Abstract
Intravenous recombinant tissue-type plasminogen activator (r-tPA, alteplase) remains the recommended therapy for acute ischemic stroke. However, several factors are limiting its practical use. It makes it urgent for us to search more efficient strategies that can save the ischemic neurons, and safely extend the time window, while in the mean time reducing the detrimental effects for stroke thrombolysis. Hyperbaric oxygen therapy (HBOT) is considered to be potentially neuroprotective. Co-administration of r-tPA and HBOT has already been proved to be effective, safe and feasible in myocardial infarction. In this article, we would like to review whether HBOT has any beneficial effects on r-tPA thrombolysis. If there is, what is the underlying possible mechanisms and how to optimize for maximal effects?
Keywords: hyperbaric oxygen therapy, tissue plasminogen activator, neuroprotection, stroke, thrombolysis, normobaric hyperoxia therapy, middle cerebral artery occlusion, matrix metalloproteinases
Introduction
With the growing size and aging of the world's population, the global burden of ischemic stroke makes it now a severe public health concern.1,2 Timely restoration of blood flow using thrombolytic therapy is the most effective management for saving those non-infarcted ischemic brain tissues. Intravenous recombinant tissue-type plasminogen activator (r-tPA, alteplase) is recommended for early thrombolysis within 4.5 hours of ischemic stroke onset. Right now, there are several factors limiting the practical use of intravenous alteplase. Firstly, there is a narrow time window. In the United States, about 22% of all ischemic stroke patients successfully present themselves to an emergency department within 3 hours; however, only 8% are eligible for alteplase treatment.3 Further, the benefits of thrombolysis decrease over time. Recently, a meta-analysis that included 26 studies and 2,063 patients identified the overall incidence of partial or complete early recanalization (≤ 3 hours after start of intravenous thrombolysis) was only 33%.4 Besides, 34% of those patients who showed initial recanalization, were found middle cerebral artery (MCA) re-occlusion by transcranial Doppler ultrasound (TCD) within 2 hours of intravenous r-tPA therapy.5 If the neurologic deterioration occurred within the first hour, it might be caused by a early recurrent ischemic stroke.6 Thirdly, the complications increase over time. In a prospective study, 52 out of 128 patients showed hemorrhagic transformation on 3-tesla MRI and susceptibility-weighted imaging, including 8 symptomatic cases, 7 days after thrombolytic therapy.7
The efforts of increasing the practical use of intravenous alteplase never stop. Aside from enhanced patient education and public awareness, extending the time window and reducing complications are the major concerns. A study showed that if alteplase was combined with argatroban, a direct thrombin inhibitor, given intravenously for 48 hours after initial alteplase treatment, the recanalization efficacy could be increased to 62%.8 In preclinical animal experiments, the combination of annexin-A2 and alteplase dissolved clots more rapidly and completely.9,10 A phase 3 trial (NCT01098981) is underway to confirm if adjunctive therapy with TCD sonothrombolysis yield better clinical outcome than alteplase alone.11 In 2015, Liang and his colleagues12 successfully expanded the therapeutic time window of r-tPA to 7 hours in MCA occlusion (MCAO) rats with early normobaric hyperoxia therapy (NBOT). Indeed, all these combined therapies request future exploration. We are still in the urgent need of searching more efficient strategies that can save the ischemic neurons, safely extend the time window, while in the mean time reducing the detrimental effects for stroke thrombolysis.13
Co-administration of R-tpa and Hyperbaric Oxygen Therapy (HBOT)
The r-tPA is physiologically synthesized by the vascular endothelial cells and released into the blood circulation. When combined with the fibrin, it initializes the enzymatic reaction and dissolves the fibrin scaffold in the clot.14 However, r-tPA has also been reported causing vascular disruption with potential cerebral hemorrhage. The toxic effect of r-tPA is associated with activation of matrix metalloproteinases (MMPs).15
HBOT is the medical use of oxygen at a level higher than atmospheric pressure.16 HBOT appears to be a potent way of oxygen delivery.13,17,18 HBOT has been applied in the treatment of cerebral vascular diseases since the 1960s.19,20 It is able to increase the oxygen partial pressure within the blood vessel and restore the oxygen supply after ischemic stroke.21,22 It has been proven that HBOT successfully reduces the infarct size,23,24 hemorrhagic transformation25 and improves neurological function in animal models of ischemic stroke.24 Thus it is considered to be potentially neuroprotective. As early as 2007, Singhal13 concluded that HBOT might extend the time window and increase the efficiency of r-tPA thrombolysis after acute ischemic stroke.
Co-administration of HBOT and R-tpa in Myocardial Infarction
To testify the feasibility of co-administration of HBOT and r-tPA, research was first done on experimental thrombogenic myocardial infarction dog in 1990.26 Simultaneous HBOT plus r-tPA, 2 hours following experimental left anterior descending coronary artery occlusion, showed synergistic effects not only in the resolution of cardiac thrombosis, but also with restoration of enzyme activity. Then more animal studies proved their safety and feasibility with reduced infarct size,27 rapid resolution of pain and ST-segment changes.28,29,30 A small clinical trial even showed favorable effects on left ventricular systolic function in patients with myocardial infarction.31
Co-administration of HBOT and R-tpa in Ischemic Stroke
Based on the experiences of myocardial infarction, a few pre-clinical studies were initiated in ischemic stroke. The first study of early simultaneous treatment with r-tPA and HBOT was done in 1999 by a research group from Germany.32 In an embolic ischemic stroke rat model, r-tPA (9 mg/kg) and HBOT (2.4 ATA (1 ATA = 100 kPa), 1 hour) were given intravenously 2 hours after stroke onset. The combination greatly improved the neurological deficits within 24 hours. Then they did more experiments to determine the timing of HBOT, using similar design and intervention. They found that the adjuvant HBOT (2.4 ATA, 1 hour) either started 45 minutes (sequential) or 2 hours (parallel) after stroke, significantly improved the functional outcome and reduced the infarct volume, than thrombolysis alone.33,34
Sun and his colleagues25 started r-tPA (9 mg/kg) and HBOT (3 ATA, 60 minutes) 1 hour after thromboembolic MCAO. Either thrombin-induced thromboemboli (TT) or calcium-induced thromboemboli (CT) model was applied. Recanalization was only observed in TT model. In both models, the adjuvant HBOT decreased the blood-brain barrier (BBB) permeability and thus hemorrhagic transformation through attenuated activation of MMPs. Interestingly, HBOT significantly reduced infarct size in TT, but not in CT model. The effect of HBOT after thromboembolic ischemia might be associated with whether the recanalization was successful.
More recently, HBOT was found to increase r-tPA-induced thrombolysis in vitro. 1 μL r-tPA was added into the clot formed from 500 μL whole-blood drawn from Sprague-Dawley mature rats. The clot was then incubated in HBOT (2.5 ATA, 90 minutes) before it weighed again to assess the percentage of clot lysis. It turned out that HBOT increased the thrombolytic effect of r-tPA by 64%, while NBOT only by 39%, as compared to controls.35 There was also one case report showing a multimodality approach of intravenous and intra-arterial r-tPA thrombolysis repeated HBOT (2.0 ATA, 90 minutes, started 6 hours after stroke onset) and hypothermia improved the symptoms in spinal cord infarction.36
Long-term Effects of Co-administration of HBOT and R-tpa
Following experimental stroke, long-lasting inflammatory responses and neuronal loss were found. The long-term inflammatory responses are considered as important for repair and neuronal plasticity. In 2011, a research team from Germany initiated r-tPA (9 mg/kg) and HBOT (2.4 ATA, 60 minutes), simultaneously 2 hours after ischemia onset in MCAO rats. They found that after 28 days, the neuron loss was not ameliorated in the four ischemia-related regions. The results may be explained by the small number of study subjects. Besides, only 60-minute HBOT was applied. And the number of reduced NeuN-positive cells alone was not adequate for differentiation neuron loss from apoptosis and necrosis in those regions.37 They also found that the administration of r-tPA with HBOT decreased the macrophage-like cell accumulation at days 14 and 28 post ischemia. Since the lack of a separate HBOT group in the study, it wants definitive proof to conclude that the decreased macrophage-like cell accumulation was related to the co-applied HBOT.15
Proposed Mechanisms: HBOT Combined with R-tpa
The follows are the proposed mechanisms why and how HBOT might add the beneficial effects of r-tPA in thrombogenic-induced ischemic stroke (Figure 1). (1) HBOT has long been demonstrated to be able to dissolve thrombosis by gas bubbles in decompression sickness.38 Then it was speculated that such a mechanical thrombolytic effect might as well be effective for atherosclerotic thrombosis. (2) Secondly, HBOT has been recommended in the treatment of central retinal artery occlusion.39 Learning from the eyes, we know when 100% oxygen is breathed under hyperbaric conditions, at 3 ATA for example, the oxygen dissolved in the plasma rises from 0.31 vol% to 6 vol%.40 Then enough plasma dissolved oxygen is available to meet metabolic needs of the infracted brain areas and earn more time for r-tPA. (3) Thirdly, study showed HBOT could up-regulate the endogenous production of r-tPA by inhibiting the plasminogen activator inhibitor-1 (PAI-1) activity which suppresses r-tPA secretion into the body.41 (4) Further, it is known to all that delayed thrombolytic therapy dramatically increases the risk of hemorrhage because of the disrupted BBB.42 NBOT has been proven to alleviate ischemic BBB damage and significantly improve outcome when r-tPA was given at 4.5 hours43 or even 7 hours12 after stroke onset. HBOT as well, significantly protect BBB integrity during cerebral ischemia via the suppression of MMPs, to reduce the reperfusion damage after recanalization.44,45
Figure 1.
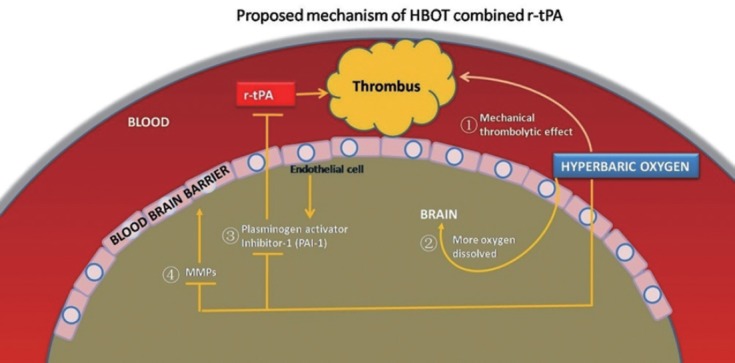
The proposed mechanisms how HBOT might add to the beneficial effects of r-tPA in thrombogenic-induced ischemic stroke.
Note: Numbers ① to ④ correlate to each listed mechanism, respectively. ① HBOT has long been demonstrated to be able to dissolve thrombosis by gas bubbles in decompression sickness. ② HBOT has been recommended in the treatment of central retinal artery occlusion. ③ Study showed HBOT could up-regulate the endogenous production of r-tPA by inhibiting the plasminogen activator Inhibitor-1 (PAI-1) activity which suppresses r-tPA secretion into the body. ④ It is known to all that delayed thrombolytic therapy dramatically increases the risk of hemorrhage because of the disrupted blood-brain barrier. HBOT: Hyperbaric oxygen therapy; r-tPA: tissue-type plasminogen activator; MMPs: matrix metalloproteinases.
Key Points of Efficient Co-administration of HBOT and R-tpa
Dose of HBOT
There is still no comprehensive study to compare the different HBOT ATA and duration, let alone in the settings of r-tPA co-administration. Many research used only one HBOT session of 60 minutes at a certain ATA. As early as 2003, Rogatsky et al.46 put forward that, in order to guarantee the neuroprotective efficacy of HBOT in clinical and experimental acute ischemic stroke (AIS), the dose of HBOT (defined as HBOT ATA, multiplied by hour of a single exposure and total number of treatments) must be maximally optimized. After analyzing retrospectively 265 patients in different hyperbaric centers, they concluded the efficacy of HBOT was closely correlated with higher level of HBOT dose. Applying at least 30–32 doses of HBOT may provide the maximum (100%) possible effects. In other words, the HBOT dose in the published literatures is unlikely to be sufficient.
Time window of HBOT
The therapeutic time window for AIS is generally believed to be started as soon as possible.47,48,49 Research showed that the oxygen therapy was neuroprotective when started either during ischemia,50,51 as early as 10 minutes,52 25 minutes,53 40 minutes,54,55 60 minutes,56 90 minutes,57 or 180 minutes after MCAO.58,59,60,61,62 Our previous study proved that delayed HBOT 48 hours after permanent MCAO (pMCAO) can still convey neuroprotection and restorative cell proliferation.63 HBOT has recently been shown to be effective when started 2–5 days after ischemic stroke onset,64,65 or even induces neuroplasticity in the chronic stage.66 A retrospective statistical analysis proved HBOT initiated within the first 3 hours post-stroke the most promise for efficacy. Pre-r-tPA administration of HBOT may also be a useful challenge to assess the improvement of r-tPA therapy.38,67 All these time points need to be tested in the context of r-tPA co-administration.
Safety in humans
In both the pilot study and randomized multicenter trial of acute myocardial infarction patients, HBOT combined with r-tPA thrombolysis was feasible and safe. Sixty-six patients with inferior acute myocardial infarction and forty-six patients with anterior acute myocardial infarction, were randomized to treatment with HBOT combined with either r-tPA or streptokinase (STK), or r-tPA or STK alone. There were two deaths in the control and one in those treated with HBOT. The HBOT resulted in more rapid resolution of pain and ST segment changes.28,30
Feasibility
Scientists from the Wake Forest, School of Medicine, North Carolina, USA have designed and built a prototype hyperbaric oxygen ambulance for stroke patients, where HBOT can be initiated before arriving at the hospital.38 As soon as it completes, it will be used in conjunction with ambulance-based telemedicine techniques,68 a CT scanner operable in the HBO environment of our mobile chamber equipped ambulance, and in clinical trials to test the safety and validity, eventually facilitate ambulance based r-tPA administration with FDA time window.
HBOT vs. NBOT
Only one study compared the effects of NBOT and HBOT in experimental stroke.34 The animals were assigned to MCAO control, NBOT, HBOT, r-tPA or HBOT + r-tPA group. Either NBOT (1 hour) or HBOT (2.4 ATA, 1 hour) was initiated 2 hours after ischemia onset. They found significant functional improvement in the NBOT, r-tPA and HBOT + r-tPA group, but not in the HBOT group. However, HBOT did tend to stabilize BBB and reduce MMP activation. Moreover, its concomitant treatment with r-tPA also provided early functional improvement. It is a pity that the study failed to add a group of NBOT + r-tPA. As a result, further studies are required to identify the beneficial effects of both NBOT and HBOT, in the setting of interactions with r-tPA, especially the optimized dose and time window of HBOT.
Summary
In embolic ischemic stroke models, HBOT is able to add to the effects of r-tPA thrombolysis with improved neurological functions. The underlying mechanisms may be associated with the mechanical effects, increased percentage of oxygen dissolved in the plasma, the up-regulated level of endogenous r-tPA and stabilization of BBB. The maximal effects can be achieved by optimizing the dose and time window of HBOT. HBOT is still a promising strategy to treat acute cerebral ischemic stroke. Future directions include experiments on different models of ischemic cerebrovascular diseases,69 cocktail treatment14,70 with NBOT,71 well designed clinical trials and more mechanism studies.
Footnotes
Funding: This work was supported by grants from National Nature Science Foundation of China (No. 81371310) and Health Bureau of Chongqing (No. 2012-2-033).
Conflicts of interest
The authors have not disclosed any potential conflicts of interest.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
References
- 1.Bejot Y, Daubail B, Giroud M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev Neurol (Paris) 2016;172:59–68. doi: 10.1016/j.neurol.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Favate AS, Younger DS. Epidemiology of ischemic stroke. Neurol Clin. 2016;34:967–980. doi: 10.1016/j.ncl.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Kissela B, Schneider A, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke. 2004;35:e27–29. doi: 10.1161/01.STR.0000109767.11426.17. [DOI] [PubMed] [Google Scholar]
- 4.Seners P, Turc G, Maier B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47:2409–2412. doi: 10.1161/STROKEAHA.116.014181. [DOI] [PubMed] [Google Scholar]
- 5.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 6.Georgiadis D, Engelter S, Tettenborn B, et al. Early recurrent ischemic stroke in stroke patients undergoing intravenous thrombolysis. Circulation. 2006;114:237–241. doi: 10.1161/CIRCULATIONAHA.105.597435. [DOI] [PubMed] [Google Scholar]
- 7.Annan M, Gaudron M, Cottier JP, et al. Functional outcome of hemorrhagic transformation after thrombolysis for ischemic stroke: a prospective study. Cerebrovasc Dis Extra. 2015;5:103–106. doi: 10.1159/000440737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barreto AD, Alexandrov AV, Lyden P, et al. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012;43:770–775. doi: 10.1161/STROKEAHA.111.625574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Fan X, Yu Z, et al. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30:1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walvick RP, Bratane BT, Henninger N, et al. Visualization of clot lysis in a rat embolic stroke model: application to comparative lytic efficacy. Stroke. 2011;42:1110–1115. doi: 10.1161/STROKEAHA.110.602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saqqur M, Tsivgoulis G, Nicoli F, et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2014;24:209–220. doi: 10.1111/jon.12026. [DOI] [PubMed] [Google Scholar]
- 12.Liang J, Qi Z, Liu W, et al. Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke. 2015;46:1344–1351. doi: 10.1161/STROKEAHA.114.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal AB. A review of oxygen therapy in ischemic stroke. Neurol Res. 2007;29:173–183. doi: 10.1179/016164107X181815. [DOI] [PubMed] [Google Scholar]
- 14.Liang LJ, Yang JM, Jin XC. Cocktail treatment, a promising strategy to treat acute cerebral ischemic stroke? Med Gas Res. 2016;6:33–38. doi: 10.4103/2045-9912.179343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalski D, Heindl M, Kacza J, et al. Spatio-temporal course of macrophage-like cell accumulation after experimental embolic stroke depending on treatment with tissue plasminogen activator and its combination with hyperbaric oxygenation. Eur J Histochem. 2012;56(2):e14. doi: 10.4081/ejh.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu J, Krafft PR, Zhang JH. Hyperbaric oxygen therapy promotes neurogenesis: where do we stand? Med Gas Res. 2011;1(1):14. doi: 10.1186/2045-9912-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Q, Manaenko A, Guo Z, Huang L, Tang J, Zhang JH. Hyperbaric oxygen therapy for post concussion symptoms: issues may affect the results. Med Gas Res. 2015;5:10. doi: 10.1186/s13618-015-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harch PG. Hyperbaric oxygen in chronic traumatic brain injury: oxygen, pressure, and gene therapy. Med Gas Res. 2015;5:9. doi: 10.1186/s13618-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Z, Tong WC, Lu XX, Peng HP. Hyperbaric oxygen therapy in acute ischemic stroke: a review. Interv Neurol. 2014;2:201–211. doi: 10.1159/000362677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai WW, Sun L, Yu ZQ, Chen G. Hyperbaric oxygen therapy in experimental and clinical stroke. Med Gas Res. 2016;6:111–118. doi: 10.4103/2045-9912.184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke - experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9:227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- 22.Mychaskiw G., 2nd Hyperbaric oxygen therapy and neurologic disease: the time has come. Undersea Hyperb Med. 2010;37:xi–xiii. [PubMed] [Google Scholar]
- 23.Yin D, Zhou C, Kusaka I, et al. Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. J Cereb Blood Flow Metab. 2003;23:855–864. doi: 10.1097/01.WCB.0000073946.29308.55. [DOI] [PubMed] [Google Scholar]
- 24.Yin D, Zhang JH. Delayed and multiple hyperbaric oxygen treatments expand therapeutic window in rat focal cerebral ischemic model. Neurocrit Care. 2005;2:206–211. doi: 10.1385/NCC:2:2:206. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Zhou W, Mueller C, et al. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1651–1660. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas MP, Brown LA, Sponseller DR, Williamson SE, Diaz JA, Guyton DP. Myocardial infarct size reduction by the synergistic effect of hyperbaric oxygen and recombinant tissue plasminogen activator. Am Heart J. 1990;120:791–800. doi: 10.1016/0002-8703(90)90194-3. [DOI] [PubMed] [Google Scholar]
- 27.Sterling DL, Thornton JD, Swafford A, et al. Hyperbaric oxygen limits infarct size in ischemic rabbit myocardium in vivo. Circulation. 1993;88:1931–1936. doi: 10.1161/01.cir.88.4.1931. [DOI] [PubMed] [Google Scholar]
- 28.Stavitsky Y, Shandling AH, Ellestad MH, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the ‘HOT MI’ randomized multicenter study. Cardiology. 1998;90:131–136. doi: 10.1159/000006832. [DOI] [PubMed] [Google Scholar]
- 29.Laden G. HOT MI pilot study. Hyperbaric Oxygen and Thrombolysis in Myocardial Infarction. Am Heart J. 1998;136:749. [PubMed] [Google Scholar]
- 30.Shandling AH, Ellestad MH, Hart GB, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the “HOT MI” pilot study. Am Heart J. 1997;134:544–550. doi: 10.1016/s0002-8703(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 31.Dekleva M, Neskovic A, Vlahovic A, Putnikovic B, Beleslin B, Ostojic M. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148:E14. doi: 10.1016/j.ahj.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Michalski D, Kuppers-Tiedt L, Weise C, et al. Long-term functional and neurological outcome after simultaneous treatment with tissue-plasminogen activator and hyperbaric oxygen in early phase of embolic stroke in rats. Brain Res. 2009;1303:161–168. doi: 10.1016/j.brainres.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Kuppers-Tiedt L, Manaenko A, Michalski D, et al. Combined systemic thrombolysis with alteplase and early hyperbaric oxygen therapy in experimental embolic stroke in rats: relationship to functional outcome and reduction of structural damage. Acta Neurochir Suppl. 2011;111:167–172. doi: 10.1007/978-3-7091-0693-8_28. [DOI] [PubMed] [Google Scholar]
- 34.Michalski D, Pelz J, Weise C, et al. Early outcome and blood-brain barrier integrity after co-administered thrombolysis and hyperbaric oxygenation in experimental stroke. Exp Transl Stroke Med. 2011;3:5. doi: 10.1186/2040-7378-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chazalviel L, Haelewyn B, Degoulet M, et al. Hyperbaric oxygen increases tissue-plasminogen activator-induced thrombolysis in vitro, and reduces ischemic brain damage and edema in rats subjected to thromboembolic brain ischemia. Med Gas Res. 2016;6:64–69. doi: 10.4103/2045-9912.184713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K, Strozyk D, Rahman C, et al. Acute spinal cord ischemia: treatment with intravenous and intra-arterial thrombolysis, hyperbaric oxygen and hypothermia. Cerebrovasc Dis. 2010;29:95–98. doi: 10.1159/000259618. [DOI] [PubMed] [Google Scholar]
- 37.Hobohm C, Laignel F, Kacza J, et al. Long-lasting neuronal loss following experimental focal cerebral ischemia is not affected by combined administration of tissue plasminogen activator and hyperbaric oxygen. Brain Res. 2011;1417:115–126. doi: 10.1016/j.brainres.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 38.McCormick JG, Houle TT, Saltzman HA, Whaley RC, Roy RC. Treatment of acute stroke with hyperbaric oxygen: time window for efficacy. Undersea Hyperb Med. 2011;38:321–334. [PubMed] [Google Scholar]
- 39.Butler FK, Jr, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea Hyperb Med. 2008;35:333–387. [PubMed] [Google Scholar]
- 40.Piantadosi CA. Physiology of hyperbaric hyperoxia. Respir Care Clin N Am. 1999;5(1):7–19, v. [PubMed] [Google Scholar]
- 41.Yamami N, Shimaya K, Sera AM, et al. Alterations of fibrinolytic activity in human during and after hyperbaric oxygen exposure. Appl Human Sci. 1996;15:239–242. doi: 10.2114/jpa.15.239. [DOI] [PubMed] [Google Scholar]
- 42.Hatcher MA, Starr JA. Role of tissue plasminogen activator in acute ischemic stroke. Ann Pharmacother. 2011;45:364–371. doi: 10.1345/aph.1P525. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009;40:2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Yang Y, Tang H, et al. Hyperbaric oxygen therapy ameliorates local brain metabolism, brain edema and inflammatory response in a blast-induced traumatic brain injury model in rabbits. Neurochem Res. 2014;39:950–960. doi: 10.1007/s11064-014-1292-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Zhang Q, Xue Y, Chen X, Haun RS. Effects of hyperbaric oxygen on the expression of claudins after cerebral ischemia-reperfusion in rats. Exp Brain Res. 2011;212:109–117. doi: 10.1007/s00221-011-2702-3. [DOI] [PubMed] [Google Scholar]
- 46.Rogatsky GG, Shifrin EG, Mayevsky A. Optimal dosing as a necessary condition for the efficacy of hyperbaric oxygen therapy in acute ischemic stroke: a critical review. Neurol Res. 2003;25:95–98. doi: 10.1179/016164103101201003. [DOI] [PubMed] [Google Scholar]
- 47.Stoller KP. All the right moves: the need for the timely use of hyperbaric oxygen therapy for treating TBI/CTE/PTSD. Med Gas Res. 2015;5:7. doi: 10.1186/s13618-015-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Yu F, Lei T, et al. Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies. Neural Regen Res. 2016;11:1015–1024. doi: 10.4103/1673-5374.184506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peplow PV. Neuroimmunomodulatory effects of transcranial laser therapy combined with intravenous tPA administration for acute cerebral ischemic injury. Neural Regen Res. 2015;10:1186–1190. doi: 10.4103/1673-5374.162687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou H, Grinberg O, Williams B, et al. The effect of oxygen therapy on brain damage and cerebral pO(2) in transient focal cerebral ischemia in the rat. Physiol Meas. 2007;28:963–976. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- 51.Yang ZJ, Xie Y, Bosco GM, Chen C, Camporesi EM. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol. 2010;108:513–522. doi: 10.1007/s00421-009-1229-9. [DOI] [PubMed] [Google Scholar]
- 52.Acka G, Sen A, Canakci Z, et al. Effect of combined therapy with hyperbaric oxygen and antioxidant on infarct volume after permanent focal cerebral ischemia. Physiol Res. 2007;56:369–373. doi: 10.33549/physiolres.930907. [DOI] [PubMed] [Google Scholar]
- 53.Sun L, Strelow H, Mies G, Veltkamp R. Oxygen therapy improves energy metabolism in focal cerebral ischemia. Brain Res. 2011;1415:103–108. doi: 10.1016/j.brainres.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 54.Veltkamp R, Siebing DA, Heiland S, et al. Hyperbaric oxygen induces rapid protection against focal cerebral ischemia. Brain Res. 2005;1037:134–138. doi: 10.1016/j.brainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Veltkamp R, Siebing DA, Sun L, et al. Hyperbaric oxygen reduces blood-brain barrier damage and edema after transient focal cerebral ischemia. Stroke. 2005;36:1679–1683. doi: 10.1161/01.STR.0000173408.94728.79. [DOI] [PubMed] [Google Scholar]
- 56.Lou M, Zhang H, Wang J, et al. Hyperbaric oxygen treatment attenuated the decrease in regional glucose metabolism of rats subjected to focal cerebral ischemia: a high resolution positron emission tomography study. Neuroscience. 2007;146:555–561. doi: 10.1016/j.neuroscience.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 57.Beynon C, Sun L, Marti HH, Heiland S, Veltkamp R. Delayed hyperbaric oxygenation is more effective than early prolonged normobaric hyperoxia in experimental focal cerebral ischemia. Neurosci Lett. 2007;425:141–145. doi: 10.1016/j.neulet.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Eschenfelder CC, Krug R, Yusofi AF, et al. Neuroprotection by oxygen in acute transient focal cerebral ischemia is dose dependent and shows superiority of hyperbaric oxygenation. Cerebrovasc Dis. 2008;25:193–201. doi: 10.1159/000113856. [DOI] [PubMed] [Google Scholar]
- 59.Liu JR, Zhao Y, Patzer A, et al. The c-Jun N-terminal kinase (JNK) inhibitor XG-102 enhances the neuroprotection of hyperbaric oxygen after cerebral ischaemia in adult rats. Neuropathol Appl Neurobiol. 2010;36:211–224. doi: 10.1111/j.1365-2990.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 60.Lou M, Wang JH, Qian QQ, Wen SQ, Ding MP. Effect of hyperbaric oxygen treatment on mitochondrial free radicals after transient focal cerebral ischemia in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008;37:437–443. doi: 10.3785/j.issn.1008-9292.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Wang RY, Chang HC, Chen CH, Tsai YW, Yang YR. Effects of hyperbaric oxygenation on oxidative stress in acute transient focal cerebral ischemic rats. Eur J Appl Physiol. 2012;112:215–221. doi: 10.1007/s00421-011-1976-2. [DOI] [PubMed] [Google Scholar]
- 62.Xue L, Yu Q, Zhang H, Liu Y, Wang C, Wang Y. Effect of large dose hyperbaric oxygenation therapy on prognosis and oxidative stress of acute permanent cerebral ischemic stroke in rats. Neurol Res. 2008;30:389–393. doi: 10.1179/174313208X300413. [DOI] [PubMed] [Google Scholar]
- 63.Mu J, Ostrowski RP, Soejima Y, et al. Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of cAMP responsive element binding protein in the rat model of MCAo-induced ischemic brain injury. Neurobiol Dis. 2013;51:133–143. doi: 10.1016/j.nbd.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee YS, Chio CC, Chang CP, et al. Long course hyperbaric oxygen stimulates neurogenesis and attenuates inflammation after ischemic stroke. Mediators Inflamm 2013. 2013:512978. doi: 10.1155/2013/512978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen CH, Chen SY, Wang V, et al. Effects of repetitive hyperbaric oxygen treatment in patients with acute cerebral infarction: a pilot study. Scientific World Journal 2012. 2012:694703. doi: 10.1100/2012/694703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Efrati S, Fishlev G, Bechor Y, et al. Hyperbaric oxygen induces late neuroplasticity in post stroke patients-randomized, prospective trial. PLoS One. 2013;8:e53716. doi: 10.1371/journal.pone.0053716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu Q, Manaenko A, Matei N, et al. Hyperbaric oxygen preconditioning: a reliable option for neuroprotection. Med Gas Res. 2016;6:20–32. doi: 10.4103/2045-9912.179337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LaMonte MP, Xiao Y, Hu PF, et al. Shortening time to stroke treatment using ambulance telemedicine: TeleBAT. J Stroke Cerebrovasc Dis. 2004;13:148–154. doi: 10.1016/j.jstrokecerebrovasdis.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Ostrowski RP, Stepien K, Pucko E, Matyja E. Hyperbaric oxygen modalities are differentially effective in distinct brain ischemia models. Med Gas Res. 2016;6:39–47. doi: 10.4103/2045-9912.179344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chazalviel L, Blatteau JE, Vallee N, Risso JJ, Besnard S, Abraini JH. Effects of normobaric versus hyperbaric oxygen on cell injury induced by oxygen and glucose deprivation in acute brain slices. Med Gas Res. 2016;6:169–173. doi: 10.4103/2045-9912.191364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi SH, Qi ZF, Luo YM, Ji XM, Liu KJ. Normobaric oxygen treatment in acute ischemic stroke: a clinical perspective. Med Gas Res. 2016;6:147–153. doi: 10.4103/2045-9912.191360. [DOI] [PMC free article] [PubMed] [Google Scholar]


