Abstract
Background
Body weight and adiposity are heritable traits. To date it remains unknown whether obesity-associated brain structural alterations are under a similar level of genetic control.
Methods
For this study we utilized magnetic resonance imaging (MRI) data from the Human Connectome Project. Voxel based morphometry (VBM) was used to investigate associations between body mass index (BMI) and regional gray matter volume (GMV) in a sample of 875 young adults with a wide BMI range (386m/489f; age 28.8 ± 3.7y; BMI 26.6 ± 5.3 kg*m-2), that included 86 pairs of monozygotic twins and 82 pairs of dizygotic twins. Twin data were analyzed by applying the additive genetic, common environmental and residual effects (ACE) model to determine heritability of brain regions that were associated with BMI.
Results
We observed positive associations between BMI and GMV in the ventromedial prefrontal cortex and the right cerebellum and widespread negative associations within the prefrontal cortex, cerebellum, temporal lobes and distinct subcortical structures. Varying degrees of heritability were found for BMI-associated brain regions, with highest heritability estimates for cerebellar GMV and subcortical structures.
Conclusions
These data indicate that brain regions associated with obesity are subject to differing levels of genetic control and environmental influences. Specific brain regions with high heritability might represent an inherent vulnerability factor for obesity.
Introduction
The rising prevalence of obesity remains unchecked in industrialized societies. As of 2011/2012 70% of the adult US population were estimated to be overweight or obese with a body mass index (BMI) exceeding 25 kg/m2 1.
Accumulation of excess adipose tissue is generally a result of unhealthy eating patterns and chronically positive energy balance, further promoted by the western “obesogenic” environment, where an abundance of unhealthy energy dense foods is readily available at any time. Hence, increasing efforts are being made to enhance our understanding of the neurobiological underpinnings that may promote and/or follow unhealthy eating patterns and the development of obesity. Both animal and human studies have documented dysfunctional dopaminergic central pathways and the effects on reward-related and motivated behavior to be key neurophysiological contributors to the behavioral phenotype leading to obesity2–4. In addition, a number of neuroimaging studies have found obesity to be associated with functional and structural alterations of the brain including prefrontal cortical, insular and striatal regions critically involved in executive functioning, reward processing and interoception5–12. Intriguingly, reduced gray matter volume (GMV) of similar brain regions (i.e. insular and prefrontal cortex) was reported in subjects with high risk for obesity, possibly indicating that structural brain differences are predisposing to obesity13. Similar results were reported by Yokum et al., linking prefrontal GMV with future weight gain in a sample of young women14; however, reported brain regions of these studies differed with respect to the exact locations. Obesity and BMI have been shown to be highly heritable, generally on a polygenic basis15,16. Certain genetic variants are common and carry significant risk of increased adiposity; for example, variations in the FTO gene (i.e., fat mass and obesity associated protein), where those homozygous for the risk allele have an increased risk for obesity (HR 1.67), on average weighing 3–4 kg more than non-carriers17. In addition, evidence for a genetically determined behavioral phenotype with altered reward sensitivity (thus favoring unhealthy eating patterns) is provided by multiple studies that have linked genetic variants of the dopamine receptor D2 (DRD2) gene (i.e. the Taq1A A1 allele) and of the μ-opioid receptor (OPRM1) to obesity, binge eating disorder and addictive behavior in general18,19.
Given evidence of volumetric brain differences and genetic influences, an important question is the degree to which obesity-related structural brain differences are heritable. It is possible that at least some regional brain volume differences reflect central endophenotypes leading to unhealthy eating and excess adiposity. Twin studies provide a powerful approach for heritability analyses of complex traits, such as obesity, by comparing monozygotic and dizygotic twins. Previous twin studies have shown varying degrees of estimated heritability for regional brain volume, thus indicating differing susceptibilities with respect to genetic and environmental influences20.
In this study, we aimed to investigate associations between regional GMV, body weight (i.e. BMI) and the heritability of obesity-associated structural brain alterations, hypothesizing high degrees of heritability – particularly for brain regions that form part in dopaminergic reward pathways. We utilized structural neuroimaging data from the Human Connectome Project, a large scale neuroimaging study with open access data that investigates human brain networks and behavioral correlates under consideration of genetic and environmental influences21. In a first step, we investigated associations between BMI and regional GMV in a sample of 875 healthy young adults, who were non-twin siblings, dizygotic or monozygotic twin pairs. In a second step, we estimated heritability of BMI and of predefined brain regions that we found to be associated with BMI, by using available twin data only.
Methods
Study population
For this study, data were drawn from the publicly available Human Connectome Project (HCP) database (i.e., S500 and S900 data releases; www.humanconnectome.org) that included a total of n=875 healthy young to middle-aged adults with structural MRI (i.e. T1 weighted MPRAGE) and BMI data. For additional information on eligibility criteria of the original study, we refer to previously published detailed descriptions21,22. The study population was composed of non-twin siblings (n=447), monozygotic (identical) twins (n=206) and dizygotic (fraternal) twins (n=222) from n=380 families. Structural MRI scans from both twin siblings were available for n=86 identical and n=83 fraternal twin pairs. All participants gave written consent and experimental procedures were approved by the institutional review board (IRB # 201204036; Title: ‘Mapping the Human Connectome: Structure, Function, and Heritability’). For our data analyses no additional approval was required by the local ethics commitee of the University of Leipzig.
Imaging procedures
Detailed descriptions of structural imaging protocols are provided on the website of the HCP (www.humanconnectome.org) and in previous publications21. In brief, two separate T1-weighted, high resolution (0.7-mm isotropic voxels) anatomical images (i.e. 3D MPRAGE) were acquired on a customized Siemens 3-T Skyra system (Siemens, Germany) with a 32-channel head coil, using the following parameters: FOV=224 mm, matrix=320, 256 sagittal slices per single slab, TR=2400 ms, TE=2.14 ms, TI=1000 ms, FA=8°, Bandwidth (BW)=210 Hz per pixel, Echo Spacing (ES) =7.6 ms. For this study we used anatomical images from the first scan only.
Data analysis
Voxel-based morphometry23 of T1-weighted MPRAGE images was performed with the Statistical Parametric Mapping package (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). Images were preprocessed with default settings of the VBM8 Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolbox, including high-dimensional DARTEL normalization algorithms and modulation for non-linear components which accounts for interindividual differences in total brain volume. First, images were segmented into gray matter (GM), white matter, and cerebrospinal fluid, followed by normalization to the DARTEL template in MNI space (voxelsize: 1.5mm × 1.5mm × 1.5mm) and smoothing of GM data with an 8mm full-width-half-maximum isotropic Gaussian kernel. All images were visually inspected prior to statistical analyses.
Multiple regression analyses of smoothed GM data were employed to investigate associations between regional GMV and BMI. As covariates of no interest we included age, gender, handedness [Edinburgh Handedness Questionnaire; ranging from −100 (lefthanded) to 100 (righthanded)], race (white/non-white), twinstatus and zygosity. ANOVA was used to test for gender*BMI interaction. In addition, we applied an absolute threshold to exclude voxels with a GM value of <0.1. Results were considered significant at a peak voxel threshold of p<0.05 corrected for the entire brain volume (FWE, family-wise error correction for multiple comparisons on the voxel-level). Since SPM8 by defaults only displays 3 peaks within a cluster we used the current cluster option to explore large clusters (>10.000 voxels).
In order to test whether associations of BMI with GMV are driven by educational background of intelligence, we ran additional post-hoc analyses including years of education (n=874) or measures of fluid intelligence (n=869; i.e. correct answers in the Penn Progressive Matrices) as further covariates.
Heritability analyses of BMI associated brain structures were performed on predefined regions of interests (ROI). These a priori ROIs were chosen based on previous publications and functional relevance and included the prefrontal cortex, including the ventromedial prefrontal cortex,5,6,12,24, cerebellum6,25,26, temporal6,25,27 and insular cortices6,25,28, anterior cingulate cortex6,25,29, hippocampal/parahippocampal cortex12,30, dorsal and ventral striatum5,9,31, midbrain3,32, amygdala3,6,33 and hypothalamus3,5. To do so, masks of the ROIs were generated with the WFU-Pickatlas toolbox (www.fmri.wfubmc.edu). Next, results of the whole brain analyses were masked for the respective ROI and GM data were extracted by centering a 3mm sphere on the peak locations34 of the overall regression analysis. Due to masking procedures, slight differences in the respective peak locations may occur, as compared to the whole brain analyses. Since no associations were found for the hypothalamus and right striatum (except for neighboring extranuclear structures), no heritability analyses were performed for these ROIs.
SAS Software (SAS Institute Inc, version 9.4, Cary, NC) was used for statistical analyses of non-imaging data and for heritability analyses of extracted GM data. For heritability analyses, we applied the additive genetic, common environmental and residual effects (ACE) model, as provided by Feng et al., with additional modifications to fit quantitative data [35. Heritability of BMI and GM data were estimated in n=336 twins (i.e. 86 monozygotic (MZ) and 82 dizygotic (DZ) twin pairs) from n=168 families using nonlinear mixed model analysis (i.e. PROC NLMIXED). Estimation of additive genetic and common environment random effects components (i.e., ACE model) was performed by the maximum likelihood method. Heritability was quantified as the ratio between estimated additive genetic variance and the total variance of the trait. Values of GM volumes were log-transformed to approximate a normal distribution prior to analyses. Due to significant group differences between MZ and DZ, sex and age were included as covariates. A p<0.05 was considered statistically significant.
In addition, we employed the Biological Parametric Mapping Toolbox (BPM; www.ansir.wfubmc.edu) within the SPM5 framework (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) to create descriptive correlation maps of twin pair GM data, thus allowing further quantification of concordance of regional GMV associated with BMI in MZ and DZ. This includes voxelwise correlation within MNI space to generate results for a homologous correlation field. BPM analyses were regionally restricted to a ROI based on the results from our VBM whole group analyses by using inclusive masks of regional GMV clusters we found to be associated with BMI. Correlational maps were not thresholded, in order to illustrate within twin pair correlations across the entire ROI. In addition we provide results of thresholded analyses (i.e. correlation coefficient of >0.5 and k=50 continuous voxels) as supplementary data.
Results
Characteristics of the study population and comparative group statistics of identical and fraternal twin pairs are listed in Table 1. No significant differences were found for BMI, race, education or fluid intelligence, yet identical and fraternal twins differed with respect to total body weight, height, age and gender.
Table 1.
Characteristics of study population
| Entire sample (N=875) | |||
|---|---|---|---|
| Age, years | 28.8 ± 3.7 (22.0 – 37.0) | ||
| Sex (m/f)* | 386/489 | ||
| Race (white/non-white)* | 638/237 | ||
| Handedness | 65.3 ± 45.0 (−100 – 100) | ||
| BMI | 26.6 ± 5.3 (16.5 – 47.8) | ||
| Height, cm | 171.0 ± 10.0 (147.3 – 203.2) | ||
| Weight, kg | 78.2 ± 18.1 (42.2 – 138.3) | ||
| Education, years (n=874) | 14.9 ± 1.8 (11.0 – 17.0) | ||
| Fluid Intelligence (PMAT; n=869) | 16.6 ± 4.9 (4.0 – 24) | ||
|
| |||
| Twin pairs only | MZ (n=172) | DZ (n=164) | p |
|
| |||
| Age, years | 29.9±3.3 | 28.9±3.4 | 0.01 |
| Gender (m/f)* | 48/124 | 71/93 | 0.004✦ |
| Race (white/non-white)* | 141/31 | 128/36 | 0.41 |
| Handedness | 68.9±45.6 | 63.9±43.9 | 0.31 |
| BMI | 26.3±4.8 | 26.6±5.3 | 0.58 |
| Height, cm | 167.8±8.8 | 172.1±9.4 | <0.001 |
| Weight, kg | 74.1±15.6 | 79.1±19.0 | 0.01 |
| Education, years | 15.0±1.8 | 15.0±1.9 | 0.81 |
| Fluid Intelligence (PMAT) | 16.2±4.6 | 17.0±4.8 | 0.13 |
Characteristics are listed as mean ± SD with corresponding ranges, except for*. Two Sample t-Tests and ✦Fisher’s Exact Test were used for group-wise statistics. PMAT Penn Progressive Matrices number of correct responses.
VBM Analyses
Detailed results of the VBM analyses are listed in Table 2. Using the entire sample of 875 subjects revealed distinct positive associations (Figure 1) within the ventromedial prefrontal cortex (VMPFC; pFWE<0.001; k=179; MNIxyz[2mm 21mm −26mm]) and the right cerebellum (pFWE=0.003; k=21; MNIxyz[27mm −45mm −42mm]). Negative associations were observed within widespread areas of the brain (Figure 2) with one large cluster comprising bilateral prefrontal and precentral cortices, cerebellum, bilateral temporal lobes including bilateral hippocampal/parahippocampal region and the bilateral amygdala, the bilateral insula, parts of the occipital cortex, bilateral thalamic regions and adjacent parts of midbrain (i.e. ventral tegmental area) and left dorsal and ventral striatum (pFWE<0.001; k=1179490; MNIxyz [-42mm 48mm −14mm]). In addition, several smaller clusters were found within the prefrontal and cingulate cortex, cerebellum and occipital cortex (for details please see Table 3). Additional adjustment for educational level (n=874) or fluid intelligence (measured by Penn Progressive Matrices; n=869) did not significantly change these results and separate analyses of n=380 non-siblings revealed a very similar pattern of positive and negative associations (data not shown).
Table 2.
Associations of regional gray matter volume with BMI
| Brain Region | cluster extent | pFWE* | T | MNI (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BMI pos. | ||||||
| R Rectal Gyrus | 179 | <0.001 | 5.70 | 2 | 21 | −26 |
| R Rectal Gyrus | 0.003 | 5.17 | 11 | 24 | −23 | |
| R Cerebellum, Tonsil | 21 | 0.003 | 5.44 | 27 | −45 | −42 |
| BMI neg. | ||||||
| L Middle Frontal Gyrus | 1179490 | <0.001 | 12.86 | −42 | 48 | −14 |
| L Superior Frontal Gyrus | <0.001 | 11.91 | −33 | 56 | −15 | |
| L Cerebellum, Inferior Semi-Lunar Lobule | <0.001 | 11.29 | −14 | −73 | −48 | |
| L Cerebellum, Uvula | <0.001 | 10.82 | −12 | −79 | −44 | |
| L Superior Frontal Gyrus | <0.001 | 10.53 | −27 | 62 | −6 | |
| R Rectal Gyrus | <0.001 | 10.48 | 6 | 51 | −27 | |
| L Middle Frontal Gyrus | <0.001 | 10.43 | −44 | 50 | −2 | |
| L Superior Frontal Gyrus | <0.001 | 10.27 | −23 | 63 | −9 | |
| L Inferior Temporal Gyrus | <0.001 | 10.16 | −33 | −7 | −50 | |
| L Cerebellum, Pyramis | <0.001 | 10.05 | −11 | −82 | −41 | |
| R Superior Frontal Gyrus | <0.001 | 9.95 | 9 | 60 | −24 | |
| R Middle Temporal Gyrus | <0.001 | 9.91 | 63 | −43 | 3 | |
| R Cerebellum, Inferior Semi-Lunar Lobule | <0.001 | 9.90 | 17 | −75 | −47 | |
| R Superior Frontal Gyrus | <0.001 | 9.86 | 32 | 56 | −15 | |
| R Middle Frontal Gyrus | <0.001 | 9.85 | 48 | 48 | −12 | |
| R Cerebellum, Tuber | <0.001 | 9.83 | 21 | −84 | −38 | |
| L Cerebellum, Pyramis | <0.001 | 9.61 | −15 | −84 | −39 | |
| R Cerebellum, Pyramis | <0.001 | 9.50 | 24 | −79 | −41 | |
| R Cerebellum, Inferior Semi-Lunar Lobule | <0.001 | 9.47 | 21 | −72 | −50 | |
| R Cerebellum, Inferior Semi-Lunar Lobule | <0.001 | 9.40 | 38 | −64 | −48 | |
| L Cerebellum, Tonsil | <0.001 | 9.37 | −27 | −58 | −56 | |
| R Uncus | <0.001 | 9.37 | 29 | −6 | −47 | |
| R Cerebellum, Inferior Semi-Lunar Lobule | <0.001 | 9.33 | 29 | −66 | −53 | |
| R Inferior Temporal Gyrus | <0.001 | 9.24 | 33 | −7 | −48 | |
| R Cerebellum, Pyramis | <0.001 | 9.16 | 29 | −76 | −44 | |
| R Cerebellum, Uvula | <0.001 | 9.04 | 35 | −79 | −32 | |
| L Middle Frontal Gyrus | <0.001 | 9.03 | −39 | 54 | 6 | |
| R Inferior Temporal Gyrus | <0.001 | 9.03 | 38 | −6 | −50 | |
| L Superior Frontal Gyrus | <0.001 | 8.94 | −30 | 59 | 7 | |
| R Cerebellum, Uvula | <0.001 | 8.90 | 15 | −79 | −42 | |
| R Parahippocampal Gyrus | <0.001 | 8.74 | 23 | −37 | −8 | |
| L Precentral Gyrus | 1275 | <0.001 | 7.16 | −36 | −22 | 55 |
| L Precentral Gyrus | <0.001 | 5.81 | −44 | −13 | 45 | |
| L Cingulate Gyrus | 116 | <0.001 | 5.66 | −9 | −13 | 33 |
| L Middle Occipital Gyrus | 52 | 0.001 | 5.56 | −38 | −76 | 4 |
| R Medial Frontal Gyrus | 26 | 0.005 | 5.09 | 17 | 38 | 30 |
| L Middle Temporal Gyrus | 46 | 0.005 | 5.08 | −35 | −84 | 24 |
| L Inferior Frontal Gyrus | 101 | 0.005 | 5.06 | −57 | 9 | 16 |
| R Anterior Cingulate | 96 | 0.006 | 5.02 | 0 | 29 | 6 |
| L Anterior Cingulate | 75 | 0.012 | 4.88 | −6 | 39 | 18 |
| L Superior Frontal Gyrus | 7 | 0.024 | 4.72 | −6 | 36 | 57 |
| R Cingulate Gyrus | 3 | 0.031 | 4.66 | 9 | −21 | 33 |
| R Inferior Frontal Gyrus | 1 | 0.036 | 4.62 | 53 | 0 | 28 |
| L Inferior Frontal Gyrus | 3 | 0.038 | 4.61 | −50 | 8 | 34 |
| L Anterior Cingulate | 2 | 0.044 | 4.57 | −9 | 29 | 28 |
| R Cerebellum, Culmen | 2 | 0.047 | 4.56 | 38 | −36 | −35 |
MNI: Montreal Neurological Institute.
Results significant at a threshold of p<0.05 FWE corrected on the voxel-level;
Bold data indicate primary peak within a cluster; Non-bold data indicate secondary peaks.
Figure 1.
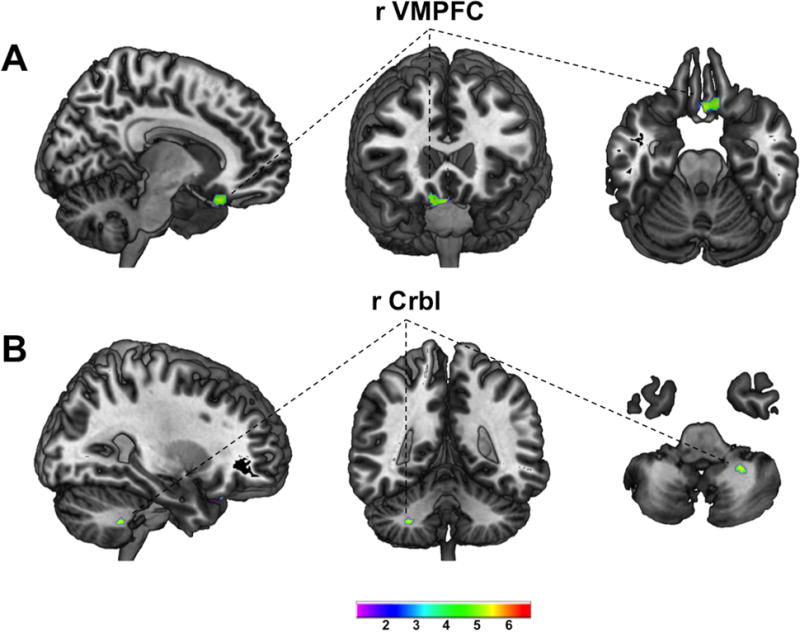
T-score maps of regional GMV positively associated with BMI (A ventromedial prefrontal cortex; B cerebellum) superimposed on high resolution rendered images of the brain within the sagital, coronal and axial plane (left to right). Maps are thresholded at p<0.05 whole brain corrected on the voxel-level (FWE); VMPFC ventromedial prefrontal cortex; Crbl cerebellum; Color bar indicates T-score;
Figure 2.
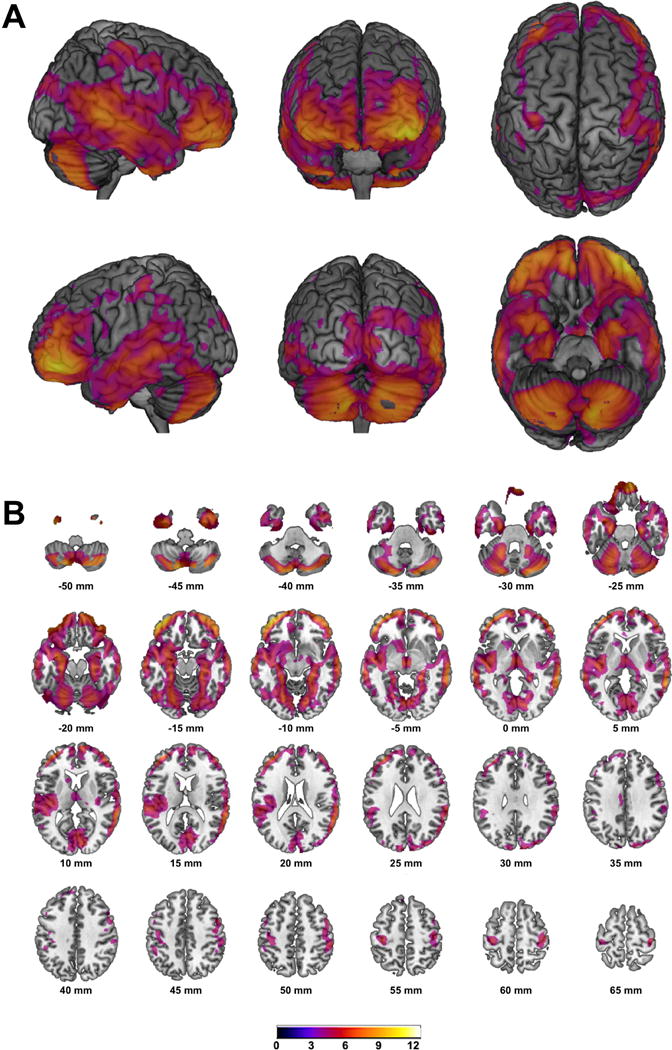
T-score maps of regional GMV negatively associated with BMI superimposed on high resolution rendered images of the brain (A) and on slices within the axial plane (B) with corresponding locations on the z-axis below; Maps are thresholded at p<0.05 whole brain corrected on the voxel-level (FWE); Color bar indicates t-score;
Table 3.
Heritability estimates for BMI and regional GMV
| Variable | Heritability (95% CI Bounds) | pACE |
|---|---|---|
| BMI | 0.73 (0.35 – 1.10) | 0.0002 |
| Regional GMV [MNIxyzmm] | ||
| L Cerebellum [−14 −73 −48] | 0.82 (0.75 – 0.88) | <0.0001 |
| R Cerebellum [17 −75 −47] | 0.79 (0.48 – 1.10) | <0.0001 |
| R Midbrain [8 −15 −5] | 0.77 (0.69 – 0.86) | <0.0001 |
| R Amygdala [−30 −3 −18] | 0.74 (0.36 – 1.13) | 0.0002 |
| L Putamen [−33 −4 3] | 0.71 (0.61 – 0.81) | <0.0001 |
| L Midbrain [−5 −12 −3] | 0.70 (0.60 – 0.81) | <0.0001 |
| L Amygdala [−30 −3 −20] | 0.68 (0.36 – 1.13) | 0.0008 |
| L Nucleus Accumbens [−11 6 −8] | 0.65 (0.54 – 0.77) | <0.0001 |
| R Orbitofrontal Cortex [9 53 −26] | 0.57 (0.13 – 1.01) | 0.01 |
| L Orbitofrontal Cortex [−42 48 −14] | 0.56 (0.13 – 1.00) | 0.01 |
| R Parahippocampal Gyrus [23 −37 −8] | 0.56 (0.10 – 1.02) | 0.02 |
| R Ventromedial Prefrontal Cortex [2 21 −26] | 0.54 (0.13 – 0.94) | 0.01 |
| L Parahippocampal Gyrus [−21 −37 −11] | 0.51 (0.04 – 0.98) | 0.03 |
| L Insula [−44 −19 3] | 0.50 (0.06 – 0.94) | 0.03 |
| R Insula [39 −19 −8] | 0.45 (0.06 – 0.83) | 0.02 |
| R Middle Temporal Gyrus [63 −43 3] | 0.42 (−0.06 – 0.89) | 0.08 |
| R Anterior Cingulate [8 54 10] | 0.38 (−0.16 – 0.92) | 0.16 |
| L Anterior Cingulate [−9 53 −2] | 0.22 (−0.31 – 0.75) | 0.41 |
| L Inferior Temporal Gyrus [−32 −3 −50] | 0.21 (−0.13 – 0.55) | 0.22 |
Heritability estimates (ACE model) for BMI and regional GMV of selected ROIs with corresponding 95% confidence intervals. The respective peak location for each ROI is specified in squared brackets. MNI: Montreal Neurological Institute.
Investigating differential effects of overweight and obesity on brain structure in males and females (i.e. gender*BMI interaction), only two small clusters were found within the left uncus/inferior temporal gyrus (pFWE=0.016; k=25; MNIxyz [−29mm 0mm −50mm]) and the right inferior temporal gyrus (pFWE=0.035; k=5; MNIxyz[32mm −6mm −45mm]) with stronger negative associations of BMI with regional GMV in males compared to females.
Heritability analyses
Detailed results of the heritability analyses are listed in Table 3. Applying the ACE model for heritability analyses yielded high heritability of BMI (i.e. h2=0.73). For BMI-associated regional GMV, highest heritability estimates were found for bilateral cerebellar GMV followed by GMV of subcortical structures (i.e. bilateral midbrain, left putamen, left nucleus accumbens) and bilateral amygdala. Overall lower, yet still statistically significant heritability estimates, were observed for GMV of prefrontal cortical regions (i.e. left lateral OFC and right medial OFC), right ventromedial PFC, bilateral parahippocampus and insula. Non-significant and low heritability estimates were found for GMV of bilateral temporal lobes and the bilateral ACC.
BPM analyses
Unthresholded correlational maps of regional GMV negatively associated with BMI within identical and fraternal twin pairs are illustrated in Figure 3. Thresholded correlational analyses (i.e. r>0.5 and k=50 continuous voxels) showed strongest within twin pair correlations for cerebellar GMV, followed by (in descending order) subcortical structures (i.e. left caudate, right thalamus), mesiotemporal regions including the bilateral parahippocampal and lingual gyrus, prefrontal and limbic structures (i.e. bilateral cingulate) for identical twins (detailed results in supplementary table S1). Within fraternal twin pairs less extensive and overall weaker correlations were observed for (in descending order) right temporal cortex, cerebellar, occipital, mesiotemporal and prefrontal cortical regions (detailed results in supplementary table S2). Brain regions positively associated with BMI showed relatively strong positive correlations within identical twins (i.e. r=0.78 for VMPFC) whereas in fraternal twins no suprathreshold correlations were observed. Supplementary information is available at IJO’s website.
Figure 3.
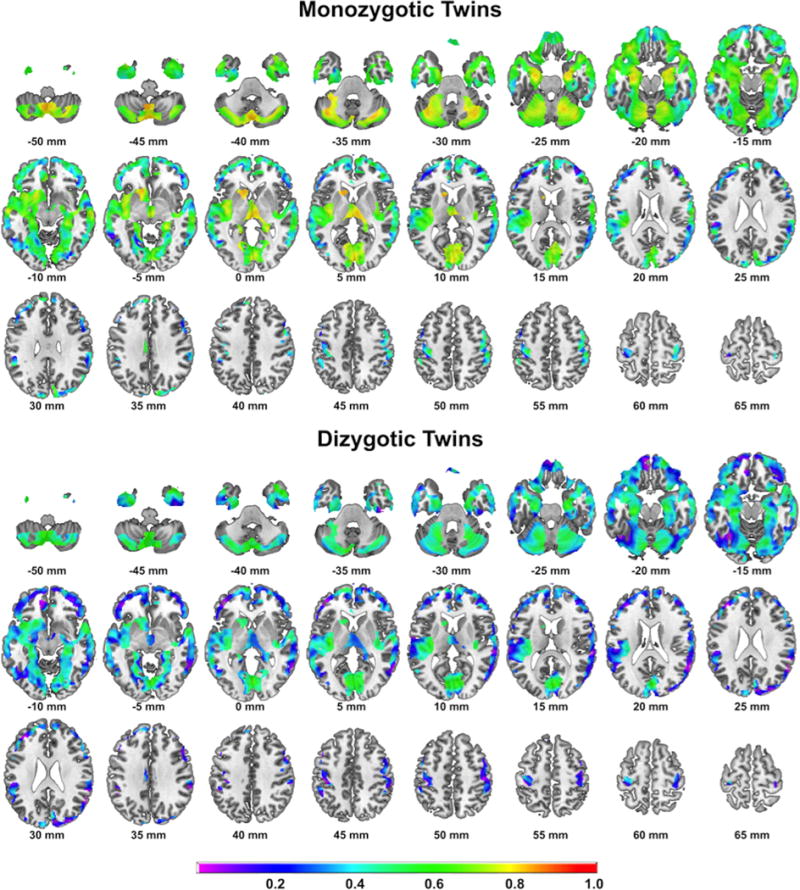
Correlational maps of within intra-pair correlations of regional GMV in identical (top) and fraternal (bottom) twin pairs. Maps are masked for brain regions we found negatively associated with BMI in the whole group VBM analysis. Color bar indicates correlation coefficient (r);
Discussion
Overall, our results show widespread and mostly negative associations of BMI with regional gray matter volume (GMV) in healthy young adults, particularly within prefrontal, temporal/mesotemporal and cerebellar brain regions as well as distinct subcortical structures with only marginal differences between males and females. In addition, we find varying degrees of heritability for these brain regions, with highest heritability estimates for cerebellar GMV and subcortical structures.
Associations of BMI with GMV
A number of previous studies investigated the relationship between obesity and brain structure, mostly showing negative associations between measures of adiposity and brain gray matter volume, but results varied significantly with respect to the brain regions involved5,7,9–11. However, two recently published studies with larger samples demonstrated comparable patterns of adiposity related brain structural alterations. Analyzing two separate community based samples (1586 adults with a mean age of 46 years and 758 adults with a mean age of 50 years), Janowitz et al. showed largely overlapping negative associations between rGMV and waist circumference for both samples, that included bilateral frontal, temporal and occipital lobes, somatosensory and motor cortex, insula, cingulate gyrus, hippocampal formation, subcortical structures (i.e., thalamus, caudate nucleus, putamen, globus pallidus) and the cerebellum6. Similar observations were made in a sample of 617 older subjects (mean age 68 years), additionally linking these structural alterations to impaired memory performance8. Importantly, results of both studies were not significantly affected by obesity-associated comorbidities, such as type 2 diabetes, hypertension and dyslipidemia. In contrast to these reports with predominantly negative associations, Horstmann et al. demonstrated exclusively positive associations between BMI and GMV in the VMPFC, including neighboring parts of nucleus accumbens (NAcc) and left putamen, in a sample of 122 healthy young and middle aged adults5. While disparities between individual studies may be attributed to differences in sample size, age range, gender distribution and comorbidities of the respective study populations, the present analysis of this large sample of young adults confirmed both positive and negative associations reported from these previous studies. Importantly, these results were not driven by intelligence or education. However, minor differences were observed between males and females with stronger associations of BMI with GMV of the inferior and mesial temporal cortex. Both increases of fat mass and fat-free mass (FFM; i.e. all non-adipose tissues) are found in obese subjects and we previously found strong negative associations of fat-free mass with GMV of similar regions within the temporal cortex25. Compared to women, men usually have more FFM, hence, gender related differences in body composition may have contributed to this finding.
Heritability of BMI and BMI-associated GMV
Applying the ACE model for estimations of heritability in monozygotic and dizygotic twins, we found high heritability for BMI. The high heritability of BMI in our study (h2=0.72) is comparable to previous studies. A meta-analysis of 88 twin studies found heritability estimates ranging between 0.47 and 0.90 with a median of 0.75, thus closely resembling the results from our analysis15.
Consistent with previous twin studies of brain morphology, our results show a wide range of heritability estimates for BMI-associated GMV (0.21 – 0.82). Previous volumetric analyses found overall high correlations within monozygotic twins (n=12) for cerebral and cerebellar volumes, followed by subcortical structures (i.e. thalamus, caudate, putamen), while measures of surface morphology showed less concordance between twins, indicating a significant impact of environmental influences on cortical brain regions36. Analyses of cerebral gray matter distribution in monozygotic and dizygotic twins (n=40) yielded highly varying heritability estimates for distinct frontal, temporal and sensorimotor cortices, again indicating region-specific genetic influences on brain structure and susceptibilities to environmental influences respectively37. In our study highest heritability estimates of BMI-associated GMV were found for the cerebellum and for distinct subcortical structures (i.e. midbrain, left putamen, left nucleus accumbens, bilateral amygdala). Numerous structural6–9,11,25,38 and functional39–42 imaging studies have highlighted the cerebellum in the context of obesity and eating behavior. Cerebellar function was once believed to be limited to motor, but increasing evidence indicates a far more diverse role including non-motor cognitive processes in the context of emotion, executive function and addictive behavior43,44.
The midbrain – and the ventral tegmental area (VTA) in particular – gives origin to the mesolimbic dopaminergic “reward pathway” via projections to the ventral striatum (i.e. NAcc) which is considered a key structure in reward related and addictive behavior3, while reward based learning – processed in the dorsal striatum – may further habituate behavioral patterns that ultimately lead to obesity45. The amygdala, on the other hand, together with the thalamus, ventral and dorsal striatum, midbrain and operculum is part of a network that guides cue-potentiated eating behavior (i.e. food intake elicited via reward predicting cues)46.
Overall lower, but still significant, heritability estimates were obtained for prefrontal cortical regions, the bilateral hippocampal region and insula, while heritability estimates for temporal cortical regions and the anterior cingulate were low and did not reach statistical significance. This comparably wide distribution of heritability estimates for BMI-associated GMV has important implications with respect to the neurobiology of obesity. First, highly heritable brain structural correlates of BMI (i.e. cerebellar and subcortical structures) represent potential endophenotypes of obesity and – starting from an early age – potentially facilitate weight gain by altered sensitivity to rewarding stimuli. On the other hand, brain regions with moderate to low heritability estimates of GMV (i.e. frontal, mesiotemporal insular and temporal cortex, ACC), appear to be more prone to environmental influence. Thus, negative associations of BMI within less heritable brain regions are more likely to represent neuroplastic changes that may occur during the time course of the developing obese phenotype. Considering the consistent detection and functional relevance of many of these regions in the context of obesity and eating behavior12, it seems plausible that obesity and the related behavioral phenotype may be further consolidated by subsequent structural and functional brain changes arising from the negative health effect of obesity.
Corroborative data are provided by longitudinal weight loss studies, demonstrating increases in hippocampal and insular GMV – brain regions with moderate to low heritability estimates – after 3 months of strenuous physical exercise in overweight and obese individuals47. Similar effects were recently demonstrated by Prehn et al. during the course of a dietary weight loss intervention with increased GMV of the HC and the inferior frontal gyrus48. Analyses of brain structural changes in obese subjects undergoing bariatric surgery yielded increases of GMV in frontal and posterior brain regions. However these brain structural changes only partially overlapped with preoperative gray matter differences49.
Since no longitudinal data are available for our study population, no final conclusion can be drawn whether these associations represent origin or consequence of obesity. Consequently, we have to acknowledge the cross-sectional nature of this study as important limitation. Other limitations of this study include the use of ROI-based approached of our heritability analyses that may have concealed other potentially relevant brain regions; therefore, these findings must be considered preliminary. Furthermore, our study is limited by the use of BMI as surrogate of adiposity due to its inability to precisely measure body fat content. Also, the inherent structure of the HCP dataset with twin and non-twin siblings may have influenced our results. Nevertheless, we did attempt to adjust for these factors in our analysis and additional analyses of a smaller sample of unrelated subjects yielded very similar results. Obesity is a complex disorder, accompanied by a variety of comorbidities that are not accounted for in our analyses. Nevertheless, our study population is comparably young (mean age = 29 years) and did not include subjects with significant health issues. However, the widespread pattern of mainly negative associations between GMV and BMI suggests a relationship between adiposity and brain structure beyond food intake, energy homeostasis and reward related behavior that could not be accounted for in these analyses.
Conclusions
In this sample of 875 healthy young adults we found widespread negative associations between BMI and regional GMV comprising the prefrontal cortex, cerebellum, temporal lobes and subcortical structures. Anatomically distinct positive associations were also observed within the right ventromedial prefrontal cortex and the right cerebellum. Heritability analyses of prespecified regions of interest showed highest heritability estimates for regional GMV of the cerebellum and subcortical structures and lowest for regional GMV of the temporal lobes. These findings indicate that brain regions associated with obesity are subject to differing levels of genetic determination and environmental influences. Some characteristics increase initial vulnerability to obesity while others arise from the negative health effects of the condition.
Supplementary Material
Acknowledgments
Data were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
This study was supported by the IFB AdiposityDiseases, Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01E01001 (http://www.bmbf.de) and the German Research Foundation (DFG) (http://www.dfg.de), within the framework of the CRC 1052 Obesity Mechanisms to Project A6 (to BP).
Footnotes
Supplementary Information: Supplementary information is available at IJO’s website
Conflict of Interest Statement: The authors declare no competing financial interests.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenny PJ. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Wang G-J, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlogl H, et al. Obesity-Related Differences between Women and Men in Brain Structure and Goal-Directed Behavior. Front Hum Neurosci. 2011;5 doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janowitz D, Wittfeld K, Terock J, Freyberger HJ, Hegenscheid K, Völzke H, et al. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. NeuroImage. 2015;122:149–157. doi: 10.1016/j.neuroimage.2015.07.086. [DOI] [PubMed] [Google Scholar]
- 7.Kurth F, Levitt JG, Phillips OR, Luders E, Woods RP, Mazziotta JC, et al. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp. 2013;34:1737–1746. doi: 10.1002/hbm.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masouleh SK, Arélin K, Horstmann A, Lampe L, Kipping JA, Luck T, et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging. 2016;40:1–10. doi: 10.1016/j.neurobiolaging.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Pannacciulli N, Del Parigi A, Chen K, Le DSNT, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: A voxel-based morphometric study. NeuroImage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, et al. Relationship Between Body Mass Index and Gray Matter Volume in 1,428 Healthy Individuals. Obesity. 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 11.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PloS One. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smucny J, Cornier M-A, Eichman LC, Thomas EA, Bechtell JL, Tregellas JR. Brain structure predicts risk for obesity. Appetite. 2012;59:859–865. doi: 10.1016/j.appet.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes. 2012;36:656–664. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinney A, Vogel CIG, Hebebrand J. From monogenic to polygenic obesity: recent advances. Eur Child Adolesc Psychiatry. 2010;19:297–310. doi: 10.1007/s00787-010-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loos RJF, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev. 2008;9:246–250. doi: 10.1111/j.1467-789X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 18.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13:279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- 20.Toga AW, Thompson PM. Genetics of Brain Structure and Intelligence. Annu Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- 21.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, et al. The Human Connectome Project: A data acquisition perspective. NeuroImage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 24.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 25.Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J-N, Wang J-J. The Cerebellum in Feeding Control: Possible Function and Mechanism. Cell Mol Neurobiol. 2007;28:469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veit R, Kullmann S, Heni M, Machann J, Häring H-U, Fritsche A, et al. Reduced cortical thickness associated with visceral fat and BMI. NeuroImage Clin. 2014;6:307–311. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Front Hum Neurosci. 2013;7:499. doi: 10.3389/fnhum.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston CSE. Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47:900–907. doi: 10.1111/j.1528-1167.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 32.Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. A potential role for the midbrain in integrating fat-free mass determined energy needs: An H2 (15) O PET study. Hum Brain Mapp. 2015;36:2406–2415. doi: 10.1002/hbm.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Rijsdijk FV, Viding E, De Brito S, Forgiarini M, Mechelli A, et al. Heritable variations in gray matter concentration as a potential endophenotype for psychopathic traits. Arch Gen Psychiatry. 2010;67:406–413. doi: 10.1001/archgenpsychiatry.2010.20. [DOI] [PubMed] [Google Scholar]
- 35.Feng R, Zhou G, Zhang M, Zhang H. Analysis of Twin Data Using SAS. Biometrics. 2009;65:584–589. doi: 10.1111/j.1541-0420.2008.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White T, Andreasen NC, Nopoulos P. Brain Volumes and Surface Morphology in Monozygotic Twins. Cereb Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- 37.Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 38.Raschpichler M, Straatman K, Schroeter ML, Arelin K, Schlögl H, Fritzsch D, et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater Corticolimbic Activation to High-Calorie Food Cues after Eating in Obese vs. Normal-Weight Adults. Appetite. 2012;58:303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci. 2014;9:932–938. doi: 10.1093/scan/nst059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasi D, Wang G-J, Wang R, Backus W, Geliebter A, Telang F, et al. Association of Body Mass and Brain Activation during Gastric Distention: Implications for Obesity. PLOS ONE. 2009;4:e6847. doi: 10.1371/journal.pone.0006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural Circuits for Cognitive Appetite Control in Healthy and Obese Individuals: An fMRI Study. PLOS ONE. 2015;10:e0116640. doi: 10.1371/journal.pone.0116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL. The Cerebellum and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 44.Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 45.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue–reward learning and food reward habituation predict future weight gain. NeuroImage. 2014;99:122–128. doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes. 2009;33:S44–S48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller K, Möller HE, Horstmann A, Busse F, Lepsien J, Blüher M, et al. Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Front Hum Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, et al. Caloric Restriction in Older Adults-Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb Cortex N Y 1991. 2016 doi: 10.1093/cercor/bhw008. [DOI] [PubMed] [Google Scholar]
- 49.Tuulari JJ. Effects of Obesity and Weight Loss Following Bariatric Surgery on Brain Function, Structural Integrity and Metabolism. 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


