Abstract
Infectious diseases are now emerging or reemerging almost every year. This trend will continue because a number of factors, including the increased global population, aging, travel, urbanization, and climate change, favor the emergence, evolution, and spread of new pathogens. The approach used so far for emerging infectious diseases (EIDs) does not work from the technical point of view, and it is not sustainable. However, the advent of platform technologies offers vaccine manufacturers an opportunity to develop new vaccines faster and to reduce the investment to build manufacturing facilities, in addition to allowing for the possible streamlining of regulatory processes. The new technologies also make possible the rapid development of human monoclonal antibodies that could become a potent immediate response to an emergency. So far, several proposals to approach EIDs have been made independently by scientists, the private sector, national governments, and international organizations such as the World Health Organization (WHO). While each of them has merit, there is a need for a global governance that is capable of taking a strong leadership role and making it attractive to all partners to come to the same table and to coordinate the global approach.
Keywords: emerging infectious diseases, vaccines, human monoclonals, Ebola, Zika
Zika’s spread reminds us that the world is still woefully unprepared to address rapidly emerging infectious diseases. EIDs are hardly new: the Black Death in the 14th century (1), smallpox and cocoliztli in the 16th century (2), and Spanish influenza in 1918–1919 (3) were among the deadliest pandemics in human history. In the last few years, severe acute respiratory syndrome (SARS), West Nile, H5N1, H1N1, Middle East respiratory syndrome (MERS), and Ebola—in addition to Zika—have emerged. Although the majority of recent EIDs have been viral, bacterial infections are also a threat, with the devastating cholera epidemic in Haiti, the foodborne Escherichia coli O104:H4 outbreak in Germany in 2011, and the invasive nontyphoidal Salmonella in Africa representing prominent examples. Today, the major risk from bacteria comes from antibiotic-resistant strains, such as multiple-drug-resistant Staphylococcus aureus (MRSA). Whether viral or bacterial, EIDs share common attributes of unpredictability, high morbidity, the potential for explosive growth of cases, and substantial social impacts.
Demographic forces amplify the risks associated with EIDs. The global population is expected to grow to 8 billion within 8 y, with nearly 60% living in relatively crowded urban areas. By 2050, half the world’s population will live in tropical environments, which bear a much larger burden of disease (4). Rapid aging of the global population will further increase susceptibility, as both ability to fight off infection and efficacy of immunization wane with age. Additionally, air travel increases annually, with more than 100,000 flights per day as of 2014. These factors favor the emergence, evolution, and spread of new pathogens.
Compelling evidence of economic effects of infectious diseases abounds. Examples range from the economic consequences of the aforementioned historical epidemics to fallout from modern epidemics of malaria (5, 6), SARS (7), Ebola (8, 9), and dengue (10). The World Bank estimates the anticipated gross domestic product (GDP) cost of a moderately severe global flu pandemic at about 5% of world income (roughly $3.5 trillion—a sum greater than Germany’s GDP) (11, 12). The full cost of such an epidemic, including the lost value of health and longevity, is nearly an order of magnitude higher (13). Initial estimates indicate that Zika—in addition to causing significant human hardship—will result in $3.5 billion in economic losses in 2016 alone (14).
Despite the massive health and economic risks posed by infectious diseases with epidemic potential, the global health community has largely failed to take a proactive approach to coping with outbreaks so far. EIDs need to be attacked on multiple fronts, including basic research on the nature and emergence of new pathogens and health systems’ strengthening in the form of epidemiological surveillance and the development of new antimicrobial agents and of faster, cheaper, and more targeted diagnostics. Many researchers and policymakers within the global health community are busy undertaking the task of identifying our shortcomings in the realm of epidemic preparedness and conceptualizing ways to enhance our capacity to respond to infectious disease threats along several of these fronts. For example, Sands et al. (15) have proposed a comprehensive approach to improving our understanding of the world’s economic vulnerability to infectious disease crises that requires data collection and analysis to be undertaken by multiple organizations at multiple levels.
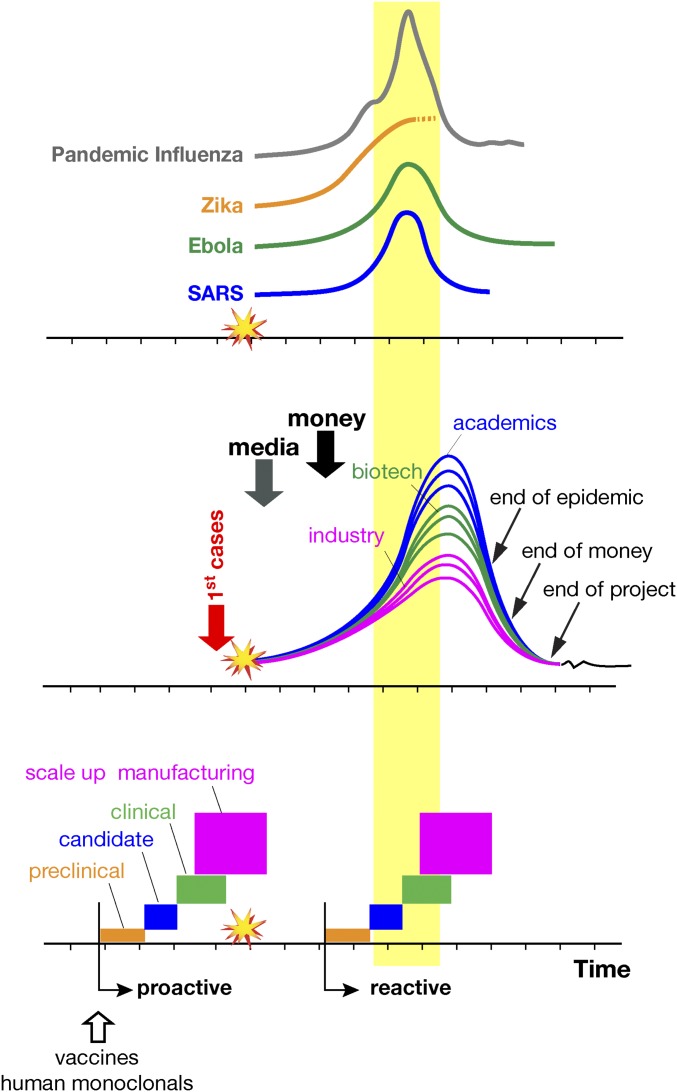
In addition, the development of new vaccines is especially needed to provide a frontline defense against disease transmission. Fig. 1 is a schematic representation of our responses to SARS, H1N1 pandemic influenza, Ebola, and Zika. Generally, a newly emerging infectious disease is met with media-driven global panic followed by a fresh wave of research funding and a push for vaccine development. Subsequently, many scientists in academia and biotechnology companies rush into the field, attracted by the new challenge and funding. Large vaccine companies, motivated mainly by public-sector pressure and social responsibility, overlook the high likelihood of failure to make a profit or even recover costs (due to low ability to pay in many locations where new infections emerge) and join the race. Meanwhile, regulatory agencies become more accessible and flexible to hasten the process of vaccine development and distribution.
Fig. 1.
Schematic representations of the progression of some emerging diseases (Top), the progression of vaccine development activities following an emerging infection (Middle), and the steps required for vaccine delivery using the present, reactive, approach compared with the proposed, proactive, approach for vaccines and human monoclonals (Bottom). Temporal scales and frequency of cases are for illustrative purposes only and differ for each disease. Industry indicates large vaccine manufacturers.
The expectation is to deliver a vaccine overnight, although the process is likely to take at least 5 y even under the most accelerated procedures. While vaccine development races on, basic quarantine procedures may eventually control the disease, as occurred in the cases of the SARS and Ebola outbreaks. Alternatively, the outbreak may resolve itself if a mild infection spreads across the population and creates a herd immunity effect. Once the outbreak is over, the media direct their attention elsewhere, donors divert funding to other priorities, and vaccine developers are left frustrated by the opportunity costs associated with their having diverted resources from normal vaccine research, development, and production to an economically and socially futile exercise. If the outbreak recurs, the global health establishment so far has not been more prepared to combat it than it was at the start. The exception has been Ebola, where a vaccine was developed and shown to be efficacious. Although the Ebola vaccine was too late to be useful in the 2014 epidemic, it will be there to face future risks. In short, the current approach has been reactive and ineffective, has paid little heed to the fact that traditional business models routinely let us down, and has done little to shore up the public’s defense against future outbreaks.
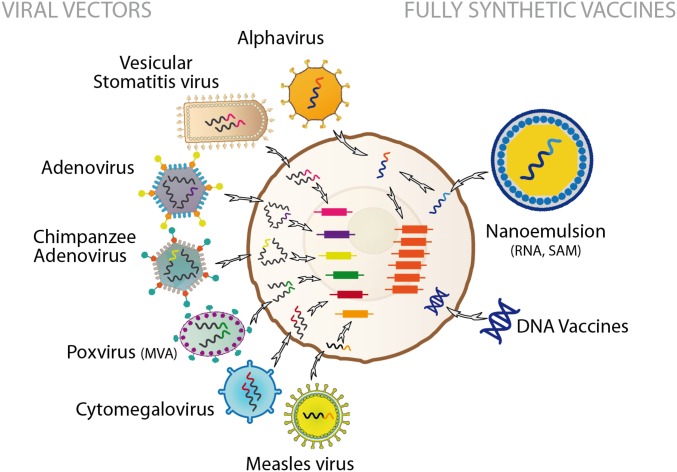
Recently, exciting progress has been made in the development of platform technologies. These platforms use a module (based on a viral vector, synthetic DNA or RNA, or a bacterial construct) to deliver a synthetic gene that encodes an immunity-inducing agent or nanoparticles containing an antigen (Fig. 2 and refs. 16 and 17). The major advantage of these platforms is that once they have been developed and licensed for one vaccine, development of the next vaccine requires only substitution of the synthetic gene, allowing a manufacturing plant to move to a new target disease with minimal changes in chemistry, manufacturing, and controls. Thus, new vaccine development can focus on the safety and efficacy of the inserted gene. Furthermore, the ability of platforms to target multiple pathogens helps justify the investment required to build and maintain manufacturing plants that specialize in one platform, because a single plant can be ready to produce multiple vaccines at any time.
Fig. 2.
Schematic representation of the most common viral vectors used to deliver synthetic gene coding for vaccine antigens into a mammalian cell (Left) or fully synthetic vaccines based on RNA and DNA (Right).
However, even with a streamlined regulatory process and platform technologies, vaccine development is inherently slow, often requiring many years. Human monoclonal antibodies could help bridge the gap. New technologies allow us to clone human B cells, sequence their Ig genes, and express large amounts of human monoclonals as full-length antibodies, single chain, or as Fab fragments. In addition, we can engineer monoclonals to obtain longer half-life, to remove the Fc receptor-mediated disease enhancement observed in the case of dengue and Zika viruses, and to enhance potency and breadth. An s.c. injection of 20–100 mg of a potent human monoclonal that induces protection from infection and persists for few weeks or months is now conceivable, as recently demonstrated in the case of HIV (18). Because antibodies are faster to develop and license than vaccines, they could represent a natural first line of protection. In the case of the Ebola outbreak, monoclonal antibodies would have reduced the mortality in health-care workers and potentially reduced the spread of disease. Because large quantities of multiple potent antibodies can be stockpiled and kept ready to deploy should an outbreak occur, monoclonals offer great promise against most known viral threats. One possible strategy could be to generate potent cross-reactive monoclonals against most known viral threats and be ready to deploy large quantities of antibody should an outbreak occur. Even in the case of an unknown pathogen, this technology, if ready to go, could allow rapid isolation of human monoclonals from convalescent individuals to produce the first tool to rapidly treat the new pathogen and prevent its transmission. Additionally, in the future, these potent monoclonals may not need to be manufactured and injected but could be produced by each individual following injection of the gene coding for the antibody, using RNA or DNA.
Although we have the technological capacity to better position ourselves in terms of epidemic preparedness and response, we currently lack an economically sustainable, proactive strategy that adequately addresses six key questions: (i) which immunologics to prioritize, (ii) which vaccine or antibody developers and platforms to select for investment, (iii) how much of a subsidy to provide to companies willing to develop prioritized immunologics, (iv) how much of any subsequent financial return on the products developed should revert to the funders, (v) what the sources of funding should be, and (vi) what the scale of operation should be. Ideally, the global health community should work together to institute, fund, and support an organization that can oversee and promote global epidemic preparedness.
Several institutional innovations that attempt to bridge the gap between technological feasibility and field-ready (or nearly field-ready) interventions are currently under discussion. The first, proposed by three eminent vaccinologists, is to raise $2 billion to establish a Global Vaccine Development Fund (GVDF) to support development up to the proof-of-concept stage of multiple vaccines that the private sector refrains from developing because they are commercially unattractive (19).
The second—which grew out of the concept for the GVDF and is being spearheaded by the governments of Norway, Japan, Germany, and India, the Wellcome Trust, and the Bill & Melinda Gates Foundation, among others—is the Coalition for Epidemic Preparedness Innovations (CEPI) (20). As part of its preliminary business plan, CEPI has identified a goal of raising $1 billion for its first 5 y with the specific goal of funding the development of at least four vaccine candidates for two to three priority pathogens (21). The objective is to help these candidates progress through the proof-of-concept stage and up to the point at which full clinical efficacy testing is feasible, advancing their readiness for scale-up in case of an outbreak. In January of 2017, CEPI announced that it had raised $460 million to date and began soliciting proposals for vaccines against MERS, Lassa, and Nipah viruses (22).
The third idea, originally proposed by GlaxoSmithKline, involves creating a dedicated biopreparedness organization (BPO). This would be a permanent, platform-based entity that is fully embedded within an experienced industrial research and development organization, with the scope to develop vaccines to clinical proof of concept and to maintain an adequate supply of clinical materials for a subsequent evaluation of efficacy, and potentially a large enough supply for an emergency intervention. The BPO would be a nonprofit, separate legal entity and work on two or three vaccines on an ongoing basis, using dedicated people and assets such as intellectual property, platform technologies, and adjuvants. Public and philanthropic organizations would provide long-term funding for the BPO, along with governance to promote monitoring and accountability.
Although the above proposals have much merit and represent a substantial step forward from the pre-Ebola era, in the most optimistic scenario they would support the development up to proof of concept of only a handful of new vaccines in the first 5 y. This would still leave us short of providing adequate protection against all 11 of the priorities identified by the WHO’s Research and Development Blueprint for Action to Prevent Epidemics (23) and would leave us well short of protecting against all 30 emerging and reemerging infectious diseases identified by Morens et al. (24). In addition, human monoclonal antibodies are not yet part of this plan.
Accessing and coordinating leading-edge global capacity and funding would make it possible to target development of vaccines and antibodies against most of the emerging and reemerging threats. For instance, if each qualified vaccine or antibody developer in a high- or low-income country would receive funding from the national government or from global funding agencies to develop one vaccine or antibody against one of the identified diseases, the world could simultaneously target 20 or 30 different diseases.
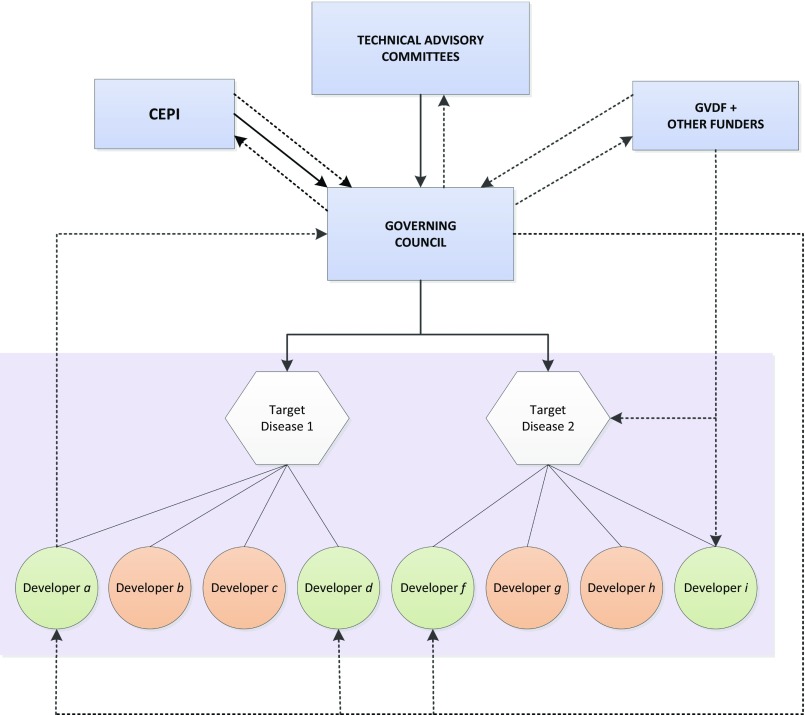
This would require an independent global agency, possibly under the authority of the WHO, G7, or both, that would be able to develop and implement a coherent and transparent global agenda and coordinate efforts among vaccine and antibody manufacturers (Fig. 3). CEPI, for example, although still nascent, could evolve into an organization well-suited to fulfilling global biopreparedness needs by adopting the following recommendations aimed at best addressing the six key questions noted above.
Fig. 3.
Schematic of a global collaborative approach to emerging infectious diseases. Dotted lines represent funding flows. Solid lines represent processes and information.
With respect to identifying which immunologics to prioritize, the agency would likely begin (as CEPI does) with the WHO’s Research and Development Blueprint for Action to Prevent Epidemics, which contains an initial list of pathogens likely to cause epidemics and a list of diseases that necessitate relatively quick action (23). Additionally, the agency would collect and review scientific opinion and analyze data regarding the disruptive potential—in terms of both population health and the global economy—of a multiplicity of infectious diseases and alternative approaches to defend against them.
The agency’s governing council would put out calls for vaccine and antibody development proposals from private companies or academic institutions. It would review the competitors’ proposals and choose vaccine and antibody developers on the basis of the technical and financial components of their bids. By defraying costs and reducing commercial risk, the agency would incentivize the development of vaccines that would otherwise go undeveloped. In case the immunologic subsequently generates some profit, these proposals could also specify, and be judged on, the share of revenues realized from sales that would be channeled back to the agency. This mechanism for sharing both risks and rewards would in part support ongoing operations and future vaccine development efforts.
Whereas the provision of long-term funding is difficult for traditional funding agencies, initially governments should represent the primary source of funding for the agency. In some countries, plausible mechanisms for generating agency funds already exist. For instance, the United States could increase its excise tax on the administration of CDC-recommended childhood vaccines from $0.75 to $1 or $1.50. The European Union’s mechanism for funding permanent research infrastructures, which is currently supported by a partnership between the European Investment Bank and the European Commission, could be used as a source of long-term funding.
The amount that each country contributes should vary according to expected benefits, as indicated by population size and income per capita. All governments throughout the world would be expected to contribute.
Beyond their baseline commitments to the agency’s general fund, donors would also have the opportunity to fund, or fund at higher levels, preferred developers and disease targets, provided that these proposals meet the needs and the quality established by the agency. This would encourage further vaccine and antibody development without undue overlap and inefficiency. Eventually, returns from products that are brought to market will contribute to the agency’s funding. Although each of the agency’s donors would commit to a certain level of funding, the financial scope of the agency’s activities should not be determined ex ante. Instead, the commitments made by national governments and other donors (e.g., philanthropic organizations) should be considered as a funding ceiling. The agency would only draw on the potential funds allocated by the donors when it approves specific proposals. That way, there will not be pressure to spend down available financial resources unless particular needs for funding and plausible solutions are identified.
The primary advantage of the proposed approach is that dedication of resources is rationally directed, proactive, and sustained, allowing more efficient development of capacity and more likely and timely availability of appropriate interventions. Central coordination of decision making and involvement of a broad coalition of governments and private sector institutions would enable development of vaccines or antibodies for many anticipated threats at once and thus allow an armamentarium of interventions against the most likely threats. In addition, targeting multiple diseases and using sharable platforms would make dedicated manufacturing infrastructure developed through this process economically viable and readily available. Also, availability of this infrastructure and knowledge of rapidly deployable platforms would allow a faster response to an unanticipated threat. Finally, the fact that some of the return on investment from each successful project would fund future research and development of publicly targeted vaccines would deflect the popular complaint that pharmaceutical companies earn monopoly profits from public investments in basic and applied research.
Ultimately, the aim of the foregoing suggestions is to produce an organization that effectively balances the efficient production of a global public good with an equitable distribution of the financial burden for creating that good, while avoiding wasteful resource allocation. As a consequence, the global population will be better positioned to rationally and efficiently defend itself against a difficult-to-define set of bacterial and viral threats to its collective future.
Acknowledgments
We thank Daniel Cadarette and Catherine Mallia for their extremely helpful research and editorial assistance.
Footnotes
D.E.B. and S.B. declare no conflict of interest. R.R. is a full-time employee of GlaxoSmithKline Vaccines.
This article is a PNAS Direct Submission.
See Editorial on page 4031.
References
- 1.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366(5):454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 2.Acuna-Soto R, Stahle DW, Cleaveland MK, Therrell MD. Megadrought and megadeath in 16th century Mexico. Emerg Infect Dis. 2002;8(4):360–362. doi: 10.3201/eid0804.010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter CW. A history of influenza. J Appl Microbiol. 2001;91(4):572–579. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 4.State of the Tropics 2014. State of the Tropics 2014 report (James Cook Univ, Cairns, Australia)
- 5.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1-2) Suppl:85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 6.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 7.Keogh-Brown MR, Smith RD. The economic impact of SARS: How does the reality match the predictions? Health Policy. 2008;88(1):110–120. doi: 10.1016/j.healthpol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartsch SM, Gorham K, Lee BY. The cost of an Ebola case. Pathog Glob Health. 2015;109(1):4–9. doi: 10.1179/2047773214Y.0000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MR, et al. 2015. The economic impact of Ebola on Sub-Saharan Africa: Updated estimates for 2015 (World Bank Group, Washington, DC)
- 10.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84(2):200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns A, van der Mensbrugghe D, Timmer H. 2008. Evaluating the economic consequences of avian influenza. Report no. 47417 (World Bank Group, Washington, DC)
- 12.Jonas OB. 2013. Pandemic risk (World Bank Group, Washington, DC)
- 13.Fan VY, Jamison DT, Summers LH. 2016. The inclusive cost of pandemic influenza risk. NBER Working Paper no. 22137 (National Bureau of Economic Research, Cambridge, MA)
- 14.World Bank 2016 The short-term economic costs of Zika in Latin America and the Caribbean (LCR) (World Bank Group, Washington, DC). Available at pubdocs.worldbank.org/en/410321455758564708/The-short-term-economic-costs-of-Zika-in-LCR-final-doc-autores-feb-18.pdf.
- 15.Sands P, El Turabi A, Saynisch PA, Dzau VJ. Assessment of economic vulnerability to infectious disease crises. Lancet. 2016;388(10058):2443–2448. doi: 10.1016/S0140-6736(16)30594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewer KJ, et al. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheid JF, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–572. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plotkin SA, Mahmoud AAF, Farrar J. Establishing a global vaccine-development fund. N Engl J Med. 2015;373(4):297–300. doi: 10.1056/NEJMp1506820. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. September 2, 2016 New vaccine coalition aims to ward off epidemics. Science. Available at www.sciencemag.org/news/2016/09/new-vaccine-coalition-aims-ward-epidemics.
- 21.Røttingen J-A, et al. New vaccines against epidemic infectious diseases. N Engl J Med. 2017;376(7):610–613. doi: 10.1056/NEJMp1613577. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. A half-billion-dollar bid to head off emerging diseases. Science. 2017;355(6322):237. doi: 10.1126/science.355.6322.237. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization (May 2016) An R&D blueprint for action to prevent epidemics. Available at apps.who.int/medicinedocs/documents/s22472en/s22472en.pdf.
- 24.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]