Abstract
Argon (Ar) is a noble gas with known organoprotective effects in rodents and in vitro models. In a previous study we failed to find a postconditioning effect of Ar during ex vivo lung perfusion (EVLP) on warm-ischemic injury in a porcine model. In this study, we further investigated a prolonged exposure to Ar to decrease cold ischemia-reperfusion injury after lung transplantation in a porcine model with EVLP assessment. Domestic pigs (n = 6/group) were pre-conditioned for 6 hours with 21% O2 and 79% N2 (CONTR) or 79% Ar (ARG). Subsequently, lungs were cold flushed and stored inflated on ice for 18 hours inflated with the same gas mixtures. Next, lungs were perfused for 4 hours on EVLP (acellular) while ventilated with 12% O2 and 88% N2 (CONTR group) or 88% Ar (ARG group). The perfusate was saturated with the same gas mixture but with the addition of CO2 to an end-tidal CO2 of 35-45 mmHg. The saturated perfusate was drained and lungs were perfused with whole blood for an additional 2 hours on EVLP. Evaluation at the end of EVLP did not show significant effects on physiologic parameters by prolonged exposure to Ar. Also wet-to-dry weight ratio did not improve in the ARG group. Although in other organ systems protective effects of Ar have been shown, we did not detect beneficial effects of a high concentration of Ar on cold pulmonary ischemia-reperfusion injury in a porcine lung model after prolonged exposure to Ar in this porcine model with EVLP assessment.
Keywords: argon, ischemia-reperfusion injury, ex vivo lung perfusion, preconditioning, postconditioning, lung transplantation, noble gases, porcine
Introduction
Lung transplantation is still hampered by a shortage of transplantable donor grafts, and those grafts that are available are often of limited quality.1 More and more, extended-criteria donor lungs are being used for organ transplantation. Although 1-year survival is similar,2 there is a higher incidence of severe primary graft dysfunction (PGD) among recipients of an extended-criteria donor lung.3 PGD is the end-result of ischemia-reperfusion injury (IRI) affecting the integrity of the capillary-alveolar membrane leading to pulmonary oedema and impaired oxygenation.4,5 Severe PGD is associated with an impaired short-term and long-term outcome.6 Therefore, there is a need to address PGD by improving donor lung quality, which is referred to as “reconditioning”. Organ reconditioning can be achieved at three different stages: prior to ischemic organ injury (preconditioning), during organ ischemia (perconditioning) or after onset of organ reperfusion (postconditioning). Preconditioning is known as classical donor management, perconditioning involves optimization of the “out of body” time (preservation) and postconditioning is seen as strategies after reinstallation of perfusion.
Our previous study, which investigated a postconditioning effect of argon (Ar) (and xenon (Xe)) on pulmonary ischemia-reperfusion injury, could not confirm such a positive reconditioning effect in lungs7; despite the fact that beneficial postconditioning effects of Ar have been shown in other injury models such as cardiac arrest,8,9 brain injury,10 and neonatal asphyxia.11 The noble gases Ar and Xe have also previously been investigated as a reconditioning strategy in IRI kidney transplant models showing beneficial effects on graft function.12,13 But since the protective effect of Ar seems to be dose-dependent,14 a higher and longer exposure to Ar to protect against pulmonary ischemia reperfusion injury seemed justified. A prolonged exposure to Ar can be achieved by introducing the gas earlier in the process of organ donation.15 During prolonged ventilation of the donor, Ar might protect and precondition the pulmonary graft prior to the ischemic insult (“preconditioning”). In addition, the lung can be inflated with the potentially protective gas mixture during cold storage (“perconditioning”). Postconditioning can be achieved by ventilating the lung after implantation in the recipient or by administrating the gas during ex vivo lung perfusion (EVLP). EVLP can be considered as a platform of reperfusion and preservation of the graft prior to transplantation. During EVLP, ventilating the donor lung prior to transplantation, in normothermic conditions with inhalational therapy is feasible.16,17 Also, the perfusion solution on EVLP could be saturated with an Ar mixture, as demonstrated with success in experimental perfusion of kidneys preceding transplantation.12,13 Replacing nitrogen (N2) with Ar in the gas mixture for the ventilator and for the gas exchanger in the EVLP system, results in a dual exposure of the pulmonary graft to Ar, on both the endothelial and epithelial sides (“postconditioning”).
This study investigated the effects of Ar on pulmonary ischemia-reperfusion injury when administered prior (preconditioning), during (perconditioning) and after (postconditioning) the ischemic insult to donor lungs. Normothermic EVLP was also used to evaluate the treatment on graft function.
Materials and Methods
Animals
Male domestic pigs n = 6/group (mean weight 35.7 kg, Topig 20, Zoötechnisch centrum KU Leuven, Lovenjoel, Belgium) were used following local ethical approval obtained at the research institute (P043/2014). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health, USA (NIH Publication No. 86-23, revised 1996).
Animal anaesthesia and study groups
Anesthesia was induced with an intramuscular injection of 5 mg/kg Zoletil 100 (Virbac, Carros, France) and 3 mg/kg xylazine 2% (VMD, Arendonk, Belgium). After placement of an 18GA peripheral line (v. auricularis), muscle relaxation and analgesia were maintained with 2 mg pancuronium boli and 20 μg/kg/h fentanyl. Anesthesia was maintained with continuous infusion of 7-10 mg/kg/h propofol. Animals were intubated with a 7.0 mm endotracheal tube and ventilated with a tidal volume (TV) of 8 mL/kg and 5 cmH2O positive end-expiratory pressure (PEEP). Respiratory rate was adjusted to obtain an end-tidal carbon dioxide (ETCO2) of 35-45 mmHg. Invasive arterial blood pressure (ABP) was measured in the right carotid artery and a 7.5 Fr Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA, USA) was introduced through the right internal jugular vein to measure pulmonary pressures. Vigilance monitor (Edwards Lifesciences) was connected to monitor mixed venous saturation (SvO2) and continuous cardiac output (CCO).
Animals (n = 6/group) were randomly divided into two groups: CONTR group and ARG group (Table 1). After baseline instrumentation, animals were preventilated (preconditioned) for 6 hours with either 21% O2 /79% N2 (CONTR group) or 21% O2 /79% Ar (ARG group). The TV of 8 mL/kg was corrected for density differences in the ARG group with the following formula:
Table 1.
Protocol outline
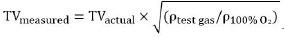
The density of the test gas (21% O2/79% Ar) was 1.505 kg/m3. Respiratory rate was adjusted to maintain an ETCO2 of 35-45 mmHg. Every hour, lungs were recruited by increasing the PEEP temporarily from 5 cmH2O to 20 cmH2O. After 6 hours of preconditioning, animals were heparinized (300 IU/kg) and 1 L blood was drained to be stored with citrate-phosphate-dextrose solution with adenine (CPDA, Sigma-Aldrich, Inc, St. Louis, MO, USA) at room temperature until the next day. Lungs were cold flushed antegradely (50 mL/kg) with OCS Solution (Transmedics, Andover, MA, USA) while ventilated with 7 mL/kg TV and 8 cmH2O PEEP. Lungs were then inflated with the same gas mixture used during the preconditioning phase, and clamped for preservation at 25 cmH2O. A retrograde flush was performed on the back table before storing the lungs on ice for a prolonged period of cold ischemia of 18 hours. The following day, lungs were cannulated for EVLP with the XVIVO Lung Cannula Set (XVIVO Perfusion, Göteborg, Sweden) and an 8.0 mm endotracheal tube. A postconditioning (after the ischemic insult) exposure to Ar was introduced for 4 hours during acellular (OCS solution + albumin) EVLP in a dual way (via ventilator and gas exchanger penetrating both epithelial and endothelial sides). In the ARG group, N2 was replaced by Ar in the gas mixture of the ventilator and the gas mixture of the gas exchanger. The gas mixture settings of the gas exchanger for Ar or N2 (3 L/min) plus carbon dioxide (CO2) and oxygen (O2) were chosen to establish equilibrium in the oxygen content at the inflow and outflow with an ETCO2 of 25-35 mmHg. Lungs were ventilated with either 12% O2 /88% N2 (CONTR) or 12% O2 /88% Ar (ARG). In the ARG group, density corrections for the tidal volume with the test gas (12% O2 /88% Ar) were calculated with the same formula (test gas density 1.533 kg/m3). Lungs were ventilated with a TV of 7 mL/kg, 7 breaths per minute respiratory rate and 5 cmH2O PEEP. After 4 hours of postconditioning, an additional 2 hours of EVLP was performed to assess the lungs. Therefore, the acellular perfusate in the reservoir was drained and the system was primed with 1 L of whole blood (stored on CPDA at room temperature). During this 2-hour evaluation period, lungs were ventilated with air in both groups with 8 mL/kg TV, 12 times per minute. The perfusate was deoxygenated in the gas mixture using 10 L/min N2 plus CO2 and O2 to obtain a mixed venous partial oxygen pressure with ETCO2 values between 25 and 35 cmH2O. A summary of the study protocol is presented in Table 1.
Sampling, assessment and statistics
During the preconditioning phase in vivo, arterial blood samples were taken hourly. Also, the following hemodynamic parameters were monitored continuously and recorded hourly: arterial blood pressure (ABP), heart rate (HR), pulmonary artery pressure (PAP), SvO2 , CCO measurement and central venous pressure (CVP). After 6 hours of preconditioning, blood samples and tissue samples of liver and kidney (both fixed in formaldehyde 6%, embedded in paraffin and stained with haematoxylin-eosin) were taken to screen for potential injury due to toxicity of prolonged Ar exposure.
During postconditioning and final evaluation on EVLP, physiological parameters were monitored and perfusate samples were taken hourly: partial oxygen pressure (PO2), left atrial pressure (LAP), pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), peak airway pressure (Ppeak). A two-way analysis of variance was used to compare physiological parameters and gas concentration in the perfusate during EVLP postconditioning. All data was analysed with Graphpad 4 (GraphPad Software Inc., La Jolla, CA, USA) and the level of statistical significance was set at P < 0.05.
Lung grafts were only evaluated at the end of the evaluation period and values were compared with a Mann Whitney test. Oxygenation was evaluated based on partial oxygen pressure over fractional inspired oxygen concentration (PaO2/FiO2). Tissue samples for histology were fixed in formaldehyde 6%, embedded in paraffin, stained with haematoxylin-eosin and scored based on injury severity by an experienced pathologist.18
Histological sections were scored from 0–3 based on severity of injury (injury severity score, ISS).19 The bronchovascular, pleuroseptal and alveolar compartment was scored individually for influx of neutrophils, mononuclear cells, presence of necrosis and congestion.
Tissue samples for wet-to-dry weight ratio (W/D) calculation were taken at the end of the protocol. A bronchoalveolar lavage (BAL) of 30 mL saline was performed in duplicate in the right middle lobe for cytokine analysis (Cytokine Swine Magnetic 7-plex Panel for Luminex, Thermo Fisher Scientific Inc, Waltham, MA, USA). Finally, the left lung was inflated at 25 cmH2O, frozen solid in liquid nitrogen vapours and scanned with Siemens Somaton computed tomography (CT) scanner (Siemens Healthcare, Erlangen, Germany). Lung mass, volume, and density were measured on the basis of the CT-scan, using imaging software (Horos™) as previously described,20 in which the lung is manually delineated and the number of voxels and mean density of the voxels within the volume is determined.
Results
In vivo assessment
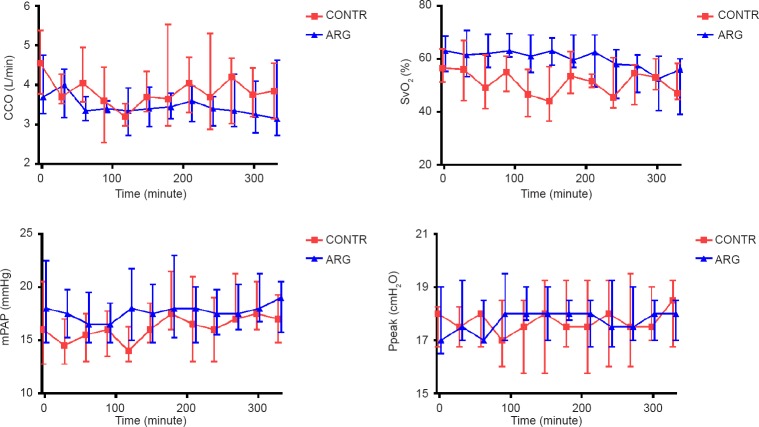
During 6 hours of preconditioning in vivo, animals showed a stable and comparable CCO, SvO2 , mean PAP (mPAP) and Ppeak in both the CONTR and the ARG groups (Figure 1).
Figure 1.
Continuous cardiac output (CCO), mixed venous saturation (SvO2), mean pulmonary artery pressures (mPAP) and peak ventilatory pressures (Ppeak) were monitored during the preconditioning period.
Note: Results are depicted as median ± IQ range; CONTR: 6 hours ventilation with air; ARG: 6 hours ventilation with argon.
After 6 hours of preconditioning, during which animals in the ARG group were exposed for 6 hours to Ar through ventilation, serum analysis for kidney and liver function did not show an elevation in liver and kidney tests compared to control animals (Table 2).
Table 2.
Liver and kidney serum tests
Histology sections of the liver showed normal structure of liver lobules with no infiltration of inflammatory cells (Figure 2). Histology sections of the kidney showed intact glomeruli and normal proximal and distal tubuli. There was no infiltration of inflammatory cells or deposition of necrotic epithelial cells.
Figure 2.
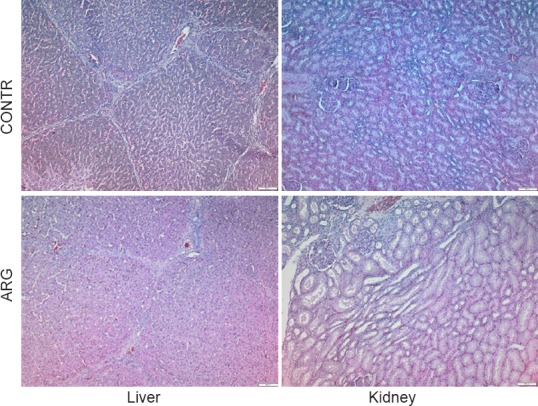
Normal histology of the liver and kidney after 6 hours ventilation with argon (ARG) or air (CONTR).
Preservation on EVLP
During 4 hours postconditioning on EVLP, PO2 was stable and similar at the inflow and outflow indicating stable gas concentrations of the perfusate (Figure 3).
Figure 3.
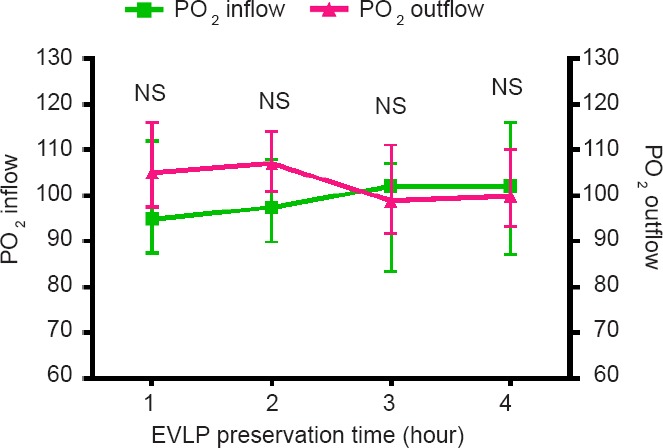
Stable PO2 shown in out- and inflow.
Note: Results are depicted as median ± IQ range and analyzed with a two-way analysis of variance (no difference between both groups). EVLP: Ex vivo lung perfusion; NS: not significant.
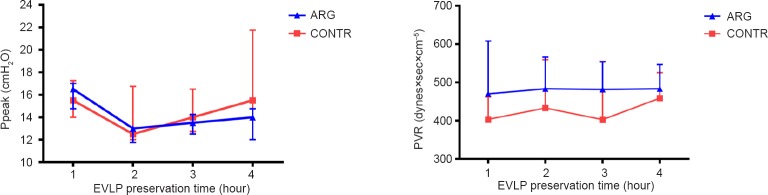
During the postconditioning on EVLP, Ppeak and PVR remained stable in both groups and were not significantly different (Figure 4).
Figure 4.
Stable airway peak pressures (Ppeak, left graph) and pulmonary vascular resistance (PVR, right graph) during postconditioning on ex vivo lung perfusion (EVLP).
Note: Data are depicted as median ± IQ range and analyzed with a two-way analysis of variance (no differences between both groups).
Evaluation of graft quality
After 2 hours of perfusion with whole blood, final evaluation of physiologic parameters on EVLP did not show a significant difference in PaO2/FiO2 , Ppeak or PVR. Also, estimation of lung oedema (W/D and CT-density) did not show a significant difference between the two groups (Table 3).
Table 3.
Final evaluation
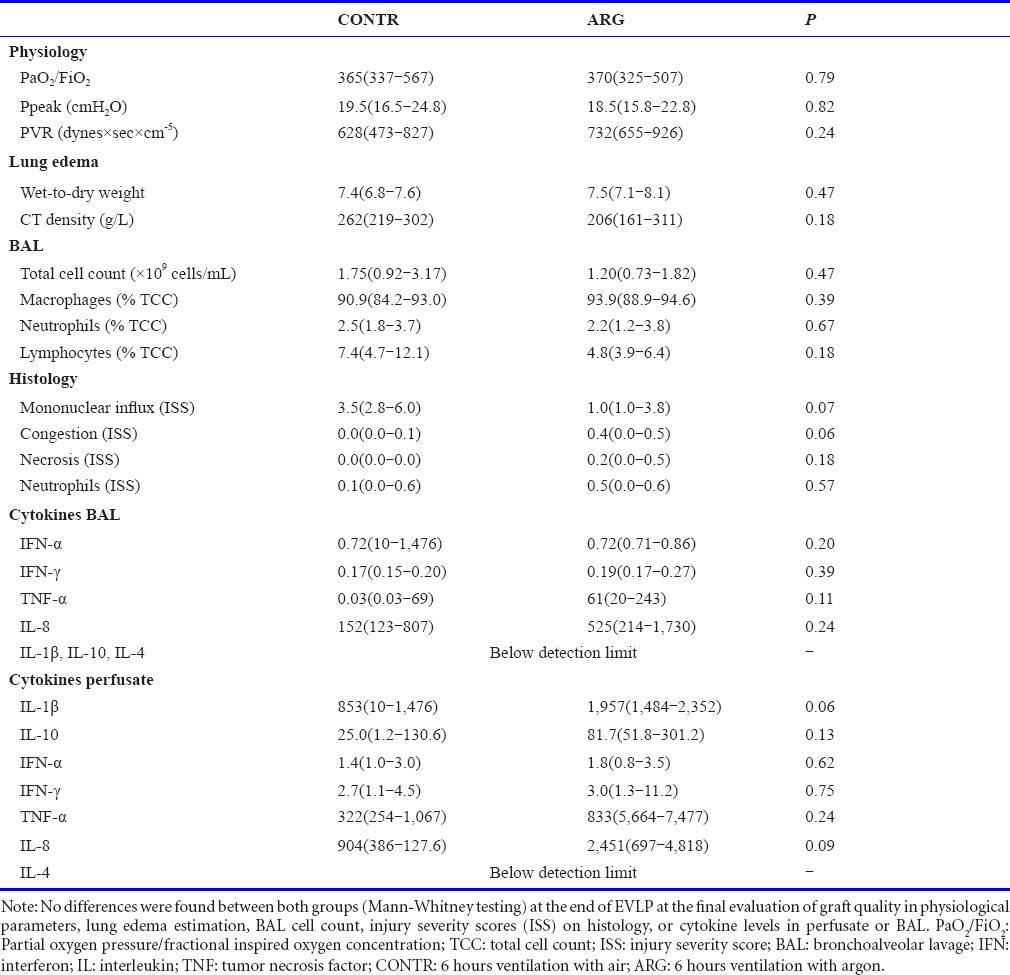
BAL total cell count and differential cell count (% of macrophages, neutrophils, lymphocytes) did not show a difference between both groups at the end of the evaluation on EVLP (Table 3). The sum of the ISS in the bronchovascular, pleuroseptal and alveolar compartment was similar for congestion, necrosis, neutrophil influx and mononuclear cell influx in both groups (Table 3). Cytokine levels of porcine interferon-α (INF-α), interferon-γ (IFN-γ), tumor necrosis factor (TNF)-α, interleukin-8, interleukin-1β, interleukin-10 and interleukin-4 in BAL and perfusate are shown in Table 3. There were no significant differences between the CONTR and ARG group.
Discussion
We report the results of a porcine study investigating the effect of prolonged exposure to Ar (prior, during and after ischemic insult) on cold-ischemic lung injury with ex vivo assessment. However, none of our investigated parameters could demonstrate a beneficial reconditioning effect on graft function.
Following a previous porcine pulmonary ischemia-reperfusion injury study where no effect was found by postconditioning with Ar, we designed an experimental protocol to allow a maximal exposure to Ar. The pulmonary graft was therefore exposed to Ar prior to the ischemic interval (preconditioning), during the cold ischemic injury (perconditioning) and after the ischemic interval during reperfusion on EVLP (postconditioning). The concentration of oxygen in the gas mixture for lung ventilation (FiO2) was chosen, to obtain the highest concentration of Ar possible (or N2) without inducing hypoxia to the lung tissue. During the preconditioning phase, while the animal was still alive, the FiO2 of air was chosen (21%) and in the ARG group the N2 in air was replaced by 79% of Ar. To allow for perconditioning, the lungs were exposed to Ar during cold preservation by inflation with the same gas mixture used as during the preconditioning phase (FiO2 21% with 79% N2 or Ar). The postconditioning phase during EVLP was optimized compared to the previous postconditioning protocol.7 Indeed: the oxygen concentration in the gas mixture of the ventilator was lowered to 12% instead of 21% to allow for a higher Ar concentration of 88% (or 88% N2 in the CONTR group). And in addition, the perfusate was saturated with Ar via the gas exchanger to expose both the epithelial and the endothelial side of the alveolar membrane during EVLP. This kind of Ar-saturated perfusion solution has also been tested in experimental kidney perfusion leading to improved graft function after transplantation.12,13
During 6 hours of preconditioning with Ar in vivo, stable hemodynamic parameters were observed. Animals had normal pulmonary and systemic blood pressures, normal heart rates and normal peak ventilation pressures during the total perfusion time of 6 hours. After 6 hours of preconditioning, tissue samples from the kidney and liver were taken and the pathology report did not reveal any sign of injury to kidney or liver due to toxicity. Serum samples tested for liver and kidney function were similar between both groups. These preliminary findings confirm that prolonged ventilation with Ar in vivo is safe.
Maintaining a steady state of oxygen delivery and gas exchange during preservation on EVLP is a technique that has been introduced by Transmedics in the OCS™ Lung protocol.21 In this set-up, partial oxygen concentration is kept stable at in- and outflow, therefore no gas exchange takes place. This allows for lower gas consumption since N2 or Ar are not continuously washed out in the gas exchanger. This technique is chosen over the complete wash-out of oxygen with high flow rates of N2 or Ar, because of the scarcity and higher price of noble gases.22,23 During EVLP, we can monitor physiologic parameters over time while lungs are exposed to Ar and compare them with the physiologic parameters of the control group.
To further assess the effect of Ar on IRI,4 after 4 hours of ex vivo postconditioning with Ar, lungs were perfused for an additional 2 hours with whole blood containing neutrophils. This concept attempted to mimic, transplantation; however, it could not replicate the complete in vivo environment. For example, the perfusion settings with a centrifugal pump result in a different shear stress environment and flow conditions when compared to the in vivo pulsatile cardiac reperfusion of the lung.
At the end of the 2-hour additional whole-blood perfusion period, the pulmonary graft was evaluated by comparing physiological data, tissue samples and BAL. A median W/D of 7.4 was observed in the CONTR group and no irreversible necrosis was seen on lung histology. In combination with an increased PVR and increased Ppeak, we can conclude that sufficient injury was inflicted to the lung graft by our injury model to study IRI. Surprisingly, no beneficial effect was detected in physiologic parameters (Ppeak, PVR, PaO2 /FiO2), histology, BAL cell count and cytokine measurements or lung edema (W/D and CT density) after prolonged exposure to argon in the ARG group. We therefore concluded that reconditioning of cold-ischemic injury by Ar is not beneficial in this setting. However, in other organ systems, both pre- and postconditioning benefits with Ar have been described and the mechanisms of these effects should be further investigated.
Evidence of an organoprotective effect in solid organ transplantation has mainly been demonstrated in kidney transplantation. Both Faure et al.13 and Irani et al.12 showed that saturation of the cold preservation medium with argon resulted in a better preserved renal architecture with improved early functional recovery, graft quality and survival after kidney transplantation in their rat and pig models, respectively. Niemann et al.24 showed that hypothermia alone, in brain-dead donors also results in a reduced rate of delayed graft function among kidney transplant recipients. The effect of hypothermia or noble gases alone, has not been thoroughly investigated in solid organ transplantation so far. It might be, that an organoprotective effect of noble gases in solid organ transplantation is based on an interaction with the hypothermic protective effect. Mechanisms of a combinational therapy of hypothermia and noble gases, in combination with the interval of exposure (pre-, per-, postconditioning) should therefore be investigated further to demonstrate a protective effect in lung transplantation.
Enhancement of the neuroprotective effect of cooling by combining it with Ar exposure, has also been shown by Broad et al.25 who demonstrated improved brain energy metabolism, faster recovery on electroencephalogram and reduced cell death on terminal deoxynucleotidyl transferase dUTP nick end labelling in a new-born piglet encephalopathy model. Notably, the reported functional and histopathological neuroprotective effect in other traumatic, hypoxic and ischemic brain injury models has also been demonstrated in normothermic conditions.11,26,27,28,29,30 A neuroprotective effect of noble gases can therefore not be explained by an augmented protective effect of hypothermia alone. It might therefore still be possible that Ar could attenuate pulmonary injury other than (cold-) ischemic injury. For example, brain-dead donors suffer from a systemic inflammation and activation of apoptosis which could trigger the onset of severe IRI.31,32 Considering the mechanisms of brain-dead induced pulmonary damage and the potential of noble gases to promote extracellular signal-regulated kinase 1/2 signalling through activation of mitogen-activated protein kinase in both normothermic and hypothermic conditions,33,34 further animal research, preferably using a brain-dead injury model combined with protective hypothermia and transplantation, seems appropriate.
These three main differences concerning in vivo reperfusion of the graft, combination of noble gases with hypothermia and a different injury model (e.g., brain death) could be the reason why argon did not improve pulmonary graft function in our model while in other organ systems a protective effect has been described.
We conclude that, although beneficial effects of Ar on ischemic injury in various other organ systems have been reported, we did not detect an improvement in pulmonary graft function after prolonged exposure to Ar in our cold-ischemic injury model with EVLP assessment. Further animal research, preferably using a brain-dead injury model, including an in-depth mechanistic investigation of the lung-protective effect of Ar, should be conducted.
Footnotes
Funding: Air Liquide Santé International Medical R&D (Paris-Saclay, France), No. MEDGAS 13-360; Clinical Research Fund UZ Leuven (Leuven, Belgium).
Conflicts of interest
None.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
References
- 1.Smits JM, van der Bij W, Van Raemdonck D, et al. Defining an extended criteria donor lung: an empirical approach based on the Eurotransplant experience. Transpl Int. 2011;24:393–400. doi: 10.1111/j.1432-2277.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.De Vleeschauwer SI, Wauters S, Dupont LJ, et al. Medium-term outcome after lung transplantation is comparable between brain-dead and cardiac-dead donors. J Heart Lung Transplant. 2011;30:975–981. doi: 10.1016/j.healun.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Somers J, Ruttens D, Verleden SE, et al. A decade of extended-criteria lung donors in a single center: was it justified? Transpl Int. 2015;28:170–179. doi: 10.1111/tri.12470. [DOI] [PubMed] [Google Scholar]
- 4.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens A, Montoli M, Faggi G, et al. Argon and xenon ventilation during prolonged ex vivo lung perfusion. J Surg Res. 2016;201:44–52. doi: 10.1016/j.jss.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Brucken A, Cizen A, Fera C, et al. Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br J Anaesth. 2013;110(Suppl 1):i106–112. doi: 10.1093/bja/aes509. [DOI] [PubMed] [Google Scholar]
- 9.Pagel PS. Cardioprotection by noble gases. J Cardiothorac Vasc Anesth. 2010;24:143–163. doi: 10.1053/j.jvca.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher PD, Rossaint J, Rossaint R, et al. Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit Care. 2009;13:R206. doi: 10.1186/cc8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang L, Yang T, Zhao H, et al. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit Care Med. 2012;40:1724–1730. doi: 10.1097/CCM.0b013e3182452164. [DOI] [PubMed] [Google Scholar]
- 12.Irani Y, Pype JL, Martin AR, et al. Noble gas (argon and xenon)-saturated cold storage solutions reduce ischemia-reperfusion injury in a rat model of renal transplantation. Nephron Extra. 2011;1:272–282. doi: 10.1159/000335197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faure A, Bruzzese L, Steinberg JG, et al. Effectiveness of pure argon for renal transplant preservation in a preclinical pig model of heterotopic autotransplantation. J Transl Med. 2016;14:40. doi: 10.1186/s12967-016-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brucken A, Kurnaz P, Bleilevens C, et al. Delayed argon administration provides robust protection against cardiac arrest-induced neurological damage. Neurocrit Care. 2015;22:112–120. doi: 10.1007/s12028-014-0029-1. [DOI] [PubMed] [Google Scholar]
- 15.Rega FR, Wuyts WA, Vanaudenaerde BM, et al. Nebulized N-acetyl cysteine protects the pulmonary graft inside the non-heart-beating donor. J Heart Lung Transplant. 2005;24:1369–1377. doi: 10.1016/j.healun.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Cypel M, Keshavjee S. Extending the donor pool: rehabilitation of poor organs. Thorac Surg Clin. 2015;25:27–33. doi: 10.1016/j.thorsurg.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 18.Martens A, Boada M, Vanaudenaerde BM, et al. Steroids can reduce warm ischemic reperfusion injury in a porcine donation after circulatory death model with ex vivo lung perfusion evaluation. Transpl Int. 2016;29:1237–1246. doi: 10.1111/tri.12823. [DOI] [PubMed] [Google Scholar]
- 19.McCormack MC, Kwon E, Eberlin KR, et al. Development of reproducible histologic injury severity scores: skeletal muscle reperfusion injury. Surgery. 2008;143:126–133. doi: 10.1016/j.surg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Verleden SE, Vasilescu DM, Willems S, et al. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med. 2014;189:292–300. doi: 10.1164/rccm.201310-1894OC. [DOI] [PubMed] [Google Scholar]
- 21.Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012;380:1851–1858. doi: 10.1016/S0140-6736(12)61344-0. [DOI] [PubMed] [Google Scholar]
- 22.Hanne P, Marx T, Musati S, Santo M, Suwa K, Morita S. Xenon: uptake and costs. Int Anesthesiol Clin. 2001;39:43–61. doi: 10.1097/00004311-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ye Z, Zhang R, Sun X. Bustling argon: biological effect. Med Gas Res. 2013;3:22. doi: 10.1186/2045-9912-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemann CU, Feiner J, Swain S, et al. Therapeutic Hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373:405–414. doi: 10.1056/NEJMoa1501969. [DOI] [PubMed] [Google Scholar]
- 25.Broad KD, Fierens I, Fleiss B, et al. Inhaled 45-50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis. 2016;87:29–38. doi: 10.1016/j.nbd.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryang YM, Fahlenkamp AV, Rossaint R, et al. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med. 2011;39:1448–1453. doi: 10.1097/CCM.0b013e31821209be. [DOI] [PubMed] [Google Scholar]
- 27.Hollig A, Weinandy A, Liu J, Clusmann H, Rossaint R, Coburn M. Beneficial properties of argon after experimental subarachnoid hemorrhage: early treatment reduces mortality and influences hippocampal protein expression. Crit Care Med. 2016;44:e520–529. doi: 10.1097/CCM.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 28.Brucken A, Kurnaz P, Bleilevens C, et al. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)-channel opening. Resuscitation. 2014;85:826–832. doi: 10.1016/j.resuscitation.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 29.David HN, Haelewyn B, Degoulet M, Colomb DG, Jr, Risso JJ, Abraini JH. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PloS One. 2012;7:e30934. doi: 10.1371/journal.pone.0030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulbrich F, Goebel U. Argon: a novel therapeutic option to treat neuronal ischemia and reperfusion injuries? Neural Regen Res. 2015;10:1043–1044. doi: 10.4103/1673-5374.160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant 2013. 2013:521369. doi: 10.1155/2013/521369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avlonitis VS, Fisher AJ, Kirby JA, Dark JH. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003;75:1928–1933. doi: 10.1097/01.TP.0000066351.87480.9E. [DOI] [PubMed] [Google Scholar]
- 33.Fahlenkamp AV, Rossaint R, Haase H, et al. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur J Pharmacol. 2012;674:104–111. doi: 10.1016/j.ejphar.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Mitchell S, Ciechanowicz S, et al. Argon protects against hypoxic-ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid-derived 2)-like 2. Oncotarget. 2016;7:25640–25651. doi: 10.18632/oncotarget.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]