Abstract
Introduction:
Study aim is to report the Magnetic Resonance Imaging (MRI) features of acute and chronic spontaneous spondylodiscitis.
Case report:
57 year old female, complaining of a fever and longstanding cervical pain worsened during physical therapy.
Methods:
MR images were acquired using superconductive magnet 1.5 T, with the following sequences: sagittal PD and T2 TSE, sagittal T1 SE, axial PD and T2 TSE (lumbar spine), axial T2 GRE (cervical spine). Axial and sagittal T1 SE after administration of (gadolinium DTPA). Examination was reviewed by three radiologists and compared to CT findings.
Results:
Patient reported cervical pain associated with fever and minimal weight loss. Blood tests were normal except hyperglycemia (DM tip II). X Ray: vertebral destruction localized at C-4 and C-5: NECT: destruction of the C-4/C-5 vertebral bodies (ventral part). MRI: Low signal of the bone marrow on T1l images, which enhanced after Gd-DTPA administration and became intermediate or high on T2 images. The steady high signal intensity of the disk on T2 images and enhancement on T1 images is typical for an acute inflammatory process. Bone Scintigrafi results: Bone changes suspicious for metastasis. Whole body CT results: apart from spine, no other significant changes.
Conclusion:
MRI is the most sensitive technique for the diagnosis of spondylodiscitis in the acute phase and comparable to CT regarding chronial stage of the disease. The present imagining essay os aimed at showing the main magnetic resonance imaging findings of tuberculous discitis.
Keywords: Spondilodiscitis, MRI, Bone Scintigrafi, CT, X Ray, destruction, abces
1. INTRODUCTION
Infectious spondylodiscitis or infectious spondylitis is an infectious process involving two vertebral bodies and the disk between them. The incidence of this disease is estimated to be around 0.4 to 2.4 per 100,000 per year and tends to increase with increasing age (1, 2). Many authors expect these numbers to increase because of better diagnostic techniques, increasing numbers of immunocompromised patients, growing IV drug use in young people, increased use of intravenous access devices, and increasing prevalence of genitourinary surgery in the elderly (3, 4). Accurate and timely diagnosis is important because a missed or delayed diagnosis of spondylodiscitis can potentially lead to the development of epidural abscess and spinal cord compression along with vertebral bone destruction and spinal instability (5). Hematogenous spread of septic emboli is generally accepted as the most common mechanism by which infection is seeded into the vertebrae (6, 7). The most common source of infection is thought to be the urinary tract (8). Classically, pyogenic spondylodiscitis presents with lesions in two adjacent vertebral bodies and the corresponding intervertebral disk. This is thought to be due to the segmental nature of the supplying arteries that bifurcate to supply two adjacent vertebral bodies (9). In children, the presence of vascular channels that directly feed the disk allows for direct hematogenous infectious seeding of the disk. In adults, the direct blood supply to the disk is reduced and thus disk infection usually arises via direct spread from the vertebral body after the end-plate has been destroyed (10). Rarely, spondylodiscitis may affect only a single vertebral body with or without disk involvement and this may lead to diagnostic confusion. In this scenario, metastatic disease and mycobacterial infection become more prominent in the differential diagnosis. Thus, awareness of the variability of imaging findings in spondylodiscitis is important in minimizing delays in diagnosis. Our purpose here is to report two cases of spondylodiscitis that presented with imaging abnormalities in only one vertebra. The early presentations of spondylodiscitis may be ignored or mistaken for a neoplasm, traumatic condition, or other acute inflammatory conditions, such as Modic change or an acute Schmorl’s node, because magnetic resonance (MR) findings can be non-specific or atypical. Furthermore, iatrogenic infectious conditions may cause abnormal MR signal intensities at unusual sites, and laboratory evaluations are not always reliable, although erythrocyte sedimentation rate (ESR) and C-reactive protein are often elevated (11). MRI should be performed as soon as infection is suspected because it has high sensitivity and specificity for the detection of early infection (12). Furthermore, when the diagnosis is uncertain, early follow-up MRI may enable visualization of early changes, which is critical because delayed diagnosis can increase the mortality and morbidity rates (13, 14, 15). Typically, spinal infection presents as involvement of two contiguous vertebrae and inflammatory change in the intervening disc, but it can take weeks or months for these findings to develop (16). The other imaging features, such as paraspinal or epidural enhancement, a hyperintense T2 disc signal, and erosion of at least one vertebral endplate have good sensitivity (> 80%) for diagnosing spinal infections (17), but they are not always seen on MR images in the early stage.
The purpose of this review was to evaluate the MR findings of early spondylodiscitis, atypical findings of iatrogenic infection, and the differentiation between spondylodiscitis and other disease entities mimicking infection.
2. CASE REPORT
A patient 57 old, female, with longstanding pain localized in cervical region and joined by episodes of fever.
During physiatrists treatment patient’s condition has been worsening due to increased pain localized in cervical region.
MR images were acquired with a superconductive magnet at 1.5 T, with the use of the following sequences: sagittal PD and T2-weighted TSE, sagittal T1-weighted SE, axial PD and T2-weighted TSE for the lumbar spine, axial T2-weighted GRE for the cervical and dorsal spine and axial and sagittal T1-weighted SE after contrast agent injection (gadolinium DTPA). MR images were reviewed by three experienced radiologists and morphological and signal intensity changes of vertebral bodies and discs were recorded on a standard form. It was possible to compare MR to CT findings. At the time of our observation the patient has reported pain at the spine level, associated with the fever and minimal weight loss. Blood tests were normal in this phase. The patient had Diabetes Mellitus Tip II.
The first imaging procedure was cervical X-ray (AP and LL). The corporal vertebral destruction localized at C-4 and C-5 was noticed (Figure 1a, 1b).
Figure 1.
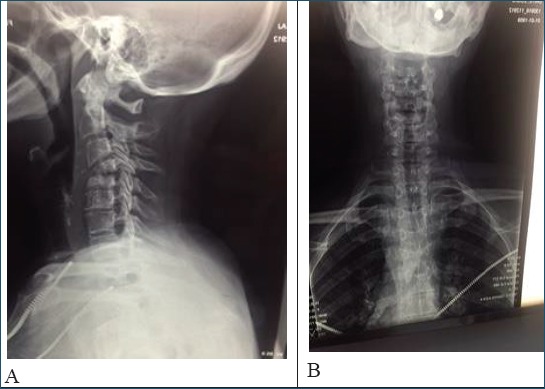
X Ray a (Latero- lateral projection) b (Antero-Posterior projection) that showed the corporal vertebral destruction localized at C-4 and C-5.
NECT-scan was recommended and, as a result, the detailed findings showed destruction with micro fractures of the C-4/C-5 vertebral bodies, more dominated at the ventral part of the C-4 (Figure 2a, 2b).
Figure 2.

NECT scan a (Sagital plane), b (axial plane) destruction of the C-4/C-5 vertebral bodies, more pronounced at the ventral part of the C-4.
Cervical MRI: heterogeneous lesion in C4 and C5 with hypersignal in T2 (Figure 3a) and STIR and hypo signal in T1 (Figure 3b). The changes are registered at bone vertebral structures (bone edema) and intervertebral disc (increased water contain).
Figure 3.

MRI examination: a (T2 WI), b (T1WI), resulted: heterogeneous lesion in C4 and C5 with hypersignal in T2 and STIR, and hypo signal in T1. The changes are registered at bone vertebral structures (bone marow edema) and intervertebral disc (increased amount of water), c (T1WI post contrast) After contrast administration, apart from diffuse pathologic enhancement of affected structures, there was another marginal form of enhancement that surrounds the water contained part of the intervertebral disc (highly suggestive for spondylodyscitis). The similar changes were discovered in other levels of spine – the thoracic vertebras (Th4-Th5).
After contrast administration, apart from diffuse pathologic enhancement of affected structures, there was another marginal form of enhancement that surrounds the part filled with water of the intervertebral disc (highly suggestive for spondylodyscitis), (Figure 3c). Pathologic enhancement was also registered in right neuro-foramina of this vertebral level. Findings correlated with inflammatory purulent process - abscess (Figure 4a, 4b, 4c). The similar changes were found in other levels of spine - the thoracic vertebras (Th4-Th5).
Figure 4.

MRI post contrast a (coronal plane), b and c (axial plane) pathologic enhancement was registered in right neuro-foramina of this vertebral level. Findings correlated with inflammatory purulent process – abscess.
Bone scintigraphy: Type of accumulations of radiopharmacs suggested neoplastic masses/metastasis (the report written by Nuclear medicine department), (Figure 5a).
Figure 5.

Bone scintigraphy: Type of accumulations of radiopharmacs suggest neoplastic masses/metastasis (the report issued from Nuclear medicine department).
Whole body CT results: CECT scan of thorax, abdomen and pelvic - no signs for neoplastic process. No signs for parenchymal pulmonary inflammatory changes. Enlarged reactive lymph nodes in anterior mediastinum and retroperitoneal space (Figure 6a, 6b, 6c). CECT in bone window shows ventral destruction thoracal vertebral bodies Th4, Th5 (Figure 7a, 7b, 7c, 7d, 7e, 7f). Laboratory essay: Quantiferon TBC gold plus positive, Agglutination brucella test and Wright test - Negative, Levenshtein test positive.
Figure 6.

a. CECT scan (Coronal plane), b (axial plane thoracal), c (axial plane abdominal) No signs for neoplastic process. No signs for parenchymal pulmonary inflammatory changes. Enlarged reactive lymphnodes in anterior mediastinum and retroperitoneal space.
Figure 7.

CECT scan; a, b (sagital plane) c,d,e,f (axial plane): bone window shows ventral destruction thoracal vertebral bodies Th4, Th5.
MRI allowed the correct diagnosis to be made in all cases, demonstrating earlier and more accurately than CT the pathological involvement of the paravertebral and spinal canal structures. A common finding in pyogenic and tuberculous spondylodiscitis was the low signal of the subcortical bone marrow on T1-weighted sagittal images, which enhanced after Gd-DTPA administration and became intermediate or high on T2-weighted images. Moreover, the steady high signal intensity of the disk on T2-weighted images and its contrast enhancement on T1-weighted images is typical for an acute inflammatory process.
3. DISCUSSION
Tuberculosis (TB) remains a major cause of morbidity and mortality in developing countries affecting millions of people worldwide (18). Skeletal TB, constituting 10–20% of all the extrapulmonary TB (ETB), is a well-recognized clinical condition that is easily diagnosed and managed with an excellent outcome (19). However, the occurrence of multifocal skeletal involvement, which is defined as osteoarticular lesions that occur simultaneously at two or more locations in the skeletal system, is exceptional and constitutes <5% of all skeletal TB cases, even in countries where TB is endemic (20-23). Tuberculosis is considered to be uncommon and only occurs in an estimated 1.5 to 3% of patients with musculoskeletal tuberculosis (24, 25).
Similar to the European Western countries and USA, the pulmonary forms of TB in Kosova have almost disappeared. The rarer pulmonary occurrence has inverted the frequency of new cases in other localizations (the incidence of extra-pulmonary forma of TB, especially localized in the thoraco-lumbar region has increased). Generally, the extrapulmonary TB is a rare condition, especially multiple TBC of spine, and most commonly is presented in thoraco-lumbal levels. Cervical segment of spine has to be also considered as a possible site of manifestation of extrapulmonary multifocal TBC – as in our case. Spondylodiscitis is a diagnostic challenge, that should be correlated with clinical and especially blood tests. Involvement of multiple intervertebral discs and neighboring vertebras include the brucellosis as another possible differential diagnosis. While plain radiography is often used as an initial imaging modality for patients with back pain, MRI is considered the most sensitive and specific imaging study for early spondylodiscitis. The typical MRI characteristics of spondylodiscitis are low T1 and high T2 signal intensity in the vertebral bodies and the intervertebral disk between them. Avid gadolinium enhancement on T1-weighted imaging in the affected tissues is also characteristic.
4. CONCLUSION
The early MR findings of infectious spondylodiscitis are non-specific and include faint bone marrow edema, paravertebral soft tissue change, and/or focal epidural enhancement, which overlap with findings of other conditions, such as Modic type 1 change, acute Schmorl’s node, disc extrusion, malignancy, or acute endplate injury. It is important to recognize these findings as the earliest signs of infectious spondylitis, and a short-term follow-up MR imaging should be performed to detect any infective changes when spinal infection is clinically suspected. In a patient with these early MR findings, correlations with clinical history and laboratory test findings should be sought. In particular, careful evaluation at or around the procedure sites, such as facet joints, epidural space, muscular structures of the back, and posterolateral portion of vertebral bodies, is essential when there is a history of a procedure or surgery. To avoid delayed diagnosis, radiologists should become familiar with the atypical MR findings of early spondylodiscitis and they should be able to differentiate these findings from those of lesions with similar findings. MRI is the most sensitive technique for the diagnosis of spondylodiscitis in the acute phase and comparable to CT regarding chronic stage of the disease. At present MRI does not allow differentiating pyogenic from tuberculous forms.
Footnotes
• Conflict of interest: none declared
REFERENCES
- 1.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–12. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerli W. Clinical Practice. Vertebral Osteomyelitis. N Engl J Med. 2010;362:1022–9. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo-de La Torre J, Bohinski R, Kuntz C. 4th Vertebral osteomyelitis. Neurosurg Clin N Am. 2006;17:339–51. doi: 10.1016/j.nec.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–7. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Meyers S, Wiener S. Diagnosis of hematogenous pyogenic vertebral osteomyelitis by magnetic resonance imaging. Arch Intern Med. 1991;151:683–7. [PubMed] [Google Scholar]
- 6.Cahill D, Love L, Rechtine G. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878–86. doi: 10.3171/jns.1991.74.6.0878. [DOI] [PubMed] [Google Scholar]
- 7.Stabler A, Reiser M. Imaging of spinal infection. Radiol Clin North Am. 2001;39:115–35. doi: 10.1016/s0033-8389(05)70266-9. [DOI] [PubMed] [Google Scholar]
- 8.Shin T, Huang K, Hou S. Early diagnosis of single segment vertebral osteomyelitis - MR pattern and its characteristics. Clin Imaging. 1999;23:159–67. doi: 10.1016/s0899-7071(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 9.Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50:777–98. doi: 10.1016/j.rcl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Leone A, Dell’Atti C, Magarelli N, Colelli P, Balanika A, Casale R, et al. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):8–19. [PubMed] [Google Scholar]
- 11.DeSanto J, Ross JS. Spine infection/inflammation. Radiol Clin North Am. 2011;49:105–27. doi: 10.1016/j.rcl.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Gürbüz MS, Berkman MZ. Spondylodiscitis occurring after diagnostic lumbar puncture: a case report. Case Rep Infect Dis. 2013;2013:843592. doi: 10.1155/2013/843592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209–212. doi: 10.1136/jnnp.65.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunbar JA, Sandoe JA, Rao AS, Crimmins DW, Baig W, Rankine JJ. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol. 2010;65:974–81. doi: 10.1016/j.crad.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Ziegelbein J, El-Khoury GY. Early spondylodiscitis presenting with single vertebral body involvement: a report of two cases. Iowa Orthop J. 2011;31:219–24. [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliffe JF. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. A microarteriographic study. J Anat. 1982;134(Pt 2):373–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;(Suppl 3):iii11–iii24. 65. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 18.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence and mortality by country. WHO global surveillance and monitoring project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 19.Moon MS, Moon YW, Moon JL, Kim SS, Sun DH. Conservative treatment of tuberculosis of the lumbar and lumbosacral spine. Clin Orthop Relat. 2002:40–49. doi: 10.1097/00003086-200205000-00007. doi: 10.1097/00003086-200205000-00007. Res. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz MH, Kantarci F, Mihmanli I, Kanberoglu K. Multifocal skeletal tuberculosis. South. Med J. 2004;97:785–7. doi: 10.1097/00007611-200408000-00023. doi: 10.1097/00007611-200408000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Khan SA, Qureshi NA. Multifocal osteoarticular tuberculosis in children. J Orthop Surg (Hong Kong) 2011;19:336–40. doi: 10.1177/230949901101900315. [DOI] [PubMed] [Google Scholar]
- 22.Hong L, Wu JG, Ding JG, Wang XY, Zheng MH, Fu RQ, Li WB, Peng WX, He WF, Sun QF. Multifocal skeletal tuberculosis: Experience in diagnosis and treatment. Med Mal Infect. 2010;40:6–11. doi: 10.1016/j.medmal.2009.03.004. doi: 10.1016/j.medmal. [DOI] [PubMed] [Google Scholar]
- 23.Marudanayagam A, Gnanadoss JJ. Multifocal skeletal tuberculosis: A report of three cases. Iowa Orthop J. 2006;26:151–3. [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Tuberculosis factsheet. Geneva, Switzerland: WHO; 2010. Nov, pp. 26–156. [Google Scholar]
- 25.Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39:203–13. doi: 10.1016/s0033-8389(05)70273-6. [DOI] [PubMed] [Google Scholar]


