Abstract
Isoflurane, a volatile and inhalational anesthetic, has been extensively used in perioperative period for several decades. A large amount of experimental studies have indicated that isoflurane exhibits neuroprotective properties when it is administrated before or after (pre-conditioning and post-conditioning) neurodegenerative diseases (e.g., hypoxic ischemia, stroke and trauma). Multiple mechanisms are involved in isoflurane induced neuroprotection, including activation of glycine and γ-aminobutyric acid receptors, antagonism of ionic channels and alteration of the function and activity of other cellular proteins. Although neuroprotection provided by isoflurane is observed in many animal studies, convincing evidence is lacking in human trials. Therefore, there is still a long way to go before translating its neuroprotective properties into clinical practice.
Keywords: isoflurane, volatile anesthetics, stroke, neuroprotection, ischemia, experimental studies, pre-conditioning, post-conditioning, mechanism
Introduction
During the perioperative period, central nervous system neurons are extremely sensitive to any impairment of substrate delivery, especially oxygen-glucose deprivation (OGD), which represents one of the major causes of irreversible brain injury.1 Neuroprotection deals with a series of mechanisms that can prevent brain tissues from cellular events (such as apoptosis, degeneration, inflammation and energy deprivation) related to chronic neurodegenerative diseases (e.g., ischemia, stroke and trauma).2 In hypoxic ischemia (HI) injury, cellular regulation is in chaos and loses balance with demand, causing the adenosine triphosphate (ATP)-dependent ion channels in dysfunction that finally results in cellular depolarization and release of excitatory neurotransmitters such as glutamate.3 As a result, this process finally leads to cell death by multiple pathways. It has been demonstrated that isoflurane is neuroprotective when used during or immediately prior to an ischemic accident in brain, which is similar to ischemic pre-conditioning.4,5 Meanwhile, isoflurane administration immediately after OGD was found to improve neuronal viability in brain cells.6 However, the mechanisms by which isoflurane pre-/post-conditioning protects brain against HI injury are still poorly understood. In this review, we will focus on the experimental studies in isoflurane pre-/post-conditioning as well as the potential mechanisms of the neuroprotective properties provided by isoflurane.
Isoflurane Shows Neuroprotection in Various Stroke Models
Stroke is a clinical syndrome that has diverse causes,7 and acute ischemic stroke has become a major problem worldwide.8 Anesthetics have made leading advances in developments of experimental models of stroke.9 Isoflurane has been demonstrated to provide neuroprotection in various animals of stroke, which includes neonatal HI, middle cerebral artery occlusion (MCAO), subarachnoid hemorrhage (SAH), and intracerebral hemorrhage (ICH) (Table 1).10
Table 1.
Isoflurane shows neuroprotection in various stroke models
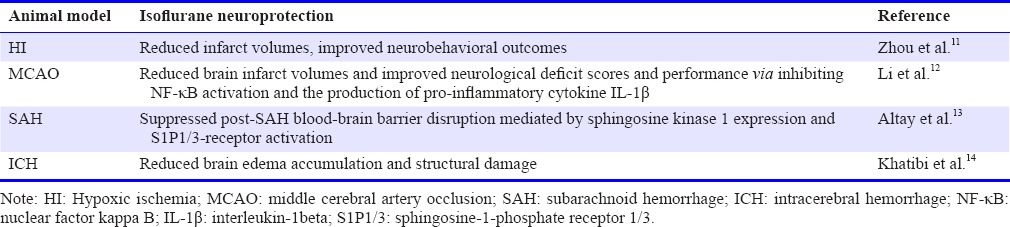
In the animal model of neonatal HI, unilateral carotid ligation and 2-hour hypoxia were used to induce HI in 10-day-old rat pups. After that, 2% isoflurane was used immediately after HI for 1 hour. As a result, isoflurane post-conditioning remarkably reduced the brain infarct volume after HI.11 Application of isoflurane at the onset of reperfusion was reported to be able to reduce infarct volumes and improved neurological outcome on rotarod at 4 weeks after MCAO.12 After SAH in mice, isoflurane suppressed the post-SAH blood-brain barrier injury.13 In a study on mouse intracerebral hemorrhagic stroke, results reveal that isoflurane may be an effective therapeutic option for ICH because of its ability to depress structural disruption and preserve brain function.14,15
Experimental Studies on Isoflurane Pre-conditioning
Pre-conditioning refers to the phenomenon that pretreatment of certain drugs or conditions prevent organs against potential lethal injury.16 Volatile anesthetics pre-conditioning in experimental works has been well documented as well as its neuroprotective properties.17
In various experimental works, isoflurane pre-conditioning has been demonstrated to reduce brain injury. (1) Isoflurane pre-conditioning significantly reduced OGD neuronal injury; and 1.5% concentration of isoflurane shows the strongest neuroprotection; hypoxia inducible factor (HIF)-1α protein and inducible nitric oxide synthase (iNOS) mRNA are activated to express in hippocampal neurons.18,19 (2) In a study of Wang and Kong,20 the role of TWIK-related K+ channel 1 (TREK1) in the neuroprotective effects of isoflurane pre-conditioning was studied in vitro and in vivo. This study suggested that isoflurane pre-conditioning activated TREK1 expression in ischemic spinal cord injury rats, which may suppress apoptosis. Also, isoflurane pre-conditioning that induces tolerance to ischemia in neurons can be reduced by down-regulation of TREAK1 via RNA interference. These findings suggest that TREK1 acts an important part in the neuroprotection of isoflurane pre-conditioning. (3) It has been already known that isoflurane treatment induces neuroprotection in adult rats. However, compared with the adults brains, neonatal brains are immature.21 Whether pre-conditioning with isoflurane in neonatal brains has neuroprotective properties is unclear. In the study of neonatal brain ischemia, 7-day-old rats received unilateral common carotid arterial ligation following 8% oxygen exposure to simulate human perinatal stroke.22 In a study of Zhao et al.,23 1) 1.5% isoflurane pre-conditioning in neonatal rats induced a delayed ischemic tolerance because isoflurane pre-conditioning reduced brain disruption in the ischemic cerebral hemisphere of rats preconditioned with isoflurane before brain HI, compared with the rats with brain HI only; 2) isoflurane pre-conditioning induced a time-dependent increase in iNOS expression in neonatal brains; and 3) iNOS may mediate the neuroprotection induced by isoflurane pre-conditioning. (4) Isoflurane pre-conditioning has been proved to protect neurons against ischemia at clinically relevant concentrations.24 However, whether pre-conditioning of isoflurane could protect other brain cells remains unknown. There is a close correlation between microglia and inflammation as well as many brain diseases including stroke.25,26 It is reported that 1%, 2% or 3% isoflurane pre-conditioning significantly weaken the microglial cell activation and injury induced by the lipopolysaccharide plus interferon γ.27 (5) In an experimental study aimed to investigate the dose-dependent effects of isoflurane pre-conditioning in cerebral ischemic injury, a rat model of transient focal ischemia was used. Forty male rats were randomly assigned to four groups (n = 10 per group): 100% oxygen 1 hour/day was given to control group for 5 days; 0.75%, 1.5%, or 2.25% isoflurane in oxygen 1 hour per day was given to isoflurane 1, isoflurane 2, isoflurane 3 groups for 5 days. The neurologic deficit scores were used to evaluate the neuron function of each group. As a result, isoflurane 2 and 3 groups had the lower neurologic deficit sores than the control group, and isoflurane 3 group showed lower scores than isoflurane 1 group (P < 0.01).28
Experimental Studies in Isoflurane Post-conditioning
It has been proved that pre-conditioning with isoflurane brain loss/injury induced by HI. However, it is still unknown that whether post-conditioning with isoflurane has a similar effect. Subsequently, some representative and valuable experimental results involving isoflurane post-conditioning are summarized as follows.
It is well known that isoflurane precondition induces protective effects in ischemia/reperfuion injury. However, brain cells respond to ischemic injury in a dynamic process, rather than an instant process.14 A study repeated the foregoing study that isoflurane post-conditioning induced protective effect on cerebral ischemic/reperfusion injury. In order to estimate the appropriate dose, 1.5%, 3.0%, and 4.0% of isoflurane postconditioned the isehcmia/reperfusion model for 60 minutes after 90 minutes of MCAO. As a result, 1.5% isoflurane post-conditioning prominently decreased the infarct volume and improved the neurobehavioral performance. However, this positive effect was not strengthened synchronously with the increasing concentration of isoflurane. In addition, the researchers applied the most appropriate concentration (1.5%) to further investigate the neuroprotection induced by isoflurane. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) apoptosis staining results showed that 1.5% concentration of isoflurane could significantly suppress the cell death as well as apoptosis.29 (2) Inspired by ischemic post-conditioning induced neuroprotection,30,31 Lee et al.6 hypothesized that volatile anesthetics can induce post-conditioning as well. They demonstrated OGD to rat corticostriatal slice in vitro study; in vivo study, the rats was given transient MCAO and then postconditioned with isoflurane. The results indicated that cell damage was decreased with the isoflurane post-conditioning after OGD. In conclusion, they successfully proved that suitable concentration of isoflurane induced the postcondtioning effect for the first time. (3) Another study showed that 1.5% and 3.0% isoflurane post-conditioning induced the strongest neuroprotective against cerebral ischemia in a rat cerebral ischemia/reperfusion model (90-minute ischemia and 60-minute reperfusion).29 (4) To further validate the proper concentration of isoflurane post-conditioning that induced the strongest neuroprotection, the researchers performed the dose-response studies. Results revealed that 1.5% isoflurane exposure for 10 minutes after a 14-minute OGD can induce neuroprotection in rat hippocampal slices. Interestingly, 3.0% isoflurane exposure for 10 minutes after a 14-minute OGD resulted in reserved neuroprotection. With the increase in isoflurane concentration, the neuroprotective effects were attenuated, and disappeared at 4.5% isoflurane exposure for 10 minutes. This finding suggests that higher concentration of isoflurane may not provide better neuroprotection.32,33 (5) Xu et al.34 investigated the concentrations and time windows of isoflurane post-conditioning in neonatal cerebral HI. In their experiment, 7-day-old rats were randomly divided into eight groups (n = 40 per group). The rats received left common carotid arterial ligation or sham surgery and exposure to 8% oxygen for 2 hours at 37°C in a thermoregulated environment. 1%, 1.5%, or 2% isoflurane was performed immediately after brain HI for 30 minutes. Others were post-conditioning with 1.5% isoflurane for 30 minutes at 3, 6, and 12 hours after brain HI. They concluded that post-conditioning with 1.5% and 2% isoflurane afforded neuroprotection in neuroprotection in neonatal rats. The time window for isoflurane post-conditioning to be effective against neonatal HI-induced brain injury was 0–6 hours after HI.
Neuroprotection of Isoflurane: Possible Mechanisms
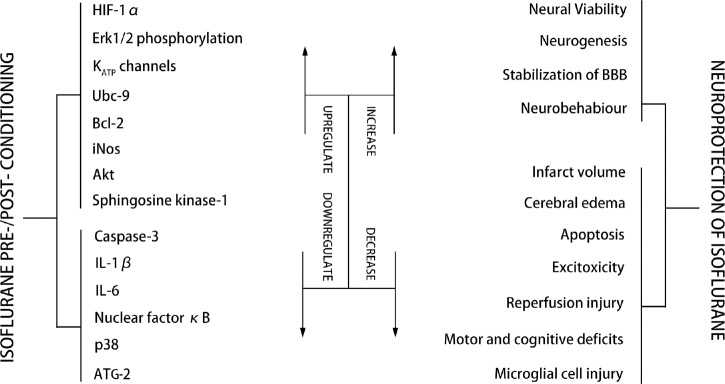
It has been reported that isoflurane, when it is applied before or after experimental neural HI, shows the ability to reduce negative outcomes. Recent studies have indicated that isoflurane appeals to be neuroprotective and increase neuronal viability through a number of mechanisms (Figure 1).
Figure 1.
Possible mechanism of isoflurane pre-/post-conditioning.
Note: HIF-1α: Hypoxia inducible factor-1α; Erk1/2: extracellular signal-related kinases 1 and 2; KATP: adenosine triphosphate-regulated potassium; Bcl-2: B-cell lymphoma 2; iNOS: inducible nitric oxide synthase; Akt: protein kinase B; IL: interleukelin; ATG-2: anti-thymocyte globulin-2; BBB: blood-brain barrier.
Mechanisms of isoflurane pre-conditioning are reviewed as follow: Isoflurane provides neuroprotection by suppressing apoptosis in HI neural injury. Isoflurane treatment decreased the hypoxia-induced release of amino acid neurotransmitters such as aspartate, glutamate and glycine induced by hypoxia, but the levels of γ-aminobutyric acid (GABA) were elevated. Morphological studies showed that isoflurane treatment attenuated edema of pyramid neurons in the CA1 region. It also reduced apoptosis as evident by lowered expression of caspase-3 and poly-ADP-ribose polymerase (PARP) genes.35 Under hypoxic situation, isoflurane was proven to be neuroprotective through the pathways that involve the release of Ca2+ and phospholipase C. Phospholipase C triggers the phosphatidylinositol-3-kinase/protein kinase B and mitogen-activated protein kinase (PI3K/Akt/MAPK) pathways, which are all anti-apoptotic.36 It was reported that the inositol triphosphate (IP3) receptor antagonist prevented the phosphorylation of MAPKs, indicating that the phenomenon after isoflurane pre-conditioning depends on the small increases in intracellular Ca2+. Meanwhile, this study indicated that isoflurane enabled neurons to avoid potentially toxic levels of Ca2+ increase.37 Akt, an anti-apoptotic protein, was observed to increase after isoflurane pretreatment. It was shown that isoflurane prevented apoptosis by activating Akt and enhancing B-cell lymphoma-2 (Bcl-2) expression.38 Meanwhile, a recent study indicated that Bcl-2 phosphorylation play a crucial role in triggering autophagy and reducing mitochondrial damage after stroke with the involvement of Akt activation.39
Neuroprotection induced by isoflurane pre-conditioning involves stimulation of ATP-regulated potassium (KATP) channels.40,41,42,43,44 A study23 was designed to evaluate the role of KATP channels in ischemic tolerance induced by isoflurane through a rat model of transient focal ischemia. Glibenclamide (GLB), a nonspecific KATP channel blocker, was administered in the experiment. The isoflurane + glibenclamide (I + G) group was given glibenclamide intraperitoneally (i.p. 5 mg/kg) (Sigma Chemical Co., St. Louis, MO, USA) before isoflurane administration; GLB group was given GLB (i.p. 5 mg/kg) once a day without isoflurane pretreatment for 5 days; control group was given 100% oxygen 1 hour/day for 5 days. The neurologic deficit scores were used to evaluate the neurological outcome of each group. As a result, the neurologic deficit scores of Iso group were lower than those of the control and I + G groups (P < 0.01 and 0.05, respectively). No statistical difference was found among the control, I + G, and GLB groups. The infarct volume of the Iso group was smaller than that of the control and I + G groups (P < 0.05). There was no statistical difference among the control, I + G, and GLB groups. The results showed that GLB that administered before isoflurane pre-conditioning could abolish the neuroprotection of isoflurane pre-conditioning. Although it was unable to make it clear whether the neuroprotection induced by isoflurane pre-conditioning is at the cellular or in the mitochondrial membrane, this study did show that the neuroprotection of isoflurane pre-conditioning is mediated by activation of KATP channels.
Recently, it has been indicated that isoflurane takes part in the modulation of several genes related to cell survival, including HIF-1, an important DNA-binding complex45 whose activity is modulated by intracellular oxygen in vitro46 and in vivo.47 The functional HIF-1 increases transcriptions of the genes that could enable cells to survive from HI conditions, such as iNOS gene.48 It has been indicated that extracellular signal-related kinases 1 and 2 (ERK1/2) is concern with the modulation of brain cell death after ischemia in both in vivo and in vitro studies.49,50 Additionally, isoflurane increased HIF-1α protein content, and PD98059 (the selective Erk1/2 inhibitor) decreased the accumulation of HIF-1α protein by isoflurane. This indicated that isoflurane activated HIF-1α expression via Erk1/2 pathway. The researchers also found that precondition of isoflurane promotes iNOS activation via HIF-1α pathway during OGD in hippocampal neurons.18 The downstream events of iNOS pathway to promote neuroprotection have been shown in the previous works include activating of protein kinase C, heat shock proteins and manganese superoxide dismutase expression.51,52
Mechanisms of isoflurane post-conditioning are reviewed as follow: Mitochondrial injury after HI conditions is a leading cause of cell damage and death.53 Therefore, the release of substances out of mitochondria resulting from the increasing permeability of mitochondria membrane has been considered to be a pathological process that leads to cell damage and death.54,55,56 Thus, Li et al.57 hypothesized that postcondition with isoflurane could reduce ischemia/reperfusion-induced mitochondrial membrane permeability increasing. As prosurvival protein kinases, such as Akt and glycogen synthase kinases 3β, are able to modulate the mitochondrial membrane permeability,58 they also hypothesize that the activation of Akt is concerned with the neuroprotection promoted by isoflurane post-conditioning. Their results turn out that isoflurane post-conditioning significantly attenuated the increase of mitochondrial membrane permeability.57
Sphingosine 1-phophate (S1P) is a bioactive sphingolipid metabolite and has been shown to bind to specific G protein-coupled receptors (the S1P receptors) and regulate multiple cellular events,59 including promoting cell proliferation, survival, migration, and inhibiting apoptosis. Prosurvival PI3K/Akt have been reported to be downstream molecules of the S1P1 receptor signaling pathway.60 Referring to a recent study which found that isoflurane ameliorates renal ischemia-reperfusion injury by initiating the S1P/S1P receptor signaling pathway,61 experiments were done in order to clarify whether the activation of S1P/S1P receptor signal pathway takes part in the neuroprotection promoted by isoflurane. In an experiment performed by Zhou et al.,11 they found that isoflurane-mediated reduction in infarct volume depends on the S1P/PI3K/Akt signaling pathway, and administration of the S1P receptor antagonist VPC23019 or the PI3K inhibitor wortmannin completely abrogated the reduction in infarct volume provided by isoflurane posttreatment. Additionally, isoflurane posttreatment not only provides short-term protection against neonatal HI brain injury, but also confers long-term neuroprotection against brain atrophy and neurological deficits, which can also be abolished by the pretreat of VPC23019 and wortmannin. What's more, isoflurane increases phosphorylation of Akt and decreases expression of cleaved caspase-3 after HI. These findings suggest that isoflurane-mediated neuroprotection against neonatal HI depends on S1P/PI3K/Akt signaling. In the animal model of germinal matrix/intraventricular hemorrhage, isoflurane was evaluated to improve motor function and prevent neural degeneration, which was mediated by S1P1/Akt pathway.62
Adverse Effects of Isoflurane Application
Isoflurane, which is widely used in clinical practice as a violate anesthetic, has been demonstrated to have neuroprotective properties. However, it is also reported that postoperative nausea and vomiting is a common complication after general anesthesia,63 and what's worse, isoflurane exposure may induce neurotoxicity including neuroapoptosis,64,65 neurodegeneration, and long-term neurocognitive deficiency66,68 via mechanisms of inhibiting glutamate receptors, activating GABA receptors and intrinsic pathway of apoptosis.69 According to a study using motor evoked potentials to monitor the spinal cord ischemia during the aortic operations, using isoflurane could increase the rate of apnea, cause prolonged hypotension, and disturb motor evoked potentials.70 It has been demonstrated that isoflurane does cause brain cells death and neurocognitive dysfunction especially in neonatal rats; however, it is difficult to extrapolate animal data to human practice. Defining the safe scope of isoflurane application may be helpful to employ isoflurane as a novel therapy.71
Conclusions
It is clear that isoflurane appeals beneficial effects no matter administrated before or after the brain injury (pre-conditioning or post-conditioning), as assessed by various outcome measurements. Although, in most experimental researches, strong positive effects have been observed, conclusive clinical studies are lacking to date. Meanwhile, there are evidences in support of isoflurane being potentially neurotoxic, but many animal studies indicate isoflurane application to be convincingly neuroprotective as discussed above.
It is difficult to explain the opposite effects of neuroprotection and neurotoxicity induced by isoflurane. One possibility for this phenomenon is that isoflurane at high concentrations or for long exposure may cause/enhance the process to kill cells, which can even overwhelms the protective properties of isoflurane.6 For further explanation for the dual effects of isoflurane, Zhang et al.72 presented the bioenergetic homeostasis hypothesis in 2011. The hypothesis was based on the evidences that isoflurane could affect mitochondrial electron transport complexes and glycolysis related pathways in cells, which could alter intracellular calcium homeostasis, ROS production and ATP synthesis, and the duration and concentration of exposure to isoflurane could play a role in severity of bioenergy inhibition. The hypothesis considered that transient or low concentration exposure to isoflurane triggers neuroprotection, and prolonged duration as well as high concentration exposure could induce neurotoxicity, even cell death.
As mentioned above, the effects of isoflurane can be described as “a double-edged sword”, but there is something we can do to enhance its neuroprotection and lighten the untoward effects. For example, cocktail treatment is a classic therapeutic strategy which is proved to be more effective than single drug treatment. A recent study showed that normobaric hyperoxia treatment together with agents enhanced its neuroprotective effects,73 so it is possible that combination therapy of normobaric hyperoxia and isoflurane could access a stronger neuroprotection. To summarize, the possibility of clinical isoflurane application seems to be promising, although much more pre-clinical or clinical work remains to be done.
Footnotes
Funding: This work was supported by the grants from Suzhou Key Medical Center of China (Szzx201501), the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), the Scientific Department of Jiangsu Province of China (No. BL2014045), the Projects of Invigorating Health Care through Science, Technology and Education, Suzhou Government (No. SZS201413, SYS201608, and LCZX201601), Jiangsu Province (No. 16KJB320008).
Conflicts of interest
The authors declare that they have no competing interests.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
References
- 1.Mortier E, Struys M, Herregods L. Therapeutic coma or neuroprotection by anaesthetics. Acta Neurol Belg. 2000;100:225–228. [PubMed] [Google Scholar]
- 2.Gadhinglajkar SV. Pharmacological Neuroprotection. Indian J Anaesth. 2003;47:8–22. [Google Scholar]
- 3.Sanders RD, Ma D, Maze M. Anaesthesia induced neuroprotection. Best Pract Res Clin Anaesthesiol. 2005;19:461–474. doi: 10.1016/j.bpa.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Miura Y, Grocott HP, Bart RD, Pearlstein RD, Dexter F, Warner DS. Differential effects of anesthetic agents on outcome from near-complete but not incomplete global ischemia in the rat. Anesthesiology. 1998;89:391–400. doi: 10.1097/00000542-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir KW, Macrae IM. Neuroimaging as a selection tool and endpoint in clinical and pre-clinical trials. Transl Stroke Res. 2016;7:368–377. doi: 10.1007/s12975-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapchak PA, Zhang JH. The high cost of stroke and stroke cytoprotection research. Transl Stroke Res. 2016 doi: 10.1007/s12975-016-0518-y. doi: 10.1007/s12975-016-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann U, Sheng H, Ayata C, Warner DS. Anesthesia in experimental stroke research. Transl Stroke Res. 2016;7:358–367. doi: 10.1007/s12975-016-0491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krings M, Hollig A, Liu J, Grusser L, Rossaint R, Coburn M. Desflurane impairs outcome of organotypic hippocampal slices in an in vitro model of traumatic brain injury. Med Gas Res. 2016;6:3–9. doi: 10.4103/2045-9912.179338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Lekic T, Fathali N, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altay O, Suzuki H, Hasegawa Y, et al. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43:2513–2516. doi: 10.1161/STROKEAHA.112.661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatibi NH, Ma Q, Rolland W, et al. Isoflurane posttreatment reduces brain injury after an intracerebral hemorrhagic stroke in mice. Anesth Analg. 2011;113:343–348. doi: 10.1213/ANE.0b013e31821f9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garton T, Keep RF, Wilkinson DA, et al. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl Stroke Res. 2016;7:447–451. doi: 10.1007/s12975-016-0480-8. [DOI] [PubMed] [Google Scholar]
- 16.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–995. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedirli N, Bagriacik EU, Emmez H, Yilmaz G, Unal Y, Ozkose Z. Sevoflurane and isoflurane preconditioning provides neuroprotection by inhibition of apoptosis-related mRNA expression in a rat model of focal cerebral ischemia. J Neurosurg Anesthesiol. 2012;24:336–344. doi: 10.1097/ANA.0b013e318266791e. [DOI] [PubMed] [Google Scholar]
- 18.Li QF, Zhu YS, Jiang H. Isoflurane preconditioning activates HIF-1alpha, iNOS and Erk1/2 and protects against oxygen-glucose deprivation neuronal injury. Brain Res. 2008;1245:26–35. doi: 10.1016/j.brainres.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 19.Xie LK, Yang SH. Brain globins in physiology and pathology. Med Gas Res. 2016;6:154–163. doi: 10.4103/2045-9912.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Kong X. Isoflurane preconditioning induces neuroprotection by up-regulation of TREK1 in a rat model of spinal cord ischemic injury. Biomol Ther (Seoul) 2016;24:495–500. doi: 10.4062/biomolther.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 22.Ashwal S, Tone B, Tian HR, Cole DJ, Liwnicz BH, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion in the rat pup. Pediatr Res. 1999;46:390–400. doi: 10.1203/00006450-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Kapinya KJ, Lowl D, Futterer C, et al. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 25.Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neuroscience. 2008;154:1002–1008. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong L, Zheng Y, Wu M, et al. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–237. doi: 10.1097/00000539-200301000-00047. table of contents. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Yin J, Ge M, et al. Transforming growth-beta 1 contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury by regulating the c-Jun N-terminal kinase signaling pathway. Biomed Pharmacother. 2016;78:280–290. doi: 10.1016/j.biopha.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 31.Burda J, Danielisova V, Nemethova M, et al. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1141–1151. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Yin J, Wang S, et al. Effects of activin A and its downstream ERK1/2 in oxygen and glucose deprivation after isoflurane-induced postconditioning. Biomed Pharmacother. 2016;84:535–543. doi: 10.1016/j.biopha.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 33.Khan MB, Hoda MN, Vaibhav K, et al. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res. 2015;6:69–77. doi: 10.1007/s12975-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Xue H, Zhao P, et al. Isoflurane postconditioning induces concentration- and timing-dependent neuroprotection partly mediated by the GluR2 AMPA receptor in neonatal rats after brain hypoxia-ischemia. J Anesth. 2016;30:427–436. doi: 10.1007/s00540-015-2132-7. [DOI] [PubMed] [Google Scholar]
- 35.Zhao DA, Bi LY, Huang Q, Zhang FM, Han ZM. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury by suppressing apoptosis. Braz J Anesthesiol. 2016;66:613–621. doi: 10.1016/j.bjane.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–429. doi: 10.1213/01.ane.0000223671.49376.b2. table of contents. [DOI] [PubMed] [Google Scholar]
- 37.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca 2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–615. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Gwak MS, Cao L, Li L, Zuo Z. Isoflurane preconditioning reduces oxygen-glucose deprivation-induced neuronal injury via B-cell lymphoma 2 protein. Environ Toxicol Pharmacol. 2011;31:262–265. doi: 10.1016/j.etap.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi Z, Dong W, Shi W, et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl Stroke Res. 2015;6:198–206. doi: 10.1007/s12975-015-0393-y. [DOI] [PubMed] [Google Scholar]
- 40.Kersten JR, Lowe D, Hettrick DA, Pagel PS, Gross GJ, Warltier DC. Glyburide, a KATP channel antagonist, attenuates the cardioprotective effects of isoflurane in stunned myocardium. Anesth Analg. 1996;83:27–33. doi: 10.1097/00000539-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Kersten JR, Schmeling TJ, Hettrick DA, Pagel PS, Gross GJ, Warltier DC. Mechanism of myocardial protection by isoflurane. Role of adenosine triphosphate-regulated potassium (K ATP) channels. Anesthesiology. 1996;85:794–807. doi: 10.1097/00000542-199610000-00015. discussion 727A. [DOI] [PubMed] [Google Scholar]
- 42.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Munch-Ellingsen J, Lokebo JE, Bugge E, Jonassen AK, Ravingerova T, Ytrehus K. 5-HD abolishes ischemic preconditioning independently of monophasic action potential duration in the heart. Basic Res Cardiol. 2000;95:228–234. doi: 10.1007/s003950050185. [DOI] [PubMed] [Google Scholar]
- 44.Yao Z, Mizumura T, Mei DA, Gross GJ. KATP channels and memory of ischemic preconditioning in dogs: synergism between adenosine and KATP channels. Am J Physiol. 1997;272:H334–342. doi: 10.1152/ajpheart.1997.272.1.H334. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li QF, Wang XR, Yang YW, Su DS. Up-regulation of hypoxia inducible factor 1alpha by isoflurane in Hep3B cells. Anesthesiology. 2006;105:1211–1219. doi: 10.1097/00000542-200612000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Weihrauch D, Schwabe DA, et al. Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in rats. Anesth Analg. 2006;103:281–288. doi: 10.1213/01.ane.0000226094.94877.98. [DOI] [PubMed] [Google Scholar]
- 48.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 49.Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 51.Nandagopal K, Dawson TM, Dawson VL. Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. J Pharmacol Exp Ther. 2001;297:474–478. [PubMed] [Google Scholar]
- 52.Zaugg M, Lucchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003;91:551–565. doi: 10.1093/bja/aeg205. [DOI] [PubMed] [Google Scholar]
- 53.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 54.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Peng L, Zuo Z. Isoflurane preconditioning increases B-cell lymphoma-2 expression and reduces cytochrome c release from the mitochondria in the ischemic penumbra of rat brain. Eur J Pharmacol. 2008;586:106–113. doi: 10.1016/j.ejphar.2008.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahnstedt H, Sweet J, Cruden P, Bishop N, Cipolla MJ. Effects of early post-ischemic reperfusion and t pa0 on cerebrovascular function and nitrosative stress in female rats. Transl Stroke Res. 2016;7:228–238. doi: 10.1007/s12975-016-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juhaszova M, Zorov DB, Kim SH, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun N, Keep RF, Hua Y, Xi G. Critical role of the sphingolipid pathway in stroke: a review of current utility and potential therapeutic targets. Transl Stroke Res. 2016;7:420–438. doi: 10.1007/s12975-016-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 61.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 62.Tang J, Tao Y, Jiang B, et al. Pharmacological preventions of brain injury following experimental germinal matrix hemorrhage: an up-to-date review. Transl Stroke Res. 2016;7:20–32. doi: 10.1007/s12975-015-0432-8. [DOI] [PubMed] [Google Scholar]
- 63.Hayase T, Tachibana S, Yamakage M. Effect of sevoflurane anesthesia on the comprehensive mRNA expression profile of the mouse hippocampus. Med Gas Res. 2016;6:70–76. doi: 10.4103/2045-9912.184715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 67.Zhu C, Gao J, Karlsson N, et al. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin YQ, Wang LF, Chen C, Gao T, Zhao ZF, Li CH. In vivo field recordings effectively monitor the mouse cortex and hippocampus under isoflurane anesthesia. Neural Regen Res. 2016;11:1951–1955. doi: 10.4103/1673-5374.197136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiao S, Zuo Z. A double-edged sword: volatile anesthetic effects on the neonatal brain. Brain Sci. 2014;4:273–294. doi: 10.3390/brainsci4020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waterford SD, Rastegar M, Goodwin E, et al. Methodology of motor evoked potentials in a rabbit model. Transl Stroke Res. 2015;6:399–406. doi: 10.1007/s12975-015-0406-x. [DOI] [PubMed] [Google Scholar]
- 71.Dixon BJ, Zhang JH. New beginnings for Medical Gas Research. Med Gas Res. 2016;6:1. doi: 10.4103/2045-9912.179336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Zhou W, Qiao H. Bioenergetic homeostasis decides neuroprotection or neurotoxicity induced by volatile anesthetics: a uniform mechanism of dual effects. Med Hypotheses. 2011;77:223–229. doi: 10.1016/j.mehy.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Liang LJ, Yang JM, Jin XC. Cocktail treatment, a promising strategy to treat acute cerebral ischemic stroke? Med Gas Res. 2016;6:33–38. doi: 10.4103/2045-9912.179343. [DOI] [PMC free article] [PubMed] [Google Scholar]