/BF01418467. [DOI] [PubMed] [Google Scholar]
- 10.Camarata PJ, Dunn DL, Farney AC, Parker RG, Seljeskog EL. Continual intracavitary administration of amphotericin B as an adjunct in the treatment of aspergillus brain abscess: Case report and review of the literature. Neurosurgery. 1992;31:575–9. doi: 10.1227/00006123-199209000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Carter E, Boudreaux C. Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J Clin Microbiol. 2004;42:5419–23. doi: 10.1128/JCM.42.11.5419-5423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy--United States, July-November 2002. JAMA. 2003;289:291–3. [PubMed] [Google Scholar]
- 13.Deb S, Khan AK, Debasish B, Subroto B. Intracranial necrotizing granuloma caused by Cladophialophora bantiana. Neurol India. 2005;53:335–6. doi: 10.4103/0028-3886.16936. [DOI] [PubMed] [Google Scholar]
- 14.Dixon DM, Walsh TJ, Merz WG, McGinnis MR. Infections due to Xylohypha bantiana (Cladosporium trichoides) Rev Infect Dis. 1989;11:515–25. doi: 10.1093/clinids/11.4.515. [DOI] [PubMed] [Google Scholar]
- 15.Elter T, Sieniawski M, Gossmann A, Wickenhauser C, Schröder U, Seifert H, et al. Voriconazole brain tissue levels in rhinocerebral aspergillosis in a successfully treated young woman. Int J Antimicrob Agents. 2006;28:262–5. doi: 10.1016/j.ijantimicag.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Fica A, Diaz MC, Luppi M, Olivares R, Saez L, Baboor M, et al. Unsuccessful treatment with voriconazole of a brain abscess due to Cladophialophora bantiana. Scand J Infect Dis. 2003;35:892–3. doi: 10.1080/00365540310016736. [DOI] [PubMed] [Google Scholar]
- 17.Garg N, Devi IB, Vajramani GV, Nagarathna S, Sampath S, Chandramouli BA, et al. Central nervous system cladosporiosis: An account of ten culture-proven cases. Neurol India. 2007;55:282–8. doi: 10.4103/0028-3886.35690. [DOI] [PubMed] [Google Scholar]
- 18.George IA, Mathews MS, Karthik R, John L, Sundar A, Abraham OC, et al. Fatal cerebral abscess caused by Cladophialophora bantiana. J Assoc Physicians India. 2008;56:470–2. [PubMed] [Google Scholar]
- 19.Harrison DK, Moser S, Palmer CA. Central nervous system infections in transplant recipients by Cladophialophora bantiana. South Med J. 2008;101:292–6. doi: 10.1097/SMJ.0b013e318164e289. [DOI] [PubMed] [Google Scholar]
- 20.Hauck EF, McGinnis M, Nauta HJ. Cerebral phaeohyphomycosis mimicking high-grade Astrocytoma. J Clin Neurosci. 2008;15:1061–6. doi: 10.1016/j.jocn.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Horré R, GS De Hoog. Primary cerebral infections by melanized fungi: A review. Stud Mycol. 1999;43:176–93. [Google Scholar]
- 22.Lakshmi V, Padmasri C, Umabala P, Sundaram C, Panigrahi M. Cerebral phaeohyphomycosis due to Cladophialophora bantiana. Indian J Med Microbiol. 2008;26:392–5. doi: 10.4103/0255-0857.43569. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence RM, Hoeprich PD, Jagdis FA, Monji N, Huston AC, Schaffner CP. Distribution of doubly radiolabelled amphotericin B methyl ester and amphotericin B in the non-human primate, Macaca mulatta. J Antimicrob Chemother. 1980;6:241–9. doi: 10.1093/jac/6.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Lee YM, Tambyah PA, Lee KH, Tan KC, Lim SG. Successful treatment of Xylohypha bantiana brain abscess mimicking invasive cerebral aspergillosis in a liver transplant recipient. J Infect. 2003;47:348–51. doi: 10.1016/s0163-4453(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 25.Levin TP, Baty DE, Fekete T, Truant AL, Suh B. Cladophialophora bantiana brain abscess in a solid-organ transplant recipient: Case report and review of the literature. J Clin Microbiol. 2004;42:4374–8. doi: 10.1128/JCM.42.9.4374-4378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DM, de Hoog GS. Cerebral phaeohyphomycosis-a cure at what lengths? Lancet Infect Dis. 2009;9:376–83. doi: 10.1016/S1473-3099(09)70131-8. [DOI] [PubMed] [Google Scholar]
- 27.Li DM, Xiu DR, Li RY, Samson RA, de Hoog GS, Wang DL. Aspergillus flavus myositis in a patient after liver transplantation. Clin Transplant. 2008;22:508–11. doi: 10.1111/j.1399-0012.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 28.Lirng JF, Tien RD, Osumi AK, Madden JF, McLendon RP, Sexton D. Cerebral phaeohyphomycosis complicated with brain abscess: A case report. Zhonghua Yi Xue Za Zhi. 1995;55:491–5. [PubMed] [Google Scholar]
- 29.Lyons MK, Blair JE, Leslie KO. Successful treatment with voriconazole of fungal cerebral abscess due to Cladophialophora bantiana. Clin Neurol Neurosurg. 2005;107:532–34. doi: 10.1016/j.clineuro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Mandell GL, Douglas RG, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. New York: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- 31.Mariné M, Pastor FJ, Guarro J. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med Mycol. 2009;47:45–9. doi: 10.1080/13693780802526840. [DOI] [PubMed] [Google Scholar]
- 32.Middleton FG, Jurgenson PF, Utz JP, Shadomy S, Shadomy HJ. Brain abscess caused by Cladosporium trichoides. Arch Intern Med. 1976;136:444–8. [PubMed] [Google Scholar]
- 33.Osiyemi OO, Dowdy LM, Mallon SM, Cleary T. Cerebral phaeohyphomycosis due to a novel species: Report of a case and review of the literature. Transplantation. 2001;71:1343–6. doi: 10.1097/00007890-200105150-00029. [DOI] [PubMed] [Google Scholar]
- 34.Palaoglu S, Sav A, Basak T, Yalcinlar Y, Scheithauer BW. Cerebral phaeohyphomycosis. Neurosurgery. 1993;33:894–7. doi: 10.1227/00006123-199311000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Pitisuttithum P, Negroni R, Graybill JR, Bustamante B, Pappas P, Chapman S, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56:745–55. doi: 10.1093/jac/dki288. [DOI] [PubMed] [Google Scholar]
- 36.Raut A, Muzumdar D, Narlawar R, Nagar A, Ahmed N, Hira P. Cerebral abscess caused by Cladosporium bantianum infection--case report. Neurol Med Chir. 2003;43:413–5. doi: 10.2176/nmc.43.413. [DOI] [PubMed] [Google Scholar]
- 37.Revankar SG. Cladophialophora bantiana brain abscess in an immunocompetent patient. Can J Infect Dis Med Microbiol. 2011;22:149–50. doi: 10.1155/2011/642517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: A review of 101 cases. Clin Infect Dis. 2004;38:206–16. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 39.Shankar SK, Mahadevan A, Sundaram C, Sarkar C, Chacko G, Lanjewar DN, et al. Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario. Neurol India. 2007;55:198–215. doi: 10.4103/0028-3886.35680. [DOI] [PubMed] [Google Scholar]
- 40.Sood P, Dogra V, Thakur A, Mishra B, Mandal A, Sinha S. Brain abscess due to Xylohypha bantiana. Scand J Infect Dis. 2000;32:708–9. doi: 10.1080/003655400459720. [DOI] [PubMed] [Google Scholar]
- 41.Suri P, Chhina DK, Kaushal V, Kaushal RK, Singh J. Cerebral Phaeohyphomycosis due to Cladophialophora bantiana-A Case Report and Review of Literature from India. J Clin Diagn Res. 2014;8:DD01–5. doi: 10.7860/JCDR/2014/7444.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velho V, Ghodgaonkar P. Cladosporium trichoides Causing Multiple Cerebral Abscesses: A Case Report. Internet J Neurosurg. 2007:4. [Google Scholar]
- 43.Vyas MC, Joshi YR, Bhargava N, Joshi KR, Tanwar RK. Cerebral chromoblastomicosis-a rare case report of cerebral abscess and brief review of literature-a case report. Indian J Pathol Microbiol. 2000;43:81–5. [PubMed] [Google Scholar]


