Abstract
Chronic exposure to cadmium compounds (Cd2+) is one of the major public health problems facing humans in the 21st century. Cd2+ in the human body accumulates primarily in the kidneys which leads to renal dysfunction and other adverse health effects. Efforts to find a safe and effective drug for removing Cd2+ from the kidneys have largely failed. We developed and synthesized a new chemical, sodium (S)-2-(dithiocarboxylato((2S,3R,4R,5R)-2,3,4,5,6 pentahydroxyhexyl)amino)-4-(methylthio) butanoate (GMDTC). Here we report that GMDTC has a very low toxicity with an acute lethal dose (LD50) of more than 10,000 mg/kg or 5000 mg/kg body weight, respectively, via oral or intraperitoneal injection in mice and rats. In in vivo settings, up to 94% of Cd2+ deposited in the kidneys of Cd2+-laden rabbits was removed and excreted via urine following a safe dose of GMDTC treatment for four weeks, and renal Cd2+ level was reduced from 12.9 μg/g to 1.3 μg/g kidney weight. We observed similar results in the mouse and rat studies. Further, we demonstrated both in in vitro and in animal studies that the mechanism of transporting GMDTC and GMDTC-Cd complex into and out of renal tubular cells is likely assisted by two glucose transporters, sodium glucose cotransporter 2 (SGLT2) and glucose transporter 2 (GLUT2). Collectively, our study reports that GMDTC is safe and highly efficient in removing deposited Cd2+ from kidneys assisted by renal glucose reabsorption system, suggesting that GMDTC may be the long-pursued agent used for preventive and therapeutic purposes for both acute and chronic Cd2+ exposure.
Keywords: Cadmium decorporation, Cadmium induced renal dysfunction, Chelating agent, GMDTC, Renal glucose reabsorption pathway
1. Introduction
Cadmium (Cd) is a naturally present and global environmental pollutant widely distributed and detected in almost all soils, surface waters and plants. The World Health Organization (WHO) has recognized chronic Cd exposure as one of the major worldwide public health problems (WHO, 2010). A wide spectrum of deleterious effects have been associated with prolonged Cd exposure (Faroon et al., 2012; IARC, 1993), but the kidney is the most sensitive and affected organ. Almost 30% of body Cd absorbed is deposited in the kidney tubule region (Bernhoft, 2013), and the biological half-life of renal Cd is unusually long and its excretion takes decades in humans (Faroon et al., 2012). Once the deposition of Cd in the kidney reaches a certain level, it causes pathological changes in the renal tubular area and the resulting renal dysfunction often persists for many years. Other Cd-associated adverse effects, including osteoporosis, diabetes, reproductive system problems and cardiovascular disease are associated with or a secondary response to Cd-caused kidney damage (Nawrot et al., 2010; UNEP, 2010).
Tremendous efforts have been made in identifying a safe drug, i.e., chelating agent, which can efficiently mobilize and excrete Cd from the kidney. Some chelating agents, e.g., Ethylene Diamine Tetraacetic Acid (EDTA), 2,3-Dimercaprol (BAL), 2,3-Dimercapto-1-propanesulfonic acid (DMPS), meso-2,3-dimercaptosuccinic acid (DMSA) and Dithiocarbamates (DTC), have been proposed for use in the treatment of patients with acute high-level Cd exposure; however, none of these chelation agents have been clinically approved so far (Bernhoft, 2013; Gale et al., 1988; Nawrot et al., 2010; Nordberg, 1984; UNEP, 2010). The application of these chelating agents in the prevention or treatment of chronic Cd exposure has proven even more disappointing due to their severe side effects and/or ineffectiveness in removing Cd from the kidney (Andersen and Nielsen, 1989; Bernhoft, 2013; Gale et al., 1988; Gale et al., 1989; Nordberg, 1984; Wang et al., 1999; Wang et al., 2004).
Here, we report that a newly synthesized chemical, Sodium (S)-2-(dithiocarboxylato((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexyl) amino)-4-(methylthio)butanoate (GMDTC), is low in toxicity and highly efficient in removing deposited Cd2+ from kidneys in all animals tested including mice, rats and rabbits. We further demonstrate that the effect of GMDTC in removing Cd2+ from the kidney is mainly assisted by glucose transporters on renal tubular cells.
2. Materials and methods
2.1. Chemicals and reagents
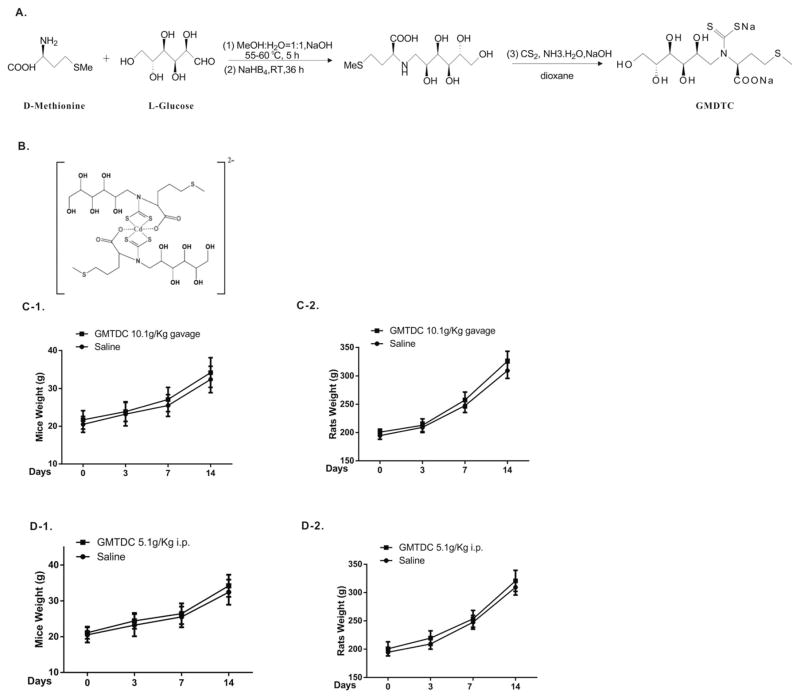
CdCl2·2.5H2O (analytical degree) are purchased from Fuchen Chemical Inc. (Tianjin, China). The GMDTC (molecular weight 433) was synthesized according to Patent ZL 200510035377 via the route depicted in Fig. 1 by WuXi AppTec (Tianjin) Co., Ltd., China. Other chemicals obtained were from authorized chemical suppliers in China. GMTDC is a water-soluble crystal with a light yellow color, and it was freshly prepared and dissolved in saline and sterilized prior to treating cells or animals. Cell culture medium and reagents were acquired from Thermo Fisher Scientific Inc. (Shanghai, China).
Fig. 1.
GMDTC synthesis and acute toxicity assessment. A). Simplified synthesis steps of Sodium (S)-2-(dithiocarboxylato((2S,3R,4R,5R)-2,3,4,5,6 pentahydroxyhexyl)amino)-4-(methylthio)butanoate (GMDTC). B). The schematic structure of the GMDTC-Cd complex was created by coordination chemistry software (molecular formula: NaCdC24H43O14N2S6). C). The body weight of mice (C-1) and rats (C-2) was shown following gavage administration of 10.1 g/kg (23.3 mmol/kg) GMDTC at 3, 7 and 14 days. D). The body weight of mice (D-1) and rats (D-2) was shown following i.p. injection of 5.1 g/kg (11.8 mmol/kg) GMDTC at 3, 7 and 14 days.
2.2. Complexometric titration experiment
A complexometric titration experiment was performed as previously described (Hafez and Khalifa, 1997). Freshly prepared GMDTC solution was titrated with twelve heavy metals and essential elements. Color changes of reactions were recorded and the proportion of complex were calculated.
2.3. Cell culture and treatment
The HK-2 cell line, an immortalized human proximal tubule epithelial cell line, was a gift from Professor Jianmin Jiang, Sun Yat-Sen University School of Pharmacy, and of American Type Culture Collection (ATCC) origin. HK-2 cells were cultured in DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. Cells were maintained at 37 °C in an atmosphere of 95% air and 5% CO2. To determine whether the glucose transporters are responsible for the transport of GMTDC and GMTDC-Cd complex across tubular cell membrane, we examined if and how GMTDC’s effect in removing intracellular Cd2+ were affected when glucose transporters SGLT2 or GLUT2 inhibitor, Canagliflozin and Phloretin, was applied. The 3-(4,5-dimethyl-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to assess HK-2 cell viability under different treatment conditions, as described previously (Zhu et al., 2015). Based on the cytotoxicity data observed from MTT assays (Fig. S2, A), 100 μM CdCl2 was selected for HK-2 cells treatment. 5 μM canagliflozin, 50 μM phloretin, and 100 μM GMDTC were selected because our data suggests that this dose does not change the variability of HK-2 cells significantly (Fig. S2, B–D).
2.4. Calcium level tracking with confocal microscopy
The effects of Cd2+ ions on cellular calcium homoeostasis have been well documented. Intracellular Cd2+ level can be monitored and measured indirectly based on the cytoplasm calcium level (Benters et al., 1997; Hinkle et al., 1992). We examined the calcium fluorescence intensity of HK-2 cells using the calcium indicator Fluo-4 AM (Life Tech, Carlsbad, CA) measured by the laser scanning confocal microscope (Zeiss LSM710). The detailed experimental steps were summarized in Table S9. Briefly, 2 × 105 HK-2 cells were seeded on the coverslips. After 12 h cultivation, cells were treated under different conditions and incubated with 5 μM Fluo-4 Am in Krebs-Ringer solution (145 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 M D-glucose). The fluorescence was excited with 488 nm by an argon ion laser and cells were scanned and images were taken using a confocal microscope (Huang et al., 2013). The average fluorescence intensity of 10 cells was calculated and used to represent the calcium concentration. All experiments were repeated three times.
2.5. Animals
Specific pathogen-free male/female Sprague–Dawley (SD, 160–180 g) rats and NIH Swiss mice (NIH, 17–20 g), and male New Zealand white rabbits weighing (2.5–3.5 kg), were purchased from Guangdong Laboratory Animal Center, China (GDMLAC, National certification No. 2006A015). All animals were provided a standard diet and housed in an approved facility with climate control and a 12 h light/12 h dark cycle. The Animal Care and Use Committee of GDMLAC in China approved animal test protocols.
2.6. Acute toxicity study of GMDTC
We investigated the acute toxicity of GMDTC in mice and rats via oral gavage and intraperitoneal injection (i.p.). Fifteen female and fifteen male of mice and rats were randomly grouped into control and two treatment groups (oral gavage and i.p.) with each consisting of 5 male and 5 female animals. The vehicle alone (0.9% saline) was administered via gavage to the control group. In gavage treatment group, we treated both male and female mice and rats with GMDTC in a single dose of 10.1 g/kg body weight. In i.p. injection, we treated both male and female mice and rats with a single dose of 5.1 g/kg body weight.
The experiment lasted for 14 days. Weights of each animal were recorded each day, and mortality/moribundity checks were performed daily. Blood was collected from the retro-orbital venous plexus at 24 h and 14 days after the initial treatment. Blood electrolytes were analyzed by a 7080 Automatic Analyzer (Hitachi Co., Tokyo, Japan). On day 14, rats and mice were euthanized individually in a CO2 chamber and all organs and tissues were observed macroscopically. Kidneys were quickly removed from all animals that were euthanized. Left kidneys were then fixed overnight in 4% (w/v) paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) at 4 °C overnight. Post-fixed kidneys were embedded in paraffin and sectioned. The paraffin-embedded kidney sections were deparaffinized and rehydrated and then stained with hematoxylin and eosin. An experienced pathologist who was blinded to the treatment allocation conducted the pathological assessment by evaluating the paraffin-embedded kidney sections.
2.7. Establish animal models of sub-chronic Cd2+ intoxication
We established Cd2+-laden animal models including mouse, rat and rabbit models. Only male animals were used given that no report indicated a different deposition of Cd2+ between male and female animals. Doses of CdCl2 used were pre-determined in pilot study or based on historical data (data not shown). To establish mouse and rat models of subchronic Cd2+ intoxication, male mice and rats were administered CdCl2 via i.p. injection for five consecutive days with a dose of 6 μmol/kg to mice or 3 μmol/kg to rats, respectively. To establish rabbit model, male rabbits were given 1.5 μmol/kg CdCl2 per day for five consecutive days via intravenous injection (i.v.). Blood samples were drawn from veins (mice/rats) or the auricular artery (rabbits). Some animals were sacrificed at different time points under pentobarbital anesthesia (i.p.). The right kidney was then taken, weighed and used to measure Cd2+ levels. Thirty-five days post the treatment, Cd2+ level in kidney reached a highest and stabilized level, and used for experiments.
2.8. Determine GMDTC’s effect in removing Cd2+ from kidney
Cd2+-laden intoxication animals were divided into 4 groups, including CdCl2 treatment alone group, EDTA positive control group, low dose and high dose GMDTC treatment groups. Same age animals without CdCl2 treatment were included as control. Each group had 10 mice and 6 rats. For rabbits, each group contained 18 rabbits except EDTA and low dose GMDTC group that had 6 rabbits each. Animals in control and CdCl2 treatment alone groups were treated with 0.9% saline, others based on the assigned treatment group were treated with 0.25 mmol/kg EDTA or GMTDC at a dose of 0.25 or 1 mmol/kg for low and high treatment group via i.p. for mice and rats and i.v. for rabbit. Mice and rats were treated for five consecutive days per week for 4 weeks, and blood, urine and biospecimens were collected at the end of treatment. For rabbits, the treatment lasted 4 weeks, and 6 rabbits in each group were randomly picked and sacrificed at the end of 1-week, 2-weeks and 4-weeks during the treatment periods. Biospecimens including urine, blood and tissues were collected at these time points.
2.9. Examine the impact of inhibiting renal glucose transporters on GMDTC’s effect in removing Cd2+ from kidney
The detailed grouping and treatment were shown in the supplement table S10. Briefly, Cd2+-laden mice were randomly divided into 8 subgroups, then treated with GMDTC and/or renal glucose transporters inhibitors (canagliflozin or phloretin) for combined 2 weeks, respectively. On day 14, mice were euthanized individually in a CO2 chamber and kidney tissues were collected for Cd2+ level analysis.
2.10. Urinary Cd2+ analysis by graphite furnace atomic absorption spectroscopy (GFAAS)
Urinary Cd2+ concentrations were obtained in an atomic absorption spectrometer as previously described (Zeini Jahromi et al., 2007). A GFAAS (PerkinElmer Pinnacle 900T) was used throughout the whole work. Working parameters (lamp current: 6.0 mA; spectral bandpass: 0.8 nm, weave length: 228.8 nm) were applied. The urine samples and the standard solutions in water were immediately were digested in 0.5 mL nitric acid at 70 °C. The digest was diluted five times with a solution containing triton X-100 and 10 μL of the solution was injected into the graphite furnace for analysis. Urinary Cd2+ concentration and excretion rate were calculated using the following equation:
2.11. Cd2+ content analysis of blood and kidney by inductively coupled plasma–mass spectrometry (ICP-MS)
Cadmium concentrations in the kidney and blood were measured by inductively coupled plasma-mass spectrometry (ICP-MS; Agilent 7500, Agilent Co. Ltd., Beijing, China) as described previously with modification (Barregard et al., 1999). Briefly, the blood was diluted ten times with a solution containing triton X-100, and then digested in 0.5 mL nitric acid at 70 °C. The kidney samples were dried at 80 °C for 1.5 h in a thermostat-controlled tube. The dried and weighted samples were digested in 0.50 mL of concentrated nitric acid for overnight at 70 °C. After further sample preparation, the ICP-MS data for each sample were generated and collected and processed using software. Cd2+ content in blood and kidney was calculated using the following equation:
3. Results
3.1. GMDTC is relatively non-toxic and has a strong chelating capacity with toxic heavy metals
We synthesized a new chemical derived from dithiocarbamates (DTC), Sodium (S)-2-(dithiocarboxylato((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexyl) amino)-4-(methylthio)butanoate (GMDTC), which was designed and prepared by using methionine and glucose as starting materials (Fig. 1, A). In a test-tube complexometric titration reaction, GMDTC showed a strong chelating capacity with toxic heavy metals, including Cd2+, Pb2+, Hg2+, Tl2+, Cr6+ and Mn2+, but had little or no complexation with body essential elements, e.g., Ca2+, Fe2+, Mg2+, Zn2+, Fe3+ and La2+ (data not shown). Utilizing HPLC-mass spectrometry (HPLC-MS), we determined that the GMDTC/Cd2+ complex contain two GMDTC molecules and one Cd2+ (data not shown). Based on the density functional theory (DFT) method, we predicted that the GMDTC/Cd complex was in a chemical structure as showed in Fig. 1, B.
As recommended by the WHO, chemicals with an acute oral lethal dose (LD50) of more than 5 g per kg (g/kg) body weight are classified as U class (unlikely to present an acute hazard) (United Nations, 2011; WHO, 2009). To reduce the number of animals used, we thus did not take steps to determine the exact LD50 of GMDTC, but instead directly tested the acute toxicity by giving a high dose of GMDTC to mice and rats, i.e., 10,100 mg/kg or 5100 mg/kg body weight, via oral gavage or intraperitoneal injection (i.p.), respectively. At these dose levels, there were no deaths or obvious adverse effects found in the mice or rats 14 days post-GMDTC administration when the blood GMDTC level was below the detection limit. GMDTC-treated mice and rats grew normally and gained weight similarly to the controls (Fig. 1, C & D). The level of key essential metal elements, including Ca2+, Mg2+, Zn2+, Cu2+ and Fe2+, in the blood was not disrupted by GMDTC treatment and kept in the normal range (Table S1). GMDTC thus belongs to the U class of chemicals and is relatively non-toxic, and has an acute oral LD50 of far more than 10,000 mg/kg body weight in mice and rats.
3.2. GMDTC is highly efficient in removing Cd2+ from kidneys among animals tested, i.e. mice, rats and rabbits, in a dose that does not cause significant side effects
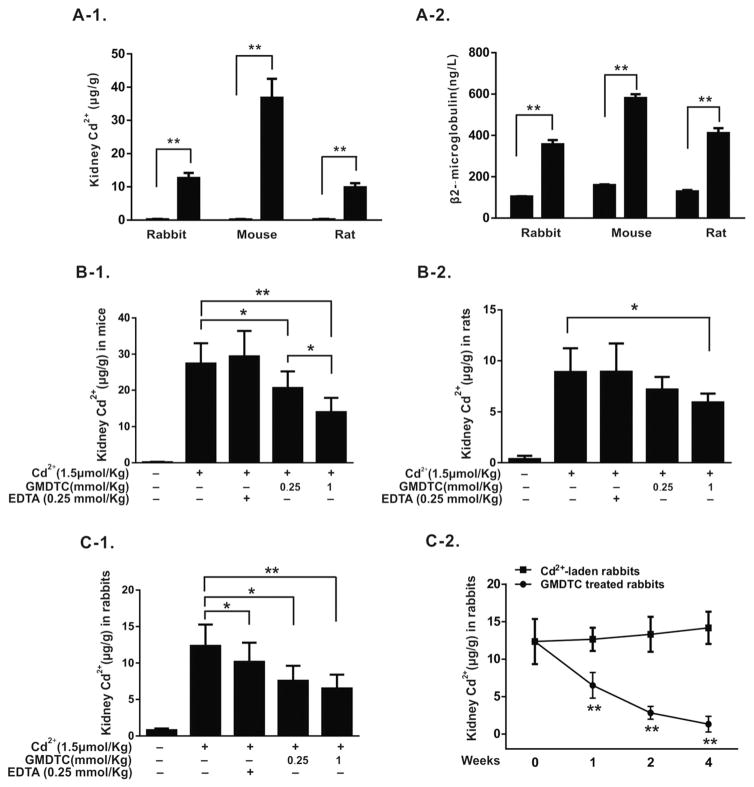
To examine GMDTC’s effects on removing deposited Cd2+ from the kidney, we established chronic Cd2+-poisoned animal models, including mice, rats and rabbits, in which a significant accumulation of Cd2+ in the kidney occurred 35 days post-final injection of Cd2+ (Fig. 2, A-1). Under the dose used, no animal dead, and body weight gain was comparable to the control group and no obvious behavioral changes were observed (data not shown). However, the increased Cd2+ deposition in the kidney did cause the change of the renal function indicated by a significant increase of β2-microglubulin amounts (Fig. 2, A-2) and minor pathological changes of renal tubular areas (Fig. S1).
Fig. 2.
GMDTC’s effect in removing Cd2+ from kidneys. Panel A, Animal models of sub-chronic Cd2+ intoxication. Cd2+ levels were significantly increased in kidneys of Cd2+-laden animals (A-1). Urinary β2-microglobulin level was significantly increased in Cd2+-laden animals (A-2); panel B, GMDTC’s effect in removing Cd2+ from kidneys of mice and rats. Renal Cd2+ levels in mice (B-1) and rats (B-2) were significantly reduced following 4-weeks GMDTC treatment. EDTA at 0.25 mmol/kg was included as a control; panel C, GMDTC’s effect in removing Cd2+ from kidneys of rabbits. A significantly reduced renal Cd2+ level was observed within a week of GMDTC treatment (C-1). EDTA at 0.25 mmol/kg was included as a control. Renal Cd2+ levels were further reduced in a time-depend manner (C-2). * P < 0.05 & ** P < 0.01.
At 35 days post last Cd2+ administration, the animals were treated with GMDTC at a dose of 0.25 or 1.0 mmol/kg via i.p. for mice and rats for 4 weeks and i.v. for rabbits for 1, 2 or 4 weeks. A group of animals treated with EDTA at 0.25 mmol/kg was included for comparison purpose. At these dose levels, GMDTC was well tolerated by all animals with 100% surviving post-administration, and no obvious toxic effects were observed. As shown in table S2, no significant impact of GMDTC on key essential metal elements was found in GMDTC treated mice. We conducted more thorough analyses of the GMDTC’s toxicity on rabbit study. The individual and organs (including brain, heart, liver, spleen, lung, kidney and testis) of each rabbit were weighted. The results showed that no significant impact of GMDTC on body weight of rabbits was observed (Fig. S2). Furthermore, organ coefficients were comparable among these different treatment groups of rabbits, suggesting that sub-chronic exposure of GMDTC has no impact of organ development (Table S3). Additionally, no significant differences of abnormal bone marrow micronuclei between GMDTC experimental group and Cd2+-laden control group were noted (Table S4). Again, we did not observe significant impact on key essential metal elements in kidney, liver, brain and lung of GMDTC treated rabbits (Table S5–S8). GMDTC treatment had no any significant effects on the status of key electrolytes such as sodium, potassium, phosphate and chloride (Table 1). Liver function blood test were used to assess GMDTC’s impact on liver function. However, no significant changes of the function of liver were observed (Table 2).
Table 1.
Key serum electrolytes level in Cd2+-laden rabbits with repeated GMDTC treatment (x̄ ± SD, n = 6).
| Time (week) | Group | P (mmol/L) | K+ (mmol/L) | Na+ (mmol/L) | Cl− (mmol/L) |
|---|---|---|---|---|---|
| 1 | Control | 1.92 ± 0.41 | 4.19 ± 0.40 | 137.83 ± 3.15 | 103.70 ± 4.46 |
| 1 | Cd2+ | 2.44 ± 0.23 | 4.47 ± 0.46 | 136.21 ± 1.66 | 103.27 ± 2.38 |
| 1 | EDTA | 2.45 ± 0.33 | 4.97 ± 0.66 | 135.52 ± 5.02 | 109.35 ± 3.86 |
| 1 | GMDTC-low | 1.77 ± 0.37 | 4.52 ± 0.27 | 136.43 ± 4.10 | 110.90 ± 6.08 |
| 1 | GMDTC-high | 2.08 ± 0.40 | 3.90 ± 0.29 | 136.00 ± 7.42 | 106.35 ± 10.42 |
| 2 | Control | 2.59 ± 0.31 | 3.80 ± 0.66 | 134.00 ± 2.26 | 102.30 ± 7.35 |
| 2 | Cd2+ | 2.57 ± 0.37 | 4.08 ± 0.22 | 140.50 ± 2.91 | 109.56 ± 7.74 |
| 2 | GMDTC-high | 2.41 ± 0.46 | 3.92 ± 0.48 | 137.73 ± 2.59 | 105.40 ± 4.06 |
| 4 | Control | 1.91 ± 0.42 | 4.10 ± 0.38 | 140.17 ± 4.19 | 107.11 ± 7.79 |
| 4 | Cd2+ | 2.52 ± 0.28 | 3.83 ± 0.31 | 139.36 ± 3.22 | 105.71 ± 5.74 |
| 4 | GMDTC-high | 2.12 ± 0.11 | 3.915 ± 0.08 | 139.050 ± 1.09 | 102.125 ± 1.36 |
Note: GMDTC-low: 0.25 mmol/kg; GMDTC-high: 1 mmol/kg.
Table 2.
The blood physiological and biochemical parameter in rabbits after chelation therapy with GMDTC (mean ± SD, n = 6).
| Time (week) | Group | Dose (mmol/kg) | ALT (U/L) | GGT (U/L) | TP (g/L) | A/G | TBIL (μmol/L) | BUN (mmol/L) | GLU (mmol/L) | TC (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | – | 60.750 ± 14.818 | 6.500 ± 1.000 | 56.875 ± 3.841 | 1.233 ± 0.099 | 0.515 ± 0.126 | 8.253 ± 1.951 | 12.735 ± 4.670 | 1.140 ± 0.196 |
| 1 | Cd2+ | – | 59.333 ± 14.418 | 6.667 ± 1.966 | 52.933 ± 3.688 | 1.222 ± 0.177 | 0.473 ± 0.070 | 8.663 ± 1.382 | 14.387 ± 4.506 | 1.153 ± 0.498 |
| 1 | EDTA | 0.25 | 56.500 ± 17.097 | 6.800 ± 1.483 | 55.667 ± 2.464 | 1.363 ± 0.151 | 0.450 ± 0.120 | 8.753 ± 1.534 | 13.105 ± 2.957 | 1.053 ± 0.193 |
| 1 | GMDTC-low | 0.25 | 60.000 ± 16.523 | 6.500 ± 2.121 | 53.700 ± 2.261 | 1.243 ± 0.170 | 0.457 ± 0.111 | 8.600 ± 1.655 | 13.373 ± 4.670 | 1.627 ± 1.289 |
| 1 | GMDTC-high | 1 | 55.333 ± 23.534 | 6.500 ± 1.643 | 53.133 ± 3.902 | 1.200 ± 0.190 | 0.444 ± 0.233 | 8.108 ± 1.202 | 14.973 ± 2.292 | 1.297 ± 1.081 |
| 2 | Control | – | 57.500 ± 2.121 | 7.000 ± 1.414 | 54.400 ± 2.263 | 1.155 ± 0.346 | 0.535 ± 0.007 | 8.240 ± 2.871 | 11.810 ± 4.101 | 1.630 ± 0.396 |
| 2 | Cd2+ | – | 50.400 ± 14.258 | 5.833 ± 2.137 | 55.100 ± 3.396 | 1.248 ± 0.079 | 0.498 ± 0.080 | 7.916 ± 1.793 | 12.505 ± 2.858 | 1.195 ± 0.623 |
| 2 | GMDTC-high | 1 | 57.000 ± 20.659 | 7.250 ± 2.217 | 57.717 ± 2.504 | 1.170 ± 0.080 | 0.422 ± 0.094 | 7.973 ± 1.082 | 12.333 ± 2.629 | 1.198 ± 0.478 |
| 4 | Control | – | 58.000 ± 5.354 | 5.667 ± 1.155 | 55.100 ± 1.407 | 1.185 ± 0.271 | 0.500 ± 0.088 | 8.510 ± 1.187 | 13.763 ± 0.542 | 1.135 ± 0.208 |
| 4 | Cd2+ | – | 52.333 ± 18.107 | 6.500 ± 1.643 | 55.683 ± 1.635 | 1.203 ± 0.111 | 0.408 ± 0.095 | 7.853 ± 1.712 | 12.912 ± 4.412 | 1.435 ± 0.837 |
| 4 | GMDTC-high | 1 | 52.833 ± 18.509 | 5.833 ± 2.229 | 53.967 ± 5.849 | 1.257 ± 0.126 | 0.423 ± 0.092 | 7.975 ± 1.249 | 12.353 ± 3.446 | 1.297 ± 0.392 |
In mice study, renal Cd2+ levels were significantly reduced from 27.4 μg/g kidney weight to 20.6 μg/g kidney weight and 14.1 μg/g kidney weight with continuous GMDTC treatment at 0.25 or 1.0 mmol/kg, respectively. We observed a similar effect in the rat models as with mouse models. Cd2+ levels of kidneys were reduced from 8.9 μg/g kidney weight to 7.1 μg/g or 5.9 μg/g kidney weights at 0.25 or 1.0 mmol/kg GMDTC treatment, respectively. The effect of GMDTC in removing Cd2+ from kidneys of Cd2+-laden rabbits was even more inspiring. Continuous i.v. injection of GMDTC at a level of 1.0 mmol/kg body weight for one week elicited a 50.6% reduction in renal Cd2+ concentration (Fig. 2, C-1 & C-2; Table 3). By 4 weeks, up to 94% of Cd2+ in the kidney was removed, and renal Cd2+ level was reduced from 12.9 μg/g kidney weight to 1.3 μg/g kidney weight (Fig. 2, C-2; Table 3). Concomitant to kidney Cd2+ reduction post GMDTC treatment, a significantly increased level of urinary Cd2+ was observed (Table 3). The highest Cd2+ excretion via urine was seen within the first 6 h after the GMDTC treatment, suggesting that GMDTC’s effect in removing Cd2+ from kidney was happening immediately and utilizing a quick excretion mechanism. At the same time, the removing of Cd2+ from kidney is associated with a significant decrease of blood Cd2+ level and the blood Cd2+ decorporation rate was 13.4%, 17.6% and 37.8% at 1, 2 and 4 weeks following GMDTC treatment, respectively, indicating that GMDTC-Cd complex was seldom reabsorbed back to blood by kidney. Moreover, GMDTC treatment could significantly reduce the amount of Cd2+ transported to brain compared with the CdCl2 group, indicating that GMDTC-Cd complex had a less capacity in passing the blood-brain barrier than Cd2+ (data not shown). The effect of EDTA in removing Cd2+ from kidney is much less significant than GMDTC’s effect. EDTA exposure had no significant effects on renal Cd2+ levels post 4 weeks treatment in both mouse and rat studies, and it also has a much lower efficiency in removing renal Cd2+ in comparison with GMDTC treatment (Fig. 2 & Table 3).
Table 3.
Blood, kidney and urine Cd2+ levels in rabbits after chelation therapy with GMDTC (mean ± SD, n = 6).
| Time (week) | Group | Dose (mmol/kg) | Kidney Cd2+ (μg/g) | Kidney Cd2+ decorporation rate (%) | Blood Cd2+ (μg/L) | Blood Cd2+ decorporation rate (%) | 0–6 h urinary Cd2+ (μg) | 0–6 h urinary Cd2+ excretion rate (μg/h) | 7–24 h urinary Cd2+ (μg) | 7–24 h urinary Cd2+ excretion rate (μg/h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | – | 0.797 ± 0.225 | – | 1.059 ± 0.387 | – | 0 | – | 0 | – |
| 1 | Cd2+ | – | 12.358 ± 3.013** | – | 88.534 ± 9.165** | – | 0.030 ± 0.037* | 0.005 | 0.017 ± 0.296* | 0.001 |
| 1 | EDTA | 0.25 | 10.173 ± 2.816**△ | 18.90 | 77.589 ± 12.967** | 12.51 | 0.284 ± 0.166**△△ | 0.042 | 0.069 ± 0.079*△ | 0.003 |
| 1 | GMDTC-low | 0.25 | 7.570 ± 2.042**△ | 41.42 | 91.502 ± 8.048** | – | 1.748 ± 1.851**△△◇◇ | 0.286 | 0.043 ± 0.067*△ | 0.002 |
| 1 | GMDTC-high | 1 | 6.506 ± 2.208**△△ | 50.62 | 76.504 ± 20.004** | 13.75 | 1.911 ± 2.580**△△◇◇ | 0.314 | 0.477 ± 1.68**△△ | 0.026 |
| 2 | Control | – | 0.192 ± 0.126 | – | 1.724 ± 7.271 | – | 0 | – | 0 | – |
| 2 | Cd2+ | – | 11.752 ± 2.102** | – | 64.981 ± 9.042**○ | – | 0.038 ± 0.028* | 0.006 | 0.039 ± 0.028* | 0.002 |
| 2 | GMDTC-high | 1 | 2.838 ± 0.852**△△ | 77.11 | 53.846 ± 12.529**△○ | 17.60 | 1.382 ± 1.720**△△ | 0.230 | 0.200 ± 0.114**△△ | 0.011 |
| 4 | Control | – | 0.600 ± 0.422 | – | 0.458 ± 0.259 | – | 0 | – | 0 | – |
| 4 | Cd2+ | – | 12.890 ± 5.087** | – | 24.066 ± 7.215**○○ | – | 0.012 ± 0.016* | 0.002 | 0.009 ± 0.012* | 0.001 |
| 4 | GMDTC-high | 1 | 1.336 ± 1.645*△△ | 94.01 | 15.143 ± 1.131**△○○ | 37.80 | 0.292 ± 0.298**△△ | 0.047 | 0.023 ± 0.027**△△ | 0.001 |
Note:
P < 0.05 vs control group at the same week;
P < 0.01 vs control group at the same week;
P < 0.05 vs Cd2+ group at the same week;
P < 0.01 vs Cd2+ group at the same week;
P < 0.05 vs GMDTC-high group at week 1;
P < 0.01 vs GMDTC-high group at week 2;
P < 0.01 vs EDTA group at week 1.
3.3. GMDTC and the GMDTC-Cd complex are transported into and out of the tubular cells via renal glucose reabsorption systems
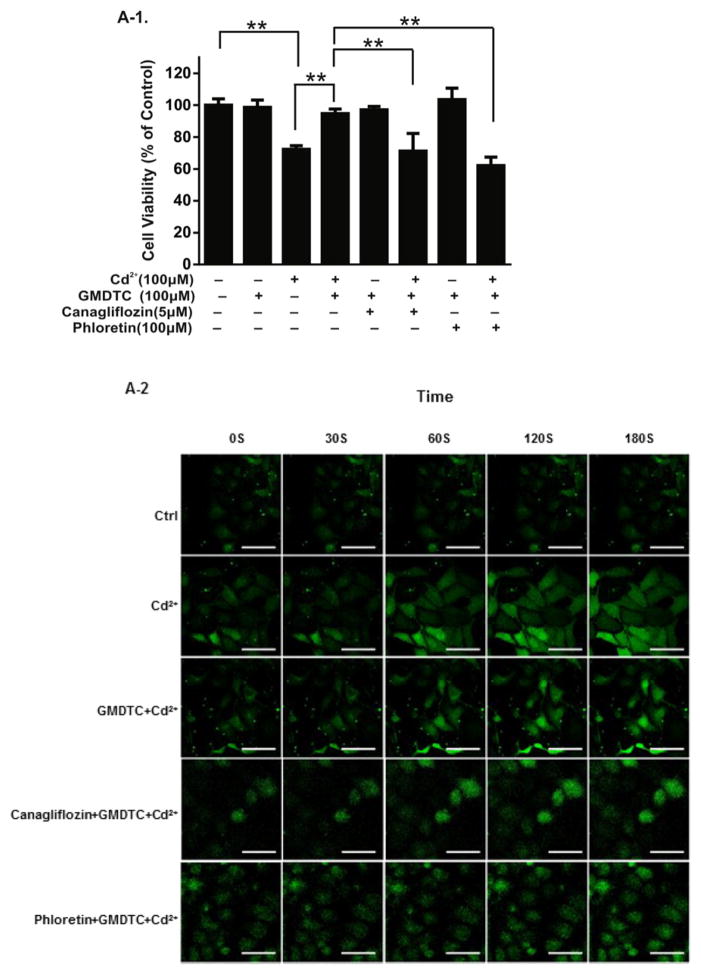
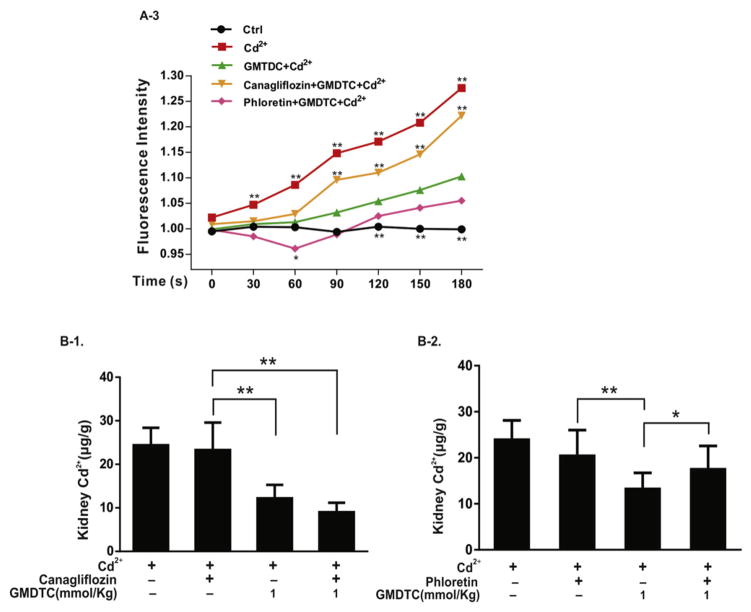
GMDTC was synthesized using glucose as one of the raw materials, and the chemical structure of GMDTC contains a glucose group. We speculated that the transport of GMDTC and GMDTC-Cd complex into and out of tubular cells may utilize glucose reabsorption systems, i.e., via the sodium-glucose linked transporter 2 (SGLT2) and glucose transporter 2 (GLUT2), respectively. To test this hypothesis, we examined the GMDTC’s effect in removing Cd2+ in vitro (Human Kidney 2 cells (HK-2)) and in the mouse model when either a SGLT2 inhibitor (Canagliflozin) or GLUT2 inhibitor (Phloretin) was applied. Cd2+ exposure induced a dose-dependent cytotoxic effect in HK-2 cells (Fig. S3, A). In comparison, GMDTC has no cytotoxic effects on HK-2 cells following 24 h exposure at the level below 400 μM (Fig. S3, B). At as low as 12.5 μM level, GMDTC was able to significantly reduce CdCl2 induced cytotoxicity on HK-2 cells (Fig. S3, E). However, when HK-2 cells were pre-treated with either canagliflozin or phloretin, the protective effects of GMDTC even at 100 μM level against CdCl2 induced cytotoxicity on the HK-2 cell were disappeared (Fig. 3, A-1). Transient spike of cytosolic Ca2+ is a common phenomenon observed when cells were exposed to Cd2+ (Benters et al., 1997; Hinkle et al., 1992) as shown in Fig. 3, A-2 (Faurskov and Bjerregaard, 1997; WHO, 2010). Co-treatment GMDTC and Cd2+ of HK-2 cells were associated with a significantly compromised reaction in spiking cytosolic Ca2+ (Fig. 3, A-2 & A-3). Pre-treatment of HK-2 cells by canagliflozin could blocked GMDTC’s effect, and intracellular Ca2+ concentration remained significantly higher post Cd2+ exposure, even with GMDTC exposure. In comparison, as we expected, GMDTC’s effect in inhibiting Cd2+-induced transient spike of cytosolic Ca2+ remained when GLUT2 was inhibited by phloretin on HK-2 cells because of temporally increased GMDTC level inside of HK-2 cells (Fig. 3, A-2&A-3). These results suggest that GMDTC and the GMDTC-Cd complex transported into and out of cells are assisted by glucose reabsorption systems, more specifically, through SGLT2 and GLUT2 at membranes of renal tubular cells. In animal study, as projected, when the activity of GLUT2 is inhibited by phloretin, the phloretin + GMDTC + Cd2+ group shows a higher renal Cd2+ level than the GMDTC + Cd2+ group (Fig. 3, B-2). However, when the activity of SGLT2 was inhibited by continuously given canagliflozin, we observed a further decrease of renal Cd2+ level in the canagliflozin + GMDTC + Cd2+ group mice compared with the GMDTC + Cd2+ group while the difference was not significant (Fig. 3, B-1).
Fig. 3.
SGLT2 and GLUT2 transporters mediate the transportation of GMDTC and the GMDTC-Cd complex into and out of renal tubular cells. Panel A, in vitro study. HK-2 cells were treated with CdCl2, GMDTC, canagliflozin or phloretin or their combinations for 24 h, and cell viability was evaluated by MTT assay (A-1). The data was from three independent experiments; time course of the cytoplasmic Ca2+ levels of HK-2 cells post Cd2+ and/or GMDTC exposure when HK-2 cells were pre-incubated with canagliflozin or phloretin. Representative images under confocal laser scanning microscope were shown (A-2, scale bar = 5 μm); the inflorescence intensity of cytoplasmic Ca2+ was recorded under confocal laser scanning microscope and presented in A-3. The data was from three independent experiments, and error bar with standard deviations was removed for clear presentation. (* P < 0.05 & ** P < 0.01, in comparison with GMDTC + Cd2+ group). Panel B, in vivo study. Canagliflozin pretreatment has a little impact on GMDTC’s effect in removing Cd2+ from kidney (B-1), n = 6; GMDTC’s effect in removing Cd2+ from kidney was compromised when Cd2+-laden mice were pretreated with phloretin (B-2), n = 6 (* P < 0.05 & ** P < 0.01).
4. Discussion
Cd2+ toxicity and treatment has attracted great interest in scientific and medical societies because of the cumulative properties of Cd2+ and subsequent damage to the kidney. When chronic toxicity, e.g., renal tubular damage, was observed, the exposure had continued for a long lag period of several years and pathological changes in the kidneys had already occurred and were usually irreversible (Satarug and Moore, 2004). If the deposited Cd2+ in kidney cannot be removed, the renal damage will be further developed and the consequences could be deleterious. Therefore, the removal of further exposure and removing deposited Cd2+ from kidneys are the most desired treatment procedures for Cd2+induced renal toxicity. However, the development of a safe chelation therapy with high efficiency in removing Cd2+ from kidney has proven extremely difficult.
Currently available chelating agents, such as EDTA, BAL, DMPS, and DMSA have not been clinically approved due to a number of safety and efficacy issues (Bernhoft, 2013), while many of these chelating agents have showed certain effects in the acceleration of Cd2+ excretion via urine (Nerudova et al., 1991; Satarug and Moore, 2004). BAL is an organic dithiol compound and associated with severe side effects (Flora and Pachauri, 2010). DMSA is also a dithiol compound with relatively fewer side effects than BAL, but its ability to access intracellular metals is weak and ineffective in removing Cd2+ from the kidney (Gale et al., 1983). EDTA is the agent most studied (Kelley, 1999). Studies in vitro and in vivo suggest that EDTA is superior to DMSA in mobilizing intracellular Cd2+ (Andersen et al., 1988; Borenfreund and Puerner, 1986). However, EDTA lacks specificity and can affect other essential metals such as zinc, iron and manganese, thus disrupting the balance of these elements in the body and leading to side effects (Flora and Pachauri, 2010). Moreover, EDTA cause nephrotoxicity (at the proximal tubule particularly), especially with repeated high-dose treatment (above 75 mg/kg) and in subjects with a previous history of kidney damage (Zhai et al., 2015).
In recent years, a new type of chelating agents has been developed, named DTC or diethyldithiocarbamape (DDC), and their derivatives, such as N-(4-methoxybenzyl)-N-dithiocarboxy-D-glucamine (4-Me-DTC) and N-(2,3,4,5, 6-pentahydroxylhexyl)-L-cysteine (GCDTC). They exhibit potent metal-binding properties and better results in accessing intracellular Cd2+. In general, they also have a lower acute toxicity than EDTA and several other chelating agents (Andersen and Nielsen, 1989; Gale et al., 1989;Wang et al., 2004; Zhao et al., 1990), thus making them an attracting target for the prevention and treatment of chronic Cd2+ intoxication. However, unwanted or unexpected side effects are also found with these new chelating agents (Andersen and Nielsen, 1989; Gale et al., 1989; O’Callaghan and Miller, 1986; Shiqi Peng et al., 2000; Wang et al., 2004; Zhao et al., 1990). Here, we demonstrated that our newly synthesized chemical, GMDTC, has almost all the properties that make it a desirable chelating agent, including its high capacity to irreversibly bind the toxic metal, low affinity for essential metals, accessibility of the kidney and bile to allow rapid elimination of the metal, low toxicity and limited metabolic transformation of itself and of the metal complex, and so on (Sinicropi et al., 2010). GMDTC has a very low acute toxicity with an acute oral LD50 far more than 10 g/kg body weight, thus can be classified as a U-class chemical based on the WHO’s standard of chemical classification. In both high dose acute exposure and sub-chronic repeated exposure, no significant side effects were produced by GMDTC in multiple animal models tested. Moreover, GMDTC treatment had no significant impacts on body key essential metal elements, a side effect that is observed in many other chelating agents. But more importantly, in a dose that didn’t cause any observed toxicity in treated animals, GMDTC treatment is accompanied with an astonishingly high efficiency in reducing renal Cd2+ concentration (Fig. 2). Renal Cd2+ concentration was reduced by 50.6%, 77.1% and 94% post a one-, two- or four-week GMDTC treatment via i.v. at dose of 1 mmol/kg in a Cd2+-laden rabbit model. Even with a much lower dose of GMDTC (0.25 mmol/kg), renal Cd2+ level was reduced by 41.1% after just one-week treatment. GMDTC’s effect in removing renal Cd2+ was lower in mouse and rat model than in rabbit model but remain highly significant. Our study showed that the binding and excretion of Cd2+ via urine from body is the most significant within 6-h after the GMDTC treatment, and significantly lower afterward. The decreased efficiency between mouse/rat studies and rabbit study is likely due to the different administration routes, as it takes a longer time for GMDTC to reach kidneys through i.p. vs. i.v. Moreover, GMDTC-Cd complex is not accumulated in body as indicated by an increased decorporation rate of blood Cd2+ following continuous GMDTC treatment.
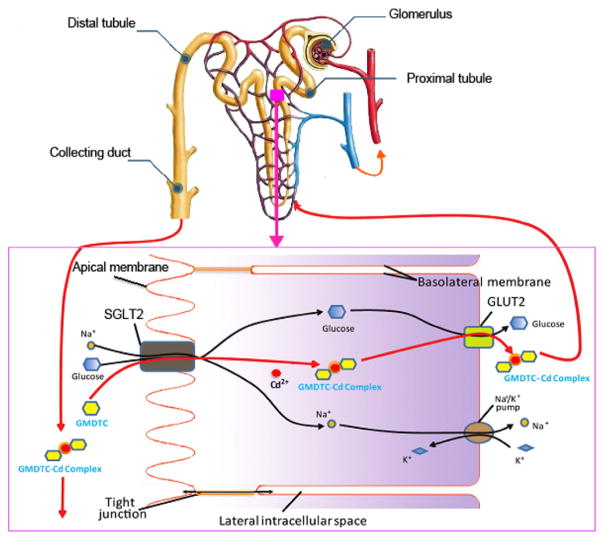
In the kidney, Cd2+ is mainly deposited in tubular cells, and the half-life of Cd2+ in kidney is up to several decades (Nordberg and Nordberg, 2002). Thus, an effective chelating agent that can be applied in clinical use has to be able to access Cd2+ in renal tubular cells, transport them out, and excrete them via urine, which was what we observed for GMDTC. GMDTC and GMDTC-Cd complex are relative large and hydrophilic molecules, it is thus unlikely that GMDTC and the GMDTC-Cd complex move into and out of tubular cells by a passive transport mechanism, i.e., via a concentration gradient. The kidney plays a central role in glucose reabsorption, in which the glucose in the proximal tubule is transported into the proximal convoluted tubule walls via SGLT2 at apical membrane (Mather and Pollock, 2011; Vallon, 2011). Once into the tubule cell, the glucose can be diffused along a glucose concentration gradient or transported utilizing a facilitative glucose transporter, GLUT2 at basolateral membrane (Chin et al., 1993). Our results, particularly in vitro study, suggested that GMDTC enters into and GMDTC-Cd complex transports out of tubular cells are mainly assisted by renal glucose reabsorption system utilizing SGLT2 and GLUT2 transporters (Fig. 3). The results in vivo studies are not completely same as the in vitro study. Although we observed a similar result in animal study as in vitro experiment when GLUT2 inhibitor was administered, the inhibition of the SGLT2 activity by canagliflozin does not affect GMDTC’s effect in removing renal Cd2+. One possible explanation is that GMDTC can get into renal tubular cells by multiple mechanisms. However, we believe that it is more likely due to the inhibition of SGLT2 activity by canagliflozin is not efficient or complete, as showed previously that SGLT2 inhibitors can only inhibit about 30–50% of renal glucose reabsorption (Liu et al., 2012). Altogether, as illustrated in Fig. 4, we believe that GMDTC’s effect in removing Cd2+ from kidney is mainly mediated by glucose reabsorption system. At the glomerulus of the nephron, GMDTC is actively transported across the epithelium of the proximal tubule via SGLT2. In renal tubule cells, two GMDTC molecules bind to one Cd2+ ions that forms the GMDTC-Cd complex, which is then released from the lumen to the blood of the tubule across the basolateral membrane facilitated by GLUT2. The majority of formed GMDTC-Cd complexes is seldom reabsorbed by SGLT2 and is consequently excreted from the body through the urine.
Fig. 4.
Schematic diagram of Cd2+ decorporation from kidney by GMDTC.
5. Conclusions
Collectively, we show that the newly synthesized chemical, GMDTC, is safe and low toxicity, but highly efficient in Cd2+ decorporation from the kidney, which is likely mediated by renal glucose transporters, SGLT2 and GLUT2. While more studies in further deciphering the mechanisms of GMDTC in removing Cd2+ from kidney and understanding the pharmacokinetics and determining the toxicity of GMDTC particularly those related to long-term exposure are warranted, all characteristics associated with GMDTC make it an ideal agent used for both preventive and therapeutic purposes for kidney damage and other adverse health effects caused by chronic Cd2+ exposure.
Acknowledgments
We thank Prof. Jianmin Jiang (School of Pharmaceutical Sciences, Sun Yat-Sen University, China) for suggestions on in vitro study, and Prof. Joseph Graziano (Mailman School of Public Health, Columbia University) for comments and preparation of the final manuscript. This work was supported by National Major New Drug Development Project of China (2015GKH-384 to X.T.), Guangdong Science and Technology Major Planning Project, China (2012A080201011 to X.T.), Guangdong Science and Technology Special Funding, China (2016A030310319 to Z.Z.) and Foundation of Foshan Cooperation Project, Guangdong, China (2012HY100302 to X.T.); and Startup fund (to X.R.) provided by the State University of New York, Buffalo.
Footnotes
Conflict interests
GMDTC is under protection by the patent, ZL 200510035377 to X.T.
Transparency document
The Transparency document associated with this article can be found, in the online version.
References
- Andersen O, Nielsen JB. Effects of diethyldithiocarbamate on the toxicokinetics of cadmium chloride in mice. Toxicology. 1989;55(1–2):1–14. doi: 10.1016/0300-483x(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Andersen O, Nielsen JB, Svendsen P. Oral cadmiumchloride intoxication in mice: effects of chelation. Toxicology. 1988;52(1–2):65–79. doi: 10.1016/0300-483x(88)90197-7. [DOI] [PubMed] [Google Scholar]
- Barregard L, Svalander C, Schutz A, et al. Cadmium, mercury, and lead in kidney cortex of the general Swedish population: a study of biopsies from living kidney donors. Environ Health Perspect. 1999;107(11):867–871. doi: 10.1289/ehp.107-1566723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benters J, Flogel U, Schafer T, Leibfritz D, Hechtenberg S, Beyer smann D. Study of the interactions of cadmium and zinc ions with cellular calcium homoeostasis using 19F-NMR spectroscopy. Biochem J. 1997;322(Pt 3):793–799. doi: 10.1042/bj3220793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoft RA. Cadmium toxicity and treatment. The Scientific World JOURNAL. 2013;2013:394652. doi: 10.1155/2013/394652. http://dx.doi.org/10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E, Puerner JA. Cytotoxicity of metals, metal-metal and metal-chelator combinations assayed in vitro. Toxicology. 1986;39(2):121–134. doi: 10.1016/0300-483x(86)90130-7. [DOI] [PubMed] [Google Scholar]
- Chin E, Zhou J, Bondy C. Anatomical and developmental patterns of facilitative glucose transporter gene expression in the rat kidney. J Clin Invest. 1993;91(4):1810–1815. doi: 10.1172/JCI116392. http://dx.doi.org/10.1172/JCI116392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroon O, Ashizawa A, Wright S, et al. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles; Atlanta (GA): 2012. [PubMed] [Google Scholar]
- Faurskov B, Bjerregaard HF. Effect of cadmium on active ion transport and cytotoxicity in cultured renal epithelial cells (A6) Toxicol in Vitro. 1997;11(5):717–722. doi: 10.1016/s0887-2333(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7(7):2745–2788. doi: 10.3390/ijerph7072745. http://dx.doi.org/10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GR, Atkins LM, Walker EM, Jr, Smith AB. Comparative effects of diethyldithiocarbamate, dimercaptosuccinate, and diethylenetriaminepentaacetate on organ distribution and excretion of cadmium. Ann Clin Lab Sci. 1983;13(1):33–44. [PubMed] [Google Scholar]
- Gale GR, Atkins LM, Smith AB, Jones SG, Jones MM. Dithiocarbamate treatment of chronic cadmium intoxication in mice. Toxicol Lett. 1988;44(1–2):77–84. doi: 10.1016/0378-4274(88)90132-4. [DOI] [PubMed] [Google Scholar]
- Gale GR, Atkins LM, Smith AB, Singh PK, Jones MM. N, N-disubstituted dithiocarbamates as cadmium antagonists: N-(4-methoxybenzyl)-N-dithiocarboxy-D-glucamine. Toxicol Lett. 1989;48(1):105–115. doi: 10.1016/0378-4274(89)90191-4. [DOI] [PubMed] [Google Scholar]
- Hafez MAH, Khalifa ME. Complexometric determination of some toxic mixtures of ions using bromo-cresol orange with visual endpoint indication. Talanta. 1997;44(5):787–796. doi: 10.1016/S0039-9140(96)02106-6. [DOI] [PubMed] [Google Scholar]
- Hinkle PM, Shanshala ED, 2nd, Nelson EJ. Measurement of intracellular cadmium with fluorescent dyes. Further evidence for the role of calcium channels in cadmium uptake. J Biol Chem. 1992;267(35):25553–25559. [PubMed] [Google Scholar]
- Huang L, Liu Y, Zhang P, et al. In vitro dose-dependent inhibition of the intracellular spontaneous calcium oscillations in developing hippocampal neurons by ketamine. PLoS One. 2013;8(3):e59804. doi: 10.1371/journal.pone.0059804. http://dx.doi.org/10.1371/journal.pone.0059804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. International agency for research on cancer monographs on the evaluation of carcinogenic risks to humans. IARC Monogr Eval Carcinog Risks Hum. 1993;58:1–444. [PMC free article] [PubMed] [Google Scholar]
- Kelley C. Cadmium therapeutic agents. Curr Pharm Des. 1999;5(4):229–240. [PubMed] [Google Scholar]
- Liu JW, Lee T, DeFronzo RA. Why do SGLT2 inhibitors inhibit only 30–50% of renal glucose reabsorption in humans? Diabetes. 2012;61(9):2199–2204. doi: 10.2337/db12-0052. http://dx.doi.org/10.2337/db12-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Supplement. 2011;(120):S1–S6. doi: 10.1038/ki.2010.509. http://dx.doi.org/10.1038/ki.2010.509. [DOI] [PubMed]
- Nawrot TS, Staessen JA, Roels HA, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals. 2010;23(5):769–782. doi: 10.1007/s10534-010-9343-z. http://dx.doi.org/10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- Nerudova J, Blaha K, Sokal A, Jehlickova H, Cikrt M. Mobilization of aged cadmium from isolated rat hepatocytes by sulfhydryl chelators. Pol J Occup Med Environ Health. 1991;4(4):349–357. [PubMed] [Google Scholar]
- Nordberg GF. Chelating agents and cadmium toxicity: problems and prospects. Environ Health Perspect. 1984;54:213–218. doi: 10.1289/ehp.8454213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg M, Nordberg GF. In: Heavy Metals in the Environment. Sarkar B, editor. Chapter 8 Marcel Dekker; New York: 2002. pp. 231–270. [Google Scholar]
- O’Callaghan JP, Miller DB. Diethyldithiocarbamate increases distribution of cadmium to brain but prevents cadmium-induced neurotoxicity. Brain Res. 1986;370(2):354–358. doi: 10.1016/0006-8993(86)90493-2. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112(10):1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiqi Peng CW, Zhao M, Zhao J, Ma D. Anti-cadmium medicines and their synthesis and medical application 2000 [Google Scholar]
- Sinicropi MS, Amantea D, Caruso A, Saturnino C. Chemical and biological properties of toxic metals and use of chelating agents for the pharmacological treatment of metal poisoning. Arch Toxicol. 2010;84(7):501–520. doi: 10.1007/s00204-010-0544-6. http://dx.doi.org/10.1007/s00204-010-0544-6. [DOI] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme) [accessed 29th January 2016];Final review of scientific information on cadmium. 2010 Available: http://www.unep.org/chemicalsandwaste/
- United Nations. [accessed 29th January 2016];Globally harmonized system of classification and labelling of chemicals (GHS) 2011 Available: http://www.unece.org.
- Vallon V. Molecular determinants of renal glucose reabsorption. Focus on “Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2”. Am J Physiol Cell Physiol. 2011;300(1):C6–C8. doi: 10.1152/ajpcell.00444.2010. http://dx.doi.org/10.1152/ajpcell.00444.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Fang Y, Peng SQ, Ma DX, Zhao JY. Synthesis of novel chelating agents and their effect on cadmium decorporation. Chem Res Toxicol. 1999;12(4):331–334. doi: 10.1021/tx970134z. http://dx.doi.org/10.1021/Tx970134z. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao M, Yang J, Li X, Peng S. Synthesis and evaluations of pentahydroxylhexyl-L-cysteine and its dimer as chelating agents for cadmium or lead decorporation. Toxicol Appl Pharmacol. 2004;200(3):229–236. doi: 10.1016/j.taap.2004.04.009. http://dx.doi.org/10.1016/j.taap.2004.04.009. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) WHO International Programme on Chemical Safety. 2009. The WHO recommended classification of pesticides by hazard and guidelines to classification; pp. 1–78. [Google Scholar]
- WHO (World Health Organization) [accessed 29th January 2016];Exposure to Cadmium: A Major Publish Health Concern. 2010 Available: http://www.who.int/ipcs/assessment/public_health/cadmium.
- Zeini Jahromi E, Bidari A, Assadi Y, Milani Hosseini MR, Jamali MR. Dispersive liquid-liquid microextraction combined with graphite furnace atomic absorption spectrometry: ultra trace determination of cadmium in water samples. Anal Chim Acta. 2007;585(2):305–311. doi: 10.1016/j.aca.2007.01.007. http://dx.doi.org/10.1016/j.aca.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Narbad A, Chen W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients. 2015;7(1):552–571. doi: 10.3390/nu7010552. http://dx.doi.org/10.3390/nu7010552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JY, Foulkes EC, Jones M. Delayed nephrotoxic effects of cadmium and their reversibility by chelation. Toxicology. 1990;64(3):235–243. doi: 10.1016/0300-483x(90)90116-x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Dubois A, Ge Y, Olson JA, Ren X. Application of human haploid cell genetic screening model in identifying the genes required for resistance to environmental toxicants: chlorpyrifos as a case study. J Pharmacol Toxicol Methods. 2015;76:76–82. doi: 10.1016/j.vascn.2015.08.154. http://dx.doi.org/10.1016/j.vascn.2015.08.154. [DOI] [PMC free article] [PubMed] [Google Scholar]