Abstract
Introduction:
Subarachnoid hemorrhage (SAH)represents hemorrhage in the space between arachnoidea and pia mater, due to aneurysm burst, spontaneously or as a consequence of trauma. It is condition that occurs more common in women than men, and its most common complications are rebleeding and vazospasm. As a result of vasospasm, develops ischemia in the portion of brain tissue that can cause additional neurological deficit. Transcranial Doppler Sonography (TCD) is a noninvasive ultrasound diagnostic method that allows monitoring of the state of intracerebral hemodynamics.
Goal:
The goal is to follow the occurrence of vasospasm after SAH, by the TCD method.
Material and methods:
We have analyzed 47 patients with SAH, by analyzing the presence of aneurysm, hypertension and smoking, and by the TCD method monitor the state of intracerebral hemodynamics during the first four days, then in the second and third week.
Results:
SAH was more common in women (61.7%) than men (38.3%), and in the age range from 22 to 64 years. Aneurism was demonstrated in 61.7% of patients, more common in women, with hypertension 68.1% also more common in women and smoking in 87.2% of patients, also more common in women. By TCD method are recorded milder, elevated blood flow velocities at a quarter of patients in the first measurement, during the second measurement at all and it had significantly greater value, and the third measurement also more increased in about a quarter of patients, so that there is a statistically significant difference in the first and second, second and third measurement for each vessel separately, but not between the first and third measurement.
Conclusion:
Predilection factors for SAH are aneurysms, hypertension and smoking. By using TCD method were recorded milder elevated blood flow velocity in the first days of SAH, with about a quarter of patients, significantly greater increase in blood flow velocity during the second week in all patients and also milder increase blood flow velocity in the third week of the start of SAH is a quarter of patients. TCD is the method of choice in the evaluation and management of vasospasm after SAH, which allows the prevention of delayed cerebral ischemia.
Keywords: SAH, vasospasm, TCD
1. INTRODUCTION
Subarachnoid hemorrhage (SAH) is the effusion of blood in the subarachnoid space (between arachnoidea and piamater). It occurs spontaneously, mainly due to rupture of a cerebral aneurysm, or as the result of traumatic injury to the head-traumatic SAH (1). The frequency SAH is 5-8% in all forms of stroke, frequently in people between 35 and 65 years, somewhat more often women. The highest incidence is found in Japan, 23 per 100,000 inhabitants and in Finland with an incidence of 22/100,000 inhabitants annually. Smoking, hypertension, and excessive consumption of alcohol remains the most important risk factors for SAH (2).
The first and most difficult complication of SAH is rebleeding, followed by the occurrence of vasospasm with subsequent infarction, particularly present between 5 and 15 days after the infarction, which is manifested at about 30% of patients. The third complication is the development of acute hydrocephalus (15%), while the chronic hydrocephalus represent complication at later stages of the disease. (3).
Vasospasm is reducing blood flow through the cerebral circulation which can be seen after aneurysmal SAH and it is a result of vasoconstriction.
Clinical vasospasm includes „deferred ischemic neurologic deficit” and „delayed cerebral ischemia” due to theprolonged vasoconstriction, resulting in an increase in blood flow velocity (4, 5). The exact mechanism of cerebral vasospasm is not fully known. Hemolysisof erythrocytes in the subarachnoid spacereleases the substances, spasmogens primarily oxyhemoglobin, which are directly or indirectly responsible for the development of vasospasm. In addition to oxyhemoglobin and other substances, such as metabolites of arachidonic acid, endothelin, free radicals, serotonin, adenosine and oxidative products of bilirubin and the very process of inflammation, play a significant role in the development of vasospasm, creating an imbalance between vasoconstrictor and vasodilator mediators (6).
Vasospasm develops in the period from the third to fifth day after the hemorrhage, and reach its maximum frequency 7- days and usually last for 2-3 weeks. Usually is focal or may have a diffuse distribution. Death as a result of vazospasmoccurs in 7% of patients, and the same number of patients having severe, permanent neurological deficit (7).
Rebleeding occurs in about 35% of cases with a mortality rate of around 42%. It is believed that the greatest risk of rebleeding is within 24 hours and that in about 22% of rebleeding occurs within the first two weeks (8).
Condition of intracerebral hemodynamics and vasospasm can be monitoredby TCD method. Sharp increase in mean blood flow velocities (MBFV) in the first few days after SAH is associated with poor prognosis (>20 cm/s per day). Studies show prognostic relevant threshold of increased MBFV in the ACM by critical MBFV of 120 cm/s or above, a very critical MBFV >140 cm/s. Increased blood flow means stronger vasospasm and often in these cases appears and consequent ischemic neurologic deficit (8-10).
2. GOAL
The goal is to follow the occurrence of vasospasm after SAH, by the TCD method.
3. MATERIAL AND METHODS
In the study are analyzed 47 patients with SAH. All respondents besides history and neurological examination was performed CT scan of the brain, which in 2 cases confirmed SAH, with special reference to the existence of an aneurysm, hypertension, smoking, etc. TCD method was used to monitor the state of cerebral circulation: on the first day (1 to 4 day) after admission, at the beginning of the second week (7-12 day) after admission and in the third week following admission.
4. RESULTS
Our study included a total of 47 patients, or 18 (38.3%) male and 29 (61.7%) female aged 22 to 64 years, with an average age of 41.43±1.62 years.
Aneurysm is proven in 29 (61.7%) patients, or in 7 males (24.1%) and 22 females (75.9%) with the difference that was statistically significant (X2=6.425; p=0.01; p <0.05) in favor of women.
Hypertension was present in 32 (68.1%) of patients or in 12 males (37.5%) and 20 females (62.5%), and smoking among 41 (87.2%) of patients or 15 males (36.6 %) and 26 females (63.4%) patients.
During the first measurement of the mean blood flow velocity (MBFV) through CS and ACM in most male respondents (88.9%) and female (75.9%) was within the reference range, and 11.1% of male patients and 24.1% of female patients had elevated MBFV through CS and ACM during the first measurement. During the first measurement of the mean blood flow velocity (MBFV) through ACA and ACP in the majority of male respondents (88.9%) and female (82.76%) the values was within the reference range. From total, 11.1% of male patients and 17.24% female patients had elevated MBFV through ACA and ACP during the first measurement. In the first measurement, a total of 9 (19.1%) patients had elevated MBFV in CS, as well as the ACM. The ACA elevated values were observed in 7 (14.8%) patients, and the same percentage was recorded also in the ACP (14.8%).
During the second measurement of the mean blood flow velocity (MBFV) through ACM and CS was increased in 100% of male patients, and in the ACA and ACP 94.4% of male patients had increased values. During the second measurement 100% of female patients had an elevated valueof MBFV through ACM and CS and 96.5% of female patients had elevated MBFV through ACA and ACP. In the second measurement, the total of 47 (100%) of the patients had elevated MBFV through CS, ACA and MCA, and the total of 45 (95.7%) patients in the ACP.
During the third measurement of MBFV through CS, ACA, ACM and ACP in most (77.8%; 77.8%; 77.8% and 72.3%) of male patient’s values was in the reference interval. A larger number of females in the third measurement had MBFV through CS, ACA, ACM and ACP refence values (69.0%, 69.0%, 69.0% and 82.8%). In the third measurement with a total of 13 (27.6%) patients were recorded elevated MBFV in CS, ACA and ACM, while elevated in ACP had a total of 10 (21.2%) patients.
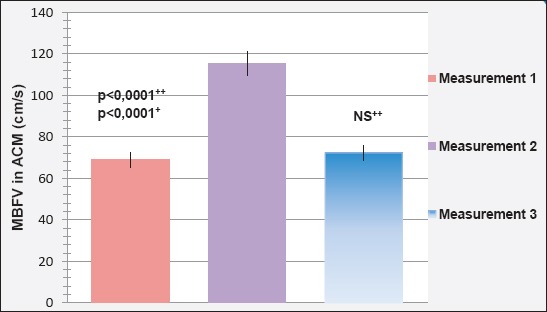
Mean blood flow velocity (MBFV) in the middle cerebral artery (ACM) during three different measurements is shown in Figure 1. The reference value MBFV the ACM, 62±12 cm/s.
Figure 1.
Mean blood flow velocity in ACM during three measurements
Mean blood flow velocity (MBFV) in the middle cerebral artery (ACM) in the first assessment amounted to 68.85±1.594 cm/s was less than the value MBFV the ACM in the second (115.53±1.567 cm/s) and third (72.30±2.158 cm/s) measurement.
Fortified is a statistically significant difference between the value MBFV the ACM in the first and second (p <0.0001), second and third (p <0.0001), but not the first and third (p = 202; p>0.05) measurement.
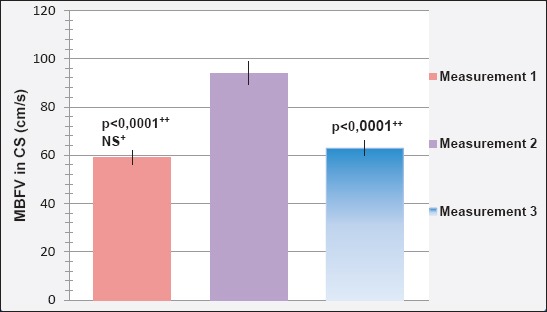
Mean blood flow velocity (MBFV) in the carotid siphon (CS) during three different measurements is shown in Figure 2. Reference value of MBFV in CS is 54±13 cm/s.
Figure 2.
Mean blood flow velocity in CS during three measurements
Mean blood flow velocity (MBFV) in the carotid siphon (CS) in the first assessment amounted to 59.09±1.708 cm/s was less than the value of MBFV in CS in second (95.04±0.958 cm/s) and third (62.87±1.996 cm/s) assessment.
Determined is a statistically significant difference between the value of MBFV in CS in the first and second (p<0.0001), second and third (p<0.0001), but not the first and third (p=0.153; p>0.05) measurement.
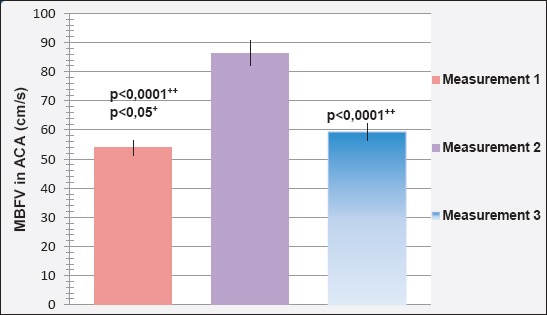
Mean blood flow velocity (MBFV) in the anterior cerebral artery (ACA) during three different measurements is shown in Figure 3. The reference value of MBFV in the ACA is 50±12 cm/s.
Figure 3.
Mean blood flow velocity in ACA during three measurements
Mean blood flow velocity (MBFV) in the anterior cerebral artery (ACA) in the first assessment amounted to 53.81±1.504 cm/s and was less than the value MBFV in the ACA in second (86.40±1.303 cm/s) and third (59.26±1.694 cm/s) measurement.
Determined is a statistically significant difference between the value of MBFV in the ACA in the first and second (p<0.0001), second and third (p<0.0001), but not the first and third (p=0.18: p>0.05) measurement.
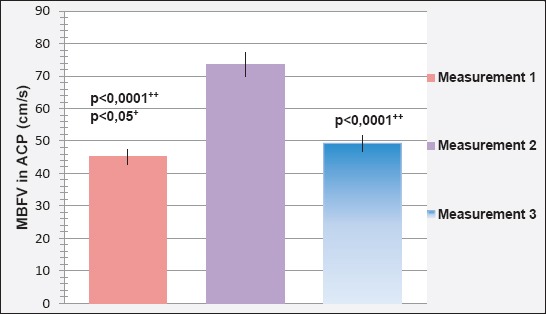
Mean blood flow velocity (MBFV) in the posterior cerebral posterior artery (ACP) during three different measurements is shown in Figure 4. The reference value of MBFV in the ACP is 42±12 cm/s.
Figure 4.
Mean blood flow velocity in ACP during three measurements
Mean blood flow velocity (MBFV) in the posterior cerebral artery (ACP) in the first assessment amounted to 45.13±1.310 cm/s was less than the value of MBFV in the ACP in the second (73.66±1.211 cm/s) and third (49.28±1.538 cm/s) measurement.
Determined is a statistically significant difference between the value of MBFV in the ACP in the first and second (p<0.0001), second and third (p=0.043; p<0.05), but not in the first and third measurement (p=0.1; p>0.05).
5. DISCUSSION
Our study included a total of 47 patients with SAH, or 18 (38.3%) male and 29 (61.7%) females, aged 22-64 years. With an average age of men of 42.56±2.79 years, and women of 40.72±2.01 years. Mean age difference between sexes was not statistically significant and corresponds to the literature by which SAH occurs predominantly in the most productive age and is more common in women than men.
Frontera et al. conducted a study on 580 patients with SAH, of which 68% were female, as percentage share roughly corresponds to our sample. Compared to the same study the age structure of our patients is younger. The average age of patients in our study was 41.43±1.62, while their average ages of patients was 53 years (11).
Aneurysm was confirmed in 29 (61.7%) of patients, or 7 (24.2%) male and 22 (75.8%) female. The difference in the prevalence of the aneurysms in patients of different sexes is statistically significant (X2=6.425; p=0.01; p<0.05).
The literature states that the cause of SAH in 80% of cases is the rupture of the aneurysm, and the presence of an aneurysm on the basis of sex is in favor of women 3:1 by literature.
Our data also confirm that the aneurysm is the leading cause of SAH in our patients and that the incidence of aneurysm was two times higher in women.
Hypertension was present in 32 (68.1%) of patients, or 12 (37.5%) male and 20 (62.5%) female.
In their study, Bonita states that the preference for SAH is three times higher in people who are treated for hypertension than among people who do not have high blood pressure. The tendency was higher in females but was not statistically significant (p>0.5) (47). Isaksen et al. in their study of risk factors for SAH state that any increase in systolic blood pressure of 20 mmHg increases the risk of SAH and that hypertension is an important independent risk factor for aneurysm SAH (12)
Smoking was represented in 41 (87.2%) patients, or 15 males (36.6%) and 26females (63.4%)
In other studies, the ratio of current smokers was significantly higher in patients with SAH (73.1%) than in the control group (41.3%). The same risk factor states Bonita in his study which involved 115 patients (45 men and 70 women). For men who are smokers, the risk of SAH was three times higher in women and even more or gender differences were not statistically significant (51% of men and 59% of women were smokers) (12).
Our results also show that smoking in patients with SAH infection was often.
We found that the deficit of motor skills (mild hemiparesis), was present in only 11 (23.4%) patients included in our study or 4 males (36.4%) and 7 females (63.6%).
The difference in the prevalence of the presence of motor control deficits in patients of different sexes was not statistically significant (X2=0.0227, p=0.88; p>0.05).
Deficit motor skills (mild hemiparesis) patients developed between 8 and 14 days after SAH.
Nakea et all. in his study on 142 patients with SAH, in 28 (19.7%) of patients recorded symptomatic vasospasm in the form of impairment of consciousness and motor deficits (13).
In his study, with 580 patients, Frontera said that 16% of patients had symptomatic vasospasm in the form of neurological deficit (impairment of consciousness, motor deficit) (11, 13).
However, Dankbar et al. in their study on 37 patients with SAH state that cerebral perfusion decreases vasospasm, or neurologic deficit did not result in their patients, although almost half of their patients had a severe vasospasm (MBFV >50% of the upper limit) (14).
Our results support the studies referred to motor deficits as a result of vasospasm because the motor deficit appeared in the period when we recorded by TCD the largest MBFV through the arteries of the front basin, especially in the ACM.
In the first measurement, elevated MBFV had 9 (19.1%) patients in the CS and ACM. Elevated levels in the ACA and ACP were observed in 7 (14.8%) patients and 20% of the upper limit for individual vessels front basin.
From baseline 11.1% of men and 24.1% of women had elevated MBFV through ACM and CS during the first measurement. Trough ACA and ACP during the first measurement 11.1% of men and 17.24% of women had elevated MBFV.
In the second measurement, the total of 47 (100%) patients had elevated MBFV in the ACM, CS and ACA, and 45 (95.7%) patients in the ACP.
MBFV were increased up to 50% of the upper limit at 74.47% of patients, and in 25.53% of patients between 50-65% of the upper limit.
During the second measurement, the mean blood flow velocity (MBFV) through ACM and CS was increased in 100% of men, and the ACA and ACP in 94.4% of men. During the second measurement 100% of female patients had elevated values of MBFV through ACM and CS and 96.5% of patients through the ACA and ACP.
In the third measurement with a total of 13 (27.6%) patients were recorded elevated MBFV in ACM, CS and ACA, while elevated values in ACP had a total of 10 (21.2%) patients. MBFV were increased up to 25% of the upper limit for individual vessels of the front basin.
In the third measurement 22.2% of men and 31.0% of women had elevated MBFV in ACM, CS and ACA, and 27.7% of men and 17.24% women in the ACP.
The obtained values of MBFV through the arteries of the front basin (CS, ACA, ACM, ACP) show a slight increase of MBFV up to 20% of the upper limit during the first measurement (first week after admission after stroke). The highest values MBFV were recorded during the second measurement (at the beginning of the second week after the stroke). In 35 (74.47%) patients the MBFV was increased up to 50% of the upper limit, and in 12 (25.53%) patients between 50-65% (120-130 cm/s in ACM). Values of MBFV in the third measurement (third week of SAH) did not exceed 25% of the upper limit.
This development of changes in MBFV in the arteries of the front basin corresponds to the information available in the literature as well as the results of other studies, which describe that vasospasm develops between the third to fifth days after the hemorrhage, reaching maximum after 6-9 days and usually last 2-3 weeks.
Mean blood flow velocity (MBFV) in artery cerebri media (ACM) in the first assessment amounted to 68.85±1.594 cm/s was less than the value of MBFV in the ACM in the second (115.53±1.567 cm/s) and third (72.30±2.158 cm/s) measurement.
Determined is a statistically significant difference between the value of MBFV in the ACM in the first and second (p<0.0001), second and third (p<0.0001), but not the first and third (p= 202; p>0.05) measurement.
The best data to link increased MBFV with vasospasm provides ACM. Condette et al. proposed, based on its work with 51 patients, the limit of MBFV to detect vasospasm in ACM of 140 cm/s. They showed that MBFV value of 130 cm/s in ACM has a sensitivity of 73% and specificity of 100% for the detection of vasospasm. In another study with 49 patients using a value of at least 130 cm/s the specificity reached 100% in detecting vasospasm in ACM (15, 16). In a larger study with more than 100 patients, MBFV less than 120 cm/s could reliably predict the absence of vasospasm (negative predictive value of 94%) and MBFV greater than 200 cm/s reliably to predict moderate to severe vasospasm (positive predictive value of 87%) (56). Condette Auliac stated that flow through the ACM directly proportional to the severity of vasospasm and vasospasm mild with values from 80 to 120 cm/sec, moderate at 130-200 cm/s and severe over 200 cm/s (15).
MBFV in ACM in our patients during the second measurement had a maximum value and was 115.53±1.567 cm/s, which means that after gradingthe severity of vasospasm most of our patients had mild vasospasm recorded by TCD in ACM. MBFV in the ACM, as the most reliable blood vessel to assess vasospasm, did not exceed the value of >140 cm/s.
Mean blood flow velocity (MBFV) in the carotid siphon (CS) in the first assessment amounted to 59.09±1.708 cm/s and was less than the value of MBFV in CS in second (95.04±0.958 cm/s) and third (62.87±1.996 cm/s) measurement.
Determined is a statistically significant difference between the value MBFV in CS between first and second (p<0.0001), second and third (p<0.0001), but not the first and third (p=0.153; p>0.05) measurement.
Vasospasm in the terminal parts of artery carotis interna, or in the carotid siphon studied several researchers. In a prospective study by Creissard and Proust, report less sensitivity of TCD (<95%) for detecting vasospasm in CS (16. 17). Older work with 49 patients have the results of specificity and predictive value of 100% when the value MBFV exceeded 130 cm/s in the CS (16).
To estimate the severity of vasospasm by MBFV in CS in our patients the most relevant was in the second measurements (95.04±0.958 cm/s).
Mean blood flow velocity (MBFV) in the anterior cerebral artery (ACA) in the first assessment amounted to 53.81±1.504 cm/s and was less than the value of MBFV in the ACA in second (86.40±1.303 cm/s) and third (59.26±1.694 cm/s) measurement.
Determined is a statistically significant difference between the value of MBFV in the ACA between the first and second (p<0.0001), second and third (p<0.0001) but not the first and third (p=0.18: p>0.05) measurement.
Vasospasm in the ACA can be difficult to detect TCD due to anatomical characteristics (18, 19). In the study by Suarez et al. which involved 199 SAH patients, the correlation between the increased flow recorded in TCD and symptomatic vasospasm was better in the CS and ACM compared with ACA (20).
To increase the sensitivity of TCD for the ACA, Vora et al. suggested that clinicians use both ACA to gain access to vasospasm on both sides (20).
The best correlation MBFV in ACM and severity of vasospasm in our patients, we found the second measurement (86.40±1.303 cm/s).
Mean blood flow velocity (MBFV) in the posterior cerebral artery (ACP) in the first assessment amounted to 45.13±1.310 cm/s and was less than the value of MBFV in the ACP in the second (73.66±1.211 cm/s) and third (49.28±1.538 cm/s) measurement.
Determined is a statistically significant difference between the value of MBFV in the ACP in the first and second (p<0.0001), second and third (p= 0.043; p<0.05) but not in the first and third measurement (p=0.1; p>0.05)
Detection of vasospasm by TCD in ACP tested Wozniak et al. Difficulty in use of TCD in screening ACA and ACP in particular is discussed in this study. In a study of 53 patients had a sensitivity of 48% and specificity of 69% in technically correct TCD in the limit value MBFV of 90cm/s. If this value is increased to 110 cm/s specificity is increased to 93% but the sensitivity is low. False positive value of 37% was attributed to anatomical factors, including occlusion and inexperience examiners. For ACP and the ACA, it has been shown that these blood vessels can be certain to confirm the criteria for vasospasm (21).
In our study MBFV in the ACP had the highest value in the second measurement (73.66±1.211 cm/s), thus the greatest prognostic significance.
6. CONCLUSION
SAH is more common in women than men, while predilection factors for it are aneurysms, hypertension and smoking.
By TCD method were recorded milder elevated blood flow velocities in the first days after SAH, in about a quarter of patients, significantly greater increase in blood flow velocity during the second week in all patients and also less severe increase in blood flow velocity in the third week afterthe SAH onset in a quarter of patients.
TCD is the method of choice in the evaluation and management of vasospasm after SAH which allows the prevention of delayed cerebral ischemia.
Footnotes
• Conflict of interest: none declared
REFERENCES
- 1.Djelilović-Vranić J. Transkranijalna doppler sonografija - TCD stetoskop za mozak, Institut za naučno istraživački rad i razvoj Kliničkog centra. Sarajevo: Univerziteta u Sarajevu; 2013. [Google Scholar]
- 2.Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–78. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 3.Valery L. Feigin, Gabriel JE. Rinkel, Carlene MM. Lawes, Ale Algra, Derrick A. Bennett, Jan van Gijn, Craig S. Anderson. Risk Factors for Subarachnoid Hemorrhage An Updated Systematic Review of Epidemiological Studies. Stroke. 2005;36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage A. Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American HeartAssociation. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Armonda RA, Bell RS, Vo AH, et al. Wartime traumatic cerebral vasospasm: recent review of combatcasualties. Neurosurgery. 2006;59:1215–25. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- 6.Marshall SA, Nyquist P, Ziai WC. The Role of Transcranial Doppler Ultrasonographyin the Diagnosis and Management of Vasospasm After Aneurysmal Subarachnoid Hemorrhage. Neurosurg Clin N Am. 2010;21:291–303. doi: 10.1016/j.nec.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Kolias AG, Sen J, Belli A. Pathogenesis of Cerebral Vasospasm Following Aneurysmal Subarachnoidhemorrhage: Putative Mechanisms and Novel Approaches. Jorunal of Neuroscience Research. 2009:87. doi: 10.1002/jnr.21823. [DOI] [PubMed] [Google Scholar]
- 8.Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anesth. 2008;55:112–23. doi: 10.1007/BF03016323. [DOI] [PubMed] [Google Scholar]
- 9.Alexandrov AV, Sloan MA, Wong LK, Douville C, Razumovsky AY, Koroshetz WJ, et al. American Society of Neuroimaging Practice Guidelines Committee. J Neuroimaging. 2007;17:11–8. doi: 10.1111/j.1552-6569.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 10.Yahia AM, Kirmani JF, Qureshi AI, et al. The safety and feasibility of continuous intravenous magnesium sulfate for prevention of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;3:16–23. doi: 10.1385/NCC:3:1:016. [DOI] [PubMed] [Google Scholar]
- 11.Frontera JA, Fernandez A, Schmidt JM, Claasser J, Wartenberg KE, Badjatia N, Connally S, Mayer SA. Defining Vasospasm After Subarachnoid Hemorrhage: What Is the Most Clinically Relevant Definition. Stroke. 2009;40:1963–8. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 12.Bonita R. Cigarette smoking, hypertension and the risk of subarachnoid hemorrhage: a population-based case-control study. Stroke. 1986;17:831–5. doi: 10.1161/01.str.17.5.831. [DOI] [PubMed] [Google Scholar]
- 13.Nakae NR, Yokota H, Yoshida D, Teramoto A. Transcranial Doppler Ultrasonography for Diagnosis of Cerebral Vasospasm After Aneurysmal Subarachnoid Hemorrhage: Mean Blood Flow Velocity Ratio ofthe Ipsilateral and Contralateral Middle Cerebral Arteries. Neurosurgery. 2011;69:876–83. doi: 10.1227/NEU.0b013e318222dc4c. [DOI] [PubMed] [Google Scholar]
- 14.Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationshipbetween vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoidhemorrhage. Neuroradiology. 2009;51:813–9. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condette-Auliac S, Bracard S, Anxionnat R, Schmitt E, Lacour JC, Braun M, Meloneto J, Cordebar A, Yin L, Picard L. Vasospasm After Subarachnoid Hemorrhage: Interest in Diffusion-Weighted MRImaging. Stroke. 2001;32:1818–22. doi: 10.1161/01.str.32.8.1818. [DOI] [PubMed] [Google Scholar]
- 16.Burch CM, Wozniak MA, Sloan MA, et al. Detection of intracranial internalcarotid artery and middle cerebral artery vasospasm following subarachnoidhemorrhage. J Neuroimaging. 1996;6(1):8–15. doi: 10.1111/jon1996618. [DOI] [PubMed] [Google Scholar]
- 17.Creissard P, Proust F, Langlois O. Vasospasm diagnosis: theoretical and realtranscranial Doppler sensitivity. Acta Neurochir (Wien) 1995;136(3-4):181–5. doi: 10.1007/BF01410623. [DOI] [PubMed] [Google Scholar]
- 18.Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationshipbetween vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoidhemorrhage. Neuroradiology. 2009;51:813–9. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barwish RS, Ahn E, Amiridze NS. Role of transcranial Doppler in optimizing treatment of cerebralvasospasm in subarachnoid hemorrhage. J Intensive Care Med. 2008;23(4):263–7. doi: 10.1177/0885066608318516. [DOI] [PubMed] [Google Scholar]
- 20.Vora YY, Suarez AM, Steinke DE, et al. Role of transcranial Doppler monitoring in the diagnosis ofcerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44(6):1237–47. [PubMed] [Google Scholar]
- 21.Wozniak MA, Sloan MA, Rothman MI, et al. Detection of vasospasm by transcranial Dopplersonography: the challenges of the anterior and posterior cerebral arteries. J Neuroimaging. 1996;6:87–93. doi: 10.1111/jon19966287. [DOI] [PubMed] [Google Scholar]


