Significance
Decreases in hunter-gatherer mobility during the Late Pleistocene altered relationships with animal communities and led to domestication. Little is known, however, about how selection operated in settlements of varying duration. This study of mice in modern African mobile settlements and ancient Levantine sites demonstrates competitive advantages for commensal mice when human mobility is low and niche partitioning with noncommensal wild mice when mobility increases. Changing mice molar shapes in a 200,000-y-long sequence from the Levant reveal that mice first colonized settlements of relatively settled hunter-gatherers 15,000 y ago. The first long-term hunter-gatherer settlements transformed ecological interactions and food webs, allowing commensal house mice to outcompete wild mice and establish durable populations that expanded with human societies.
Keywords: house mouse, sedentism, Natufian hunter-gatherers, commensalism, niche construction
Abstract
Reductions in hunter-gatherer mobility during the Late Pleistocene influenced settlement ecologies, altered human relations with animal communities, and played a pivotal role in domestication. The influence of variability in human mobility on selection dynamics and ecological interactions in human settlements has not been extensively explored, however. This study of mice in modern African villages and changing mice molar shapes in a 200,000-y-long sequence from the Levant demonstrates competitive advantages for commensal mice in long-term settlements. Mice from African pastoral households provide a referential model for habitat partitioning among mice taxa in settlements of varying durations. The data reveal the earliest known commensal niche for house mice in long-term forager settlements 15,000 y ago. Competitive dynamics and the presence and abundance of mice continued to fluctuate with human mobility through the terminal Pleistocene. At the Natufian site of Ain Mallaha, house mice displaced less commensal wild mice during periods of heavy occupational pressure but were outcompeted when mobility increased. Changing food webs and ecological dynamics in long-term settlements allowed house mice to establish durable commensal populations that expanded with human societies. This study demonstrates the changing magnitude of cultural niche construction with varying human mobility and the extent of environmental influence before the advent of farming.
Reductions in hunter-gatherer mobility during the Late Pleistocene and development of sedentary ways of life altered human relations with plant and animal communities and played a pivotal role in domestication. Niche construction activities of settled hunter-gatherers, such as building, food storage, and waste accumulation, influenced food webs and exerted novel selection pressures on animal populations in human settlements (1–3). These factors resulted in changes in the abundance, ecology, and evolutionary trajectories of a range of animal species. Wild boars and wolves are thought to have been attracted to food sources in settlements following commensal pathways before developing long-term mutualism characteristic of domestication (4–6). Domestication of other animals, like goats or cattle, may have followed from hunting pressures exerted by settled communities and consequent shifts in hunting strategies (7–9). There is limited empirical evidence, however, on when pivotal shifts in mobility and selection pressures on animals in human settlements first occurred. Little is known about changing selection dynamics or competition among commensal taxa with changing occupational pressures. Despite acknowledged relationships between hunter-gatherer sedentism and domestication, scholars also hold widely divergent views regarding the level of hunter-gatherer versus early farmer impacts on ancient landscapes.
Our study of the beginnings of mouse commensalism in the Levantine region offers high-resolution data on the effect of fluctuations in human mobility on selection in human settlements, niche partitioning among mice species, and niche dynamics through time. The Levant is the likely place of origin of the commensal niche of house mice (Mus musculus domesticus; hereafter, domesticus) and the springboard for global human-mediated expansion of this species (10–14). Sites dating to the Early Holocene (ca. 12,000 B.P., all dates are calibrated radiocarbon years before present), a short time before the Neolithic, furnish the earliest known fossil evidence of domesticus (11, 13). Evidence for Early Holocene house mice was preceded by several millennia of decreased settlement mobility, social elaboration, and subsistence shifts among complex hunter-gatherers in the southern Levant (15). Wild grain collection, hunting, and reliance on small mammals intensified from as early as 25,000–23,000 B.P (16, 17). The appearance of the Natufian culture ca. 15,000 B.P. has been perceived by many as marking a punctuated transition to sedentism and built settlement environments in the region (18). As a result, it has been suggested that rather than emerging with farming, preagricultural human sedentism led to commensalism in the domesticus lineage (19, 20). This trajectory of reduced mobility was not unidirectional, however, and the intensities with which Natufian settlements were occupied and degree of human mobility continued to fluctuate substantially over ca. 3,500 y before the Neolithic (18, 21, 22).
The Levantine Natufian-Neolithic archaeological sequence provides an exceptional opportunity to examine the evolution of mouse commensal adaptations in relation to human niche construction. Toward the end of the Pleistocene, domesticus and the short-tailed (ST) mouse Mus macedonicus (hereafter, macedonicus) were both present in the Levant (23). Sympatry between the closely related and competitive species may have depended on differential use of habitats in intensely settled human environments. However, few studies have so far investigated commensal dynamics in relation to competitive interactions among mice in hunter-gatherer settlements. Mechanisms of habitat partitioning are not well understood in contemporary or ancient temporary households. To address these questions, specific ecological and archaeological data are needed, because although cities are well studied, there is currently little empirical information on rodent interactions in small-scale settlements of any age.
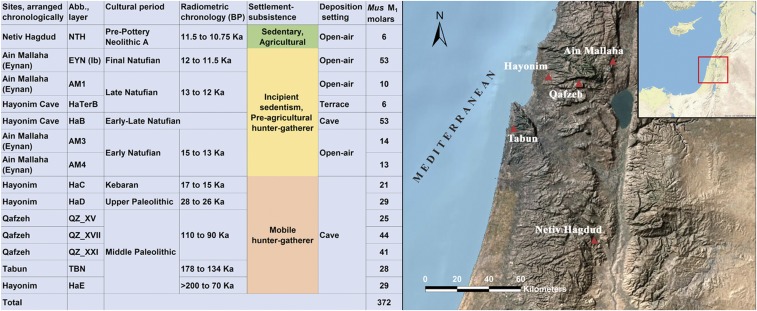
To examine competitive interactions and habitat partitioning in human settlements occupied for varying periods, we collected ethnoarchaeological data in southern Kenya, live-trapping 192 mice in six small-scale settlements (<20 people each) occupied for varied durations. Here, a sympatric pair of species of spiny mice of the genus Acomys display variability in commensal habits and morphology (24, 25) relevant to interpretation of ancient sympatric Mus species in the southern Levant. This study provides the basis for the mouse interaction model that we use in interpretation of the archaeological data. We studied the influence of ancient hunter-gatherer settlement fluctuations on niches for commensal mice in a continuous sequence of Middle-Late Pleistocene and Early Holocene occupations in the southern Levant >200,000–10,200 B.P. (Fig. 1). To determine the presence and proportions of domesticus and macedonicus species in the archaeological deposits, we used a high-resolution geometric morphometric approach for our analysis of modern and archeological Mus molars (26). Mice specimens were studied from cave and open-air sites that preserved a record of fluctuations in the intensity of human occupation over time and the Natufian transition to sedentism (27–29): Middle Paleolithic levels at Tabun, Qafzeh, and Hayonim; Kebaran levels at Hayonim; Natufian levels from Ain Mallaha and Hayonim; and Pre-Pottery Neolithic A (Early Neolithic) from Netiv Hagdud (Fig. 1).
Fig. 1.
Chronology (Left) and location (Right) of the archaeological contexts providing Mus zooarchaeological samples used in this study. Sizes of Mus M1 samples are given in the last column of the table. The base map was generated from Environmental Systems Research Institute (ESRI) map data using ArcGIS v.9.1; Esri, GEBCO, DeLorme, NaturalVue, United States Geological Survey, NASA, Esri Inc.
Our findings reveal niches for mice in human settlements 15,000 y ago, fluctuations in competitive dynamics among commensal mice with mobility, and the presence of dominant populations of house mice in settled hunter-gatherer households. This study also provides long-term perspectives on the variable nature of hunter-gatherer influences on commensal niches and niche construction occurring at the threshold of domestication and agriculture.
Results
Modern Referential Model.
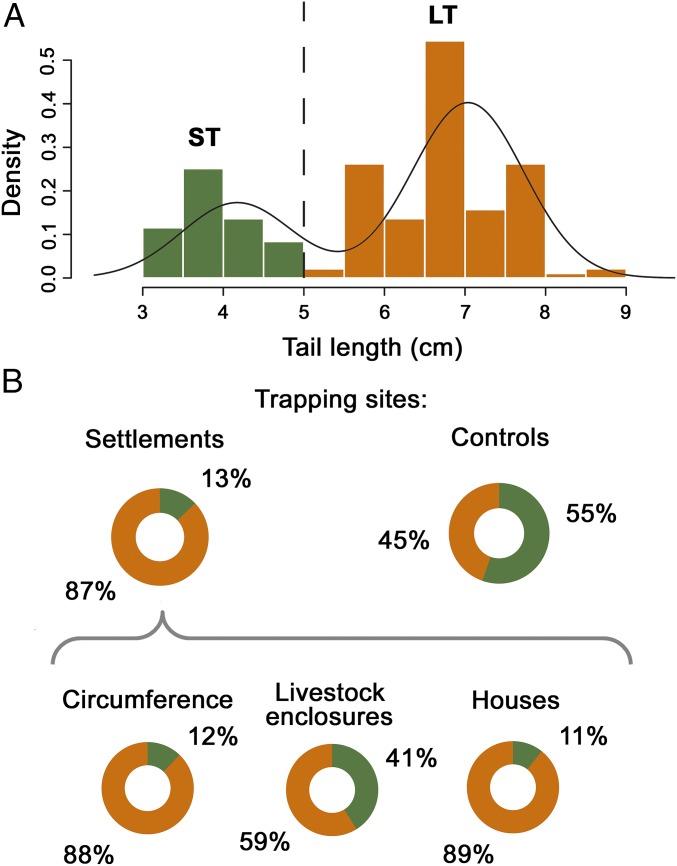
Maasai settlements studied were occupied by seasonally mobile but sedentizing herders. Over the past 40 y, the average settlement use life has increased from 3.7 to 6.4 y (30). Families stored little grain, which contributed to a neutral rather than amensal relationship with rodents. Householders did not have a negative attitude to rodents and did not kill them. We observed no intentional selection on mice in settlements. Acomys spp. were the most abundant species in the study, representing 60% of small mammals trapped inside the six study settlements and 72% of small mammals trapped in adjacent control sites 200–400 m from settlements. Mixture analysis and unsupervised classification with Bayesian modeling performed on tail length measurements taken from the captured Acomys individuals demonstrate the occurrence of two taxa (Fig. 2A and Statistical Analyses): Acomys wilsoni, a ST species, and Acomys ignitus, a long-tailed (LT) species.
Fig. 2.
Species composition of Acomys in Maasai settlements. (A) Mixture analysis combined with Bayesian modeling performed on Acomys tail length measurements showing the existence of two taxonomic groups: ST group and LT group. (B) Pie charts showing the combined proportions of the two taxa (ST/LT) in the settlements and control sites, as well as in different parts of settlements (circumference fence, animal enclosures, and houses).
A. wilsoni and A. ignitus occurred in both the control sites and within the settlements. The level of association between these two species and the settlements varies significantly (χ2 = 39.668, Monte Carlo P = 0.0001, Cramer’s V = 0.456; Statistical Analyses). A. ignitus accounts for 87% (n = 101) of individuals captured inside the settlements but only 45% (n = 33) of individuals in the control sites, suggesting that among the two Acomys species, the LT A. ignitus is also the more commensal of the two (Fig. 2B). The difference in proportions is most pronounced when examining results of trapping inside dwellings, where 23 A. ignitus individuals but only one A. wilsoni individual were trapped (Fig. 2B).
Our Maasai modern reference dataset reveals notable sharing of the commensal habitat by Acomys species. Moreover, numbers of commensal Acomys do not increase with increasing use intensity of settlements within the Maasai system. Here, settlements remain small-scale and seasonal mobility involves periodic complete or partial abandonment of settlements. Using the age and extent of seasonal human occupation of the six settlements in our case study to rank-order the settlements (Table S1), only a weakly significant negative correlation exists between these settlement ranks and numbers of A. ignitus (Spearman’s rank correlation, r s = −0.9; P = 0.03 using the exact permutation test; Statistical Analyses). Additionally, numbers of A. wilsoni in the settlements and of both Acomys species in the control sites show no correlation with Maasai settlement intensity (A. wilsoni settlements: r s = −0.8, P = 0.08; A. ignitus controls: r s = −0.2, P = 0.81; A. wilsoni controls: r s = −0.2, P = 0.65).
Table S1.
Structural, social, and economic characteristics of the six study settlements
| Settlement | S1 | S2 | S3 | S4 | S5 | S6 |
| Age, y | 2 | 8 | 14 | 21 | 43 | 45 |
| Seasonal use, % of y | 0.75 | 0.75 | 0.75 | 0.75 | 0.25 | 1 |
| Combined index of occupation intensity, age × seasonal use | 1.5 | 6 | 10.5 | 15.75 | 10.75 | 45 |
| No. of households | 3 | 1 | 1 | 1 | 2 | 1 |
| No. of houses | 6 | 9 | 4 | 6 | 3 | 9 |
| Diameter, m | 80 | 60 | 50 | 70 | 40 | 70 |
| Adult population size and composition | ||||||
| Men | 3 | 4 | 1 | 4 | 6 | 6 |
| Women | 4 | 11 | 2 | 6 | 4 | 4 |
| Young adults | 3 | — | 0 | 3 | 3 | 3 |
| Estimated livestock holdings (cattle, sheep/goats, and donkeys) | 200 | 500 | 50 | 500 | 500 | 500 |
Values presented in the table refer to numbers generally observed in the settlements during the study period or retrieved through conversations with local residents.
These results indicate that the LT A. ignitus possesses a competitive advantage over the ST A. wilsoni in the settlements, thereby gaining access to preferred resources. However, because Maasai settlement use remains seasonal, A. wilsoni is only partially outcompeted by A. ignitus, indicating that absolute competitive exclusion and dominance of the commensal niche require longer term and more permanent human occupation. Dietary niche overlap and competition are thought to influence relationships among coexisting species of Acomys (31, 32). In human settlements, access to more optimal dietary resources for Acomys, including seeds and plant matter, can be enhanced due to release from competition with wild herbivores (24). Enhanced resource provisioning for mice in Maasai settlements, including herbaceous biomass and seeds, is the expected result of elevated productivity of soils fertilized by the dung from livestock herds (33).
Archaeological Samples.
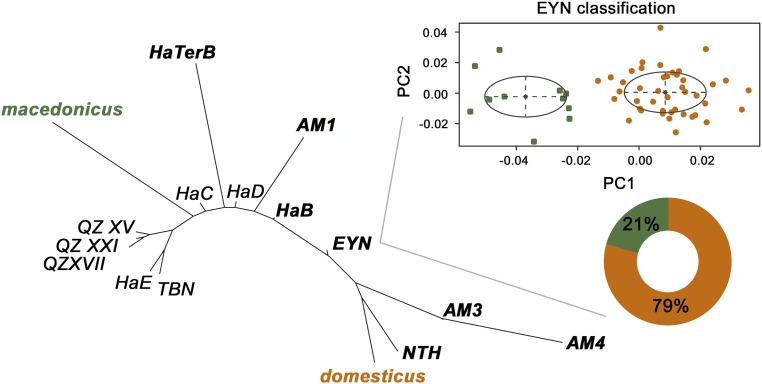
Samples were internally consistent and differed with stratigraphic context. The molar shape relationship among the archaeological Mus samples and the two modern sympatric species of Mus from Israel (Statistical Analyses and Fig. S1), showing the mean shape for each sample, is displayed by an unrooted neighbor-joining tree (Fig. 3). This phenogram clearly polarizes the sequence, showing that a single Mus species, macedonicus, was present in the entire Pre-Natufian sequence (>15,000 B.P.), including Tabun, Hayonim (HaE-C), and Qafzeh (XV, XVII, XXI) caves, as well as in the Natufian samples from Hayonim Cave (HaB, HaTerB) and the Late Natufian phase of Ain Mallaha (AM1). The presence of a single species in these samples is confirmed by Bayesian analysis, which finds only one most likely group (Statistical Analyses and Table S2). The earliest documented remains of domesticus are the remains from Early Natufian Ain Mallaha (AM3–4), where they comprise 100% of the Mus remains. This finding indicates a sharp replacement of macedonicus by domesticus and the presence of house mice that did not share the commensal niche with macedonicus (Fig. 4). A second sharp replacement is shown in the Late Natufian occupation phase of Ain Mallaha, when macedonicus again comprises 100% of the remains. Archaeological evidence from Ain Mallaha suggests a shift from sedentary to more ephemeral human occupation between the early and late phases of the Natufian, as evidenced by decreases in repeated use and size of stone structures and burials of individuals with personal adornments (Archaeological Interpretation of Fluctuations in Hunter-Gatherer Site Use Intensity in the Ain Mallaha Sequence).
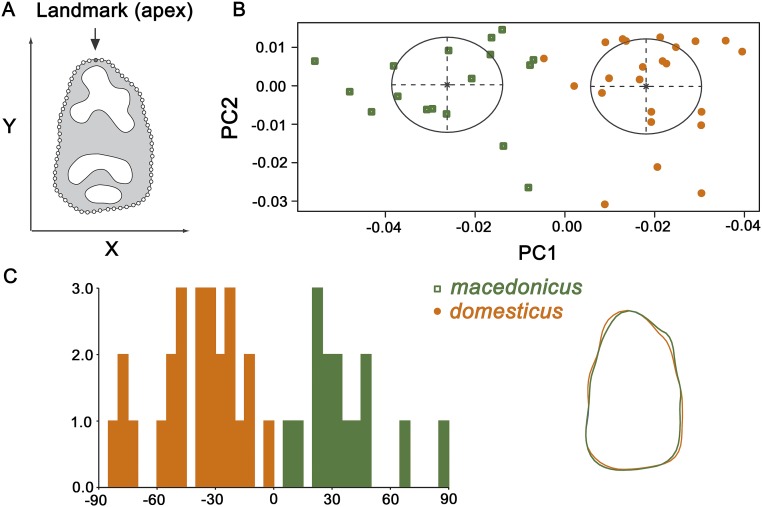
Fig. S1.
Molar shape differentiation among modern sympatric species in Israel, Mus macedonicus (macedonicus) and Mus musculus domesticus (domesticus). (A) Landmarks and semilandmarks digitized on the external outline of the crown of M1. (B) Model-based clustering of shape variables (PC1 and PC2). (C) Histogram of discriminant factors on shape variables after cross-validation and visualization of shape changes from domesticus to macedonicus along the discriminant axis. A geometric morphometric analysis for distinguishing the two sympatric Mus species in Israel, domesticus and macedonicus, found no significant molar size differences (ANOVA, Welch F test: F = 3.148, df = 36.43, P = 0.08436), but did detect significant differences in molar shape (multivariate ANOVA: Wilk’s Lambda = 0.087, df1 = 13, df2 = 26, F = 20.78, P = 0.0001). Supervised classification found a correct classification of 100% for samples of the two species after cross-validation. Moreover, an unsupervised approach with Bayesian modeling found clear separation between the two taxa.
Fig. 3.
(Left) Combined neighbor-joining tree analysis of modern and archaeological Mus specimens (domesticus, orange; macedonicus, green). TBN, Tabun; Ha, Hayonim; QZ, Qafzeh; AM and EYN, Ain Mallaha; NTH, Netiv Hagdud. Natufian neolithic sites in are shown in boldface. (Right) Species composition of EYN is shown.
Table S2.
Parameterization of the covariance matrix and result of Gaussian finite mixture analysis with an expectation-maximization model on multiple shape variables (PC1 and PC2 coefficients) for each archaeological sample
| Site abbreviation, layer | Model | Distribution type | Volume | No. of components | Log-likelihood | n | df | BIC |
| EYN (Ib) | EII | Spherical | Equal volume | 2 | 291.2446 | 54 | 6 | 558.5554 |
| HaB | XXI | Diagonal multivariate normal | 1 | 262.7917 | 52 | 4 | 509.7784 | |
| HaC | XII | Spherical multivariate normal | 1 | 110.9673 | 21 | 3 | 212.801 | |
| HaD | XXI | Diagonal multivariate normal | 1 | 159.4504 | 29 | 4 | 305.4316 | |
| HaE | XII | Spherical multivariate normal | 1 | 153.4476 | 29 | 3 | 296.7933 | |
| HaTerB* | EEI | Diagonal | Equal volume and shape | 5 | 51.70151 | 6 | 16 | 74.73488 |
| AM3 | XII | Spherical multivariate normal | 1 | 77.67928 | 14 | 3 | 147.4414 | |
| AM4 | XII | Spherical multivariate normal | 1 | 70.02207 | 13 | 3 | 132.3493 | |
| AM1* | EEI | Diagonal | Equal volume and shape | 9 | 101.5368 | 10 | 28 | 138.6011 |
BIC, Bayesian Information Criterion; AM, Ain Mallaha (Eynan); Ha, Hayonim.
Sample size likely too small for obtaining the model-based parameters reliably.
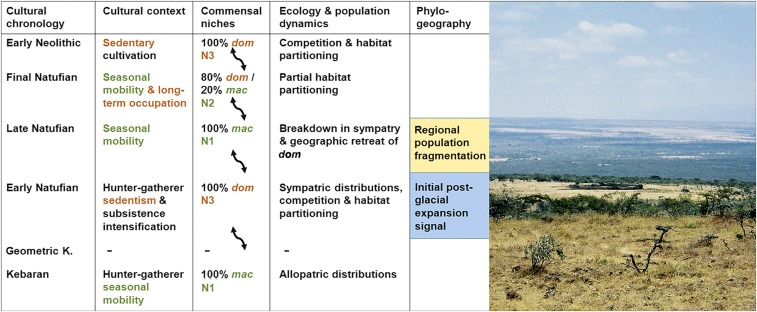
Fig. 4.
(Left) Connections between cultural, ecological, and phylogeographic factors in commensal niche dynamics in the terminal Pleistocene southern Levant (dom, domesticus; mac, macedonicus; double-headed arrows indicate transitions in commensal niches). Lack of data for the geometric Kebaran precludes determining between patterns of short-term or multiyear seasonal mobility for this period. Phylogeographic information is based on data in the study by Bonhomme et al. (14). Niche development stages N1–N3 are described in the main text. (Right) Maasai settlement in southern Kenya at the center of the photograph is surrounded by a grassy buffer zone, corresponding to human occupation intensity.
Unlike any of the other samples, the sample of the Final Natufian at Ain Mallaha [Eynan (EYN)] occupies an intermediate position between the two poles of our ancient Mus molar shape continuum. It is the only sample where, according to Bayesian clustering models (Statistical Analyses and Table S2), both species occur in sympatry, but the house mouse was in the majority (79%; Fig. 3). The relative abundances of domesticus and macedonicus in this sample are closely congruent with the structure of sympatry between the two Acomys species within Maasai settlements (87% A. ignitus, the more commensal of the two species, compared with only 13% A. wilsoni). This finding suggests habitat partitioning in both cases and a more loose-knit facultative association of commensal mice and the human environment when the intensity of human occupation lies between sedentism and frequent seasonal movements, as seen in the Maasai context.
Combining Zooarchaeological and Referential Data.
We propose three stages of niche development in commensal interactions of the house mouse (Fig. 4): N1, nonexistence or rare and opportunistic presence of house mice (archaeologically undetectable); N2, facultative association of house mice with the settlements where there is only relative dominance of house mice and competitive exclusion of macedonicus; and N3, obligate association of house mice with the settlements where there is absolute dominance of domesticus and competitive exclusion of macedonicus. Early Natufian occupations at Ain Mallaha show a well-developed N3 pattern, which was due to increased intensity of human occupation and dampened seasonality, and is thought to have resulted in the exclusion of macedonicus. It appears that increased human mobility, which has been suggested for the late phase of the Natufian, including at sites such as Ain Mallaha (Archaeological Interpretation of Fluctuations in Hunter-Gatherer Site Use Intensity in the Ain Mallaha Sequence), favored the return of macedonicus and temporary exclusion of domesticus, reverting to a stage N1 pattern. The fluctuating abundances of domesticus between the occupations of Final Natufian Ain Mallaha and Early Neolithic Netiv Hagdud seem to mirror a resumed but more gradual trend of sedentization, which is documented in the archaeological record (Archaeological Interpretation of Fluctuations in Hunter-Gatherer Site Use Intensity in the Ain Mallaha Sequence) and fits with a stage N2 pattern of sharing of the commensal niche among a pair of sympatric species of mice.
Statistical Analyses
Modern Referential Model.
To assess the presence of one or both Acomys species in the different trapping sites, we analyzed variation in tail length measurements using a model-based clustering approach and an iterative expectation-maximization (EM) method for maximum likelihood estimation in parametrized Gaussian mixture models (54). Classification within the EM Gaussian mixture model was used to assess the relative proportion of each Acomys taxon for each trapping site. The difference in the relative proportion of Acomys taxa between settlements and control sites was assessed with a two-by-two χ2 test; a Monte Carlo permutation test of significance; and the Cramer’s V estimator of effect size, which shows a moderate-strong effect size. Correlation between numbers of Acomys species and intensity of human settlement use, represented as settlement ranks, was assessed using Spearman’s nonparametric rank-order correlation coefficient, with an exact permutation test of significance.
Geometric Morphometrics (GMM).
Overall differences in size and shape among modern Mus taxa from Israel were tested with an ANOVA (F test) and multivariate ANOVA (Wilk’s Lambda test). The efficiency of accurately distinguishing the two sympatric species of mice in Israel based on their M1 outlines was assessed with both supervised [canonical variate analysis (CVA)] and unsupervised model-based clustering approaches as presented above. CVA was performed on a reduced dataset of shape variables, using principal components (PCs) with 99% variance, coupled with a leave-one-out cross-validation analysis to calculate the percentage of correct classification. Model-based classification was performed on the first two PCs computed on the matrix of variance/covariance of the shape variables. The unsupervised model-based clustering was performed on two shape variables (PC1 and PC2 scores) to assess the clustering for both modern species of mice and the archaeological samples (Fig. S1 and Table S2).
An unrooted neighbor-joining tree was constructed from the Mahalanobis distances calculated among means of shape in the different samples to examine the relationship between modern and ancient samples of mice from Israel. Procrustes superimposition was performed with tpsRelw 1.59 (53). GMM statistical analysis was performed with R (55), using libraries from mclust (56) and Modern Applied Statistics with S (MASS) (57).
Archaeological Interpretation of Fluctuations in Hunter-Gatherer Site Use Intensity in the Ain Mallaha Sequence
Archaeological material evidence from Ain Mallaha demonstrate changes in intensity of human occupation, which are shown mainly by shifts in the extent of investment in stone construction as well as through changes in social customs of burial. It has long been thought that the initial emergence of permanently built villages as seen at Ain Mallaha as well as other Natufian sites marked an early transition to a sedentary way of life among hunter-gatherers before the introduction of farming (34). A reduction in the size of stone structures together with a pronounced shift in burial customs between the early and late phases of the Natufian, which involved increased frequency of group burial and complete disappearance of the practice of body decoration and inclusion of grave goods in several key sites, has broadly been interpreted as evidence for a change from a sedentary to more mobile pattern of settlement use (18).
Use of material culture evidence to infer changes in human mobility reliably in the Natufian has also been debated (35), and it is likely that sequences of changes in mobility varied from site to site (21, 39). In the long and continuous archaeological sequence of Natufian occupations in Ain Mallaha, the Early Natufian phase (levels 2–4) reveals a cluster of relatively large structures with preserved sections of some of the walls measuring up to 1 m in height. Human burials were unearthed in association with some of the structures, apparently entered below floors. In a subsequent phase of occupation during the Late Natufian (level Ic), there is a reduction in the size of structures, accompanied by the occurrence of multiple pits used for burial, where more than one individual was entered successively (29). Terminating the Natufian sequence of Ain Mallaha, the Final Natufian (level Ib) reveals a number of stone structures comparable in their relatively small size to stone structures of the preceding Late Natufian, although showing evidence for repeated occupation of structures in the form of successions of tightly packed living floors as were detected in structures of the Early Natufian phase at the site.
The Mus spp. material from Ain Mallaha was excavated during the 1972–1976 (Early and Late Natufian) and 1996–2005 (Final Natufian) field seasons. It was collected through wet sieving in areas selected for their excellent stratigraphic context. Stratigraphic subdivision and assignment of features to phases within the Natufian period were based on analyses of material culture attributes, which are well-established as sensitive chronological markers for this period. In particular, studies of typological and metrical characteristics of microlithic implements, especially lunates, were used to ensure stratigraphic control on contexts providing fossil remains for this study. Rodent specimens were recovered from well-defined Early and Late Natufian features, including densely packed floors of structures, pits, and burials. Lunates of similar size as mouse mandibles used in this study were associated with them in the dense stony layers of the Final Natufian. We saw no evidence for mixing. This assessment of the integrity of the samples was reinforced by taphonomic study of the rodent subfossils and considerations of weathering. Assemblages were consistent within stratigraphic contexts and differed from one another.
Discussion
Our findings from the 200,000-y sequence studied fill a critical gap in current reconstructions of the origins of commensal interactions between humans and the house mouse (domesticus) with reductions in human mobility. The data reveal the presence of dominant commensal populations of house mice in southern Levantine sedentary hunter-gatherer settlements as early as 15,000 B.P. Assemblages from occupations of Natufian hunter-gatherers and Early Neolithic farmers, which span the period of sympatry of house mice and the congeneric macedonicus between 15,000 and 11,000 B.P., show strong temporal fluctuations in relative proportions of sympatric mice. These ecological shifts mirror documented events in the cultural record, involving alternations between periods of human sedentism and increased mobility over this period (18, 21, 22). Integrating archaeological findings with the findings from our modern referential model provides insight into the nature of early interactions between humans and commensal mice.
Commensal Origins of House Mice.
Our findings demonstrate that the initial development of a commensal niche and its maintenance over time depended on long-term human occupation of settlements and sustained habitat alteration. The house mouse commensal niche appeared full-blown at the beginning of the Natufian sequence at the site of Ain Mallaha (levels 3–4, Early Natufian). Interestingly, sympatric macedonicus was absent in early occupation levels at Ain Mallaha and reappeared in later ones (levels 1c and Ib, Late and Final Natufian, respectively), a reconstruction that covaries with architectural shifts. This finding demonstrates that in the context of newly established sympatry between the two species, populations of house mice outcompeted their macedonicus counterparts, completely excluding them from the commensal niche.
Ecologists have suggested a pattern of habitat partitioning and competitive exclusion among modern populations of ST macedonicus and house mice in the Levant (13, 23). However, due to the urbanized and intensely agrarian nature of present-day landscapes, it has been difficult to establish whether this pattern initially emerged among early sedentary hunter-gatherers or later Neolithic famers. Evidence for the dominance of house mice in early occupations of the Natufian culture speaks to absolute habitat partitioning and competitive exclusion among populations of commensal mice. This evidence is consistent with widespread archaeological evidence for an abrupt hunter-gatherer transition to a sedentary way of life and initial construction of permanently built environments (18, 34, 35). At this time, settled hunter-gatherers introduced a new suite of selective pressures with unintentional impacts on mouse populations. Strong dependence of house mice on the commensal niche is indicated at the population level (36).
However, the establishment of a commensal niche in Natufian settlements like Ain Mallaha did not build to a trajectory of sustained dominance of commensal mice. Toward the end of the Ain Mallaha sequence, the two sympatric mouse species share the commensal niche, with house mice showing only relative dominance. This pattern is rare and occurs nowhere else in the 200,000-y sequence studied (also ref. 37). In light of the correspondence that we demonstrate between our archaeological and modern reference datasets, a pattern of seasonal occupation is highly probable. This pattern may have persisted from a few years to several decades. Archaeologically, distinct occupation lenses within floors at Ain Mallaha indicate repeated use of the Final Natufian (EYN) structures despite reduced architectural investment during this period (28, 29, 38). Strong links between limited mobility, occupation pressure, and competitive advantages for domesticus are indicated by successive population replacements of macedonicus during periods of increased human sedentism. Conversely, a return to a more mobile existence in later phases of the Natufian (18, 22, 39) correlates with replacement of domesticus by macedonicus.
Fine-grained data from sites spanning a long time period allow us to evaluate the strength of ecological interactions and the resulting array of new selective pressures introduced by sedentary hunter-gatherers of the Natufian culture at a range of different scales. Direct human selection and mutualism occurred only in the very recent stages in the formal domestication of the house mouse (40), indicating the strength of indirect selection in the human niche. The response of mice to habitat alteration by sedentary Natufian hunter-gatherers was a manifest behavioral shift to a largely commensal existence and is an excellent index of the changing magnitude of cultural niche construction with varying human mobility.
Ecological Footprint of Pleistocene Hunter-Gatherers.
Hunter-gatherer construction and long-term occupation of stone dwellings led to an ecological buffering effect for mice with increased resources available to them through people storing and discarding food in settlements. This activity provided a release from nonanthropogenic selection pressures, such as seasonally fluctuating food abundances and competition. Underlying mechanisms of food provisioning vary (e.g., wild grain storage, cultivation, enhanced soil fertility as in our Maasai case study), but all impacts increase with human occupation intensity. Previous research suggested that house mice became established within the commensal niche during the Early Neolithic, with the introduction of farming (13). We demonstrate that Pre-Neolithic hunter-gatherer sedentism altered selective environments for commensal species. Intensive accumulation of wild grains combined with protocultivation is now known from as early as 23,000 B.P. at the Levantine site of Ohalo II (17). Putative storage facilities are known from Natufian sites at Ain Mallaha and Hayonim Cave (41–43). Our results suggest that increased storage and greater accumulation and discard of food waste played a role in altered ecological webs, leading to the development of permanent and dominant house mouse populations in sedentary hunter-gatherer settlements.
Phenotypic traits of house mice selected for within intense anthropogenic habitats across Asia included a longer tail, shorter skull, and darker pelage relative to the phenotypic traits of sympatric mice of the same genus (44, 45). Such shifts in size, cranial morphology, and color-based phenotypic traits have been linked to brain functions organized toward reduced wariness, reactivity, and aggression, a key requirement in adaptation of domestic and commensal animals to intensely anthropized environments (46, 47). Further research is needed on trait selection in commensal habitats (48); however, the similarity in the greater tail-to-body ratio trait in commensal species from different continents, modern house mice, and Acomys in Maasai settlements supports links between morphological shifts and selection on mice in anthropogenic habitats. Selection for longer tails, important for agility in locomotion and possibly flight response in mice, was a likely outcome of intensified human activity in permanently settled habitats.
The beginning of sedentary living marked a turning point in human and environmental history when permanent settlement began to exert lasting impacts and ecological legacies on ancient landscapes. The contribution of commensal rodents to dampening agricultural productivity and spread of disease in later history is testimony that the transition to sedentism had a profound, long-lasting, and unpredictable influence on the human niche. Our results demonstrate that relatively large-scale and sustained anthropogenic alteration of ecological dynamics in settlements was initiated considerably earlier than previously thought. They provide evidence for the timing of and competitive interactions among mouse populations during one of the earliest processes of human-mediated biological invasion, which transpired among preagricultural societies. This study emphasizes the significance of human niche construction activities in sedentary settlements for commensal pathways to domestication (2, 3). The commensal nature of relationships between humans and mice through time, mouse behavioral flexibility, and morphological shifts also strengthen assertions that animal domestication may have relied to an important extent on indirect selection in the human niche, acting on existing variation and behavioral and phenotypic plasticity of target species (49).
Materials and Methods
Modern Referential Model.
Ecological monitoring of small mammals and investigation of commensal use of settlements by local species of Acomys were conducted in six small-scale settlements of seasonally mobile Maasai herders in southern Kenya. These settlements were occupied for durations varying from 3 to 12 mo seasonally over 2–45 y. The study area is located within the administrative district of Kajiado in the Maasai locality of Eselenkei (50). Eselenkei is a semiarid region of southern Kenya characterized by average annual rainfall of 425–675 mm and dominated by Acacia-Commiphora bush that is associated with shallow red sandy substrates. Mobile pastoralism is the predominant land use in the study area, where rain-fed farming is not sustainable due to low and unpredictable rainfall. The different ages of six settlements selected for this study, from 2 to 45 y old, span the entire age range of Maasai settlements in the region (30).
We used a standard ecological field technique for studying small rodents and shrews, capture-mark-release trapping, which was used inside the six villages and in adjacent control sites of similar size. At each site, we deployed an orthogonal 25-trap (large folding aluminum Sherman live traps: 3 × 3.5 × 9 in) grid for a period of 5–7 d, and this trapping technique was repeated three more times to produce a total trapping effort of 7,350 trap days (number of traps × number of trapping days). Data on taxonomic identification and body measurements were taken for each trapped individual. Nine different taxa of small rodents and insectivores were documented in traps (Acomys, Gerbillus, Mastomys, Elephantulus, Grammomys, Crocidura, Taterillus, Tatera, and Lemniscomys), of which Acomys provided the most frequently trapped individuals across settlement and control sites. The presence of two species of Acomys in our trapping area accords with published measurements, which distinguish the species based on tail lengths (A. wilsoni is a ST species, and A. ignitus is a LT species; 3–5 cm and 5.5–9 cm, respectively) (table 1 in ref. 25), and with maps of species distribution in the International Union for Conservation of Nature Red List of Threatened Species (maps.iucnredlist.org). We note that the study was approved by the Animal Studies Committee of Washington University in St. Louis.
Rodent Samples and Geometric Morphometrics of Molar Form in Modern and Fossil Mice.
Research focused on rodent mandibles. Samples were retrieved using flotation and were selected from well-stratified contexts, including at Ain Mallaha features, such as densely packed floors, walls, and pits. Architecture is reduced in the Late and Final Natufian phases (last 50 cm of a 3-m section), but distinct occupation lenses within floors provided sampling contexts. Taphonomic analysis investigated variation in fossilization and weathering patterns, finding no evidence for such variation.
Total sample sizes were 64 modern samples and 372 fossil samples. Geometric morphometrics provide a more accurate and reliable technique for specific and subspecific differentiation within the Mus group based on cranial remains than previously used linear morphometric methods. Improved accuracy in comparison to earlier studies of Mus fossil material in the Levant (20) is due to the use of high-resolution digitizing, quantification, and partitioning of shape and size information.
To compare modern and archaeological samples of mice, we used the first lower molar (M1) as a phenotypic marker and a geometric morphometric approach to quantify and analyze morphological variation (26). In this study, we captured the form of the M1 with a 2D outline of its crown in occlusal view. This technique employs 64 equidistant Cartesian coordinates starting from the apex of the M1 and digitized from photographs using tpsDig 2.12 (51). The starting point is considered a landmark (type 3), and the 63 other points are considered semilandmarks (52). To extract comparable shape variables out of these outlines, we used Procrustes superimposition based on a generalized least square approach, which aligns the outlines to remove size, position, and orientation components from the coordinates and to obtain a new set of coordinates called Procrustes coordinates or shape variables used in subsequent statistical analyses. Semilandmarks were aligned with a bending energy method (53). The centroid size (square root of the summed distances between each point and the outline centroid) is also computed after alignment and is used as a size variable for each specimen.
Acknowledgments
We thank the late Prof. Eitan Tchernov and Dr. Rivka Rabinovich from the National Natural History Collections at the Hebrew University of Jerusalem for providing T.C. access to Mus molars from archaeological sites included in this study. We also thank the excavators, including Ofer Bar-Yosef, Avi Gopher, Arthur Jelinek, Donald Henry, and Bernard Vandermeersch, for meticulous collection of the skeletal remains of small mammals. The ethnoarchaeological study was supported by National Science Foundation Grant BCS-0536507 (to L.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619137114/-/DCSupplemental.
References
- 1.Smith BD. A cultural niche construction theory of initial domestication. Biol Theory. 2011;6(3):260–271. [Google Scholar]
- 2.Zeder MA. Core questions in domestication research. Proc Natl Acad Sci USA. 2015;112(11):3191–3198. doi: 10.1073/pnas.1501711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin NL, et al. Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc Natl Acad Sci USA. 2016;113(23):6388–6396. doi: 10.1073/pnas.1525200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morey DF. The early evolution of the domestic dog. Am Sci. 1994;82(4):336–347. [Google Scholar]
- 5.Vigne J-D, et al. Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. Proc Natl Acad Sci USA. 2009;106(38):16135–16138. doi: 10.1073/pnas.0905015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigne J-D. The origins of animal domestication and husbandry: A major change in the history of humanity and the biosphere. C R Biol. 2011;334(3):171–181. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Helmer D, Gourichon L, Monchot H, Peters J, Saña Segui M. Identifying early domestic cattle from prepottery Neolithic sites on the middle Euphrates using sexual dimorphism. In: Vigne J-D, Peters J, Helmer D, editors. The First Steps of Animal Domestication. Oxbow; Oxford: 2005. pp. 86–95. [Google Scholar]
- 8.Zeder MA. A critical assessment of markers of initial domestication in goats (Capra hircus) In: Zeder M, Bradley D, Emshwiller E, Smith B, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Univ of California Press; Berkeley: 2006. pp. 181–208. [Google Scholar]
- 9.Marom N, Bar-Oz G. The prey pathway: A regional history of cattle (Bos taurus) and pig (Sus scrofa) domestication in the northern Jordan Valley, Israel. PLoS One. 2013;8(2):e55958. doi: 10.1371/journal.pone.0055958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auffray J-C, Vanlerberghe F, Britton-Davidian J. House mouse progression in Eurasia: A palaeontological and archaeozoological approach. Biol J Linn Soc Lond. 1990;41:13–25. [Google Scholar]
- 11.Cucchi T, Vigne JD, Auffray JC. First occurrence of the house mouse (Mus musculus domesticus Schwarz & Schwarz, 1943) in the Western Mediterranean: A zooarchaeological revision of sub-fossil house mouse occurrences. Biol J Linn Soc Lond. 2005;84(3):429–445. [Google Scholar]
- 12.Cucchi T. Uluburun shipwreck stowaway house mouse: Molar shape analysis and indirect clues about the vessel’s last journey. J Archaeol Sci. 2008;35(11):2953–2959. [Google Scholar]
- 13.Cucchi T, Auffray J-C, Vigne J-D. On the origin of the house mouse synanthropy and dispersal in the Near East and Europe: Zooarchaeological review and perspectives. In: Macholán M, Baird SJ, Munclinger P, Piálek J, editors. Evolution of the House Mouse. Cambridge Univ Press; Cambridge, UK: 2012. pp. 65–93. [Google Scholar]
- 14.Bonhomme F, et al. Genetic differentiation of the house mouse around the Mediterranean basin: Matrilineal footprints of early and late colonization. Proc Biol Sci. 2011;278(1708):1034–43. doi: 10.1098/rspb.2010.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher LA, Richter T, Stock JT. The Pre-Natufian Epipaleolithic: Long-term behavioral trends in the Levant. Evol Anthropol Issues Rev. 2012;21(2):69–81. doi: 10.1002/evan.21307. [DOI] [PubMed] [Google Scholar]
- 16.Munro ND, Kennerty M, Meier JS, Samei S. Human hunting and site occupation intensity in the Early Epipaleolithic of the Jordanian western highlands. Quat Int. 2016;396:31–39. [Google Scholar]
- 17.Snir A, et al. The origin of cultivation and proto-weeds, long before Neolithic farming. PLoS One. 2015;10(7):e0131422. doi: 10.1371/journal.pone.0131422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belfer-Cohen A, Bar-Yosef O. Early sedentism in the Near East: A bumpy ride to village life. In: Kujit I, editor. Life in Neolithic Farming Communities. Kluwer Academic; Boston: 2002. pp. 19–38. [Google Scholar]
- 19.Tchernov E. Biological evidence for human sedentism in Southwest Asia during the Natufian. In: Bar-Yosef O, Valla FR, editors. The Natufian Culture in the Levant. International Monographs in Prehistory; Ann Arbor, MI: 1991. pp. 315–340. [Google Scholar]
- 20.Auffray J-C, Tchernov E, Nevo E. Origine du commensalisme de la souris domestique (Mus musculus domesticus) vis-à-vis de l’homme. Comptes Rendus l’Academie des Sci Paris. 1988;307(9):517–522. French. [Google Scholar]
- 21.Weinstein-Evron M. Archaeology in the Archives: Unveiling the Natufian Culture of Mount Carmel. BRILL; Boston: 2009. [Google Scholar]
- 22.Bar-Yosef O. Climatic fluctuations and early farming in West and East Asia. Curr Anthropol. 2011;52(S4):S175–S193. [Google Scholar]
- 23.Auffray J-C, et al. Presence and ecological distribution of Mus “spretoides” and Mus musculus domesticus in Israel, circum-Mediterranean vicariance in the genus Mus. Z Saugetierkd. 1990;55(1):1–10. [Google Scholar]
- 24.Canova L, Fasola M. Population density and diet of the spiny mouse Acomys cf. cahirinus in a desertic area of northern Kenya. Rev Ecol. 1994;49(1):87–90. [Google Scholar]
- 25.Denys C, Gautun J-C, Tranier M, Volobouev V. Evolution of the genus Acomys (Rodentia, Muridae) from dental and chromosomal patterns. Isr J Zool. 1994;40(2):215–246. [Google Scholar]
- 26.Cucchi T, et al. On the trail of Neolithic mice and men towards Transcaucasia: Zooarchaeological clues from Nakhchivan (Azerbaijan) Biol J Linn Soc Lond. 2013;108(4):917–928. [Google Scholar]
- 27.Valla FR. 1987. Chronologie absolue et chronologies reIatives dans le Natoufie. Chronologies in the Near East, British Archaeological Reports International Series 379, eds Aurenche O, Evin J, Hours F (British Archaeological Reports, Oxford), pp 267–294. French.
- 28.Valla FR. The first settled societies: Natufian (12,500–10,200 BP) In: Levy T, editor. The Archaeology of Society in the Holy Land. Leicester Univ Press; London: 1995. pp. 170–187. [Google Scholar]
- 29.Valla F, Khalaily H, Samuelian N, Bocquentin F. What happened in the Final Natufian? Journal of the Israel Prehistoric Society. 2010;40:131–148. [Google Scholar]
- 30.Worden JS. 2007. Fragmentation and settlement pattern in Maasailand—Implications for pastoral mobility, drought vulnerability, and wildlife conservation in an East African Savanna. PhD dissertation (Colorado State University, Fort Collins, CO)
- 31.Neal BR. The breeding pattern of two species of spiny mice, Acomys percivali and A. wilsoni (Muridae: Rodentia), in central Kenya. Mammalia. 1983;47(3):311–321. [Google Scholar]
- 32.Alibhai SK, Key G. A preliminary investigation of small mammal biology in the Kora National Reserve, Kenya. J Trop Ecol. 1985;1(4):321–327. [Google Scholar]
- 33.Muchiru AN, Western DJ, Reid RS. The impact of abandoned pastoral settlements on plant and nutrient succession in an African savanna ecosystem. J Arid Environ. 2009;73(3):322–331. [Google Scholar]
- 34.Perrot J. Premiers villages de Syrie et de Palestine. Comptes Rendus des Séances l’Académie des inscriptions. Série B, Lett. 1968;112(2):161–177. French. [Google Scholar]
- 35.Edwards PC. Problems of recognizing earliest sedentism: The Natufian example. J Mediterr Archaeol. 1989;2(1):5–48. [Google Scholar]
- 36.Hulme-Beaman A, Dobney K, Cucchi T, Searle JB. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol Evol. 2016;31(8):633–645. doi: 10.1016/j.tree.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Cucchi T, Vigne JD, Auffray JC, Croft P, Peltenburg E. Introduction involontaire de la souris domestique (Mus musculus domesticus) à Chypre dès le Néolithique précéramique ancient (fin IXe et VIIIe millénaires av. J.-C.) C R Palevol. 2002;1(4):235–241. French. [Google Scholar]
- 38.Valla FR, Khalaily H. The frst sedentary peoples in Israel: Mallaha (Eynan) 1996. Bulletin du Centre de recherche français à Jérusalem. 1997;1:72–82. [Google Scholar]
- 39.Grosman L, et al. Nahal Ein Gev II, a Late Natufian community at the Sea of Galilee. PLoS One. 2016;11(1):e0146647. doi: 10.1371/journal.pone.0146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor T. Animals as Neighbors: The Past and Present of Commensal Species. Michigan State Univ Press; East Lansing, MI: 2013. [Google Scholar]
- 41.Perrot JF, Ladiray D, Solivères-Masséi O. 1988. Les Hommes de Mallaha, Eynan (Association Paléorient, Paris). French.
- 42.Bar-Yosef O. The Natufian culture in the Levant. Evol Anthropol. 1998;6(3):159–177. [Google Scholar]
- 43.Kuijt I. What do we really know about food storage, surplus, and feasting in preagricultural communities? Curr Anthropol. 2009;50(5):641–644. doi: 10.1086/605082. [DOI] [PubMed] [Google Scholar]
- 44.Schwarz E. Commensalism and domestication. Am Nat. 1939;73(746):270–278. [Google Scholar]
- 45.Slábová M, Frynta D. Morphometric variation in nearly unstudied populations of the most studied mammal: The non-commensal house mouse (Mus musculus domesticus) in the Near East and Northern Africa. Zool Anz. 2007;246(2):91–101. [Google Scholar]
- 46.Zeder MA. Pathways to animal domestication. In: Gepts P, Damania A, editors. Biodiversity in Agriculture: Domestication, Evolution, and Sustainability. Cambridge Univ Press; Cambridge, UK: 2012. pp. 227–259. [Google Scholar]
- 47.Wilkins AS, Wrangham RW, Fitch WT. The “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics. 2014;197(3):795–808. doi: 10.1534/genetics.114.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sánchez-Villagra MR, Geiger M, Schneider RA. The taming of the neural crest: A developmental perspective on the origins of morphological covariation in domesticated mammals. R Soc Open Sci. 2016;3(6):160107. doi: 10.1098/rsos.160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeder MA. Domestication as a model system for niche construction theory. Evol Ecol. 2016;30(2):325–348. [Google Scholar]
- 50.Weissbrod L. Biological indicators of occupation intensity: An environmental ethnoarchaeology of Maasai settlements. In: Dean RM, editor. The Archaeology of Anthropogenic Environments. Center for Archaeological Investigations, Southern Illinois University; Carbondale, IL: 2010. pp. 295–320. [Google Scholar]
- 51.Rohlf FJ. 2010. tpsDig 2—Thin Plate Spline Digitizer Version 2.16 (Department of Ecology and Evolution, State University of New York at Stony Brook, NY)
- 52.Bookstein FL. Landmark methods for forms without landmarks: Localizing group differences in outline shape. In: Amini A, Bookstein F, Wilson D, editors. Proceedings of the IEEE Workshop on Mathematical Methods in Biomedical Image Analysis. IEEE Computer Society; San Francisco: 1996. pp. 279–289. [Google Scholar]
- 53.Rohlf FJ. 2005. tpsRelw—Thin Plate Spline Relative Warp Version 1.41 (Department of Ecology and Evolution, State University of New York at Stony Brook, NY)
- 54.Fraley C, Raftery AE, Murphy TB, Scrucca L. 2012. mclust Version 4 for R: Normal Mixture Modeling for Model-Based Clustering, Classification, and Density Estimation, Technical Report No. 597 (University of Washington, Seattle)
- 55.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2016. [Google Scholar]
- 56.Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. J Am Stat Assoc. 2002;97(458):611–631. [Google Scholar]
- 57.Venables WN, Ripley BD. Modern Applied Statistics with S. 2nd Ed Springer; New York: 2002. [Google Scholar]