Abstract
Inhibitory A type γ-aminobutyric acid receptors (GABAARs) play a pivotal role in orchestrating various brain functions and represent an important molecular target in neurological and psychiatric diseases, necessitating the need for the discovery and development of novel modulators. Here, we show that a natural compound curcumol, acts as an allosteric enhancer of GABAARs in a manner distinct from benzodiazepines. Curcumol markedly facilitated GABA-activated currents and shifted the GABA concentration-response curve to the left in cultured hippocampal neurons. When co-applied with the classical benzodiazepine diazepam, curcumol further potentiated GABA-induced currents. In contrast, in the presence of a saturating concentration of menthol, a positive modulator for GABAAR, curcumol failed to further enhance GABA-induced currents, suggesting shared mechanisms underlying these two agents on GABAARs. Moreover, the benzodiazepine antagonist flumazenil did not alter the enhancement of GABA response by curcumol and menthol, but abolished that by DZP. Finally, mutations at the β2 or γ2 subunit predominantly eliminated modulation of recombinant GABAARs by curcumol and menthol, or diazepam, respectively. Curcumol may therefore exert its actions on GABAARs at sites distinct from benzodiazepine sites. These findings shed light on the future development of new therapeutics drugs targeting GABAARs.
The γ-aminobutyric acid (GABA) system is essential for the orchestration of local networks and the functional interaction between different brain regions1. As major executors in the GABAergic system, A-type GABA receptors (GABAARs) are pentameric protein complexes that form Cl−-permeable ion channels that are widely distributed across the central nervous system, and primarily confer fast inhibitory control over neural activity, thus participating in almost every aspect of physiological and pathophysiological brain function2. GABAARs are made up of 19 known subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3), and many contain two α subunits, two β subunits, and one γ subunit3. Despite the large repertoire resulting from various combinations of these subunits, the main subunit configuration is α1-β2-γ2, at a ratio of 2:2:1, constituting approximately 60% of all GABAARs in the brain4. There are two GABA-binding sites5, formed at two interfaces between α and β subunits. By contrast, the binding site6 for benzodiazepines7 is formed by one of the α subunits6,8,9 and the γ subunit6,10,11,12. The benzodiazepine as a broad spectrum of positive allosteric modulators of the GABAAR has been in clinical use for decades and is still among the most widely prescribed drugs for the treatment of insomnia and anxiety disorders.
The clinical use of classical benzodiazepines is limited by their side effects7 and the risk of drug dependence13,14. Identification of receptor subtype-selective compounds, and the discovery of novel modulators beyond benzodiazepines, are necessary to overcome these limitations. Indeed, GABAARs are also major targets15 for barbiturates16, steroids17, and anaesthetics18,19,20,21,22,23,24,25,26, all of which are positive modulators. Moreover, given the increasing evidence that targeting GABAARs improves treatment in a broad range of neuropsychiatric disorders1,27,28, continued efforts are necessary to discover or develop novel GABAAR modulators, including agonists and antagonists29.
Natural compounds isolated from plants are a rich source of novel GABAAR ligands. Some natural flavonoids, first isolated from plants used as tranquilizers in folkloric medicine, together with their synthetic derivatives, possess selective affinity for the benzodiazepine-binding site of GABAARs with a broad spectrum of central nervous system effects30. In addition, a few natural terpenoids containing ether31,32 or hydroxyl groups33,34,35 have been identified as positive modulators of GABAARs (Fig. 1a), potentiating GABAergic transmission33,36 and thereby suppressing aberrant excitability as seen during epileptiform activity33,37. Two compounds isolated from the Chinese medicinal herb Acorus gramineus, α- and β-asarone (1-propenyl-2,4,5-methoxybenzol)31,32, act on endogenous and recombinant GABAARs, activating the receptor and alleviating epileptic seizures. The widely-used cooling and flavouring agent menthol (5-methyl-2-propan-2-ylcyclohexan-1-ol, Fig. 1a), the best-known monoterpene extracted from the essential oil of the genus Mentha of the Lamiaceae family, suppresses hippocampal neuronal excitation and epileptic activity by enhancing GABAergic inhibition37. Menthol also enhances GABAAR-mediated currents in midbrain periaqueductal grey neurons36, suggesting a broader spectrum of GABAAR-related pharmacotherapy in future, using menthol and related compounds. Interestingly, menthol has an alike general anaesthetic activity and similar sites of action on the GABAARs to the intravenous agent propofol (2,6-di-isopropylphenol), but not to benzodiazepines, steroids or barbiturates34. Curcumol38 [(3 S,5 S,6 S,8aS)-3-methyl-8-methylidene-5-(propan-2-yl)octahydro-6H-3a,6-epoxyazulen-6-ol] is a sesquiterpene compound and a major bioactive component of Rhizoma Curcumae oil. Notably, it induces minimal activation of GABAARs on its own, but facilitates the GABA-activated current in hippocampal neurons and cell lines, which express endogenous and recombinant GABAARs33, respectively. As a result, curcumol suppresses basal and epileptic activity in animals33, strengthening its pharmacological efficacy as a novel allosteric GABAAR modulator. However, the molecular mechanisms underlying curcumol modulation on GABAARs remain to be established. By comparing the electrophysiological effects of curcumol with other known modulators, and performing mutagenesis analysis on recombinant GABAARs, here we identify that curcumol as an allosteric modulator of GABAARs in a manner distinct from benzodiazepines, but through sites shared with menthol.
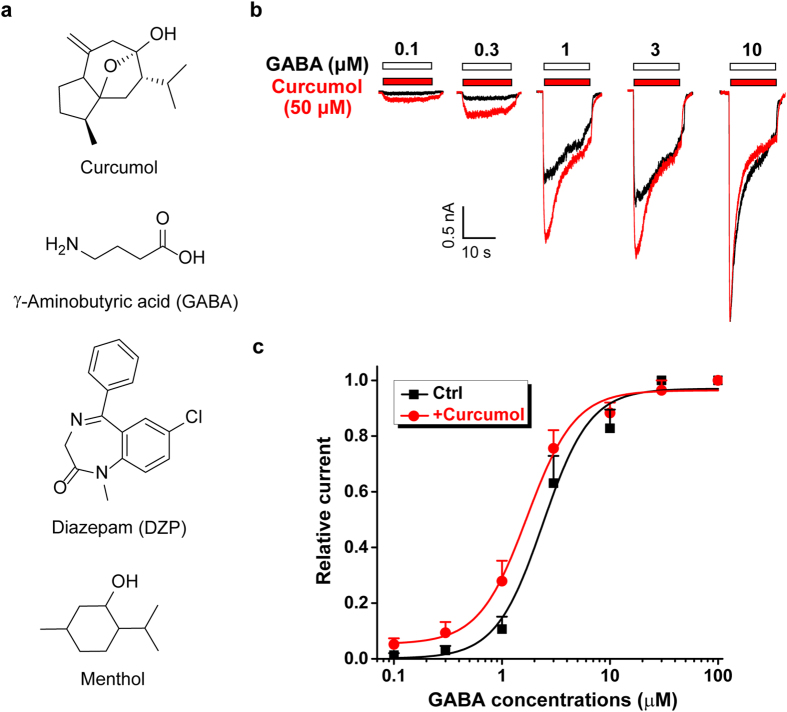
Figure 1. Modulation of GABA response by curcumol in cultured hippocampal neurons.
(a) Chemical structures of curcumol and other GABAAR ligands or modulators used in the present study. (b) Representative traces showing the currents evoked by different concentrations of GABA (black) alone, or curcumol (50 μM) plus various concentrations of GABA (red) as indicated. (c) Concentration–response curves of GABA for currents evoked in the absence (black squares) or presence (red circles) of 50 μM curcumol. Current amplitudes were normalized to the maximal response. These values were derived from previously published data33, regraphed here in a different way to assess the effect of curcumol on GABA concentration-response curve. The EC50 and Hill coefficient values were 2.4 ± 0.4 μM, 2.0 ± 0.6 without curcumol and 1.7 ± 0.2 μM, 1.9 ± 0.3 with curcumol, respectively. n = 6 each group.
Results
Characterization of curcumol on the GABA concentration-response curve in hippocampal neurons
A previous study33 showed that curcumol (Fig. 1a), a bioactive component of Rhizoma Curcumae oil39,40,41, enhanced GABA response in a concentration-dependent manner. In that study33, we established that at the agonist (i.e. GABA) concentration of 1 μM, curcumol facilitated the GABA-induced current with an EC50 of 34.4 ± 2.9 μM. To make an obvious and significant effect of curcumol on GABAARs, we chose 50 μM as the effective concentration in the present study.
We assessed the effects of curcumol on GABA concentration-response curve in hippocampal neurons by re-examination of the effect of 50 μM curcumol on the currents induced by a wide range of GABA concentrations shown in the previous study33. In contrast to the previous purpose to identify the operational range of GABA concentrations by curcumol33, here we perform data re-analysis to generate the concentration-response curves of GABA in the absence and presence of curcumol. As shown in Fig. 1b,c, the concentration-response curves to GABA were shifted to the left by curcumol. The EC50 (the agonist concentration that induces the half-maximal response) values in the absence and presence of curcumol were 2.4 ± 0.4 μM and 1.7 ± 0.2 μM, respectively. Mechanistically, the 1 μM GABA used in the following study falls an approximate EC10 and EC30 (the agonist concentrations that give rise to the 10 and 30% of maximal response, respectively) concentration of GABA, in the absence and presence of curcumol, respectively (Fig. 1c). Meanwhile, the Hill coefficients in the absence or presence of curcumol were 2.0 ± 0.6 and 1.9 ± 0.3, respectively. This increase of the apparent affinity to GABA implies a potentially allosteric regulation by curcumol of GABA-mediated GABAAR response; however, the precise mechanisms underlying the action of curcumol on GABAARs remain not fully understood.
Interplay of curcumol and diazepam on GABA-activated currents in hippocampal neurons
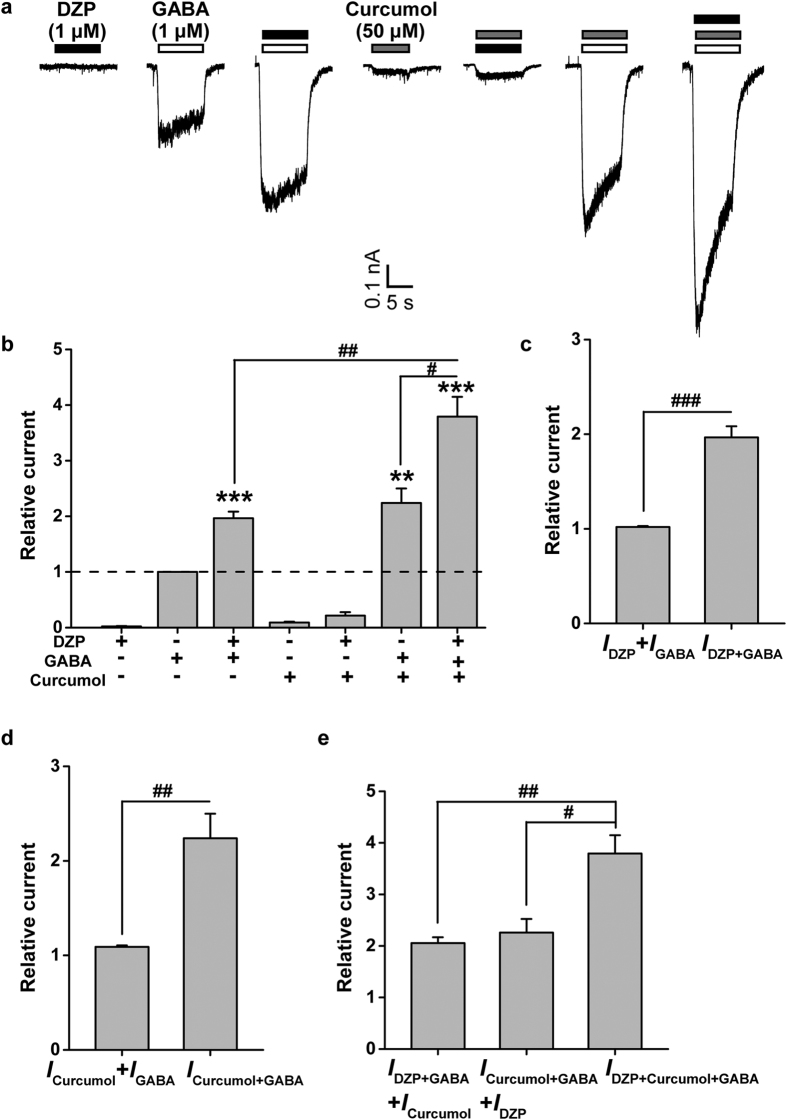
To decipher the underlying mechanisms of curcumol on GABAARs, we sought to determine the potential interaction between curcumol and other known GABAAR modulators, such as the classical benzodiazepine, diazepam (DZP, Fig. 1a). Cultured hippocampal neurons were exposed to GABA, DZP, and curcumol, alone or combination with each other (Fig. 2). DZP (1 μM) alone induced negligible inward currents but significantly potentiated GABA (1 μM)-evoked currents (Fig. 2a,b), consistent with its allosteric modulatory nature7. Likewise, curcumol (50 μM) produced minimal currents on its own but substantially enhanced GABA (1 μM)-induced currents (Fig. 2a,b), consistent with the previous observation33. We also compared the enhancement of GABA-activated currents by DZP or curcumol (i.e. IDZP+GABA and ICurcumol+GABA, respectively) with the sum of the independent currents induced by GABA (IGABA) and DZP (IDZP) or curcumol (ICurcumol), and found that the potentiation of GABA-mediated currents by DZP or curcumol was more than additive (Fig. 2c,d). This confirmed that curcumol, like DZP, allosterically potentiates the GABAAR activation in hippocampal neurons.
Figure 2. Interplay between diazepam (DZP) and curcumol on GABA-induced currents in cultured hippocampal neurons.
(a) Representative traces of GABA (1 μM)-induced currents in the absence or presence of DZP (1 μM) or curcumol (50 μM). (b) Pooled data from (a). (c–e) Histograms showing relative IDZP, IGABA, IDZP+GABA, ICurcumol, ICurcumol+GABA, and IDZP+Curcumol+GABA. ICurcumol, curcumol-activated current; ICurcumol+GABA, current activated by curcumol and GABA; IDZP, diazepam-activated current; IDZP+GABA, current activated by DZP and GABA; IDZP+Curcumol+GABA, current activated by DZP, curcumol and GABA; IGABA, GABA-activated current. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 6 each group. **P < 0.01, ***P < 0.001, compared with the current induced by GABA alone (dashed line); #P < 0.05, ##P < 0.01, ###P < 0.001, compared as indicated, paired Student’s t-test.
Interestingly, curcumol further increased the current induced by the combination of GABA and DZP (Fig. 2a,b), and the increase (IDZP+Curcumol+GABA) was more than additive (IDZP+GABA + ICurcumol; Fig. 2e), supporting the notion that curcumol causes an additional enhancement of the DZP-potentiated GABAAR activation. Consistent with this, DZP also led to a further increase in the current induced by the combination of GABA and curcumol (Fig. 2a,b), and the increase (IDZP+Curcumol+GABA) was more than additive (ICurcumol+GABA + IDZP; Fig. 2e). Thus, GABA, DZP, and curcumol act together to facilitate the GABAAR activation in hippocampal neurons. This suggests that curcumol, as a positive allosteric modulator of GABAARs, likely acts at a site distinct from the benzodiazepine-binding site.
Interplay between curcumol and menthol on GABA-activated currents in hippocampal neurons
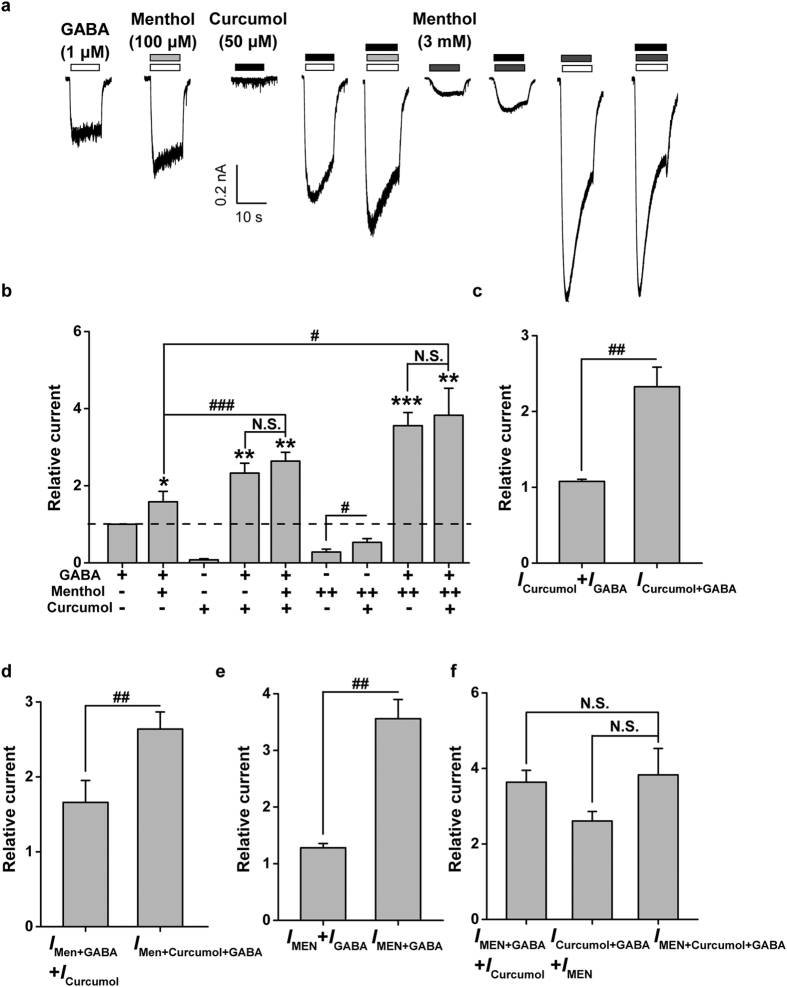
To understand in more depth molecular mechanisms underlying curcumol modulation of GABAARs, we further investigated the interplay of curcumol and menthol34,37, both belonging to terpenoid compounds carrying hydroxyl groups (Fig. 1a). Menthol at lower concentrations (up to 100 μM) did not activate a tangible inward current (IMen = 0; data not shown), but significantly potentiated GABA (1 μM)-evoked currents (Fig. 3a,b), consistent with the previous observation37. Similarly, in an independent set of experiments from that shown in Fig. 2, curcumol (50 μM) significantly enhanced the GABA (1 μM)-induced currents (Fig. 3a,b), and the compound current (ICurcumol+GABA) was more than additive (ICurcumol + IGABA; Fig. 3c). Interestingly, curcumol-mediated enhancement (ICurcumol+GABA) occluded the further action of menthol (100 μM) (IMen+Curcumol+GABA; Fig. 3a,b), with menthol unable to improve the current (IMen+Curcumol+GABA) to more than that induced by GABA and curcumol (ICurcumol+GABA). Conversely, the compound current (IMen+GABA+Curcumol) amplitude to the combination of GABA, curcumol, and menthol (100 μM) was much higher than that of GABA and menthol (IMen+GABA) (Fig. 3a,b) and, again, more than additive (IMen+GABA + ICurcumol; Fig. 3d). These observations, in contrast to the non-overlapping effects between curcumol and DZP (1 μM) (Fig. 2), raise the possibility that curcumol has a similar mechanism to menthol but not DZP, and that curcumol holds a much higher efficacy than menthol (100 μM). Curcumol would thereby occlude further action of menthol, but would have no similar effects on the modulation by DZP at GABAARs (Fig. 2).
Figure 3. Interplay between menthol and curcumol on GABA-induced currents in cultured hippocampal neurons.
(a) Representative current traces induced by GABA (1 μM) in the absence or presence of menthol (100 μM or 3 mM) or curcumol (50 μM). (b) Pooled data from (a). Menthol: (+), 100 μM; (++), 3 mM. (c–f) Histograms showing relative ICurcumol, IGABA, ICurcumol+GABA, IMen+GABA, IMen+Curcumol+GABA, IMEN, IMEN+GABA, and IMEN+Curcumol+GABA. ICurcumol, curcumol-activated current; ICurcumol+GABA, curcumol plus GABA-activated current; IGABA, GABA-activated current; IMen+GABA, menthol (100 μM) plus GABA-activated current; IMen+Curcumol+GABA, menthol (100 μM), curcumol, plus GABA-activated current; IMEN, menthol (3 mM)-activated current; IMEN+GABA, menthol (3 mM) plus GABA-activated current; IMEN+Curcumol+GABA, menthol (3 mM), curcumol, plus GABA-activated current. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 5 each group. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the current induced by GABA alone (dashed line); N.S., not significant, #P < 0.05, ##P < 0.01, ###P < 0.001, compared as indicated, paired Student’s t-test.
To characterize the interplay between curcumol and menthol more comprehensively, we increased the concentration of menthol up to 3 mM. Menthol (3 mM) alone activated a significant inward current (Fig. 3a, referred to as IMEN) that was blocked by a selective GABAAR inhibitor, bicuculline methiodide (1 μM), (data not shown)37, and enhanced by curcumol (Fig. 3a,b). Moreover, co-application of menthol (3 mM) and GABA enhanced GABAAR activation (Fig. 3a,b) in a more than additive manner (IMEN+GABA > IMEN + IGABA, Fig. 3e). In the simultaneous presence of curcumol and menthol (3 mM) with GABA, although the overall current (IMEN+Curcumol+GABA) was significantly greater than that induced by GABA and curcumol (ICurcumol+GABA), there was no difference between IMEN+Curcumol+GABA and IMEN+GABA (Fig. 3a,b). This shows that curcumol did not further increase the current induced by GABA and menthol (3 mM) together. In addition, the overall current induced by GABA, curcumol, and menthol (3 mM) did not differ from the sum of IMEN+GABA + ICurcumol, or ICurcumol+GABA + IMEN (Fig. 3f). Namely, menthol at higher concentrations saturates an allosteric site for GABAAR modulation and more likely precludes further action by curcumol. This suggests that curcumol and menthol share similar binding sites on GABAARs for modulation.
Actions of GABA-activated currents by curcumol and menthol, but not DZP, are resistant to benzodiazepine antagonist in hippocampal neurons
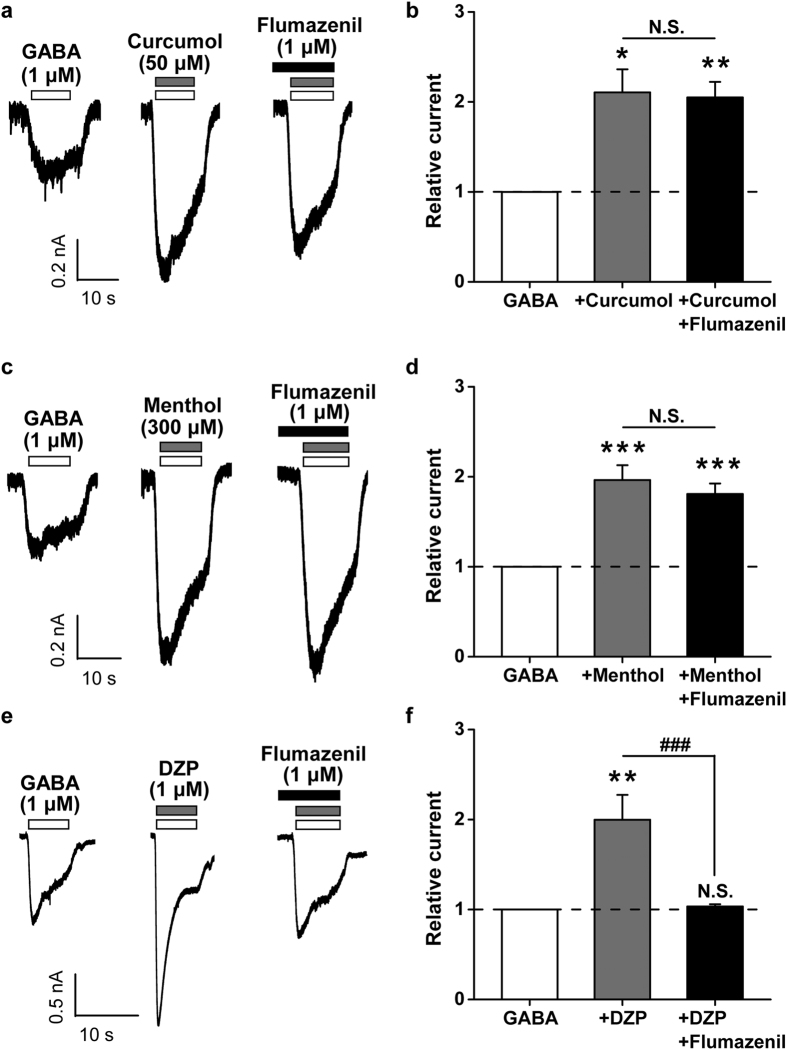
To underline the differential interplay between curcumol and menthol or DZP, we then examined whether actions of the above compounds were differentially affected by flumazenil (1 μM), a benzodiazepine antagonist. When flumazenil (1 μM) was coapplied with curcumol and GABA (Fig. 4a), curcumol still enhanced the GABA-induced current to a comparable extent (210.6 ± 25.7% vs. 204.9 ± 17.4% of GABA-induced currents by curcumol in the absence and presence of flumazenil, respectively, n = 5–6 per group, P > 0.05, Fig. 4b). Likewise, the effect of menthol was also not altered by flumazenil (196.3 ± 16.5% vs. 180.8 ± 11.7% of GABA-induced currents by menthol in the absence and presence of flumazenil, respectively, n = 10–13 per group, P > 0.05, Fig. 4c,d), which was consistent the previous study performed on Xenopus oocytes expressing the α1-β2-γ2 subtype of GABAAR34. By contrast, in the presence of flumazenil, DZP failed to enhance the GABA-induced current in hippocampal neurons (199.8 ± 27.6% vs. 103.4 ± 2.5% of GABA-induced currents by DZP in the absence and presence of flumazenil, respectively, n = 10 per group, P < 0.01, Fig. 4e,f), verifying flumazenil as a benzodiazepine antagonist. Together, these results strengthen the notion that curcumol and menthol do not share sites of action with benzodiazepines on GABAARs.
Figure 4. Effects of flumazenil on the modulation of GABA response by curcumol, menthol, or DZP in cultured hippocampal neurons.
(a, c, e) Representative current traces induced by GABA (1 μM) alone, or in the absence or presence of curcumol (100 μM, a), or menthol (300 μM, c), or DZP (1 μM, e), or in the simultaneous presence of flumazenil (1 μM). (b, d, f) Pooled data from (a), (c) and (e), respectively. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 5–13 each group. N.S., not significant, *P < 0.05, **P < 0.01, ***P < 0.001, compared with GABA (1 μM) alone (dashed line), paired Student’s t-test; N.S., not significant, ###P < 0.001, compared as indicated, unpaired Student’s t-test.
Curcumol shares site of action with menthol, but not DZP, on the α1-β2-γ2 subtype of GABAAR
To investigate binding sites for the modulatory action of curcumol over other known modulators on the GABAARs (Fig. 1a), we turned to confirm the effects of curcumol, menthol, and DZP on recombinant GABAARs expressed in HEK-293T cells. As the α1-β2-γ2 subtype constitutes the largest proportion (~60%) of GABAARs in the brain4,7 and is primarily responsible for phasic GABAergic inhibition in hippocampal CA1 pyramidal neurons, we therefore used this subtype firstly to examine the actions by different modulators. Curcumol (50 μM), or menthol (300 μM), or DZP (1 μM) each significantly enhanced currents induced by GABA (1 μM) on HEK-293T cells expressing wild-type (WT) α1-β2-γ2 GABAARs (Fig. 5a,b). This was analogous with the observation on the cultured hippocampal neurons shown above (Figs 1, 2, 3, 4), and consistent with previous reports on the α1-β2-γ2 subtype of GABAARs expressed in various recombinant systems8,33,34. These results therefore lay a foundation on which to examine the specific site(s) responsible for the modulation of GABAARs by curcumol and other modulators.
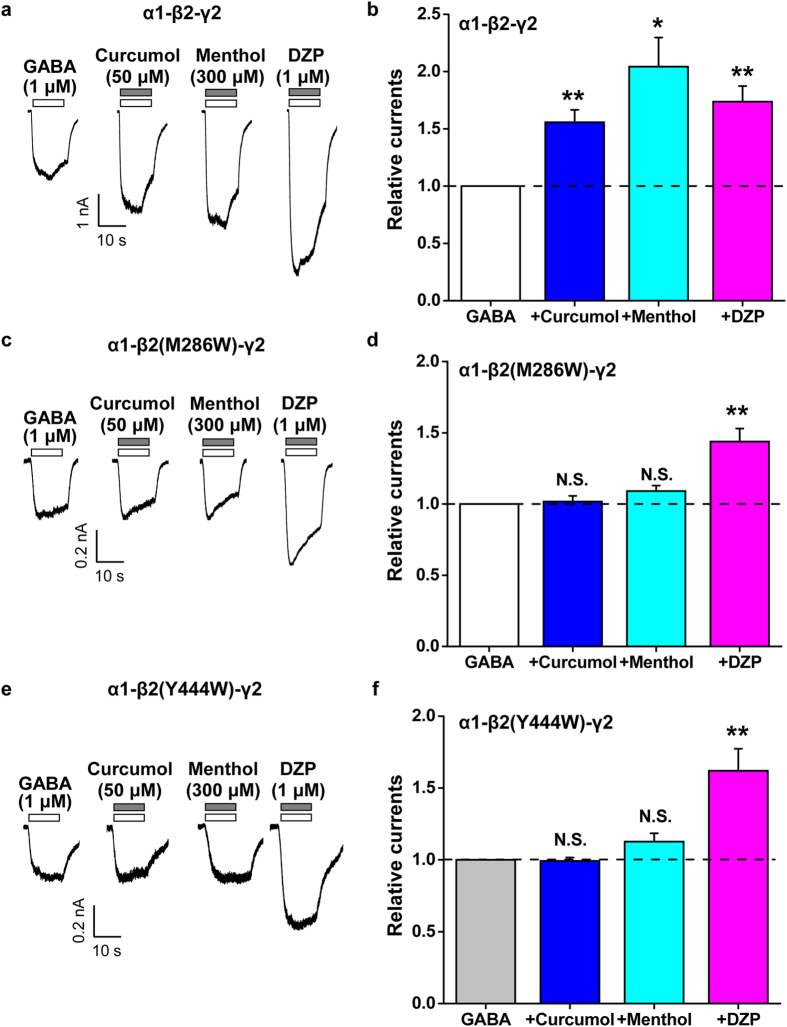
Figure 5. Effects of point mutations in β2 subunit of GABAAR on the modulation of α1-β2-γ2 GABAAR subtype by curcumol, menthol, or DZP.
(a, c, e) Representative current traces induced by GABA (1 μM) in the absence or presence of curcumol (50 μM), menthol (300 μM), or DZP (1 μM) in HEK-293T cells that expressed α1, β2, β2-M286W, or β2-Y444W, and γ2 GABAAR subunits. (b, d, f) Pooled data from (a), (c) and (e), respectively. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 3–8 each group. N.S., not significant, *P < 0.05, **P < 0.01, compared with the current induced by GABA alone (dashed line), paired Student’s t-test.
It has been established that a methionine residue at amino acid position 286 [transmembrane domain (TM) 3] and a tyrosine residue at position 444 (TM4) at the β2 subunit are important for the anaesthetic actions18,19,20,21,22,23,24,25,26, including menthol34, but not benzodiazepines, on the α1-β2-γ2 subtype of GABAAR. Mutations at either one of these residues to a tryptophan (i.e. M286W or Y444W) both selectively abolished menthol-mediated enhancement of GABAAR function. Given the structural similarity between curcumol and menthol (both are terpenoid compounds carrying hydroxyl groups; Fig. 1a), in addition to previous identification of the interplay between curcumol and menthol over DZP (Figs 2 and 3), we expected that these sites important for menthol would also be essential for the curcumol action. To investigate this, we exposed these modulators (Fig. 1a) to HEK-293T cells expressing mutant [α1-β2(M286W)-γ2 or α1-β2(Y444W)-γ2] GABAARs. Previous studies suggested that the GABA concentration–response relationships (i.e. the agonist concentration that induces the half-maximal response, EC50 and Hill coefficient) for both mutant receptors are similar to those for the WT GABAAR19,20,34. Therefore, GABA (1 μM) was also used to screen for modulation by curcumol (50 μM), menthol (300 μM), and DZP (1 μM). We found no enhancement of either type of mutant receptor current by menthol (Fig. 5c–f), consistent with the previous study in Xenopus oocytes expressing these mutant receptors34. Notably, the modulation by curcumol was also abolished by inclusion of the mutations in the β2 subunits (Fig. 5c–f). By contrast, the enhancement of mutant β2-M286W or β2-Y444W currents by DZP (Fig. 5c–f) was not significantly different from the WT α1-β2-γ2 GABAAR (173.7 ± 13.6%, 143.8 ± 9.2%, and 162.0 ± 15.3% of GABA-induced currents by DZP on the WT, β2-M286W, and β2-Y444W GABAARs, respectively, n = 4–6 per group, P > 0.05 vs. WT). These results were comparable with the previous report studied in Xenopus oocytes34, which showed that flunitrazepam, another type of benzodiazepine, also reserved its allosterically modulatory effect. The lack of mutation effects on these sites to benzodiazepines34 (Fig. 5c-f) agrees with a previous study showing that the α subunit adjacent to the γ2 subunit determines the sensitivity to benzodiazepines in the recombinant receptors8. Together, these results collectively point to a notion that curcumol is an allosteric modulator for GABAARs in a manner distinct from benzodiazepines.
Variant mechanisms underlying actions of curcumol over menthol or DZP on the α5-β2-γ2 subtype of GABAAR
Next, we extended the mechanistic study of curcumol over menthol or DZP to another GABAAR subtypes. While the α1-containing GABAARs primarily govern the phasic GABAergic inhibition4,42, the α5-containing are the major isoforms underlying tonic inhibition43,44,45 in hippocampal neurons. Accordingly, the effects of curcumol over menthol or DZP were examined on the HEK-293T cells expressing either WT or mutant α5-β2-γ2 GABAARs. As expected, curcumol (50 μM), or menthol (300 μM), or DZP (1 μM) each significantly potentiated the currents induced by GABA (1 μM) on HEK-293T cells expressing WT α5-β2-γ2 GABAARs (Fig. 6a,b), all of which are similar with the α1-β2-γ2 subtype (Fig. 5a,b).
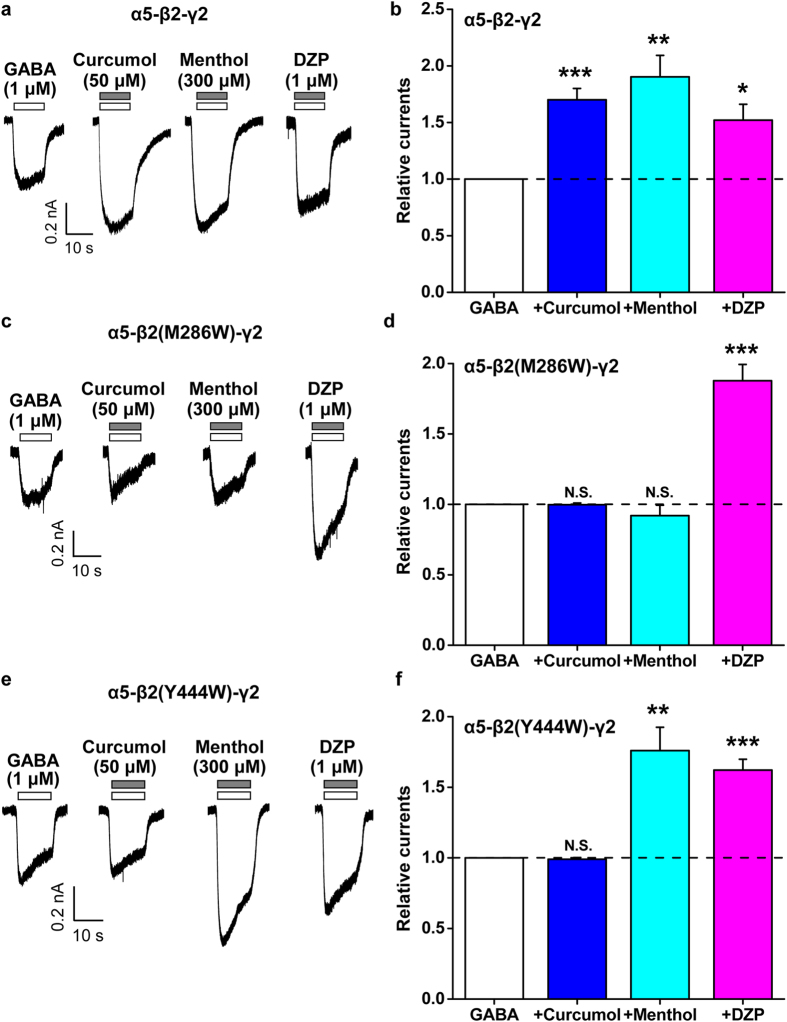
Figure 6. Effects of point mutations in β2 subunit of GABAAR on the modulation of α5-β2-γ2 GABAAR subtype by curcumol, menthol, or DZP.
(a, c, e) Representative current traces induced by GABA (1 μM) in the absence or presence of curcumol (50 μM), menthol (300 μM), or DZP (1 μM) in HEK-293T cells that expressed α5, β2, β2-M286W, or β2-Y444W, and γ2 GABAAR subunits. (b, d, f) Pooled data from (a), (c) and (e), respectively. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 6–8 each group. N.S., not significant, *P < 0.05, **P < 0.01, ***P < 0.001, compared with the current induced by GABA alone (dashed line), paired Student’s t-test.
Then, we exposed curcumol, menthol, and DZP, respectively, to HEK-293T cells expressing the mutant [α5-β2(M286W)-γ2 or α5-β2(Y444W)-γ2] GABAARs. In line with the α1-β2-γ2 subtype of GABAAR (Fig. 5c–f), the modulation by curcumol was also abolished by inclusion of either the M286W (Fig. 6c,d) or Y444W (Fig. 6e,f) mutations in the β2 subunit of the α5-β2-γ2 subtype of GABAAR. Interestingly, the enhancement of the α5-β2-γ2 GABAAR response by menthol was eliminated in the β2-M286W (Fig. 6c,d), but not β2-Y444W (Fig. 6e,f)-containing receptors. As expected, the enhancement of GABA-induced currents in β2-M286W or β2-Y444W mutants by DZP (Fig. 5c–f) was not significantly different from the WT α5-β2-γ2 GABAAR (152.1 ± 13.9%, 187.8 ± 11.5%, and 176.0 ± 16.5% of GABA-induced currents by DZP on the WT, β2-M286W, and β2-Y444W GABAARs, respectively, n = 4–8 per group, P > 0.05 vs. WT). The differential responsivities to curcumol over menthol or DZP in the α5-β2-γ2 GABAAR mutants bring up variant mechanisms underlying the actions of these modulators.
A mutation in γ2 subunit of GABAAR resistant to benzodiazepine preserves the actions of curcumol and menthol
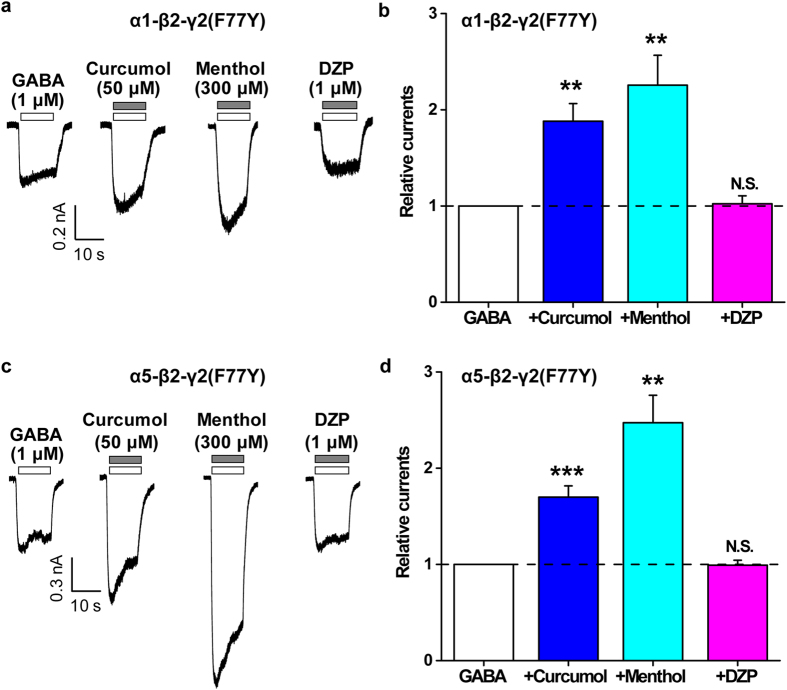
Finally, to underpin the differential mechanisms conferring the modulatory actions of curcumol over menthol or DZP (Fig. 1a), we then examined the effects of these modulators on the mutant GABAARs resistant to benzodiazepine modulation. It has been established that a phenylalanine at position 77 in the γ2 subunit is essential for the binding of benzodiazepine and the resultant regulation of GABAARs6,46. Consistent with the previous report studied in Xenopus oocytes46, inclusion of the F77Y mutation (Phe → Tyr) in the γ2 subunit indeed abolished the enhancement of GABA-induced currents by DZP (1 μM) in α1-containing GABAARs (Fig. 7a,b). Similarly, the α5-β2-γ2(F77Y) GABAAR also became insensitive to DZP (Fig. 7c,d). Notably, the effect of curcumol was completely preserved (α1-containing: 155.7 ± 10.9% and 188.1 ± 18.3% of GABA-induced currents by curcumol on the WT and γ2-F77Y GABAARs, respectively, n = 4–5 per group, P > 0.05, Figs 5b and 7b; α5-containing: 170.1 ± 10.0% and 169.9 ± 11.7% of GABA-induced currents by curcumol on the WT and γ2-F77Y GABAARs, respectively, n = 7–8 per group, P > 0.05, Figs 6b and 7d). Similarly, the effect of menthol on the GABA-induced response was also largely retained (α1-containing: 204.3 ± 25.4% and 225.5 ± 31.1% of GABA-induced currents by menthol on the WT and γ2-F77Y GABAARs, respectively, n = 3–4 per group, P > 0.05, Figs 5b and 7b; α5-containing: 190.3 ± 18.9% and 247.3 ± 28.5% of GABA-induced currents by menthol on the WT and γ2-F77Y GABAARs, respectively, n = 5–8 per group, P > 0.05, Figs 6b and 7d). Thus, the mutation in the γ2 subunit of GABAAR resistant to benzodiazepine by no means affect the actions of curcumol and menthol. In summary, our results collectively establish the notion that curcumol exerts its facilitatory actions on GABAARs at sites distinct from benzodiazepine sites (Fig. 8).
Figure 7. Effects of point mutations in γ2 subunit of GABAAR on the modulation of α1-β2-γ2 or α5-β2-γ2 GABAAR by curcumol, menthol, or DZP.
(a, c) Representative current traces induced by GABA (1 μM) in the absence or presence of curcumol (50 μM), menthol (300 μM), or DZP (1 μM) in HEK-293T cells that expressed α1 or α5, β2, and γ2-F77Y GABAAR subunits. (b, d) Pooled data from (a) and (c), respectively. Data represent peak current amplitude normalized to that induced by GABA (1 μM) alone (dashed line). n = 4–8 each group. N.S., not significant, **P < 0.01, ***P < 0.001, compared with the current induced by GABA alone (dashed line), paired Student’s t-test.
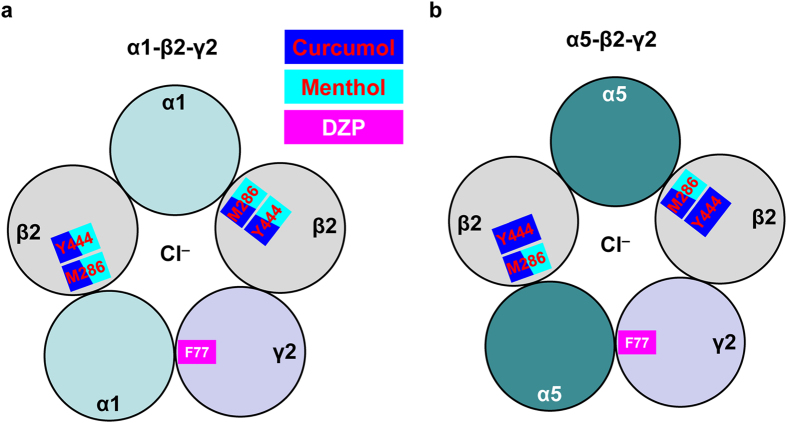
Figure 8. A hypothetical scheme for the modulation of GABA(A) receptors by curcumol, menthol, or DZP through different mechanisms.
(a) For the α1-β2-γ2 GABAAR, while curcumol and menthol but not DZP act the receptor through the sites of Met-286 (M286) and Tyr-444 (Y444) in the β2 subunit, DZP but not curcumol nor menthol acts the receptor through Phe-77 (F77) in the γ2 subunit. (b) For the α5-β2-γ2 GABAAR, while curcumol acts the receptor through the sites of M286 and Y444, menthol acts the receptor through the site of M286 but not that of M444 in the β2 subunit, DZP but not curcumol nor menthol acts the receptor through Phe-77 (F77) in the γ2 subunit. Please see the text for more details.
Discussion
In the present study, we have shown that curcumol (Fig. 1a), a natural compound and major bioactive component of Rhizoma Curcumae oil, acts as an allosteric modulator of GABAARs (Fig. 1b,c) in a manner different from that of the classical benzodiazepines. Curcumol significantly potentiated the GABAAR activation in neurons in a way that did not overlap with modulation by DZP, a well-characterized benzodiazepine, but acted together with DZP to enhance receptor function (Fig. 2). By contrast, curcumol occluded the effects of menthol, another type of GABAAR modulator, at the concentration of 100 μM, and was occluded by this compound at the concentration up to 3 mM, indicative of a shared binding site between curcumol and menthol (Fig. 3). Moreover, the benzodiazepine antagonist flumazenil had no impact on the enhancements of GABA response by curcumol and menthol, but abolished that by DZP (Fig. 4). Finally, while single mutations (M286W or Y444W) in the β2 subunit abolished the effects of curcumol and menthol, but not DZP (Figs 5 and 6), single mutation (F77Y) in the GABAAR γ2 subunit abolished the effects of DZP, but not curcumol nor menthol (Fig. 7). Curcumol therefore exerts its actions on GABAARs at sites distinct from those of benzodiazepines (Fig. 8). These findings shed more light on the modulation of GABAARs and could guide the development of new drugs targeting this receptor.
In line with the multifaceted physiological and pathophysiological roles of GABAARs in the central nervous system, the pharmacology9,47 and the drug development48 on these receptors have also advanced considerably in recent decades. In addition to the natural agonist GABA5, positive GABAAR modulators include benzodiazepines6,7, barbiturates16, steroids17, and anaesthetics18,19,20,21,22,23,24,25,26, each of which has specific binding sites on GABAARs. Several lines of evidence from the present study support that curcumol shares mechanisms with anaesthetics in the allosteric modulation of GABAARs. First, although curcumol and DZP enhanced each other’s allosteric modulation (Fig. 2), curcumol and menthol reciprocally and concentration-dependently occluded each other’s effects (Fig. 3), suggesting that curcumol acts on GABAARs via a mechanism different from that of benzodiazepines, but similar to that of menthol. Second, menthol and curcumol are both terpenoid compounds (monoterpene and sesquiterpene, respectively) with a functional hydroxyl group (Fig. 1a), a characteristic stereochemical configuration that differs from that of DZP, providing the structural basis of ligands for curcumol action independent of benzodiazepine binding sites. It is noteworthy that the structure–effect relationship of menthol indicates the importance of the hydroxyl group in these ligands34,37. Likewise, curdione [(3 S,6E,10 S)-6,10-dimethyl-3-propan-2-ylcyclodec-6-ene-1,4-dione], an analogue of curcumol, predominantly lacks the hydroxyl group and exhibits greatly reduced potency at the GABAAR33. Third, mutagenesis analysis of the GABAAR demonstrated that the TM3 and TM4 regions in the β2 subunits are important for the potentiating effects of curcumol and menthol, but not DZP. Together with a previous study34 showing that menthol shares general anaesthetic activity and GABAAR site of action with the intravenous agent propofol, but not with benzodiazepines, steroids or barbiturates, we determined that curcumol likely represents a new member of the anaesthetic family for allosteric modulation of GABAARs.
Belonging to the non-classical anaesthetic subclass of GABAAR modulators, curcumol not only shares an obvious chemical scaffold with menthol and propofol, but also contains new information about the structure–activity relationship for this particular form of GABAAR pharmacology18,19,20,21,22,23,24,25,26. As discussed earlier, the hydroxyl group in these compounds33,34,37 is essential for the positive modulation of GABAARs. The ortho positioning of an aliphatic chain is also a prerequisite for the activity of propofol or menthol analogues, including both the allosteric modulation19,34,49 and direct activation of GABAARs50. Accordingly, curcumol shares equivalent positioning of an isopropyl adjacent to their respective hydroxyl groups (Fig. 1a), which likely plays a major part in the interaction with GABAARs. Notably, curcumol preferentially enhances receptor function, which is different from propofol and menthol that hold both efficacies of allosterically enhancing and directly activating GABAARs. In the present study, together with the previous report33, curcumol at the concentrations even up to its water solubility limit (~300 μM)38 induced only minimal direct activation of GABAARs. Moreover, curcumol was more potent than menthol, but probably less than propofol19,34. Curcumol (50 μM) could significantly occlude the action of menthol (100 μM, Fig. 3). These pharmacological efficacy differences would be ascribed to the backbone structure of these compounds: propofol is a phenol (pKa 11.0, planar ring structure) and menthol is a neutral cyclohexanol (chair structure), but curcumol is an epoxy azulen (a more complex structure). A better understanding of the structure–function relationship of curcumol interaction with GABAARs will aid the design of new drugs with higher efficacy and specificity for GABAARs.
Curcumol does not always run parallel with menthol on the modulation of GABAARs (Fig. 8). In the α5-β2(Y444W)-γ2 mutated GABAARs, while the action of curcumol was eliminated, that of menthol kept intact (Fig. 6e,f). These effects were α subunit specific, as in the α1-β2(Y444W)-γ2 mutated GABAARs, the actions of curcumol and menthol were both abolished (Fig. 5e,f). In addition, these effects were dependent on the specific residue(s) in the β2 subunit. In the β2-M286W mutated GABAARs, both α1- (Fig. 5c,d) and α5-containing subtypes (Fig. 6c,d) became unresponsive to curcumol in addition to menthol. The more consensus involvement of β2-M286 residue located at TM3 region in the GABAAR modulation implies a more direct role of this site19,34 in conferring the anaesthetic modulation of GABAARs19,34. This is also reminiscent of an observation that GABA-induced inter-subunit conformational movements in the α1-ΤM1-β2-ΤM3 transmembrane subunit interface are necessary to gate the GABAAR channels21,25. Of note, the β2-Y444 residue located at TM4 region is also important for anaesthetic modulation20,34, of which the dynamic structural arrangements15,25 are still being actively investigated. It is definitely meaningful to further dissect these subtle variances, including the possibility that different subunit interfaces are being used in the α1-β2-γ2 and α5-β2-γ2 GABAARs for anaesthetic modulation, which would be helpful for identification of receptor subtype-selective compounds for drug development in the future. In fact, many compounds, including propofol, etomidate, avermectin, and many others have been reported to mediate their effects through the same anaesthetic site15. Not only, multiple propofol-binding sites18,19,20,21,22,23,24,25,26,51 have also been identified. Nevertheless, the present identification of curcumol working in a similar way to menthol through acting at anaesthetic sites distinct from the benzodiazepine site will inspire more structural and functional studies using this novel compound.
Curcumol preferentially enhances GABA-induced GABAAR activation, its prominent feature over other known anaesthetic modulators (i.e. propofol and menthol). However, it is unlikely to open the chloride channel considerably in the absence of GABA, which gives this compound its intriguing potential to be an ideal candidate GABAAR drug. This self-limiting property of curcumol for GABAAR modulation is also reminiscent of the widely-prescribed benzodiazepines in current therapeutic use. In contrast to barbiturates, benzodiazepines6,7 do not directly activate GABAARs in the absence of GABA (Fig. 2). Nevertheless, the clinical use of benzodiazepines is currently limited because their various pharmacological effects are not clearly separable by dosing. For instance, although the anxiolytic actions of benzodiazepines are observed at lower doses than their sedative actions, sedation is still a problem if benzodiazepines are used as daytime anxiolytics. Benzodiazepines also have addictive properties and are liable to be abused13,14, which limits their long-term use, and physical dependence and tolerance are areas of concern7. Considering this, curcumol holds a potential promise for the future development of novel GABAAR drugs. Importantly, curcumol not only potentiates GABA-induced GABAAR activation, but also amplifies the modulation of GABAARs in the presence of benzodiazepines (i.e. DZP) (Fig. 2). Therefore, as a non-classical anaesthetic modulator, curcumol and its derivatives might represent an alternative or supplementary strategy to alleviate or remove the side-effects that limit long-term and high-dose administration of benzodiazepines. However, the assumption remains under-developed yet, which needs to be carefully investigated in the future.
Curcumol is a natural compound isolated from Rhizoma Curcumae oil. Used alone or mixed in a specific type of traditional Chinese medicine, knowledge of its pharmacological effects on the central nervous system is increasing. Rhizoma Curcumae (rhizome of Curcuma; Ezhu) has been used as a condiment and home remedy in China for thousands of years, illustrating its lack of prominent toxicity in human. Rhizoma Curcumae oil has been suggested to possess pharmacological efficacy in a number of domains, including neuroprotection39, cognitive enhancement40, and anti-seizure efficacy41. Of the three main ingredients in Rhizoma Curcumae oil (curcumol, curcumin, and curdione), curcumol is the most potent GABAAR modulator, and probably confers, at least in part, the pharmacological effects reported above. Moreover, like most naturally derived substances, curcumol is lipophilic and readily crosses the blood–brain barrier52, with the maximal concentration of curcumol after intravenous injection of Rhizoma curcuma oil up to 108.85 ± 65.91, 92.38 ± 17.63 μg/g in the liver and brain, equivalent to 458.43 ± 278.87 and 390.86 ± 74.59 μM (both the densities of liver and brain tissue were assumed to be 1.0 g/ml), respectively. Using the radioactive [3H]-curcumol, a previous study53 demonstrated that curcumol can be rapidly and completely absorbed orally in rats; it emerged in the blood at 5 min and peaked at 15 min, respectively, after the oral administration. In addition, tissue distribution (including the penetration into the brain), drug stability and metabolism, expressing as the area under concentration time curve of curcumol, under oral administration all were comparable with that by intravenous injection53, supporting a more easily administration way for using this drug. Based on the pharmacokinetics of curcumol, together with the pharmacological effects on GABAARs, it is not surprising that curcumol is capable of targeting against the central nervous system to treat neurological diseases. Indeed, curcumol alone decreased basal locomotor activity and chemically induced seizure activity in mice33, confirming its effectiveness as a GABAAR modulator to target the central function. However, despite curcumol belonging to the anaesthetics class of GABAAR modulators, its anaesthetic effects remain unexplored. Of note, whether the long-term use of curcumol would produce dependence or tolerance, as with benzodiazepines, remains to be determined in the future studies. Nevertheless, the present study has contained new information about the pharmacological nature of curcumol on the central nervous system, and provides a primary basis for further in-depth studies regarding the pharmacological development of curcumol and its related drugs.
In summary, we have identified the natural compound curcumol as an allosteric modulator of GABAARs. Curcumol possesses an intriguing self-limiting efficacy at GABAARs, in addition to its mechanisms being similar to anaesthetics but independent on benzodiazepine binding sites. This work therefore suggests a novel approach to the development of drugs targeting GABAARs.
Methods
Animals
Animal procedures reported in the present study were approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine, Shanghai, China. All efforts were made to minimize animal suffering and to reduce the number of animals used. Mice were housed under standard laboratory conditions (12/12 h light/dark, temperature 22–26 °C, air humidity 55–60%) with food and water ad libitum. Animal procedures were carried out in accordance with the guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University School of Medicine, and approved by the Institutional Animal Care and Use Committee (Department of Laboratory Animal Science, Shanghai Jiao Tong University School of Medicine) (Policy Number DLAS-MP-ANIM. 01–05).
Cell culture
Primary cultures of mouse hippocampal neurons were prepared according to previously described techniques33. In brief, 15-day-old embryonic C57BL/6 J mice were anesthetized with halothane. Brains were removed rapidly and placed in ice-cold Ca2+- and Mg2+-free phosphate buffered solution. Tissues were dissected and incubated with 0.05% trypsin-EDTA for 10 min at 37 °C, followed by trituration with fire-polished glass pipettes, and plated on poly-D-lysine-coated 35 mm culture dishes at a density of 1 × 106 cells per dish. Neurons were cultured with Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen) and maintained at 37 °C in a humidified 5% CO2 atmosphere incubator. Cultures were fed twice a week and used for electrophysiological recording 10–20 days after plating. For neuron cultures, glial growth was suppressed by addition of 5-fluoro-2-deoxyuridine (20 μg/ml; Sigma-Aldrich) and uridine (20 μg/ml; Sigma-Aldrich).
Human embryonic kidney (HEK)-293T cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 1 mM L-glutamine, 10% foetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin (all from Invitrogen).
Site-directed mutagenesis
Mutations of receptor cDNA were generated with the QuikChange® mutagenesis kit (Stratagene, La Jolla, CA) in accordance with the manufacturer’s protocol using high-pressure-liquid-chromatography-purified or PAGE-purified oligonucleotide primers (Sigma-Genosys, The Woodlands, TX). All mutants were verified by DNA sequence analysis.
Functional expression of the recombinant GABAARs
The rat α1, β2, and γ2 subunit cDNA of GABAAR were obtained from Dr. Yu Τian Wang (University of British Columbia, Vancouver, BC, Canada). The rat α5 subunit cDNA was kindly provided by Dr. David H. Farb (Boston University School of Medicine, Boston, Massachusetts, USA). Transient transfection of HEK-293T cells was carried out using HilyMax liposome transfection reagent (Dojindo Laboratories). Cotransfection with a green fluorescent protein expression vector, pEGFP-C3, was used to enable identification of transfected cells for patch clamp recording by monitoring the fluorescence of green fluorescent protein. Electrophysiological measurements were performed 24–48 h after transfection.
Electrophysiology
Whole-cell recordings were made using an Axon 700A patch-clamp amplifier (Axon Instruments, Foster City, CA, USA). Membrane currents were sampled and analysed using a Digidata 1440 interface and a personal computer running Clampex and Clampfit software (Version 10, Axon Instruments). In voltage clamp mode, the membrane potential was held at −60 mV for whole-cell current recording. All electrophysiological experiments were carried out at room temperature (23 ± 2 °C).
The standard external solution contained (in mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 N-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES), and 10 glucose (pH 7.4 with Tris-base, 325–330 mOsm/L). The pipette solution was composed of (in mM): 120 KCl, 30 NaCl, 1 MgCl2, 0.5 CaCl2, 5 ethylene glycol tetraacetic acid (EGTA), 2 Mg-ATP, 10 HEPES, pH 7.2 adjusted with Tris-base.
Chemicals and drugs
The chemicals used in the present study curcumol [(3 S,5 S,6 S,8aS)-3-methyl-8-methylidene-5-(propan-2-yl)octahydro-6H-3a,6-epoxyazulen-6-ol], menthol [5-methyl-2-propan-2-ylcyclohexan-1-ol], and diazepam (DZP) [7-chloro-1-methyl-5-phenyl-3H-1,4-benzodiazepin-2-one] were purchased from Sigma-Aldrich (St. Louis, MO). Curcumol, menthol, and DZP were initially dissolved as concentrated stock solutions in dimethyl sulfoxide and subsequently diluted to the desired concentration in the standard external solution. The final concentration of dimethyl sulfoxide was lower than 0.1% and was confirmed to be ineffective alone at the same concentration in control experiments (data not shown). Other drugs were either first dissolved in deionized water and then diluted to a final concentration in standard external solution just before use or dissolved directly in the standard external solution. Drugs were applied using a rapid application technique termed the “Y-tube” method as described previously54,55,56. The tip of the drug tube was positioned 50–100 μM away from the patched cells. This system allows a complete exchange of external solution surrounding a cell within 20 ms. Throughout the experiment, the bath was superfused continuously with the standard external solution.
Data analysis
Values are expressed as the mean ± S.E.M. Groups are compared using Student’s t test. P < 0.05 was considered to be statistically significant. P and n represent the value of significance and the number of neurons or cells, respectively. Clampfit 10.5 (Molecular Devices) was used for data analysis. The smooth concentration-response curves of curcumol on facilitation of the GABA response in hippocampal neurons were drawn according to a modified Michaelis-Menten equation by the method of least squares (the Newton-Raphson method) after normalizing to the maximal GABA response: I = Imax × Ch/(Ch + EC50h), where I is the normalized value of the current, Imax is the maximal response, C is the drug concentration, EC50 is the concentration which induces the half-maximal response and h is the apparent Hill coefficient.
Additional Information
How to cite this article: Liu, Y.-M. et al. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci. Rep. 7, 46654; doi: 10.1038/srep46654 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank Dr. James J. Celentano for critical reading of the manuscript. We thank all groups that provided us with GABAAR cDNAs. We also thank Ms. Ying Li, Mr. Jin Wang, and Mr. Xing-Lei Song for their technical assistance. This study was supported by grants from the National Basic Research Program of China (2013CB835100 and 2014CB910300), the National Natural Science Foundation of China (81400870, 81500941, 81571031, and 91632304), the Shanghai Committee of Science and Technology (14DZ2272200 and 17XD1403200), the Shanghai Municipal Education Commission (Research Physician Project: 20152234).
Footnotes
The authors declare no competing financial interests.
Author Contributions T.L.X., W.H.G., W.G.L., and F.L. designed the project. Y.M.L., H.R.F., J.D., C.H., S.D., and T.Z. performed cell culture. Y.M.L., J.D., and C.H. carried out electrophysiological recordings. Y.M.L., J.D., and W.G.L. performed data analysis. Y.M.L., W.G.L., and F.L. wrote the manuscript. All authors read and approved the final manuscript.
References
- Rudolph U. & Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol 54, 483–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. & Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56, 141–148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. & Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60, 243–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan R. M. & Whiting P. J. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19, 139–143 (1996). [DOI] [PubMed] [Google Scholar]
- Lummis S. C. Locating GABA in GABA receptor binding sites. Biochem Soc Trans 37, 1343–1346, doi: 10.1042/BST0371343 (2009). [DOI] [PubMed] [Google Scholar]
- Sigel E. & Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18, 425–429 (1997). [DOI] [PubMed] [Google Scholar]
- Rudolph U. & Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10, 685–697 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minier F. & Sigel E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101, 7769–7774 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. The rise of a new GABA pharmacology. Neuropharmacology 60, 1042–1049 (2011). [DOI] [PubMed] [Google Scholar]
- Pritchett D. B. et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature 338, 582–585 (1989). [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Mohler H. & Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron 5, 703–711 (1990). [DOI] [PubMed] [Google Scholar]
- Gunther U. et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 92, 7749–7753 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. R. et al. Neural bases for addictive properties of benzodiazepines. Nature 463, 769–774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto E. GABA withdrawal syndrome: GABAA receptor, synapse, neurobiological implications and analogies with other abstinences. Neuroscience 313, 57–72 (2016). [DOI] [PubMed] [Google Scholar]
- Puthenkalam R. et al. Structural studies of GABAA receptor binding sites: which experimental structure tells us what? Front Mol Neurosci 9, 44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L. & Olsen R. W. GABAA receptor channels. Annu Rev Neurosci 17, 569–602 (1994). [DOI] [PubMed] [Google Scholar]
- Hosie A. M., Wilkins M. E., da Silva H. M. & Smart T. G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444, 486–489 (2006). [DOI] [PubMed] [Google Scholar]
- Mihic S. J. et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389, 385–389 (1997). [DOI] [PubMed] [Google Scholar]
- Krasowski M. D., Nishikawa K., Nikolaeva N., Lin A. & Harrison N. L. Methionine 286 in transmembrane domain 3 of the GABAA receptor beta subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology 41, 952–964 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. E. et al. A conserved tyrosine in the beta2 subunit M4 segment is a determinant of gamma-aminobutyric acid type A receptor sensitivity to propofol. Anesthesiology 107, 412–418 (2007). [DOI] [PubMed] [Google Scholar]
- Bali M., Jansen M. & Akabas M. H. GABA-induced intersubunit conformational movement in the GABAA receptor alpha 1M1-beta 2M3 transmembrane subunit interface: experimental basis for homology modeling of an intravenous anesthetic binding site. J Neurosci 29, 3083–3092 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nury H. et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 469, 428–431 (2011). [DOI] [PubMed] [Google Scholar]
- Yip G. M. et al. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol 9, 715–720 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L. et al. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4, 1697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakar S. S. et al. Multiple propofol-binding sites in a gamma-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog. J Biol Chem 289, 27456–27468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldifassi M. C., Baur R. & Sigel E. Functional sites involved in modulation of the GABAA receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology 105, 207–214 (2016). [DOI] [PubMed] [Google Scholar]
- Braat S. & Kooy R. F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86, 1119–1130 (2015). [DOI] [PubMed] [Google Scholar]
- Knabl J. et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451, 330–334 (2008). [DOI] [PubMed] [Google Scholar]
- Fernandez F. et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci 10, 411–413 (2007). [DOI] [PubMed] [Google Scholar]
- Wasowski C. & Marder M. Flavonoids as GABAA receptor ligands: the whole story? J Exp Pharmacol 4, 9–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. et al. alpha-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology 65, 1–11 (2013). [DOI] [PubMed] [Google Scholar]
- Zaugg J. et al. Positive GABA(A) receptor modulators from Acorus calamus and structural analysis of (+)-dioxosarcoguaiacol by 1D and 2D NMR and molecular modeling. J Nat Prod 74, 1437–1443 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J. et al. Curcumol from Rhizoma Curcumae suppresses epileptic seizure by facilitation of GABA(A) receptors. Neuropharmacology 81, 244–255 (2014). [DOI] [PubMed] [Google Scholar]
- Watt E. E. et al. Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. Eur J Pharmacol 590, 120–126 (2008). [DOI] [PubMed] [Google Scholar]
- Hall A. C. et al. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur J Pharmacol 506, 9–16 (2004). [DOI] [PubMed] [Google Scholar]
- Lau B. K., Karim S., Goodchild A. K., Vaughan C. W. & Drew G. M. Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br J Pharmacol 171, 2803–2813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. B. et al. A-type GABA receptor as a central target of TRPM8 agonist menthol. PLoS One 3, e3386 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. Subunit-specific inhibition of glycine receptors by curcumol. J Pharmacol Exp Ther 343, 371–379 (2012). [DOI] [PubMed] [Google Scholar]
- Dohare P., Garg P., Sharma U., Jagannathan N. R. & Ray M. Neuroprotective efficacy and therapeutic window of curcuma oil: in rat embolic stroke model. BMC Complement Altern Med 8, 55 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Y. et al. Effect of Rhizoma curcumae oil on the learning and memory in rats exposed to chronic hypoxia and the possible mechanisms. Acta Physiologica Sinica 60, 228–234 (2008). [PubMed] [Google Scholar]
- Wang Y. & Zhao X. J. Experimental study on the antiepileptic properties of Rhizonla curcumae oil in different epilepsy models. Pharmacol Clin Chin Materia Medica 20, 11–12 (2004). [Google Scholar]
- Pirker S., Schwarzer C., Wieselthaler A., Sieghart W. & Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850 (2000). [DOI] [PubMed] [Google Scholar]
- Caraiscos V. B. et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101, 3662–3667 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski D. R. et al. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol 499 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J., Mann E. O. & Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28, 1421–1426 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr A., Baur R. & Sigel E. Subtle changes in residue 77 of the gamma subunit of alpha1beta2gamma2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J Biol Chem 272, 11799–11804 (1997). [DOI] [PubMed] [Google Scholar]
- Johnston G. A. GABA(A) receptor channel pharmacology. Curr Pharm Des 11, 1867–1885 (2005). [DOI] [PubMed] [Google Scholar]
- Froestl W. An historical perspective on GABAergic drugs. Future Med Chem 3, 163–175 (2011). [DOI] [PubMed] [Google Scholar]
- Krasowski M. D., Hong X., Hopfinger A. J. & Harrison N. L. 4D-QSAR analysis of a set of propofol analogues: mapping binding sites for an anesthetic phenol on the GABA(A) receptor. J Med Chem 45, 3210–3221 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B. et al. Structural requirements of phenol derivatives for direct activation of chloride currents via GABA(A) receptors. Eur J Pharmacol 421, 85–91 (2001). [DOI] [PubMed] [Google Scholar]
- Moraga-Cid G., Yevenes G. E., Schmalzing G., Peoples R. W. & Aguayo L. G. A Single phenylalanine residue in the main intracellular loop of alpha1 gamma-aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology 115, 464–473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. et al. Tissue distribution of curcumol in rats after intravenous injection of zedoary turmeric oil fat emulsion. Asian J Pharmacodynamics Pharmacokinetics 9, 51–57 (2009). [Google Scholar]
- Su C. Y., Liu X. Y., Xu H. X. & Zhu X. Y. The metabolism of 3H-curcumol in normal rats and tumour bearing mice. Acta Pharmaceutica Sinica 15, 257–262 (1980). [PubMed] [Google Scholar]
- Murase K., Randic M., Shirasaki T., Nakagawa T. & Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res 525, 84–91 (1990). [DOI] [PubMed] [Google Scholar]
- Dong X. P. & Xu T. L. Radix paeoniae rubra suppression of sodium current in acutely dissociated rat hippocampal CA1 neurons. Brain Res 940, 1–9 (2002). [DOI] [PubMed] [Google Scholar]
- Li Y., Wu L. J., Legendre P. & Xu T. L. Asymmetric cross-inhibition between GABAA and glycine receptors in rat spinal dorsal horn neurons. J Biol Chem 278, 38637–38645 (2003). [DOI] [PubMed] [Google Scholar]