Abstract
The therapeutic effects of hydrogen-rich saline (HRS) have been reported for a wide range of diseases mainly via selectively reducing the amount of reactive oxygen species. Oxidative stress plays an important role in the pathogenesis of uveitis and endotoxin-induced uveitis (EIU). In this study, we investigated whether HRS can mitigate EIU in rats. Sprague-Dawley rats were randomly divided into Norm group, Model group, HRS group, dexamethasone (DEX) group, and rats in the latter three groups were injected with equal amount of lipopolysaccharide (LPS) to induce EIU of different severities (by 1 mg/kg of LPS, or 1/8 mg/kg of LPS). Rats in HRS group were injected with HRS intraperitoneally at three different modes to purse an ameliorating effect of EIU (10 mL/kg of HRS immediately after injection of 1 mg/kg of LPS, 20 mL/kg of HRS once a day for 1 week before injection of 1 mg/kg of LPS and at 0, 0.5, 1, 2, 6, 8, 12 hours after LPS administration, or 20 mL/kg of HRS once a day for 1 week before injection of 1/8 mg/kg of LPS, and at 0, 0.5, 1, 2, 6, 8, 12, 24 hours and once a day for 3 weeks after LPS administration). Rats of DEX group were injected with 1 mL/kg of DEX solution intraperitoneally immediately after LPS administration. Rats in Norm and Model groups did not receive any treatment. All rats were examined under slit lamp microscope and graded according to the clinical signs of uveitis. Electroretinogram, quantitative analysis of protein in aqueous humor (AqH) and histological examination of iris and ciliary body were also carried out. Our results showed that HRS did not obviously ameliorate the signs of uveitis under slit lamp examination and the inflammatory cells infiltration around iris and cilliary body of EIU induced by 1 mg/kg or 1/8 mg/kg of LPS (P > 0.05), while DEX significantly reduced the inflammation reflected by the above two indicators (P < 0.05). The impaired retinal function of mild EIU induced by 1/8 mg/kg of LPS, showed by delay of peak time of b-wave of Dark adapted 3.0 electroretinogram, was not significantly restored by HRS (P > 0.05), while DEX had an obvious therapeutic effect (P < 0.05). However, HRS exerted an inhibition trend on elevation of protein in AqH of EIU induced by 1 mg/kg of LPS, and significantly reduced the increasing amount of protein in AqH of mild EIU induced by 1/8 mg/kg of LPS (P < 0.05). In conclusion, HRS could not obviously mitigate EIU in rats, while it could inhibit the elevation of AqH protein.
Keywords: hydrogen, hydrogen-rich saline, uveitis, lipopolysaccharide, slit lamp, aqueous humor protein, inflammatory cells infiltration, electroretinogram
Introduction
Molecular hydrogen (H2), a new kind of antioxidants, is effective in the prevention and treatment of many diseases by virtue of its selective anti-oxidative, anti-inflammatory and anti-apoptotic activities,1,2 and hydrogen-rich saline (HRS) is a safe, portable, and easy approach for therapeutic H2 delivery.3 Ischemia injury, inflammation, cancer and allergy have been reported to be alleviated to some extent by H2.4,5,6,7 Specifically, H2 could mitigate inflammation in lung, liver and other organs.8,9,10 The mechanism by which H2 alleviates inflammation may be that it directly reduces the amount of media accounting for incurring inflammation,11 or it directly combines with cytotoxic reactive oxygen species (ROS), such as the ·OH and ONOO– radicals, to ameliorate the oxidative stress, which is deemed to initiate and promote inflammation.12,13
Uveitis, a particular inflammation of uvea, may involve the iris, choroid, optic nerve, retina, vitreous body, or a combination thereof, and can be caused by numerous ocular diseases and systemic conditions. It is a major cause of severe visual handicap and is responsible for an estimated 10% of all blindness in the United States.14 The etiology of uveitis is complicated and its pathological mechanism is not clear up to date, in which oxidative stress may play an important role.15,16 Steroids, cyclosporine A and other immunosuppressors are all effective drugs to treat uveitis; however, long-term use is precluded because of significant side effects, such as osteoporosis and renal toxicity.17 Efforts towards developing a non-steroidal therapy for uveitis have been made by many researchers.18
Endotoxin-induced uveitis (EIU) is a commonly used animal model that shares essential pathological features with human uveitis.19 EIU can be induced by systemic injection of endotoxin (also known as lipopolysaccharide, LPS) and then exhibits an acute ocular inflammation with iris hyperaemia and inflammatory cells infiltration.20 Studies have shown that oxidative stress is one of the main mechanisms in the pathogenesis of EIU.21 Quite a few drugs, such as anthocyanin, resveratrol and sea urchin pigment, have been reported to mitigate EIU through reducing the amount of ROS.22,23,24,25 Until now, it has not been elucidated whether H2 has an alleviating effect on uveitis. Considering the antioxidant activity of H2 , and the role of oxidative stress playing in the pathogenesis of uevitis and EIU, we launched this study to explore whether HRS can somehow ameliorate uveitis in a rat model of EIU.
Materials and Methods
Animals and materials
Healthy adult male Sprague-Dawley (SD) rats (6 to 8 weeks old, weighing 180–220 g) were obtained from the Laboratory Animal Center of the Fourth Military Medical University in Xi’an, Shaanxi Province, China (license No. 2014270138S). All animal experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. Furthermore, all experiments were approved by the Animal Care and Use Committee of the Fourth Military Medical University. All animals were kept under dim cyclic light (5 lux, 12 hours on/off, 6 a.m.–6 p.m.), with food and water available ad libitum.
Lipopolysaccharide (LPS, L6511, Sigma-Aldrich, St. Louis, MO, USA) was stored in the dark at 4°C, and was dissolved into saline before use.26 HRS was prepared as described previously.27 Briefly, H2 was dissolved in saline under high pressure (0.4 MPa) for 6 hours to achieve a supersaturated solution. HRS was then stored in an aluminum bag without dead volume at 4°C under atmospheric pressure, and was freshly prepared every week to maintain the concentration above 0.6 mM. Hydrogen content in the saline was confirmed using a previously described gas chromatographic method.28
Experimental design
Part 1
To observe the effects of normal dose of HRS on EIU, rats were equally divided into Norm, Model, dexamethasone (DEX), and HRS groups at random, and rats in the latter three groups were induced EIU by subcutaneously injecting 1 mg/kg of LPS into the footpads.26 Rats in DEX group were injected with 1 mg/kg DEX (dexamethasone sodium phosphate injection, #H41020055, 5 mg/mL, Zhuofeng Pharmaceutical Co., Ltd., Zhengzhou, Henan Province, China) intraperitoneally (i.p.) immediately after injection of LPS, while rats of HRS group were injected with 10 mL/kg HRS i.p. Rats in Norm and Model groups did not receive any treatment. Slit lamp examination, quantitative analysis of protein in aqueous humor (AqH) and histological examination with inflammation cell counting around iris and ciliary body (ICB) were carried out 24 hours after LPS administration.
Part 2
To investigate the effects of large dose of HRS on EIU, 24 rats were equally assigned into Norm group, Model group, DEX group, HRS group randomly, and rats in the latter three groups were induced EIU by subcutaneous injection of 1 mg/kg of LPS into the footpads. Rats of DEX group were injected with 1 mg/kg of DEX i.p. immediately after injection of LPS. Rats in HRS group were injected with 20 mL/kg HRS i.p. once a day for 1 week before injection of LPS and at time points of 0, 0.5, 1, 2, 6, 8, 12 hours after LPS administration. Slit lamp examination, quantitative analysis of protein in AqH and histological examination with inflammation cell counting around ICB were carried out 24 hours after LPS injection.
Part 3
To obtain mild EIU models, 18 rats were induced to develop mild EIU by subcutaneously injecting 1/2, 1/3, 1/4, 1/6, 1/7 and 1/8 mg/kg of LPS into the footpads (3 rats for each dose) respectively. Signs of uveitis were estimated under slit lamp 24 hours after injection of LPS.
Part 4
To observe the effects of large dose of HRS on mild EIU, rats were randomly divided into Norm group, Model group, DEX group, HRS group, and rats in the latter three groups were induced EIU by subcutaneously injecting 1/8 mg/kg of LPS into the footpads. Rats of DEX group were injected with 1 mg/kg DEX i.p. immediately after injection of LPS. Rats in HRS group were injected with 20 mL/kg HRS i.p. once a day for 1 week before LPS injection, and at 0, 0.5, 1, 2, 6, 8, 12, 24 hours and once a day for 3 weeks after injection of LPS. All rats were examined under slit lamp microscope and graded according to the clinical signs of uveitis at 12, 24, 48, 72, 96 hours after LPS administration. Besides, electroretinogram (ERG), AqH protein quantitative analysis and histological examination with inflammation cell counting around ICB were carried out on days 1, 4, 7, 10, 14 and 21 after LPS administration. In this part, 24 rats (6 for each group) were used for the examination of uveitis under slit lamp and by ERG within 21 days after LPS administration, because these two indicators were obtained when the rats were alive. These rats were killed for histopathological observation by hematoxylin and eosin staining and aqueous humor protein measurement at 21 days after LPS administration. Besides, another 72 rats were used for histopathological observation by hematoxylin and eosin staining and aqueous humor protein measurement at 1, 4, 7, and 10 days after LPS administration, with 6 rats from each group at each time, except for the Norm group, in order to minimize the number of animals used.
Evaluation of EIU under a slit lamp
Slit lamp examination was conducted under anesthetization of rats. Pictures of the rat eyes with the slit on the middle of the cornea were taken. Clinical scoring of uveitis was graded as previously reported29: iris hyperemia (0–2), flare (0–2), hypopyon (0–2), and miosis (0–2). The maximum possible score was 8.
ERG recording
ERG recording was carried out as previous reported.3 After overnight dark adaption, animals were anesthetized with intraperitoneal injections of 1% sodium pentobarbital (3 mL/kg, Sigma-Aldrich) and Sumianxin II (a compound preparation of xylidinothiazoline, EDTA, dihydroetorphine hydrochloride and haloperidol, 0.025 mL/kg, Jilin Shengda Animal Pharmaceutical Co., Ltd., Dunhua, Jilin Province, China), and placed on a platform to keep fixed. Their pupils were dilated with 0.5% tropicamide-phenylephrine ophthalmic solution (Shenyang Xingji Corporation, Shenyang, Liaoning Province, China). The corneas were covered with medical sodium hyaluronate gel (Bausch & Lomb Freda, Shandong Province, China). The reference electrode and ground electrode were inserted beneath the skin of the cheek around the tested eye and tail respectively. Full-field (Ganzfeld) stimulation and a computer system (RETI port, Roland Consult GmbH, Brandenburg, Germany) were applied to record ERG. All operations were conducted under a dim red light to maximize retinal sensitivity. Dark-adapted 0.01 ERG, Dark-adapted 3.0 ERG, Dark-adapted oscillatory potentials, Light-adapted 3.0 ERG and Light-adapted flicker ERG were recorded and analyzed according to the ISCEV guidelines,30 by a technician unaware of animal grouping and treatments.
Protein concentration in AqH
AqH was collected by anterior chamber puncture with an insulin syringe immediately after euthanization of rats. All the AqH samples were stored in the ice until tested. The samples were centrifuged at 12,000 r/min for 10 minutes at 4°C to obtain the supernatant. The total protein concentration in the AqH was measured by bicinchonininc acid (BCA) assay (W041, Jiancheng, Nanjing, Jiangsu Province, China) according to the manufacturer's instructions.
Histopathological evaluation
Eyes of rats of all groups were enucleated rapidly by intraperitoneal injection of lethal dose of sodium pentobarbital (Sigma-Aldrich), and were stored in 4% paraformaldehyde solution at 4°C overnight. Tissues were dehydrated using graded ethanol, and then paraffin embedded. The 4 μm sagittal sections were cut along the pupil-optic nerve axis. For each eye, three sections that included the optic nerve were stained with hematoxylin and eosin, images of which were taken using a digital imaging system (DP71, Olympus, Japan) and analyzed by counting the infiltrating cells around ICB at high magnification (× 400).
Statistical analysis
All data were expressed as the mean ± SE and analyzed by one-way analysis of variance (ANOVA) followed by contrast analysis (least significant difference-t test when equal variances assumed, and Dunnett's T3 test when equal variances not assumed) using the SPSS 16.0 software (SPSS, Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
Effects of normal dose of HRS on EIU
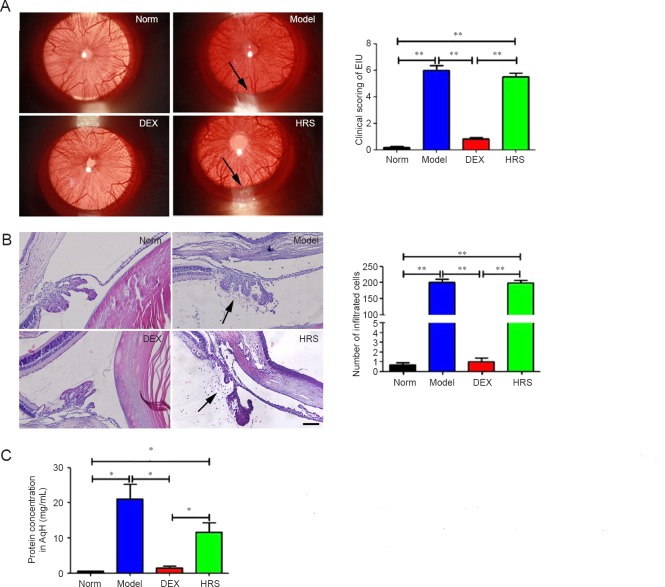
Rats of Norm group had no signs of uveitis, while rats of Model group manifested typical signs of uveitis such as iris hyperemia and hypopyon at 24 hours after injection of 1 mg/kg of LPS. The quantitative score of uveitis signs of Model group was 6.00 ± 0.37, with the number of inflammation cells around ICB being 200.33 ± 10.54, and the total protein concentration in AqH being 21.17 ± 4.15 mg/mL. All the indexes were higher compared with those of Norm group (0.17 ± 0.11, 0.67 ± 0.21 and 0.48 ± 0.05 mg/mL) with significant differences (P < 0.01), indicating that a rat model of uveitis was established successfully. Rats of HRS group had obvious uveitis signs, with the quantitative score of uveitis signs and the number of inflammation cells around ICB being 5.50 ± 0.2 and 198.50 ± 8.73, both of which had no significant differences compared to those of Model group (P > 0.05). The total protein concentration in the AqH of HRS group was 14.65 ± 2.95 mg/mL, which was somewhat lower than that of Model group, but without any significant difference between the two groups (P > 0.05). Rats of DEX group displayed no obvious signs of uveitis, with almost no inflammatory cells infiltrating around ICB and protein in AqH (Figure 1).
Figure 1.
Effects of normal dose of hydrogen-rich saline (HRS) on endotoxin-induced uveitis (EIU).
Note: (A) Findings under slit lamp examination and scoring of uveitis 24 hours after LPS administration. Arrows show hypopyon. (B) Histology of ICB and numbers of inflammation cells infiltrating around ICB 24 hours after LPS administration (× 200). Arrows show infiltrated cells. Scale: 100 μm. (C) Protein concentration in AqH at 24 hours after LPS injection. *P < 0.05, **P < 0.01. LPS: Lipopolysaccharide; ICB: iris and ciliary body; AqH: aqueous humor; DEX: dexamethasone.
Effects of large dose of HRS on EIU
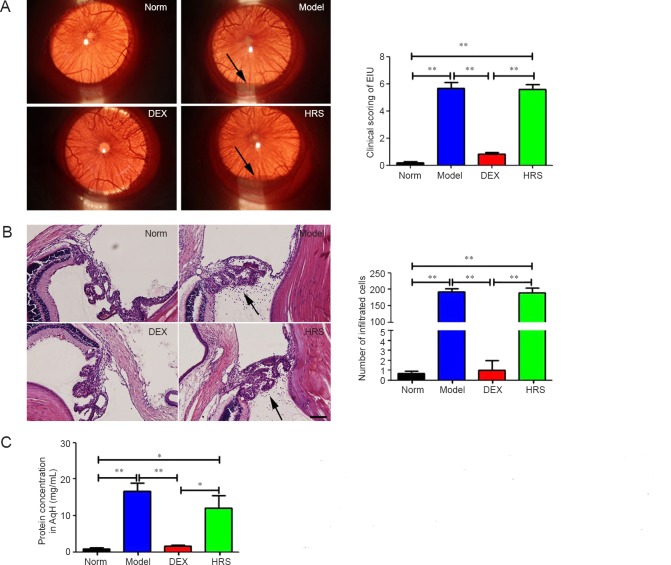
Rats injected with multiple injections of large dose of HRS exhibited similar signs of uveitis to those of Model group 24 hours after LPS administration. The quantitative score of uveitis signs and the number of inflammation cells around ICB in HRS and Model groups were 5.58 ± 0.35 vs. 5.61 ± 0.44, and 189.50 ± 13.93 vs. 192.33 ± 9.45 respectively, with no significant differences existing between them (P > 0.05). The protein concentration in AqH of HRS group was lower than that of Model group (12.17 ± 3.23 vs. 16.60 ± 2.30 mg/mL), but the difference was not statistically significant (P > 0.05). There was no obvious iris hyperemia or hypopyon in rats of DEX group, with almost no inflammatory cells infiltrating around ICB and almost no protein in AqH (Figure 2).
Figure 2.
Effects of large dose of hydrogen-rich saline (HRS) on endotoxin-induced uveitis (EIU).
Note: (A) Findings under slit lamp examination and scoring of uveitis 24 hours after LPS administration. Arrows show hypopyon. (B) Histology of ICB and numbers of inflammation cells infiltrating around ICB 24 hours after LPS administration (× 200). Arrows show infiltrated cells. Scale: 100 μm. (C) Protein concentration in AqH at 24 hours after LPS injection. *P < 0.05; **P < 0.01. LPS: Lipopolysaccharide; ICB: iris and ciliary body; AqH: aqueous humor; DEX: dexamethasone.
EIU of various severities
Since we could not find the relieving effects of HRS, even by multiple injections of large dose, on EIU induced by 1 mg/kg of LPS in rats, rats were subcutaneously injected with 1/2, 1/3, 1/4, 1/6, 1/7 and 1/8 mg/kg of LPS into the footpads respectively to establish mild EIU models. From findings under slit lamp examination of uveitis, we discovered that the severity of uveitis (the iris hyperemia and hypopyon) lessened with the reducing dose of LPS. A relatively mild uveitis in rats was observed when 1/8 mg/kg of LPS was administered (Figure 3).
Figure 3.
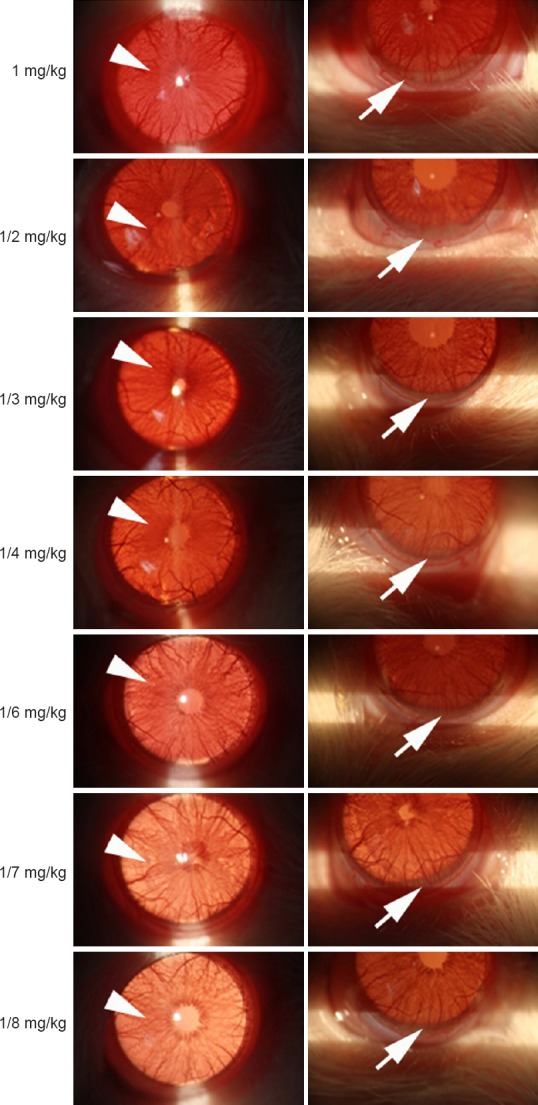
Findings under slit lamp examination of uveitis at 24 hours after injection of various doses of lipopolysaccharide.
Note: Arrowheads show hyperaemia of iris. Arrows show hypopyon.
Effects of large dose of HRS on mild EIU
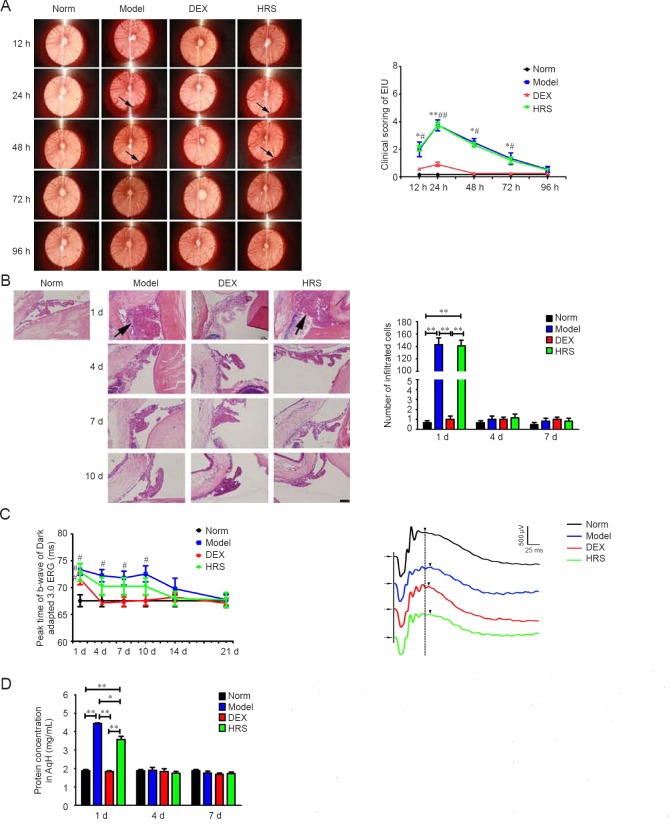
Rats of Model group had signs of uveitis at 24 hours after injection of 1/8 mg/kg of LPS, with the quantitative score of uveitis signs, number of inflammation cells around ICB and protein concentration in AqH being 3.82 ± 0.40, 143.33 ± 10.54 and 4.44 ± 0.05 mg/mL, respectively. All these indexes were smaller than those induced by 1 mg/kg of LPS, with significant differences (P < 0.05), suggesting that a smaller dose of LPS could result in a relative mild EIU. The number of inflammation cells around ICB and protein concentration in AqH returned to normal at 4 days after LPS administration. However, rats of HRS group with multiple injections of large dose exhibited similar signs of uveitis to those of Model group, with no significant differences existing between the two groups in the quantitative score of uveitis signs within 96 hours and the number of inflammation cells around ICB within 21 days after injection of LPS (P > 0.05) (parts of data were shown in Figure 4B). The protein concentration in the AqH of HRS group at 24 hours after injection of LPS was 3.57 ± 0.19 mg/mL, which was lower than that of Model group, with the difference being statistically significant (P < 0.05). The peak time of b-wave of Dark-adapted 3.0 ERG of HRS group was slightly shorter than those of Model group from 4 to 14 days after injection of LPS. However, the differences between the two groups had no statistical significances (P > 0.05). No obvious signs of uveitis were found in rats of DEX group during the whole process, with almost no inflammatory cells infiltrating around ICB and almost no protein in AqH. The peak time of b-wave of DEX group was delayed compared to that of Norm group at 1 day after LPS administration (P < 0.05). However, it returned to normal from 4 days after injection of LPS (Figure 4).
Figure 4.
Effects of large dose of hydrogen-rich saline (HRS) on mild endotoxin-induced uveitis (EIU).
Note: (A) Findings under slit lamp examination and scoring of uveitis within 96 h after LPS administration. Arrows show hypopyon. (B) Histology of ICB and numbers of inflammation cells infiltrating around ICB within 21 d after LPS administration (× 200). Arrows show infiltrated cells. Scale: 100 μm. (C) The peak time of b-wave of Dark-adapted 3.0 ERG of EIU within 21 d after LPS administration and typical ERG waveform of 1 d after LPS injection. The b-wave peak time is measured from the time of the flash to the peak of the wave, which follows the oscillatory potentials. Arrows showed start of flash; Arrowheads show peak of b-wave. (D) Protein concentration in AqH 1, 4 and 7 d after LPS injection. *P < 0.05; **P < 0.01; #P < 0.05, vs. Norm group. LPS: Lipopolysaccharide; ICB: iris and ciliary body; AqH: aqueous humor; ERG: electroretinogram; h: hours; d: day(s); DEX: dexamethasone.
Discussion
HRS, a convenient and safe way to provide H2, has been reported in a large number of studies.1,31,32 H2 can easily pass through biological tissue barriers, such as blood-brain barrier and blood-eye barrier, to reach target organs. It has been proved to reduce oxidative stress in various organs and tissues, such as the brain, eyes, lungs, liver, kidney.33,34 EIU is an animal model of human uveitis, and oxidative stress also plays an important role in its pathogenesis, with many drugs proved to be effective in ameliorating EIU through anti-oxidation.22,23,35 Because of the antioxidant property of HRS,36 we expected that it could somehow alleviate EIU.
HRS is usually administrated by intraperitoneal injection at the dose of 5 mL/kg or 10 mL/kg by most studies.3,37 We first chose the dose of 10 mL/kg to guarantee a high concentration of H2. Our results showed that 24 hours after LPS injection, HRS did not ameliorate the clinical signs and histological changes of EIU, while the protein concentration in AqH exhibited a declined trend. We supposed that the amount of H2 provided by a single injection of 10 mL/kg of HRS might not be enough to achieve an obvious ameliorating effect on EIU.
The severity of EIU in rats peaks at 24 hours after LPS injection,26 and the blood-AqH barrier breaks within 2 hours, with protein leakage and cell infiltration into anterior chamber 6 hours later.21 This means that the first two hours after injection of LPS may be a key time to inhibit EIU. Therefore, we next administered HRS within 2 hours after LPS administration at a dose of 20 mL/kg multiple times before and after the LPS injection. However, results of this part still did not show an obvious mitigating effect of HRS on EIU, while the elevation of protein concentration in AqH was somehow inhibited by HRS administration.
Cai et al.38 reported that H2 had protective and therapeutic effects on mild brain injury in newborn rat induced by ischemic and hypoxia, while Matchett et al.39 found that H2 did not reduce the area of brain ischemia and the corresponding oxidation products on moderate and severe ischemic and hypoxia-induced brain injury of newborn rat. One of the reasons they put forward for the different effects of H2 on brain injury induced by ischemic and hypoxia was that the tissue damage severity of moderate and severe brain injury might surpass the short-term ameliorating effect of H2.
Thus, we speculated that EIU with an obvious iris hyperemia and hypopyon induced by 1 mg/kg of LPS might likewise be a bit too serious and beyond the antioxidant activity of H2 to achieve a relieving effect. So, we tried to reduce the severity of EIU by gradually reducing the dose of LPS and discovered that a relative mild EIU with less obvious iris hyperemia and hypopyon was developed with administration of 1/8 mg/kg of LPS. Multiple intraperitoneal injections of 20 mL/kg of HRS were then given to rats with mild EIU in a hope that H2 could ameliorate the severity of EIU. Besides, we also recorded ERG 21 days within LPS injection to seek the effect of HRS on functional change of retina in EIU, as the retinal function was also reported to be affected in EIU rat.23,40,41 The results showed that the large dose of HRS failed to ameliorate the iris hyperemia and hypopyon due to mild EIU shown by slit lamp examination and histological evaluation. Dark-adapted 3.0 ERG is a combined response arising from photoreceptors and bipolar cells of both the rod and cone systems (rod dominated), and the b-wave of this reaction comes from bipolar cells. Our data showed that retinal function of EIU rats was damaged by LPS administration as showed by the delay of b-wave peak time of Dark-adapted 3.0 ERG. HRS could not obviously rescue the affected retinal function of EIU rat. What was interesting was that the elevation of protein concentration in AqH of mild EIU was somewhat inhibited by large dose of HRS.
A variety of antioxidants, such as vitamin E, were reported to ameliorate muscle atrophy via antioxidant activity,42 while Fujita et al.43 found that oxidative stress levels and the corresponding muscle function in a rat model of muscle atrophy did not be improved after drinking hydrogen-rich water for a long time, which also possessed antioxidant activity. The reason, they argued, might be that H2 provided by hydrogen-rich water could not maintain for a long time in the body, thus unable to alleviate muscle atrophy. In accordance with our results, EIU, which could somewhat be alleviated by antioxidants,22,23,24,25 was not obviously ameliorated even by multiple injections of large dose of HRS. The reason for our results might be the same with that of Ryo's. Maybe, continuous hydrogen gas inhalation could achieve a more obvious ameliorating effect on EIU, for it can provide H2 maintaining in the body for a longer time.
One of our results showed that HRS could not relieve the infiltrated cells in EIU, while it could, to some extent, reduce the increasing concentration of protein in AqH. This phenomenon might due to the protein and inflammatory cells separation in EIU.44,45 Some drugs have been reported to significantly reduce protein leakage and cell infiltration of EIU (such as glucocorticoids,20 in accordance with the result of DEX in our study), while some other drugs prevented only protein leakage or cell infiltration. For example, indometacin can significantly reduce the increasing protein concentration in AqH in EIU, but has no obvious effect on the inflammatory cells infiltration.20 The protein and inflammatory cells separation of EIU might due to the fact that media accounting for protein exudation and cell infiltration are different. Studies found that prostaglandin E2 is responsible for protein exudation, while leukotriene B4 for inflammatory cells infiltration.46,47,48 HRS might, to some degree, inhibit the medium responsible for protein exudation (prostaglandin E2), while have no effect on the medium for inflammatory cell infiltration (leukotriene B4), which needs further confirmation in the future study.
Footnotes
Funding: This study was supported by a grant from the Foundation of Open Sharing Platform of Science and Technology of Shaanxi Province, China (No. 2015FWPT-02 to ZMZ).
Conflicts of interest
There is no competing, commercial, or conflicts of interests regarding this paper.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
References
- 1.Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–279. doi: 10.1055/s-0034-1395509. [DOI] [PubMed] [Google Scholar]
- 3.Chen T, Tao Y, Yan W, et al. Protective effects of hydrogen-rich saline against N-methyl-N-nitrosourea-induced photoreceptor degeneration. Exp Eye Res. 2016;148:65–73. doi: 10.1016/j.exer.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Yu P, YongYang, et al. Hydrogen-rich saline resuscitation alleviates inflammation induced by severe burn with delayed resuscitation. Burns. 2015;41:379–385. doi: 10.1016/j.burns.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Runtuwene J, Amitani H, Amitani M, Asakawa A, Cheng KC, Inui A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ. 2015;3:e859. doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh T, Fujita Y, Ito M, et al. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389:651–656. doi: 10.1016/j.bbrc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Yu P, Zeng Q, et al. Neuroprotective effect of hydrogen-rich saline in global cerebral ischemia/reperfusion rats: up-regulated tregs and down-regulated miR-21, miR-210 and NF-kappaB expression. Neurochem Res. 2016;41:2655–2665. doi: 10.1007/s11064-016-1978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong Y, Sun LI, Sun R, Chen H, Yu Y, Xie K. Combination therapy of molecular hydrogen and hyperoxia improves survival rate and organ damage in a zymosan-induced generalized inflammation model. Exp Ther Med. 2016;11:2590–2596. doi: 10.3892/etm.2016.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie K, Yu Y, Huang Y, et al. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37:548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 10.Gharib B, Hanna S, Abdallahi OM, Lepidi H, Gardette B, De Reggi M. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C R Acad Sci III. 2001;324:719–724. doi: 10.1016/s0764-4469(01)01350-6. [DOI] [PubMed] [Google Scholar]
- 11.Tian R, Hou Z, Hao S, et al. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 2016;1637:1–13. doi: 10.1016/j.brainres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Shi Q, Chen C, Deng WH, et al. Hydrogen-rich saline attenuates acute hepatic injury in acute necrotizing pancreatitis by inhibiting inflammation and apoptosis, involving JNK and p38 mitogen-activated protein kinase-dependent reactive oxygen species. Pancreas. 2016;45:1424–1431. doi: 10.1097/MPA.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 13.Guo SX, Fang Q, You CG, et al. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J Transl Med. 2015;13:183. doi: 10.1186/s12967-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves SW, Sloan FA, Lee PP, Jaffe GJ. Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006;113:307.e1. doi: 10.1016/j.ophtha.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Turk A, Aykut M, Akyol N, et al. Serum anti-carbonic anhydrase antibodies and oxidant-antioxidant balance in patients with acute anterior uveitis. Ocul Immunol Inflamm. 2014;22:127–132. doi: 10.3109/09273948.2013.830753. [DOI] [PubMed] [Google Scholar]
- 16.Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000;19:41–68. doi: 10.1016/s1350-9462(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K, Bajwa A, Freitas-Neto CA, Metzinger JL, Wentworth BA, Foster CS. A comprehensive review and update on the biologic treatment of adult noninfectious uveitis: part II. Expert Opin Biol Ther. 2014;14:1651–1666. doi: 10.1517/14712598.2014.947957. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 20.Cousins SW, Guss RB, Howes EL, Jr, Rosenbaum JT. Endotoxin-induced uveitis in the rat: observations on altered vascular permeability, clinical findings, and histology. Exp Eye Res. 1984;39:665–676. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76:497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 22.Lennikov A, Kitaichi N, Noda K, et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol Vis. 2014;20:171–177. [PMC free article] [PubMed] [Google Scholar]
- 23.Miyake S, Takahashi N, Sasaki M, Kobayashi S, Tsubota K, Ozawa Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Lab Invest. 2012;92:102–109. doi: 10.1038/labinvest.2011.132. [DOI] [PubMed] [Google Scholar]
- 24.Bosch-Morell F, Roma J, Puertas FJ, Marin N, Diaz-Llopis M, Romero FJ. Efficacy of the antioxidant ebselen in experimental uveitis. Free Radic Biol Med. 1999;27:388–391. doi: 10.1016/s0891-5849(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 25.Kubota S, Kurihara T, Mochimaru H, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 26.Keles S, Halici Z, Atmaca HT, et al. The ocular endothelin system: a novel target for the treatment of endotoxin-induced uveitis with bosentan. Invest Ophthalmol Vis Sci. 2014;55:3517–3524. doi: 10.1167/iovs.14-14193. [DOI] [PubMed] [Google Scholar]
- 27.Tian L, Zhang L, Xia F, An J, Sugita Y, Zhang Z. Hydrogen-rich saline ameliorates the retina against light-induced damage in rats. Med Gas Res. 2013;3:19. doi: 10.1186/2045-9912-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 29.Lajavardi L, Bochot A, Camelo S, et al. Downregulation of endotoxin-induced uveitis by intravitreal injection of vasoactive intestinal Peptide encapsulated in liposomes. Invest Ophthalmol Vis Sci. 2007;48:3230–3238. doi: 10.1167/iovs.06-1305. [DOI] [PubMed] [Google Scholar]
- 30.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 31.Shen M, Zhang H, Yu C, Wang F, Sun X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med Gas Res. 2014;4:17. doi: 10.1186/2045-9912-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JL, Zhang QS, Zhu KD, et al. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen Res. 2015;10:958–964. doi: 10.4103/1673-5374.158361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang Z, Sun XJ, Zhang X, et al. Nuclear factor-kappaB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. doi: 10.1002/jnr.23281. [DOI] [PubMed] [Google Scholar]
- 34.Sun JC, Xu T, Zuo Q, et al. Hydrogen-rich saline promotes survival of retinal ganglion cells in a rat model of optic nerve crush. PLoS One. 2014;9:e99299. doi: 10.1371/journal.pone.0099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XQ, Liu HL, Wang GB, et al. Effect of artesunate on endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2011;52:916–919. doi: 10.1167/iovs.10-5892. [DOI] [PubMed] [Google Scholar]
- 36.Qin ZX, Yu P, Qian DH, et al. Hydrogen-rich saline prevents neointima formation after carotid balloon injury by suppressing ROS and the TNF-alpha/NF-kappaB pathway. Atherosclerosis. 2012;220:343–350. doi: 10.1016/j.atherosclerosis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Wang X, Xue W, et al. Beneficial effects of hydrogen-rich saline against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2013;1517:150–160. doi: 10.1016/j.brainres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Cai J, Kang Z, Liu WW, et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 39.Matchett GA, Fathali N, Hasegawa Y, et al. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia-ischemia rat models. Brain Res. 2009;1259:90–97. doi: 10.1016/j.brainres.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto T, Ozawa Y, Kamoshita M, et al. The neuroprotective effect of rapamycin as a modulator of the mTOR-NF-kappaB axis during retinal inflammation. PLoS One. 2016;11:e0146517. doi: 10.1371/journal.pone.0146517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki M, Ozawa Y, Kurihara T, et al. Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci. 2009;50:1433–1439. doi: 10.1167/iovs.08-2493. [DOI] [PubMed] [Google Scholar]
- 42.Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med. 2007;42:627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita R, Tanaka Y, Saihara Y, Yamakita M, Ando D, Koyama K. Effect of molecular hydrogen saturated alkaline electrolyzed water on disuse muscle atrophy in gastrocnemius muscle. J Physiol Anthropol. 2011;30:195–201. doi: 10.2114/jpa2.30.195. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacherjee P, Williams RN, Eakins KE. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Invest Ophthalmol Vis Sci. 1983;24:196–202. [PubMed] [Google Scholar]
- 45.Okumura A, Mochizuki M. Endotoxin-induced uveitis in rats: morphological and biochemical study. Jpn J Ophthalmol. 1988;32:457–465. [PubMed] [Google Scholar]
- 46.Herbort CP, Okumura A, Mochizuki M. Immunopharmacological analysis of endotoxin-induced uveitis in the rat. Exp Eye Res. 1989;48:693–705. doi: 10.1016/0014-4835(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 47.Herbort CP, Okumura A, Mochizuki M. Endotoxin-induced uveitis in the rat. A study of the role of inflammation mediators. Graefes Arch Clin Exp Ophthalmol. 1988;226:553–558. doi: 10.1007/BF02169204. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacherjee P, Hammond B, Salmon JA, Eakins KE. Effect of lipoxygenase products on leukocyte accumulation in the rabbit eye. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:325–330. [PubMed] [Google Scholar]