Polycystic ovary syndrome (PCOS) afflicts ∼15% of women in their reproductive years (1). PCOS women exhibit high circulating levels of testosterone (T), intermittent or absent menstrual cycles, and polycystic ovaries on ultrasound, or at least two of these three diagnostic criteria (2). Hyperandrogenism is at the core of PCOS, its ∼70% heritability, and likely its pathogenic origin (1). PCOS pathophysiology, however, extends well beyond hirsutism and infertility to encompass hypothalamic neuroendocrine dysfunction, and of particular concern, increased risks for metabolic disease and cancer associated with long-term morbidity and mortality (3). PCOS origins are currently considered polygenic (4) and prepubertal (5), with over 21 replicated candidate genes affecting gonadotropin secretion and action, extracellular matrix formation, and various common cellular functions. Such PCOS risk genes, however, account for <10% of patient presentation. A definitive etiology for PCOS remains elusive and delays progress toward a cure.
In contrast, evidence from animal models has consistently indicted T in the pathogenic origins of PCOS-like traits (6). Naturally occurring hyperandrogenic female monkeys spontaneously exhibit PCOS-like traits (7), and early development T or dihydrotestosterone (DHT) exposure in female rodents, sheep, and monkeys reproduces a variety of PCOS-like traits (8), demonstrating potential genetic, epigenetic, and developmental contributions to PCOS pathogenesis mediated by androgen receptor (AR) signaling.
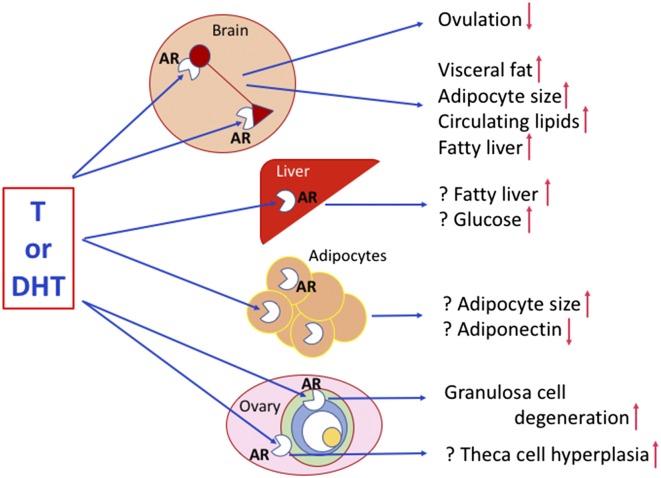
Caldwell et al. (9) recently took this molecular understanding to a new level by blocking AR gene expression in female mice and preventing PCOS-like trait development after fetal DHT exposure, specifically implicating AR action in PCOS developmental pathogenesis. In PNAS, the same group (10) refine this molecular precision and convincingly demonstrate how neuronal AR genomic excision, alone, protects female mice from certain PCOS-like traits induced by prepubertal onset of DHT excess. AR-enabled conversion to a PCOS-like phenotype therefore occurs predominantly in neurons, as protection provided by neuron-specific AR deletion (NeuroARKO) and not granulosa cell-specific AR deletion (GCARKO) closely emulates protection afforded by global AR knockout (ARKO), and thus designates the brain as a potential “ground zero” for much of PCOS-like pathogenesis (Fig. 1).
Fig. 1.
Schematic diagram of hypothetical sites for AR-mediated conversion to PCOS-like traits derived from the molecular modeling of Caldwell et al. (10). Question marks indicate deductive speculation based on Caldwell et al. (10). In the ovarian preovulatory follicle, green indicates theca cells, blue represents granulosa cells, white is the fluid-filled antrum, and yellow is the oocyte. While providing a potential molecular blueprint for sites of androgen induction of PCOS-like traits, Caldwell et al. (10) provide no clues as to the molecular mechanisms generating the androgen excess.
Caldwell et al. (10) bred ARflox mice (AR exon 3 flanked by loxP sites inserted into the genome) to Sox2-Cre mice to delete AR from every cell in the body to derive the global ARKO mice (Sox2-Cre enables excision of AR exon 3 at loxP sites, blocking AR expression). Substitution of the universal deletor, Sox2-Cre, for the well-validated CamKIIα-Cre mouse produced NeuroARKO (Cre-enabled deletion of AR exon 3 only occurs in neurons), and substituting an antimullerian hormone-Cre mouse resulted in ovarian GCARKO (AR exon 3 deletion in granulosa cells only). Postnatally, and before puberty, wild-type and all types of ARKO female mice were housed in female-only groups and subcutaneously implanted with DHT-filled or empty (control) capsules for 14 wk to elicit PCOS-like traits in DHT-implanted animals (11). This commentary will focus on trait protection from DHT conversion in ARKO and NeuroARKO mice, given the minimal protection afforded by ovarian GC deletion of AR.
An obvious first point is the distinct phenotype expressed by the different AR gene block models without DHT exposure (12). Caldwell et al. (10) recognize this and include separate groups of each different type of ARKO female implanted with empty capsules as specific controls for their contemporaries implanted with DHT. For example, global ARKO without DHT treatment exhibit elevated circulating levels of T, DHT, and estrogenic metabolites of T, diminished estradiol (E2) levels, diminished E2-mediated negative feedback on luteinizing hormone (LH), deficient ovulatory LH surges, normal pituitary LH responses to exogenous gonadotropin-releasing hormone (GnRH), diminished number of ovulation sites per estrus cycle and litter sizes, and increased numbers of abnormal ovarian follicles (12). Second, with the exception of elevated systolic blood pressure, AR signaling is required for all DHT-induced PCOS-like traits. Absence of cardiovascular protection may suggest DHT-induced changes in estrogen alter estrogen receptor-mediated regulation of blood pressure (13). Third, this is a prepubertal pathogenic onset model for PCOS, testing one developmental origin hypothesis for PCOS (14), and different results might be achieved with discrete, prenatal DHT exposure during a fetal “developmental window” for many organ systems, including the brain (8). Fourth, and perhaps most telling, hyperandrogenism is used to generate a pathogenic origin. Hyperandrogenic pathogenesis is implicit in most animal models of PCOS.
In examining the role of neuronal AR in mediating reproductive dysfunction, Caldwell et al. (10) pinpoint the brain as the site of AR-mediated PCOS-like conversion: ovaries in both ARKO and NeuroARKO DHT-treated females still exhibit fresh corpora lutea, and wild-type ovaries transplanted to ARKO females at the onset of DHT treatment retain normal estrus cyclicity. Commendably, Caldwell et al. (10) are not misled by DHT-minimized changes in vaginal cytology across the estrus cycle providing a false indication of anovulatory cycles in NeuroARKO females. DHT is likely disrupting ovulation-inducing gonadotropin surges by diminishing kisspeptin secretion (12) in AR-expressing kisspeptin/neurokinin B neurons in the hypothalamus (15, 16), key neuronal regulators of GnRH release. Because diestrus levels of gonadotropins, T, and progesterone are not elevated in any DHT treatment group, there may be no additional AR-mediated disruption of E2 and progesterone-mediated negative feedback at the hypothalamic-pituitary level. DHT-induced defects in NeuroARKO folliculogenesis, however, may indicate gonadotropin abnormalities unrelated to ovulation. Inability to demonstrate an ovulatory gonadotropin surge is not typical of PCOS women, as short-duration circumvention (17) of negative feedback defects in gonadotropin regulation (14) reliably induce ovulation.
A neuronal site for PCOS reproductive pathogenesis would be entirely consistent with recent neurokinin B NK3-receptor antagonist treatment of women with PCOS. In a recent clinical trial (18), the NK3 receptor antagonist AZD4901 diminishes basal LH, LH pulse frequency, and basal T, eliciting an 18% versus placebo 6% incidence of ovulation. Novel therapeutic targeting of PCOS neuroendocrine reproductive pathophysiology may thus yield fertility dividends and dispense with current systemic estrogen receptor agonists/antagonists or aromatase inhibitors (17).
Ovarian manipulation, nevertheless, contributes a modicum of insight into pathogenic molecular mechanism. CGARKO, alone, protects against GC degeneration typical of PCOS ovaries (19), implicating AR-mediated antagonism of folliculogenesis. ARKO, in contrast, provides the only protection against theca cell layer hypertrophy, despite inducing hyperandrogenism relative to wild-type controls (12). This theca cell result implicates
Caldwell et al. have established a rich molecular tool box from which future studies can dissect out organ-specific PCOS-like pathogenesis in fetal to prepubertal mouse models of DHT-induced traits.
AR-mediated action in theca cell hyperplasia observed in polycystic ovaries (19).
The standout result of the Caldwell et al. paper (10) involves NeuroARKO (and ARKO) protection against DHT-induced metabolic dysregulation, including abolition of increases in visceral fat, pronounced adipocyte hypertrophy and fatty liver, as well as hyperlipidemia. It is interesting to speculate whether blocking AR expression in hypothalamic neurons releasing agouti-related peptide, neuropeptide Y, or prepro-opiomelanocortin (20), neuronal peptides regulating food intake and metabolism, prevents AR-mediated conversion to PCOS-like metabolic dysfunction. Such an interpretation would be consistent with flutamide (AR antagonist) prevention of prenatal DHT programming of agouti-related peptide neuronal changes in PCOS-like sheep (20) and flutamide-improved lipid profiles in women with PCOS (21). Although flutamide can result in hepatoxicity, the prospect of novel directed AR antagonism, active solely within the central nervous system, and pioneered by inclusion of optogenetic and DREADD approaches in Cre-lox–generated mouse models (22), including those generated here (10), hold tremendous promise for application to PCOS-related metabolic dysregulation.
Aspects of metabolic dysregulation prevented by ARKO, but not by NeuroARKO, set up an interesting dichotomy and imply an additional AR-mediated PCOS conversion site outside the brain. In contrast to ARKO, NeuroARKO fails to prevent DHT-induced hyperglycemia, diminished adipocyte release of adiponectin, and only partially prevents fatty liver and adipocyte hypertrophy. In the absence of insulin resistance, Caldwell et al. (10) propose a causal contribution from AR-mediated dysregulation of hepatic glycogen. In addition, a recent series of studies in women demonstrating AR-mediated constraint on adipocyte maturation, as well as T-associated increases in visceral fat and hyperlipidemia in PCOS women with minimal loss of insulin sensitivity (23), implicate the adipocyte alongside the hepatocyte as potential future sites for organ specific blockade of AR expression, identifying specific contributions to PCOS-like lipid dysregulation.
In sum, Caldwell et al. (10) have established a rich molecular tool box from which future studies can dissect out organ-specific PCOS-like pathogenesis in fetal to prepubertal mouse models of DHT-induced traits. The challenge now becomes application of analogous molecular precision to nonhuman primate models to develop appropriately targeted therapeutics for women with PCOS.
Footnotes
The author declares no conflict of interest.
See companion article on page E3334.
References
- 1.Dumesic DA, et al. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauser BC, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Wild RA, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Perspectives in polycystic ovary syndrome: From hair to eternity. J Clin Endocrinol Metab. 2016;101:759–768. doi: 10.1210/jc.2015-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchen LC, et al. Evidence for increased 5α-reductase activity during early childhood in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101:2069–2075. doi: 10.1210/jc.2015-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakim C, Padmanabhan V, Vyas AK. Gestational hyperandrogenism in developmental programming. Endocrinology. 2016;158:199–212. doi: 10.1210/en.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott DH, et al. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017;32(4):923–936. doi: 10.1093/humrep/dex036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters KA. Androgens in polycystic ovary syndrome: Lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23:257–263. doi: 10.1097/MED.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell AS, et al. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156:1441–1452. doi: 10.1210/en.2014-1887. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell ASL, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114:E3334–E3343. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell AS, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155:3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 12.Cheng XB, et al. Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am J Physiol Endocrinol Metab. 2013;305:E717–E726. doi: 10.1152/ajpendo.00263.2013. [DOI] [PubMed] [Google Scholar]
- 13.Milner TA, et al. Cellular and subcellular localization of androgen receptor immunoreactivity relative to C1 adrenergic neurons in the rostral ventrolateral medulla of male and female rats. Synapse. 2007;61:268–278. doi: 10.1002/syn.20370. [DOI] [PubMed] [Google Scholar]
- 14.Burt Solorzano CM, et al. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–337. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of neurokinin-3 and κ-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157:752–763. doi: 10.1210/en.2015-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20:485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legro RS. Ovulation induction in polycystic ovary syndrome: Current options. Best Pract Res Clin Obstet Gynaecol. 2016;37:152–159. doi: 10.1016/j.bpobgyn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 18.George JT, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101:4313–4321. doi: 10.1210/jc.2016-1202. [DOI] [PubMed] [Google Scholar]
- 19.Chang RJ, Cook-Andersen H. Disordered follicle development. Mol Cell Endocrinol. 2013;373:51–60. doi: 10.1016/j.mce.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol. 2011;23:401–411. doi: 10.1111/j.1365-2826.2011.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamanti-Kandarakis E, Mitrakou A, Raptis S, Tolis G, Duleba AJ. The effect of a pure antiandrogen receptor blocker, flutamide, on the lipid profile in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2699–2705. doi: 10.1210/jcem.83.8.5041. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JL, Enriori PJ. A talk between fat tissue, gut, pancreas and brain to control body weight. Mol Cell Endocrinol. 2015;418:108–119. doi: 10.1016/j.mce.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Dumesic DA, et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab. 2016;101:4178–4188. doi: 10.1210/jc.2016-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]