Significance
Protein secretion is an essential determinant of bacterial physiology and virulence. Members of the Corynebacteriales order have evolved a complex cell envelope containing two membranes, a plasma membrane and an outer membrane, called the mycomembrane, which harbors mycolic acids and outer membrane proteins (OMPs) of unusual structure. Here, we have investigated the biogenesis of OMPs in Corynebacterium glutamicum and deciphered the role of O-mycoloylation in targeting OMPs to the mycomembrane. Partially enabled by our methodology, we found that the posttranslational state of major OMPs determined their presence in the outer membrane vs. the extracellular medium. We have also uncovered a short linear amino acid motif for O-acylation of proteins that seems to be preserved throughout the kingdoms.
Keywords: Corynebacteriales, O-acylation, sequence motif, top-down proteomics, NMR
Abstract
The outer membranes (OMs) of members of the Corynebacteriales bacterial order, also called mycomembranes, harbor mycolic acids and unusual outer membrane proteins (OMPs), including those with α-helical structure. The signals that allow precursors of such proteins to be targeted to the mycomembrane remain uncharacterized. We report here the molecular features responsible for OMP targeting to the mycomembrane of Corynebacterium glutamicum, a nonpathogenic member of the Corynebacteriales order. To better understand the mechanisms by which OMP precursors were sorted in C. glutamicum, we first investigated the partitioning of endogenous and recombinant PorA, PorH, PorB, and PorC between bacterial compartments and showed that they were both imported into the mycomembrane and secreted into the extracellular medium. A detailed investigation of cell extracts and purified proteins by top-down MS, NMR spectroscopy, and site-directed mutagenesis revealed specific and well-conserved posttranslational modifications (PTMs), including O-mycoloylation, pyroglutamylation, and N-formylation, for mycomembrane-associated and -secreted OMPs. PTM site sequence analysis from C. glutamicum OMP and other O-acylated proteins in bacteria and eukaryotes revealed specific patterns. Furthermore, we found that such modifications were essential for targeting to the mycomembrane and sufficient for OMP assembly into mycolic acid-containing lipid bilayers. Collectively, it seems that these PTMs have evolved in the Corynebacteriales order and beyond to guide membrane proteins toward a specific cell compartment.
Protein lipidation, once assumed to act as a stable membrane anchor for soluble proteins, is now attracting widespread attention for its emerging role in diverse signaling pathways and regulatory mechanisms (1). Among the different classes of posttranslational protein fatty acid modifications, O-acylation is quite rare. Only a few examples have been reported, mostly in eukaryotes. For example, the O-octanoylation of ghrelin, a secreted 28-aa peptide, is essential for growth hormone, orexigenic, metabolic, and insulin secretion effects (2, 3), whereas the O-palmitoleylation of Wingless (Wnt) signaling proteins is required for targeting to the endoplasmic reticulum and secretion and activation of cell surface receptors (4, 5). In contrast, O-mycoloylation recently discovered in Corynebacteriales (6) is the only type of O-acylation found in bacteria. This modification consists of the addition of a mycoloyl residue, a C32-C36 α-alkyl, β-hydroxy-fatty acyl chain, to serine residues through an O-ester linkage. O-mycoloylation is catalyzed by a well-conserved mycoloyltransferase C (cMytC), which was found to be associated with the bacterial cell envelope and secreted into the extracellular medium (7). cMytC belongs to the α/β hydrolase superfamily and uses trehalose dimycolate and protein as substrates.
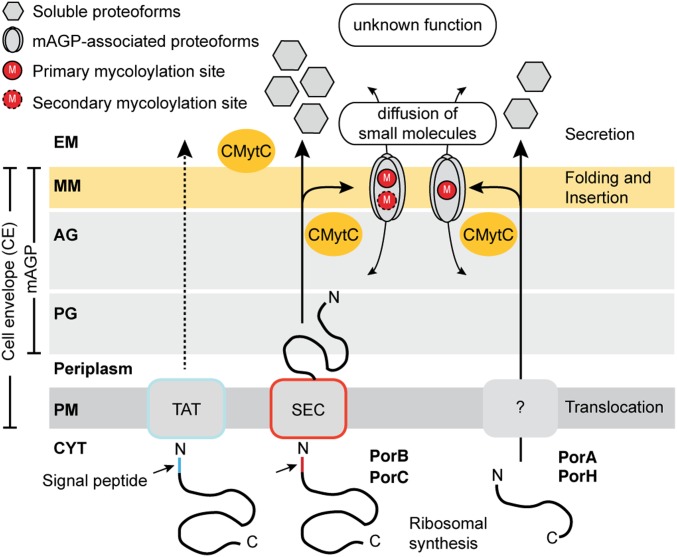
Corynebacteriales, which are diderm Gram-positive bacteria, include species like Corynebacterium glutamicum, which has been harnessed for industrial production of amino acids, as well as Corynebacterium diphtheriae and Mycobacterium tuberculosis, which cause devastating human diseases. Corynebacteriales contain a unique structure termed the mycoloyl–arabinogalactan–peptidoglycan (mAGP) complex. The mAGP complex consists of a heteropolymer of peptidoglycan covalently linked to arabinogalactan, which in turn, is covalently associated with mycolic acids of the inner leaflet of the outer membrane. The collection of mycolic acids and associated membrane proteins is referred as the mycomembrane. To date, bacterial O-mycoloylation has been reported on small porins located in the cell envelope of C. glutamicum and homologs, including the cation-selective channels PorA (45 residues), PorH (57 residues), and protein of unknown function, ProtX, all of which are small polypeptides (6). Although the function and underlying mechanisms of O-mycoloylation remain elusive, the biological consequences of having long acyl moieties attached to proteins include enhancement of membrane binding as well as protein–protein interactions. For example, in vitro studies revealed that protein posttranslational modification (PTM) was essential for PorA/PorH pore-forming activity (8). Interestingly, the mycoloylated PorA, PorH, and ProtX proteins all lack dedicated signal sequences for export but are only found in the mycomembrane. Hence, it can be assumed that posttranslational O-mycoloylation may provide a structural element for subcellular targeting of secreted outer membrane proteins (OMPs).
Thus far, studies have failed to identify a well-defined sequence in mycomembrane-associated proteins that can function as a targeting signal. To decipher the putative role of O-mycoloylation in the biogenesis of mycomembrane-associated proteins, we used a strategy based on homologous expression and structural characterization of the four major porins in C. glutamicum, all of which are found or thought to be found in the mycomembrane. This set includes the cation-selective channels PorA and PorH, which do not contain any leader sequence and are posttranslationally modified by a mycolic acid on serine residues S15 and S56 (6, 9), respectively. In addition, we selected the anion-selective channel PorB and its homolog PorC containing 99 and 97 residues, respectively, after proteolytic cleavage of the N-terminal signal sequence specific for the Sec apparatus. The α-helical PorB (10) is known to be targeted to the cell wall, whereas PorC, by virtue of its colocation in the same operon, is thought to be similarly targeted, although no evidence currently exists for this (11). We first investigated the partitioning of endogenous or recombinantly expressed PorA, PorH, PorB, and PorC porins between bacterial compartments. Next, we purified proteins from each cell compartment and identified proteoforms containing PTMs that are associated with specific cellular compartments. We determined the nature and the position of the PTMs by combining site-directed mutagenesis, top-down electrospray ionization (ESI)-MS/MS, and NMR spectroscopy and propose short linear motifs for O-acylation of proteins from different kingdoms. Finally, we showed that the presence of one or multiple mycoloyl residue(s) constitutes the main driving force for membrane association and is essential for targeting porins to the mycolic acid-containing lipid bilayer.
Results
C. glutamicum Porins Are Targeted to the mAGP Complex and Secreted into the Extracellular Medium.
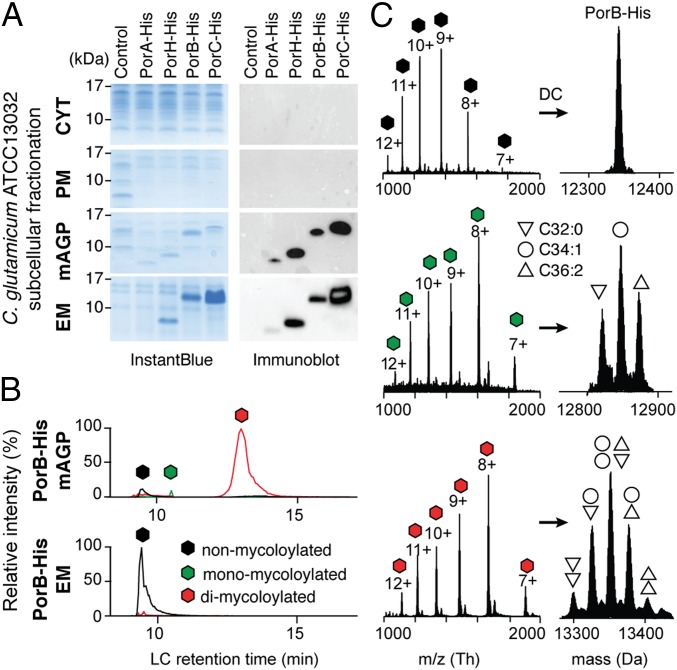
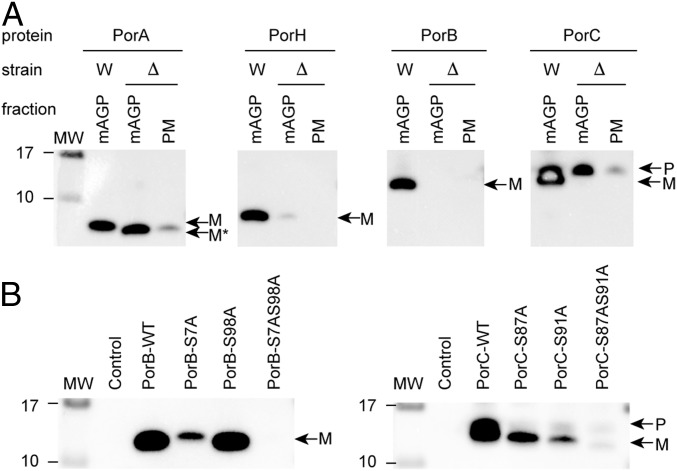
We first created recombinant expression systems for C. glutamicum porins in the native host and compared the distribution of His-tagged PorA, PorH, PorB, and PorC within the bacterial compartments. These experiments were carried out in both exponential and stationary growth phases of WT C. glutamicum (ATCC13032) expressing individual porA, porH, porB, and porC genes with native signal sequences using an isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible Ptac promoter on high-copy plasmids (Table S1). After cell fractionation, the cytosol (CYT), the plasma membrane (PM), the mAGP comprising the mycomembrane-associated components, and the extracellular medium containing secreted proteins were isolated. Pure PM and mAGP fractions were obtained by sucrose density ultracentrifugation using a procedure described elsewhere (12). Cell debris was removed from CYT and extracellular medium fractions by two cycles of ultracentrifugation. SDS/PAGE followed by Western blot analysis of subcellular CYT, PM, and mAGP fractions showed that PorA, PorH, and PorB were correctly and specifically targeted to the outer membrane, and no residual signal was observed in other cell compartments (Fig. 1A). PorC was also found associated with the mAGP, constituting evidence for its specific targeting. Surprisingly, significant amounts of PorH, PorB, and PorC and traces of PorA were also detected in the extracellular medium, indicating the existence of soluble proteoforms secreted by the bacteria (Fig. 1A).
Table S1.
Plasmids and strains used in this study
| Materials | Properties | Source |
| Plasmids | ||
| pAN6 | KanR, C. glutamicum/E. coli shuttle vector with LacIq, Ptac, pBL1 oriV C. glutamicum, pUC18 oriV E. coli | Laboratory collection of R. Freudl and M. Bott (Institut für Bio- und Geowissenschaften 1, Biotechnologie, Forschungszentrum Jülich GmbH, D-52425 Jülich, Germany) |
| pXMJ19 | CmR, C. glutamicum/E. coli shuttle vector with LacIq, Ptac, pBL1 oriV C. glutamicum, pUC18 oriV E. coli | Laboratory collection of M.D. |
| pAN6-porA-thr-His | KanR, pAN6 containing porA with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porB-thr-His | KanR, pAN6 containing porB with thr cleavage site and 8xHis Tag in C terminus | This study |
| pAN6-porB-S7A-thr-His | KanR, pAN6 containing porB-S7A mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porB-S98A-thr-His | KanR, pAN6 containing porB-S98A Mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porB-S7AS98A-thr-His | KanR, pAN6 containing porB-S7A-S98A double mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porC-thr-His | KanR, pAN6 containing porC with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porC-S87A-thr-His | KanR, pAN6 containing porC-S87A mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porC-S91A-thr-His | KanR, pAN6 containing porC-S91A mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porC-S87AS91A-thr-His | KanR, pAN6 containing porC-S87A-S91A double mutant with thr cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porH-Xa-His | KanR, pAN6 containing porH with xa cleavage site and 8xHis tag in C terminus | This study |
| pAN6-porH-porA-thr-His | KanR, pAN6 containing porH porA operon with modified porA in C terminus with thr cleavage site and 8xHis tag in C terminus | This study |
| pXMJ19-porA-thr-His | CmR, pXMJ19 containing porA with thr cleavage site and 8xHis Tag in C terminus | Ref. 8 |
| pXMJ19-porB-thr-His | CmR, pXMJ19 containing porB with thr cleavage site and 8xHis tag in C terminus | This study |
| pXMJ19-porC-thr-His | CmR, pXMJ19 containing porC with thr cleavage site and 8xHis tag in C terminus | This study |
| pXMJ19-porH-Xa-His | CmR, pXMJ19 containing porH with xa cleavage site and 8xHis tag in C terminus | Laboratory collection of R. Benz (Department of Life Sciences and Chemistry, Jacobs University Bremen, 28759 Bremen, Germany) |
| Strains | ||
| C. glutamicum ATCC 13032 | WT strain, biotin auxotroph | Laboratory collection |
| C. glutamicum ATCC 13032 ΔCmytC | KanR, ATCC 13032 derivative with an in-frame inactivation of the cmytC gene | Ref. 7 |
| E. coli mc1061 | MC1061 recA− | Laboratory collection |
Fig. 1.
Identification of distinct proteoforms for C. glutamicum OMPs associated with the mAGP complex and secreted in the extracellular medium. (A) C. glutamicum ATCC13032 cells expressing recombinant PorA-His, PorH-His, PorB-His, and PorC-His were cultured under identical conditions. Subcellular fractions corresponding to the CYT, the PM, the mAGP complex, and the extracellular medium were analyzed by SDS/PAGE after staining with InstantBlue (Left) and Western blotting (Right) with antibodies against the protein His tag. Fractions isolated from WT, untransformed cells were coanalyzed as control. Molecular mass markers (in kilodaltons) are indicated next to the gel. (B) Extracted ion chromatograms of PorB-His purified from mAGP (Upper) and extracellular medium (Lower) fractions containing nonmycoloylated (black), monomycoloylated (green), and dimycoloylated (red) proteoforms. (C) Representation of the multicharged MS spectra (Left) and deconvoluted (DC) spectra obtained for PorB-His proteoforms with isotopic resolution (Right). The mycolic acid compositions of each proteoform are indicated by triangle and circle symbols. EM, extracellular medium.
Posttranslational O-Acylation Is a Common Feature of Mycomembrane-Associated Porins, Whereas Secreted Porins Are Not Modified.
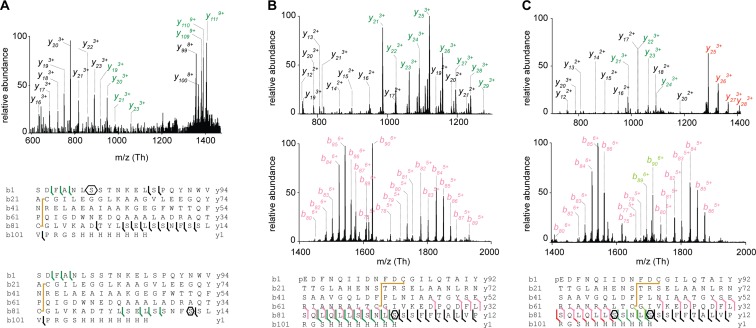
Next, we wanted to test whether mAGP-associated PorA, PorH, PorB, and PorC proteins possess specific targeting and sorting signals that were absent in the secreted proteoforms. In this context, we carried out top-down nanoliquid chromatography (nano–LC)-MS analysis on the recombinant porins purified from the extracellular medium (extracellular medium fraction) and the mAGP complex. We were able to separate the different proteoforms of both secreted and mAGP-associated porins using reverse-phase C4 chromatography (Fig. 1B), thus enabling subsequent detection and characterization by MS (Fig. 1C). The analysis of purified mAGP-associated PorB yielded three resolved chromatographic peaks (Fig. 1B, Upper) and high-quality MS spectra from multicharged species (Fig. 1C, Left). Because of the high resolution and mass accuracy of the MS instrumentation, the molecular weight determination of each proteoform was sufficient to unambiguously decipher the protein PTM. The first chromatographic peak (elution time of 9 min) corresponded to a mature, unmodified form of PorB (Fig. 1C, Top), whereas the second peak (elution time of 10.5 min) contained three proteoforms with mass increments of 478.5, 504.6, and 530.6 Da that could readily be assigned as monoacylated species with C32:0, C34:1, and C36:2 mycolic acyl residues, respectively (Fig. 1C, Middle). The third chromatographic peak (elution time of 13.5 min) consisted of dimycoloylated proteoforms with five distinct acylation patterns: C32:0/C32:0, C34:1/C32:0, C34:1/C34:1 or C32:0/C36:2, C34:1/C36:2, and C36:2/C36:2 (Fig. 1C, Bottom), reflecting the mycolic acid composition of the C. glutamicum mycomembrane under standard bacterial growth conditions (6). Overall, nine distinct proteoforms of PorB were found to be associated with the mAGP complex compartment. Assuming that the ionization yields of the mycoloylated and nonmycoloylated species were roughly similar, we could compare the signal intensities of a given charge state (9+) obtained for each species, showing a significant prevalence of dimycoloylated PorB species in the mAGP complex (Figs. 1B and 2C, Top). Next, we performed the analysis of secreted PorB under identical experimental conditions (Fig. 1B, Lower) and found a single proteoform that was consistent with mature, nonacylated PorB (Fig. 1C, Top). These results prompted us to extend the analysis to other recombinant porins and check whether the O-acylation represented the major structural discrepancy between secreted and mAGP-associated porins. Following identical protocols, we found that PorA, PorH, and PorC purified from the extracellular medium were all consistent with nonmycoloylated species, whereas mAGP-associated proteoforms were acylated by one or two mycolic acids (Fig. S1). Together, these results confirmed that posttranslational O-acylation is a common feature of mAGP-associated porins, whereas secreted proteins are not acylated (Table S2). We show that (i) PorB and PorC have been shown to be mycoloylated, (ii) polymycoloylation is evidenced at the molecular level, and (iii) any of four porins have been found in a secreted, soluble form.
Fig. 2.
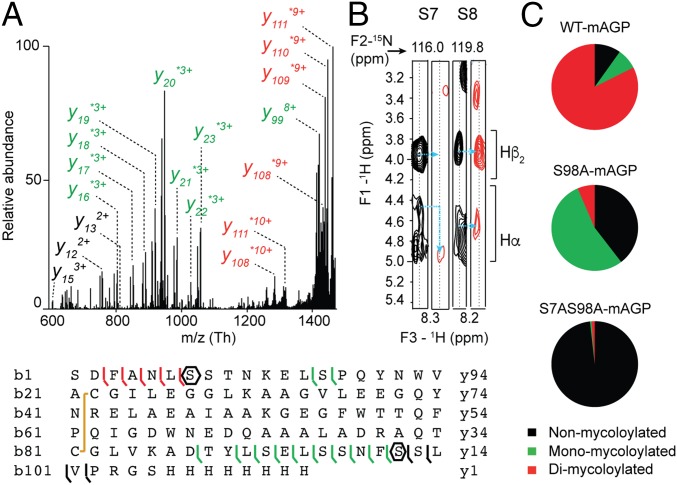
Characterization of protein modifications by MS/MS, NMR, and site-directed mutagenesis. Example of PorB. (A) Top-down CID of dimycoloylated PorB-His-10+ charge state (m/z 1,336.10 Th) with nonmycoloylated, monomycoloylated, and dimycoloylated y and b fragments colored black, green, and red, respectively. The sequence coverage was obtained by fragmenting the 8+, 9+, and 10+ charge states of PorB-His, identifying S98 and S7/S8 residues (polygons) as putative mycoloylation sites and a disulfide bond between C22 and C81 (yellow line). (B) Solution NMR analysis of nonmycoloylated (black) and mycoloylated (red) PorB-His. Selected strips extracted from 3D 1H, 15N, 1H heteronuclear single quantum coherence–total correlation spectroscopy (HSQC-TOCSY) spectra obtained on (U-15N)–labeled PorB-His showing Hα and Hβ2 chemical shifts of S7 and S8 residues for the two proteoforms. Although 1H resonances from S8 were not affected, significant spectral changes were observed for Hα of residue S7 (blue arrows), thus identifying the O-acylation of the S7 hydroxyl of PorB-His. (C) The positions of PTM within the protein sequence were validated by site-directed mutagenesis of S7 and S98 residues and subsequent MS analysis of PorB-His WT (WT-mAGP; Top) and its mutant derivatives PorB-S98A (S98A-mAGP; Middle) and PorB-S7AS98A (S7AS98A-mAGP; Bottom). Nonmycoloylated (black), monomycoloylated (green), and dimycoloylated (red) proteoforms were semiquantified from extracted ion chromatograms of the corresponding 9+ charge states.
Fig. S1.
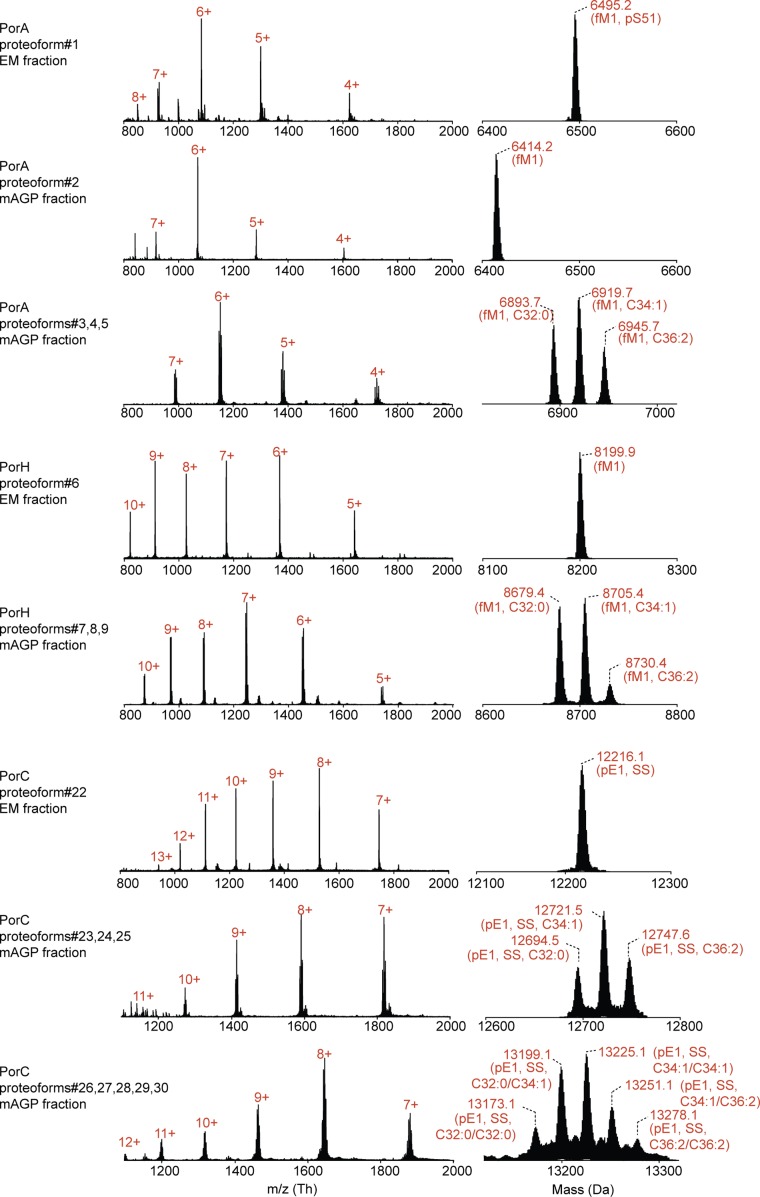
Nano–LC-ESI-MS analysis of recombinant PorA, PorH, and PorC proteoforms purified from extracellular medium (EM) and mAGP fractions. The multicharged MS spectra are shown in Left together with their corresponding isotopic patterns deconvoluted with MagTran. The charge states are indicated in red. C32:0/C34:1/C36:2, mycolic acid composition; ESI, electrospray ionization; fM1, formylation of N-terminal methionine; pE1, N-terminal pyroglutamylation; pS51, phosphorylation of S51 residue; SS, disulfide bridge.
Table S2.
Complete set of 30 proteoforms observed by top-down MS for recombinant C. glutamicum OMPs
| Proteoform | Protein | Length, AA | AA position range | Average mass, Da | Exp. mass, Da | Δ, ppm | Δ, Da | O-mycoloylation | Other PTMs (nature and site) | Disulfide bond | Cell fraction | UniProt accession no. | ||
| Site 1 | Site 2 | Nature | ||||||||||||
| 1 | PorA | 59 | 1–59 | 6,495.1 | 6,495.2 | 13.86 | 0.1 | — | — | — | fM1; pS51 | — | Extracellular medium | Q79VC8 |
| 2 | PorA | 59 | 1–59 | 6,415.1 | 6,415.2 | 10.91 | 0.1 | — | — | — | fM1 | — | mAGP | Q79VC8 |
| 3 | PorA | 59 | 1–59 | 6,893.7 | 6,893.7 | 3.48 | 0.0 | S15 | — | C32:0 | fM1 | — | mAGP | Q79VC8 |
| 4 | PorA | 59 | 1–59 | 6,919.8 | 6,919.7 | 19.22 | 0.1 | S15 | — | C34:1 | fM1 | — | mAGP | Q79VC8 |
| 5 | PorA | 59 | 1–59 | 6,946.0 | 6,945.7 | 41.61 | 0.3 | S15 | — | C36:2 | fM1 | — | mAGP | Q79VC8 |
| 6 | PorH | 74 | 1–74 | 8,200.8 | 8,200.9 | 14.63 | 0.1 | — | — | — | fM1 | — | Extracellular medium | Q5K031 |
| 7 | PorH | 74 | 1–74 | 8,679.4 | 8,679.4 | 2.77 | 0.0 | S56 | — | C32:0 | fM1 | — | mAGP | Q5K031 |
| 8 | PorH | 74 | 1–74 | 8,705.5 | 8,705.4 | 15.28 | 0.1 | S56 | — | C34:1 | fM1 | — | mAGP | Q5K031 |
| 9 | PorH | 74 | 1–74 | 8,731.7 | 8,731.4 | 33.10 | 0.3 | S56 | — | C36:2 | fM1 | — | mAGP | Q5K031 |
| 10 | PorB | 113 | 28–140 | 12,342.3 | 12,341.9 | 34.03 | 0.4 | — | — | — | — | C22-C81 | Extracellular medium | Q8NRS3 |
| 11 | PorB | 113 | 28–140 | 12,820.4 | 12,820.4 | 1.87 | 0.0 | S7 | — | C32:0 | — | C22-C81 | mAGP | Q8NRS3 |
| 12 | PorB | 113 | 28–140 | 12,846.5 | 12,846.5 | 2.57 | 0.0 | S7 | — | C34:1 | — | C22-C81 | mAGP | Q8NRS3 |
| 13 | PorB | 113 | 28–140 | 12,872.7 | 12,872.5 | 14.68 | 0.2 | S7 | — | C36:2 | — | C22-C81 | mAGP | Q8NRS3 |
| 14 | PorB | 113 | 28–140 | 12,820.4 | 12,820.4 | 1.87 | 0.0 | — | S98 | C32:0 | — | C22-C81 | mAGP | Q8NRS3 |
| 15 | PorB | 113 | 28–140 | 12,846.5 | 12,846.5 | 2.57 | 0.0 | — | S98 | C34:1 | — | C22-C81 | mAGP | Q8NRS3 |
| 16 | PorB | 113 | 28–140 | 12,872.7 | 12,872.5 | 14.68 | 0.2 | — | S98 | C36:2 | — | C22-C81 | mAGP | Q8NRS3 |
| 17 | PorB | 113 | 28–140 | 13,298.9 | 13,299.9 | 78.80 | 1.0 | S7 | S98 | C32:0/C32:0 | — | C22-C81 | mAGP | Q8NRS3 |
| 18 | PorB | 113 | 28–140 | 13,325.0 | 13,325.9 | 66.87 | 0.9 | S7 | S98 | C32:0/C34:1 | — | C22-C81 | mAGP | Q8NRS3 |
| 19 | PorB | 113 | 28–140 | 13,351.2 | 13,351.9 | 54.98 | 0.7 | S7 | S98 | C34:1/C34:1 or C32:0/C36:2 | — | C22-C81 | mAGP | Q8NRS3 |
| 20 | PorB | 113 | 28–140 | 13,377.3 | 13,377.9 | 43.21 | 0.6 | S7 | S98 | C34:1/C36:2 | — | C22-C81 | mAGP | Q8NRS3 |
| 21 | PorB | 113 | 28–140 | 13,403.5 | 13,403.9 | 31.48 | 0.4 | S7 | S98 | C36:2/C36:2 | — | C22-C81 | mAGP | Q8NRS3 |
| 22 | PorC | 111 | 46–156 | 12,216.3 | 12,216.1 | 19.65 | 0.2 | — | — | — | pE1 | C12-C69 | Extracellular medium | Q8NRS4 |
| 23 | PorC | 111 | 46–156 | 12,694.6 | 12,694.5 | 5.99 | 0.1 | S91 | — | C32:0 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 24 | PorC | 111 | 46–156 | 12,720.7 | 12,720.5 | 18.32 | 0.2 | S91 | — | C34:1 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 25 | PorC | 111 | 46–156 | 12,746.9 | 12,746.6 | 22.67 | 0.3 | S91 | — | C36:2 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 26 | PorC | 111 | 46–156 | 13,173.1 | 13,173.1 | 3.64 | 0.0 | S91 | S87 | C32:0/C32:0 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 27 | PorC | 111 | 46–156 | 13,199.2 | 13,199.1 | 8.26 | 0.1 | S91 | S87 | C32:0/C34:1 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 28 | PorC | 111 | 46–156 | 13,225.4 | 13,225.1 | 20.11 | 0.3 | S91 | S87 | C34:1/C34:1 or C32:0/C36:2 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 29 | PorC | 111 | 46–156 | 13,251.5 | 13,251.1 | 31.85 | 0.4 | S91 | S87 | C34:1/C36:2 | pE1 | C12-C69 | mAGP | Q8NRS4 |
| 30 | PorC | 111 | 46–156 | 13,277.7 | 13,277.1 | 43.53 | 0.6 | S91 | S87 | C36:2/C36:2 | pE1 | C12-C69 | mAGP | Q8NRS4 |
Protein modification sites are indicated with one-letter code amino acid and residue numbers from the mature protein. AA, amino acid; Exp. Mass, experimental mass; fM1, N-formylmethionine; pE1, N-terminal pyroglutamic acid; pS51, phosphorylation of S51.
The Secretion of Nonacylated Porins Is Physiologically Relevant.
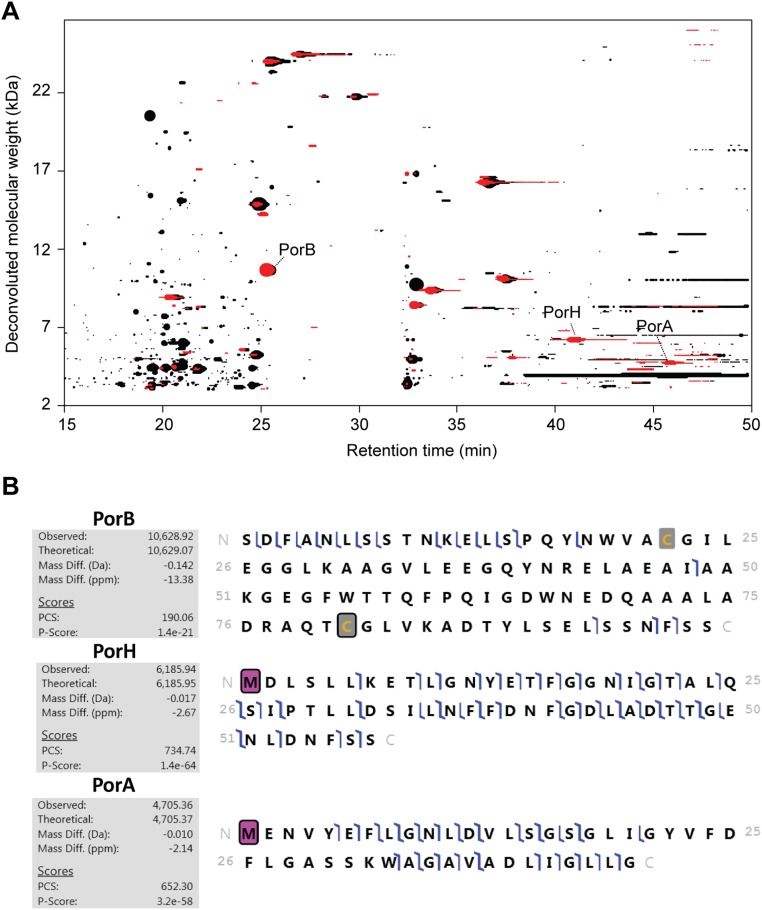
Whereas O-mycoloylated porins have been previously identified from natural extracts of C. glutamicum and other Corynebacteriales (6), the detection of soluble proteoforms in the extracellular medium has remained elusive. In this context, we wanted to further investigate the modifications on secreted proteoforms using top-down nano–LC-MS/MS. Although collision-induced dissociation (CID) fragmentation may prevent the detection of most labile modifications, such as phosphorylation and O-glycosylation, it generally yields better fragmentation. In the case of O-mycoloylation, we did not see any neutral loss of the mycolic acid residue, allowing their precise localization. All of the proteoforms of PorA-His and PorH-His were systematically found with a mass increment of +28 Da that was readily assigned to the formylation of the N-terminal methionine. Interestingly, secreted PorA-His was alternatively found with an additional mass difference of +80 Da, consistent with the phosphorylation of a hydroxyl residue in addition to the N-terminal formylation (Fig. S1). However, this phosphorylation was further localized on the S51, which is part of the additional residues of the His-Tag (Table S2). PorC proteoforms were characterized by a mass discrepancy of −19 Da, in agreement with the presence of a disulfide bond (−2 Da) and the cyclization of the N-terminal glutamyl residue on signal peptide cleavage, yielding a pyroglutamyl residue (−17 Da) (Table S2). Although such N-terminal protein modifications (i.e., N-formylation and the formation of pyroglutamyl residues) have been identified in bacterial secreted proteins and OMPs (13, 14), we sought additional evidence about the natural relevance of secreted porin species. To check whether nonacylated soluble porins resulted from a physiological secretion process, endogenous proteins secreted from stationary-phase, untransformed C. glutamicum cells were isolated using an identical protocol. We then conducted a discovery mode data-dependent analysis, in which the three most intense precursors of each MS scan were selected and fragmented. The resulting MS/MS spectra were then automatically searched against the C. glutamicum protein database in discovery mode (SI Experimental Procedures). This unbiased approach enabled the identification of 24 proteins in WT C. glutamicum, among which the nonacylated PorB, PorH, and PorA were unambiguously identified with good mass accuracy (<20 ppm) and sequence coverage (30–50% of the fragments explained) (Fig. S2), thus confirming that secretion of nonacylated porins is physiologically relevant. Furthermore, the N-formylation of PorA and PorH together with PorB disulfide bridge formation were also confirmed. We found identical proteoforms at the same retention times for PorB, PorH, and PorA in the extracellular medium extract of the C. glutamicum ΔcMytC strain for which the cMytC responsible for the O-acylation of mAGP-associated PorA and PorH porins has been deleted. However, in contrast to recombinantly expressed PorC, endogenous protein was not detected in the extracellular medium. This result suggests that PorC is not readily expressed by the bacterium under standard in vitro conditions, consistent with a previous publication (11).
Fig. S2.
Nonacylated OMP isoforms revealed from crude extracellular medium extracts of C. glutamicum using top-down proteomics in discovery mode. (A) Proteoform footprint of the LC-MS run generated with the RoWinPro program, showing the deconvoluted molecular masses obtained along the gradient for extracellular medium extracts of untransformed C. glutamicum WT (red) and ΔcMytC mutant (black). The size of the dots reflects the relative signal intensity for each one of the detected proteins. PorB, PorH, and PorA are indicated with arrows. (B) MS/MS CID spectra were then automatically searched against the C. glutamicum protein database using ProteomeDiscoverer (ThermoFisher Scientific) with the dedicated ProSight-PD nodes. N-formylmethionine is indicated by pink squares, and disulfide bonds are indicated by gray squares. PCS, protein characterization score.
Identification of O-Acylation Sites by Combining MS/MS, NMR, and Site-Directed Mutagenesis.
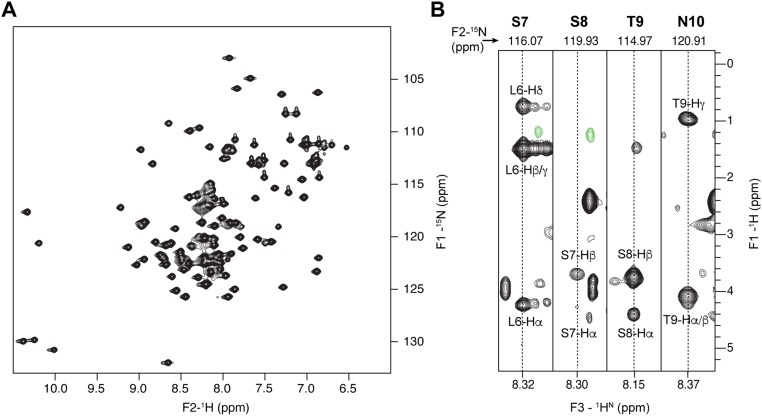
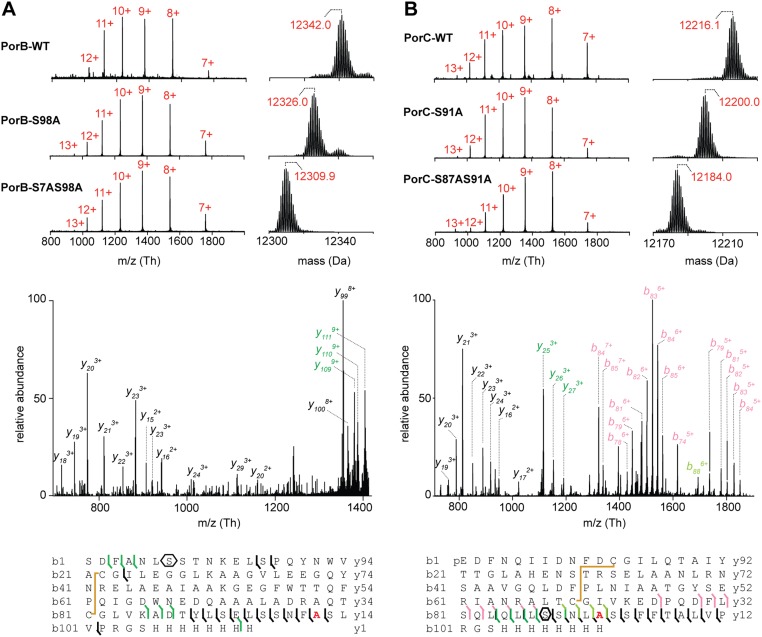
Previously, we have shown that PorA and PorH were O-mycoloylated on S15 (6) and S56 residues (9), respectively. The identification of O-acylation sites within mono- and multiacylated proteoforms of PorB and PorC represents a new challenge considering the protein size, the large number of acylated proteoforms, and the putative heterogeneity of O-mycoloylation sites. As shown in Fig. 2A, CID fragmentation of PorB dimycoloylated proteoforms yielded a set of unmodified, monomycoloylated, and dimycoloylated fragment ions consistent with the presence of a first O-acylation site on S98 and a second site located between S7-L13. The analysis of fragment ions stemming from monomycoloylated PorB proteoforms confirmed these results but did not allow determining whether S7 or S8 was the site of modification (Fig. S3A). To decipher the position of the O-acylation within this protein segment, we analyzed and compared the 1H sidechain chemical shifts of serine residues S7 and S8 in nonacylated and acylated (U-15N)–labeled PorB (Fig. 2B). As expected, well-defined 1H resonances were observed at expected chemical shifts for both S7 and S8 residues of the nonacylated PorB sample [Fig. 2B (note that the Hα resonance of S8 is obscured by overlap with the peak from the Hα of residue F3) and Fig. S4]. However, both Hα and Hβ resonances of residue S7 exhibited significant differences between nonacylated and acylated PorB species. In particular, the Hα chemical shift of S7 was strongly perturbed by acylation and shifted from 4.45 to a different frequency, likely at 4.90 ppm. The Hβ resonance was broadened beyond detection, probably because of the presence of conformational exchange in the millisecond range. However, the S8 1H resonances of acylated PorB were not affected, thus suggesting the S7 hydroxyl group as the sole site of O-acylation within the protein segment S7-L13. To confirm the O-acylation sites, we generated a set of single and double mutants in which the native serine was substituted by an alanine (i.e., PorB-S98A, PorB-S7A, and PorB-S7AS98A). We purified both secreted and mAGP-associated PorB mutants and confirmed the presence of the mutations and a concomitant loss of PTM in a semiquantitative manner (Fig. 2C and Fig. S5A). This strategy was also successfully applied to the mono and dimycoloylated proteoforms of PorC, leading to the unambiguous identification of acylation sites on residues S87 and S91 (Figs. S3 B and C and S5B).
Fig. S3.
MS/MS fragmentation and product ion maps from CID spectra for mono- and multiacylated PorB-His and PorC-His species. (A) Full MS/MS spectrum of monomycoloylated PorB-His (proteoforms 12 and 15; C34:1) 10+ charge state at m/z 1,285.66 Th showing mycoloylation on either S98 or S7 residues (polygons). (B) Low and high m/z ranges of the MS/MS spectrum of the monomycoloylated PorC-His (proteoform 24; C34:1) 9+ charge state (m/z 1,414.40) indicating a pyroglutamylation at position 1, a disulfide bond between C12 and C69, and a mycolic acid on S91 (polygon). The sequence coverage was obtained by fragmentation of the charge states 8+ to 11+. (C) Low and high m/z ranges of the MS/MS spectrum of dimycoloylated PorC-His (proteoform 28; 2xC34:1) 9+ charge state at m/z 1,470.57 Th showing a pyroglutamylation at position 1, a disulfide bond between C12 and C69, and mycolic acids on S91 and S87 residues (polygons). The sequence coverage was obtained by fragmentation of the charge states 8+, 9+, and 10+. The color code used for fragmentation ions y and b is unmodified fragments (black), disulfide bond (−2 Da; yellow), pyroglutamylation (−17 Da; light pink), monomycoloylation (dark green), monomycoloylation and pyroglutamylation (light green), and dimycoloylation (red). Mycoloylations are associated with mass discrepancies of +478 Da (C32:0), +504 Da (C34:1), and +530 Da (C36:2).
Fig. S4.
NMR assignments of backbone and sidechain 1H and 15N resonances within the PorB L6-N10 protein segment. (A) 2D (15N-1H)-band-selective optimized-flip-angle short-transient (SOFAST) heteronuclear multiple quantum correlation spectroscopy (HMQC) spectrum obtained on nonacylated (U-15N)–labeled PorB (black). (B) Sequential strips extracted from the 3D (15N, 1H, 1H)-HSQC-TOCSY spectrum (80 ms mixing time) of nonacylated (U-15N)–labeled PorB at 15N and 1H amide frequencies indicated at the top and the bottom of the strips, respectively. Interresidue correlations between backbone amide resonances of residue i and sidechain proton resonances of residue i-1 are observed, and the corresponding assignments are indicated.
Fig. S5.
MS and MS/MS analysis of PorB-His and PorC-His mutants by using top-down CID. (A, Upper) Multicharged MS spectra of secreted PorB WT (PorB-WT, proteoform 10), single (PorB-S98A) and double (PorB-S7AS98A) mutants, and the corresponding isotopic patterns deconvoluted with MagTran. The charge states are indicated in red. (A, Lower) Full MS/MS spectrum of mAGP-associated PorB-S98A 10+ charge state (m/z 1,284.05) showing the PTM loss in the C-terminal region and the remaining mycoloylation in the S7-L13 protein stretch. The sequence coverage was obtained by fragmentation of the charge states 8+, 9+, and 10+. (B, Upper) Multicharged MS spectra of secreted PorC-His WT (PorC-WT, proteoform 22), single (PorC-S91A) and double (PorC-S87AS91A) mutants, and the corresponding isotopic patterns deconvoluted with MagTran software. The charge states are indicated in red. (B, Lower) Full MS/MS spectrum of mAGP-associated PorC-S91A 10+ charge state (m/z 1,271.46) indicating a unique mycoloylation on S87 residue, thus confirming the PTM loss in the most distal position. The sequence coverage was obtained by fragmentation of the charge states 8+, 9+, and 10+. The color code used for fragmentation ions y and b is unmodified fragments (black), disulfide bond (−2 Da; yellow), pyroglutamylation (−17 Da; light pink), monomycoloylation (dark green), and monomycoloylation and pyroglutamylation (light green). Mycoloylations are associated with mass discrepancies of +478 Da (C32:0), +504 Da (C34:1), and +530 Da (C36:2). The mutated residue is colored in red in the protein sequence.
Sequence Analysis of PTM Sites Reveals Consensus Patterns for O-Acylation.
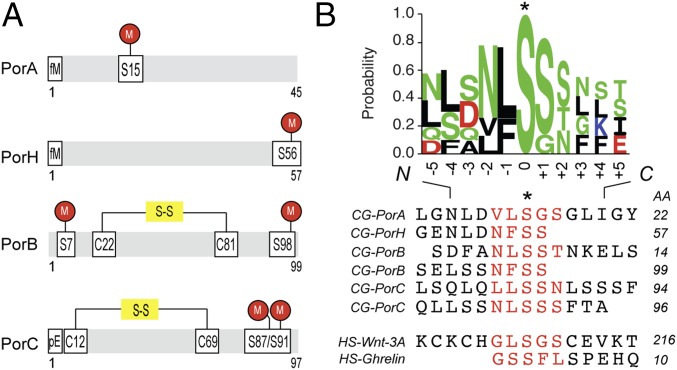
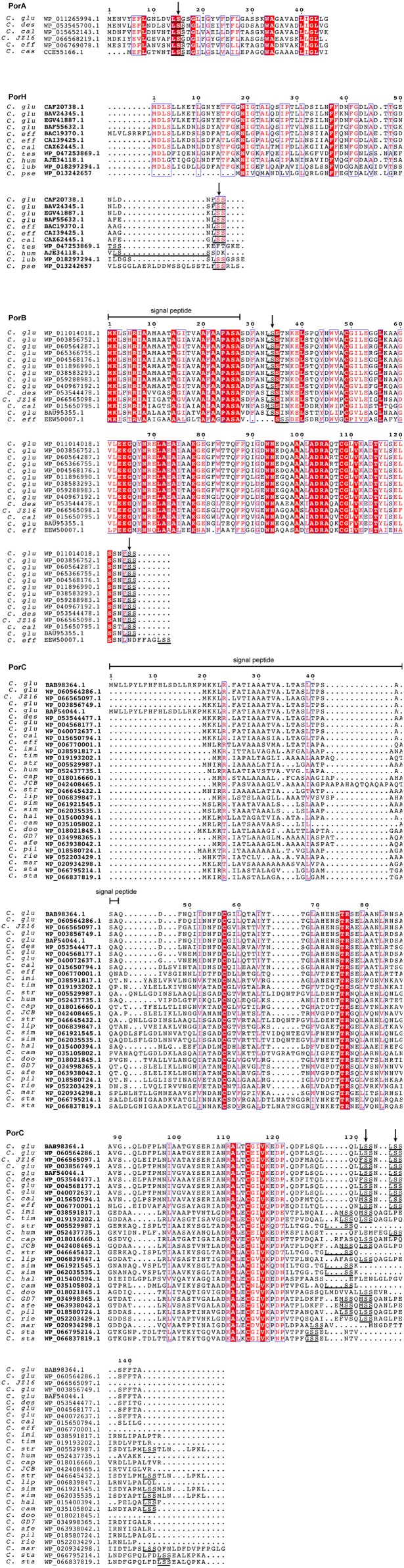
After complete PTM mapping of secreted and mAGP-associated C. glutamicum porins PorA, PorH, PorB, and PorC had been obtained (Fig. 3A and Table S2), we examined the amino acid composition of the O-acylation sites. The data were analyzed using the Motif-X algorithm, but no specific motif was found, indicating that the conservation is not strong (15). Therefore, we constructed an alignment of known O-acylated peptides comprising C. glutamicum porins and human Ghrelin and Wnt-3a (Fig. 3B), centered on the modified serine residue, that revealed moderately conserved short linear motifs around the PTM site. The frequency of residue- and position-specific amino acid occurrences was calculated. These O-acylation sites exhibited an increased occurrence of certain amino acids (e.g., S, L, N, and F) and the total absence of others (R, M, W, and Y) for both C. glutamicum and human proteins. The enrichment factor depended on the proximity to the modified serine, suggesting a putative role of these residues in enzyme–substrate interactions. Subsequently, we examined if such short linear amino acid stretches were conserved among PorA, PorH, PorB, and PorC orthologs (Fig. S6). Each query sequence was searched using BLAST against the Corynebacteriales proteome, and only sequences having at least 30% global similarity to the original query were kept for inclusion. Orthology was detected in more than 10 different organisms, including C. diphtheria and Corynebacterium efficiens, species known to possess mycoloyltransferase activity or mycoloylated proteins associated with the cell envelope (6, 7). We found that the motif was well-conserved between orthologs in contrast to generally nonconserved surrounding regions of the protein. Focusing on regions with evolutionarily conserved residues, we discovered putative O-acylation sites within cell envelope-associated proteins in C. glutamicum, such as lipocalin, flotillin, esterase, and mycoloyltransferase (Table S3). These proteins are known to interact with the outer membrane in their nonacylated (mycoloyltransferase and esterase) or acylated (lipocalin and flotillin) forms in Corynebacteriales and Gram-negative bacteria. These results strongly suggest the presence of short linear motifs that can be O-acylated and in the case of C. glutamicum proteins, result in mAGP association. These sequences may have been evolutionarily conserved across kingdoms, because they have also been detected in two human O-acylated proteins.
Fig. 3.
O-acylation of OMPs occurs in short linear motifs. (A) Schematic representation of protein modifications along the protein sequences of C. glutamicum PorA, PorH, PorB, and PorC. The sites of modifications are reported onto the sequence using the following code: M, O-mycoloylation; pE, pyroglutamate; and S-S, disulfide bond. (B) Sequence alignments of selected O-acylated proteins from C. glutamicum and human genomes within a region surrounding the fatty acylation residue. The sequence logo was calculated from the C. glutamicum peptide sequences only and displays the position-specific frequency of each amino acid composing the motif. AA, amino acid; CG, C. glutamicum; HS, Homo sapiens. *Fatty acylation residue.
Fig. S6.
Multiple sequence alignments identify O-acylation short linear motifs within porin orthologs. Multiple sequence alignments for PorA, PorH, PorB, and PorC proteins. O-acylation motifs are underlined, and PTM sites are indicated by arrows. As a reference, the query protein sequences from C. glutamicum ATCC13032 are indicated in the first line, with residue numbers at the top of the alignment. Sequence similarity is shown by red letters, whereas sequence identity is highlighted by white letters on a red background. Orthologous sequences are represented with their respective GenBank accession numbers, and corresponding species are indicated as follows: C. afe (Corynebacterium afermentas); C. cal (Corynebacterium callunae); C. cam (Corynebacterium camporealensis); C. cap (Corynebacterium capitovis); C. cas (Corynebacterium casei); C. des (Corynebacterium deserti); C. dip (Corynebacterium diphtheriae); C. doo (Corynebacterium doosanense); C. eff (Corynebacterium efficiens); C. glu (C. glutamicum); C. hal (Corynebacterium halotolerans); C. hum (Corynebacterium humireducens); C. imi (Corynebacterium imitans); C. lip (Corynebacterium lipophiloflavum); C. lub (Corynebacterium lubricantis); C. mar (Corynebacterium maris); C. pilosum (Corynebacterium pilosum); C. pse (Corynebacterium pseudotuberculosis); C. rie (Corynebacterium riegelii); C. sim (Corynebacterium simulans); C. sta (Corynebacterium stationis); C. str (Corynebacterium striatum); C. tes (Corynebacterium testudinoris); C. tim (Corynebacterium timonense); C. ulc (Corynebacterium ulcerans).
Table S3.
C. glutamicum proteins with conserved O-acylation pattern
| Name (motif) | Protein | Sequence | AA | Localization | Function | Refs. |
| Lipocalin (LSS) | NP_599863.1 | MRISSKLVTTALLAAISLFGISTAQAQDIFDGGRLAGGSSQVSNLSSVPENLALPEIENSIDLERYKGKWYQVAAIPQPFSLQCSHDVTADYGVIDSDTISVTNKCGTFFGPSVIEGSAKVVSNASLKVSFPGIPFQSEDNQANYRVTYIEDDYSLAIVGSPSRSSGFILSRTPQLSSDQWSHVRNITEDSGWWPCAFITVPATGGLNTATPLCTL | 216 | Outer membrane lipoprotein | Maintenance of outer membrane structure (N-acylation of Cys1 for E. coli ortholog) | 26, 27 |
| Peptidoglycan hydrolase(LSS) | NP_600753.1 | MTRALIALAVSGALLSSMTPAVAQPQNPDDAAIAQAEENVSAGDGEVARLAGSLSSTDAEINRVELEMGALREEVNKSLVDLHDAQAIAEQARQDALAAKKDLDDSQAQIEAAQERLDEISRAAYRQNGTSKGLSGISGNGNSEDALDRQTYLRTSAEKQQAAVEELDRLRTENANKESVLRQARIVAEQREAEAVEKQVQTEAAIAANSEQLNVLTNNRSTLVAQRDGAERNLAIARAQADNLQGQRAEYEEFQQAEQARIQAEAEAQAAAEEKRRADEAAAQAAAEAQEAAQQAQAAEEAQAAQAAETAQAQAAQAAETQAAQAAQAQAEANDRAAAQQRAAEAQAAAEQAQREADAQAANDAQAQALREQALTAASIAAAALIAASQSSHATTQNPYPTDEDADPTDIADIQGPTQPGTGESGDSQSNSSDNDSTGNDSTGSDSSDSDSSGNDSSEVISGDRSAQIETVIARAMSQLGVQYAWGGGNANGPTLGIRDGGVADSYGDYNKVGFDCSGLTLYAFAGVGISLPHYTGYQYQHGTKVSPSEMQRGDLIFYGPGASQHVAIYLGDGQMIEAPNSGSVVKISPVRWSGMTESVVRLI | 604 | Cell wall | Hydrolysis of peptidoglycan, hydrolase from NlpC family | |
| Alkaline phosphatase(LSS) | NP_601655.2 | MSFKRRSLAITCAITSSIVLAGISGTAAAQSSYVSGSSGLLSSSELHPETPAPLDAPKNIIYMIGDGMGYNHVSATNLFETGQTMHQVEGEPGSVTPVEGGTPVQAFEGAEWTQLAQSTFQDGNSYDPERAWADHNYVNENFTDSAAAGTAMATGVKTTNGMIGINPANEPAKNTSEYAIEKGKAAGVVSSVPFNHATPAAWAAHNSNRNDLHAMAEEMINSDLNVIMGAGHPFFDNNGNPITEADEDYMQASQYERLASGETDFTFVEEDADFEALANGQVESDKYFGLAQVEDPLQHDRDGDSVTPYDVPLNDVVDLSTMSKAALNVLNQDEDGFHIMIEGGAIDWAGHANDMARDIEEVQEFNKAVETVIEWVETNSSWDETLVIVTADHETGYMTGPNDDPNWSAMTGAAGIVPNHGWHSGNHINQLVPVFARGAGVSDIVAAADQVDPIRGNYIDNIEIANLTFNKWW | 473 | Periplasm | Domain PhoA: nonspecific membrane-bound phosphormonoesterases that catalyze the hydrolysis reaction via a phosphoseryl intermediate to produce inorganic phosphate and the corresponding alcohol, optimally at high pH | |
| Prenyltransferase (LSS) | NP_599855 | MMEKIRLILLSSRPISWINTAYPFGLAYLLNAGEIDWLFWLGIVFFLIPYNIAMYGINDVFDYESDMRNPRKGGVEGAVLPKSSHSTLLWASAISTIPFLVILFIFGTWMSSLWLTLSVLAVIAYSAPKLRFKERPFIDALTSSTHFTSPALIGATITGTSPSAAMWIALGSFFLWGMASQILGAVQDVNADREANLSSIATVIGARGAIRLSVVLYLLAAVLVTTLPNPAWIIGIAILTYVFNAARFWNITDASCEQANRSWKVFLWLNYFVGAVITILLIAIHQI | 287 | Periplasm | ||
| EsteraseCop1, cMytA (LSS) | AAP23202.1 | MRDTAFRSIKAKAQAKRRSLWIAAGAVPTAIALTMSLAPMASAQSSNLSSDAVVGSIAQGVTDGLTDYLKPRVEELPAGEVTYPEIAGLPDGVRVISAEWATSKHVILTIQSAAMPERPIKVQLLLPRDWYSSPNREFPEIWALDGLRAIEEQSGWTIETNIEQYYADKNAIVVLPVGGESSFYSDWEGPNNGKNYQWETFLTQELAPILDKGFRSNTDRAITGISMGGTAAVNIATHHPDMFKFVGSFSGYLDTTSAGMPIAISAALADAGGYDANAMWGPVGSERWQENDPKSNVDKLKGKTIYVSSGNGADDFGKEGSVAIGPANAAGVGLEVISRMTSQTFVDRASQAGVEVVASFRPSGVHSWEYWQFEMTQAFPHIANALGMSTEDRGVECAPVGAIADAVADGAMGTCLTNEYDVTGGKAQDFANGRAYWSANTGAFGLVGRINARYSELGGPASWLGYPTSSELKTPDGRGRFVTFEHGSIYWTATTGPWEIPGDMLAAWGTQDYEKGSLGYPTGAAVEYNGGLRQQFEGGYVFRTSNNQSYWVRGEISKKYAEDGIFAQLGFPTGNEKLINGGAFQEFEKGNIYWSASTGAHVILHGDIFDAWGAKGWEQGEYGFPTSDQTAITAGGQTIDFQNGTIRQVNGRIEESR | 657 | Extracellular and associated (noncovalently) to the CE of C. glutamicum | Mycoloyltransferase A α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity. Transfers a mycoloyl residue on trehalose monomycolate and cell wall arabinogalactan | 30, 31 |
| Esterase Cmt1, cMytC | AAP23203.1 | MKLLRRIAAPAIALGIAMSTIVTPSTAGAAEVTPADVAGDTALSTISDSAPADEASAPRWRAHVNAADERVKEMWAYSPSMDRNVPLVVITADESAGPRPVIYLLNGGDGGEGAANWVMQTDVLDFYLEKNVNVVIPMEGKFSYYTDWVEENASLGGKQMWETFLVKELPGPLEEKLNTDGQRAIAGMSMSATTSLLFPQHFPGFYDAAASFSGCAATSSLLPWEYLKLTLDRGNATPEQMWGPRGGEYNIYNDALINSDKLRGTELYVSNASGLAGEWESVDSPRFEGLNQQVQSIAMAETVVTGGIIEAATNKCTHDLKAKLDSAGIPADWNLRPTGTHSWGWWQDDLRGSWTTFARAFELEA | 365 | Extracellular and associated (noncovalently) to the CE of C. glutamicum | cMytC α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity and an extended loop involved in substrate specificity. Transfers a mycoloyl residue onto PorA, PorH, PorB, and PorC proteins | 7, 30, 31 |
| Esterase Cmt2, cMytB (LSS) | AAP23204 | MSVFTRAGEASRKLVALVVALATAAALMVVGQGTAQAANRDWLRADNSGYCDWDAVGFWVQRCDVWSPAMGRNIPVQIQPAGRGGNAGLYLLDGMRATEYSNAWLVDTNAARLYAPNNITLVMPVGGAGSFYADWNSQASLSSSDPVIYMWETFLTQELPAYLEQNFGVARNNNSIGGLSMGGTAALNLAAKHPDQFRQAMSWSGYLNTTAPGMQTLLRLAMLDTGGFNVNAMYGSIINPRRFENDPFWNMGGLANTDVYISAASGLWSPQDDGVRVDHRLTGSVLEFVAMTSTRIWEAKARLQGLNPTADYPMYGIHGWAQFNSQLERTQGRVLDVMNAW | 341 | Extracellular and associated (noncovalently) to the CE of C. glutamicum | Mycoloyltransferase B α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity. Transfers a mycoloyl residue on trehalose monomycolate and cell wall arabinogalactan | 30, 31 |
| Esterase Cmt3, cMytE | AAP23205.1 | MRKGISRVLSVAVASSIGFGSVLSGTGIAAAQDSAFDYGMDPSMNYNPIDDIKDRPQGLSNLPYFGSKLTSWGSSDATASSGIVTSALPQYTDPRYPLGKDDLPKATIDMEPEALARLERFVGVDGDRIRQINAYSPSMGRTIPLVWVVPEDNTVPGPTVYALGGGDGGQGGQNWVTRTDLDELTSEKNINLIMPMLGSFSFYADWAGESESMGGAQQWETFLMHELPEPLEAAIGADGQRSIVGMSMSGGSVLNFATHDPNFYSSVGSFSGCAETNSWMGRRGIAATAYNGNVVPEQIFGEVDSDYSRYNDPLLNAAKLEEQDNLYIFAGSGVFSELDVIGDNAPIDEDAFKNRVLVGFEIEAMSNTCTHNLKAATDQMGIDNINYDFRPTGTHAWDYWNEALHRFFPLMMQGFGLDGGPIPIYNPNGVTSSESSSELSSDVSLGTVIGSVAGSSGSSAGSSVREFIAGSSGSSQSAGSFYE | 483 | Not expressed in CGL2005 | Mycoloyltransferase E α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity but not expressed in all C. glutamicum strains. | 30, 31 |
| Esterase Cmt4, cMytF (LSS) | AAP23206.1 | MRKGISRVLSVAVASSIGFGTVLTGTGIAAAQDSAFDYGMDPNMNYNPIDDIKDRPEGLSNLPYFGSKLTSWGSSDATASSGVVTSALPQYTDPRYPLGKDDLPKATIDMEPEVLARLERFVGVDGDRIRQINAYSPSMGRTIPLVWVVPEDNTVPGPTVYALGGGDGGQGGQNWVTRTDLEELTSDNNINLIMPMLGSFSFYADWAGESESMGGAQQWETFLMHELPEPLEAAIGADGQRSIVGMSMSGGSVLNFATHDPNFYSSVGSFSGCAETNSWMGRRGIAATAYNGNVVPEQIFGEVDSDYSRYNDPLLNAAKLEEQDNLYIFAGSGVFSELDVIGDNAPIDEDAFKNRVLVGFEIEAMSNTCTHNLKAATDQMGIDNINYDFRPTGTHAWDYWNEALHRFFPLMMQGFGLDGGPIPVYNPNGVSSSESSSELSSDVSLGTVIGSVAGSSGSSEGSSVREFLAGSSGSSQSTGSFYE | 483 | Mycoloyltransferase F α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity. Transfers a mycoloyl residue on trehalose monomycolate and cell wall arabinogalactan | 30, 31 | |
| Esterase Cmt5, cMytD (LSS) | AAP23207.1 | MKLFISRALIAGTVSLTLFSSPLASAQSSGLSSVLSSEDSSATNSEQDFEKSSESGSSAQDFIALSTANPDLTGGSVEGLLSSMSLIGSSQLPLGGPLLSSDSNYPLETDPSITEARIVEKRVLNGLRLEKWSVASPSMQRNVDVQIMKSAEADSPAPMLYMLDGIGGNKNSSGWINGGEGPKVFADENVTVVMPLGAASSMYSDWLEEDPALGRIKWETFIVEELAPLLEAEEELNFNGHRGIGGLSMGATGAVHLANSNPDLFDGVIGISGCYSTLDPIGQTTVSLIVNSRGGNVENMWGPTGSETWKAHDVTSNPEGLRDMAVYLSAANGVVDDIDLADSEKEPFYNLLAGVVLERGSLSCTEALDESMSRAGMNHQVVDYKDSGTHNWRNFNPQLQPGWDAIKHALY | 411 | Mycoloyltransferase D α/β hydrolase family with a conserved catalytic triad (Ser, His, Gly) for mycoloyltransferase activity. Transfers a mycoloyl residue on trehalose monomycolate and cell wall arabinogalactan | 30, 31 | |
| Flotillin (VLSGG) | ANU32924.1 | MEAIAILFVIGAILVVAVIVLGIFFLTSRTWIKVAAADEALIVSAKKKGESQVIVHGKAVVMPITQTHQKISLRSRQVNMQVTAQSDDNVTLNVEAVALVKIGSEAEFIRRAAQRFASSDKEIVRFTQDQLEGVLRGVVAQQTVTSLMRERKKFSEQIAETVIPELEKQGLILDSFQIRGITDDVGYIKSLGAPEIQAKKQAAEIAETEAARAIAKSRIANQEADLVEQTQLDANKAAADAQVGEARAQAMQAERLADEKARLEVLRQQAENKQIELEAEVNKVADAERYRRKQEVEADTFEQTRRAQAQVEIAEAEATAAKVRAMAEAEAVRLKGQAEADAIKAKAEAYRENQEALLAQQAMEILPELMSNFASGYANIGSMTVLSGGEGSENSVGSRFAGEQALGLKSIIESVKQTTGIDLAEIIQGRAAGHAQGSAQGAAIAEALSHDETVEDRSEK | 460 | Eukaryotes—PM microdomains—acylated by myristate or palmitate Prokaryotes (acylated in E. coli) | Regulator of membrane protein trafficking | 28, 29 |
| Acyltransferase (VLSGG) | NP_599609.1 | MTTSTATAKQQASKSHFRPDIQGLRAVAVLLVLIFHAGDGSVLSGGFTGVDIFFVISGYLITGHLIRSCLEKGKISLINFYAGRIRRILPAATAVLVFTALITILVLPDTRWMLIGAEIIASSVYLVNWLFASNTNYLNAEAAASPVQHYWTLSVEEQFYILWPALLIGLLYFSRHRFTLDSEETSGTKLNKVRWLQRYIALAAVLITLVSLCFSIYFSYTNPAPAYFITSTRLWELGIGAILASFSFQLERISAPIGYALGVMGLASIVAAGVTFDSQTVFPGYAALVPTLGAAAVIIGGMGGRAEKGVGVLLKVKPMRWIGDLSYSLYL | 331 | |||
| Membrane-associated Zn-dependent protease 1 (VLSGG) | NP_601220 | MAAYLLGVVLFFLGIAVTIALHEWGHFITARIFGMKVRRFFIGFGPTVFAKRRGETVYGLKAIPVGGFCDIAGMTAQDELDPEDLPRAMYLKPWWQRIIVLSGGVIMNLIVGFLVLYGVAVSSGIPNPDVDTTATVDTVQCVPETQISATELSSCVGSGPAGDAGIEHGDKILAVNGQEMASFTAIRDAILELPGETATLTIEREGTLFDVDLQVASVTRLASDGSEITVGAVGMSSLPPTDVYKKYGPIEGVGATARFTGDMISATWDGLKAFPAKIPGVVASIFGAERDVESPMSVVGASRIGGEFVERSMWDMFMMMLASLNFFLALFNLVPLPPLDGGHIAVVIYEKIRDFFRKLRGKPAGGPADYTKLMPVTVAVAALLMTVGGLVIVADVVNPIRLFG | 404 | Membrane protein | Domain (4–401): RseP. Domain (8–119): S2P-M50_PDZ_RseP-like. Domain 138–215: PDZ metalloprotease. Domain (316–398): S2P-M50 super family. Membrane-associated protease RseP, regulator of RpoE activity; Sigma-E pathway of extracytoplasmic stress response, regulation of prokaryotic lipid biosynthesis and membrane composition |
C. glutamicum proteins with conserved O-acylation pattern are underlined. N-terminal signal sequences, identified using signal4P program, are indicated in bold. AA, amino acid; LSS, Leu-Ser-Ser; PDZ, PDZ domain; VLSGG, Val-Leu-Ser-Gly-Gly.
Targeting to the mAGP Complex Is Dependent on the Presence of the O-Mycoloylation.
Protein acylation often regulates trafficking, segregation, or clustering of proteins within membrane compartments (16). To ascertain that the O-mycoloylation plays a role in protein targeting to the mycomembrane, we expressed PorA, PorH, PorB, and PorC in WT and cmytC-deleted C. glutamicum strains using an IPTG-inducible Ptac promoter on a high-copy plasmid (pXmj19) and compared the distribution of recombinant porins within the mAGP complex and the PM (Fig. 4A). Results showed the absence of nonacylated mature His-tagged PorH, PorB, and PorC in both the mAGP complex and the PM when cMytC is depleted, confirming that O-acylation plays an essential role in the targeting and/or the retention of proteins to the mycomembrane. However, a significant amount of PorA was detected in the mycomembrane and to a lesser extent, the PM when O-mycoloylation activity was abolished. The PorA sequence clearly contains a stretch of 45 predominantly hydrophobic residues that is not present in the other porins. This inherent hydrophobicity likely explains the residual membrane association of the unmodified protein. Interestingly, the loss of the mycoloyl residue was associated with a nonspecific membrane association with both the mAGP complex and the PM. Furthermore, because multiacylated proteoforms were characterized for both PorB and PorC, we questioned whether both PTMs were mandatory for mAGP localization (Fig. 4B). Consequently, we monitored the presence of WT PorB and PorC and mutant derivatives PorB-S7A, PorB-S98A, PorB-S7AS98A, PorC-S87A, PorC-S91A, and PorC-S87AS91A in mAGP fractions when expressed in the WT C. glutamicum strain. Although mature forms of PorB and PorC were predominantly found associated with the mAGP fraction, aggregated forms of nonacylated precursor were copurified in the case of PorC (Fig. S7). As depicted in Fig. 4B, mutation of the S7 site of PorB and S91 site of PorC significantly reduced the association of these proteins with mAGP complex. In contrast, mutation of S98 in PorB and S87 in PorC resulted in minimal or no change in mAGP association, suggesting that, in both proteins, association is driven by predominantly one mycoloylation site (i.e., PorB-S7 and PorC-S91). Dual mutants of these proteins totally abrogated mAGP association and in both cases, result in enhanced secretion of nonacylated PorB and PorC into the extracellular medium.
Fig. 4.
The O-acylation is essential for retention of PorH, PorB, and PorC at the mycomembrane but not PorA. (A) The mAGP complex and the PM were isolated from WT (W) and cMytC-deficient (Δ) C. glutamicum strains expressing recombinant PorA-His, PorH-His, PorB-His, and PorC-His and analyzed by SDS/PAGE after immunodecoration with antibodies against protein His tag. (B) Analysis of the mAGP complex purified from WT C. glutamicum cells expressing native recombinant proteins (WT) or the mutant derivatives of PorB-His [i.e., PorB-S7A, PorB-S98A, and PorB-S7AS98A (Left)] and PorC-His [i.e., PorC-S87A, PorC-S91A, and PorC-S87AS91A (Right)] by SDS/PAGE and immunodecoration with antibodies against protein His tag. Fractions isolated from untransformed WT cells were coanalyzed as control. Bands corresponding to the recombinant proteoforms are indicated with arrows using the following code: M, mature form corresponding to the recombinant protein without its N-terminal signal sequence; M*, mature form without O-mycoloylation; P, recombinant protein precursor containing the N-terminal signal sequence. Molecular mass (MW) markers (in kilodaltons) are indicated next to the gel.
Fig. S7.
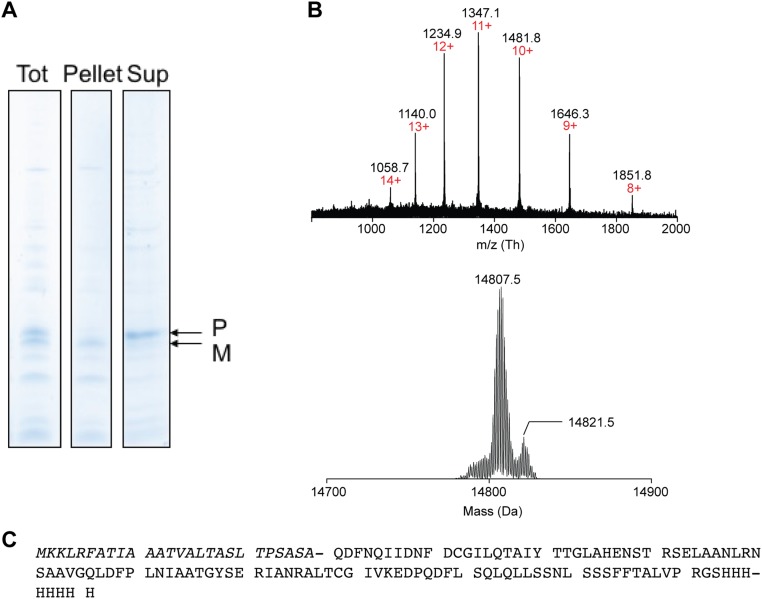
Characterization of aggregated recombinant PorC-His precursor within mAGP and PM fractions. (A) SDS/PAGE analysis of PorC-His containing C. glutamicum CE fractions before and after treatment with 8 M urea buffer and ultracentrifugation (200,000 × g for 1 h at 4 °C). M, mature form corresponding to the recombinant protein without its N-terminal signal sequence; P, recombinant protein precursor containing the N-terminal signal sequence. (B) Multicharged MS spectrum (Upper) and the corresponding isotopic pattern (Lower) of PorC-His found in the 8 M urea soluble fraction deconvoluted with MagTran software. The charge states are indicated in red. (C) Protein sequence of PorC-His precursor with the N-terminal sequence (italics). The molecular mass is expected to be 14,809.1 Da. The deconvoluted spectrum shows one major species at 14,807.5 Da, which corresponds to the nonmycoloylated PorC-His precursor containing a disulfide bond.
SI Experimental Procedures
DNA Manipulations and Bacterial Growth Media.
Chromosomal DNA purification was performed by using the DNeasy Blood & Tissue Kit (Qiagen). PCR, DNA preparations, ligations, and restrictions were performed by using Phusion High-Fidelity DNA Polymerase (ThermoScientific), QIAprep Spin Miniprep Kit (Qiagen) or QIAquick PCR Purification Kit (Qiagen), T4 DNA Polymerase (ThermoScientific), and FastDigest enzymes (ThermoScientific) all according to the recommendations of the suppliers. The bacterial strains and plasmids used in this study are listed in Table S1. The recombinant plasmids were introduced in Escherichia coli MC1061 recA− by classical heat shock transformation. DNA was introduced in Corynebacterium glutamicum cells by electrotransformation (200 ohms, 25 μF, 2.5 V). E. coli was grown in Luria Bertani medium (AthenaES) at 37 °C with aeration or on Luria Bertani medium agar plates containing the appropriate antibiotic. C. glutamicum strains were grown in Brain Hearth Infusion (BHI) medium (BD Biosciences) at 30 °C with aeration or on BHI agar plates with appropriate antibiotics. Alternatively, C. glutamicum liquid cultures were grown in CGXII minimal medium composed of 41 g of 4-morpholinepropanesulfonic acid, 1 g of KH2PO4, 1 g of K2HPO4, 0.25 g of MgSO4 × 7 H2O, 10 mg of MnSO4 × H2O, 10 mg of FeSO4 × 7 H2O, 1 mg of ZnSO4 × H2O, 0.2 mg of CuSO4, 0.02 mg of NiCl2 × 6 H2O, 10 mg of CaCl2, 0.2 mg of biotin, 1% (wt/vol) glucose, 1% (wt/vol) trehalose, 0.5% (wt/vol) 15N-NH4Cl, and 0.1 mM protocatechuic acid for 1 L growth medium. When necessary, the following antibiotics were used: kanamycin (Kan), 50 μg/mL; and chloramphenicol (Cm), 12.5 µg/mL.
Construction of Plasmids.
DNA fragments encoding WT PorA and PorH proteins with C-terminal thrombin (thr) or Xa factor (xa) cleavage sites and 8xHis-tag (His) were amplified by PCR by using the plasmids pXMJ19-porA-thr-His and pXMJ19-porH-xa-His as templates and the following primers: PorA-forward (5′-aaaactgcagaaggagatatacatatggaaaacgtttacgagttccttggaaacc-3′), PorA-reverse (5′-cgacggagctcgaattctagccaatcagccaaatcggatttc-3′), PorH-forward (5′-aaaactgcagaaggagatatacatatggatctttcccttctcaaggaaaccc-3′), and PorH-reverse (5′-cgacggagctcgaattctagccaatcagccaaatcggatttc-3′). The amplified fragments were cloned in pAN6 vector by using NdeI and EcoRI restriction enzymes, thus generating pAN6-porA-thr-His and pAN6-porH-xa-His plasmids. DNA fragments encoding native PorB and PorC precursors (i.e., with N-terminal signal sequence) were amplified by PCR by using purified C. glutamicum ATCC13032 chromosomal DNA as a template and the following primers containing the initiation codon (bold letters) or the nucleotide sequence encoding the thr cleavage site fused with the poly(his)8 tag and the codon stop (bold letters): porB-forward (5′-cctaaaacatatgaagctttcacaccgc-3′), porB-reverse (5′-cgatatgctagcttagtgatggtgatggatggtgatgggatccacgcggcaccagggaagagaagttggaggacagc-3′), porC-forward (5′-aaggaaacatatgaagaaactacgtttcg-3′), and porC-reverse (5′-cgatatgctagcttagtgatggtgatggatggtgatgggatccacgcggcaccagagcagtgaagaaggaagaagatag-3′). The amplified fragments were cloned into pAN6 vector by using NdeI and NheI restriction enzymes, thus yielding to pAN6-porB-thr-His and pAN6-porC-thr-His plasmids, respectively. Alternatively, DNA fragments encoding native PorB and PorC precursors (i.e., with N-terminal signal sequence) with a C-terminal 8His tag cleavable by thr were subcloned in pXMJ19 vector. In this context, the PCR amplifications were performed by using pAN6-porB-thr-His and pAN6-porC-thr-His plasmids and the following primers: forward (5′-gccggtctagagcatgcctgcagaaggagatatac-3′) and reverse (5′-gcgagtttgaattcttagtgatggtgatggtgatggtgatggg-3′). DNA fragments were inserted into the pXMJ19-porH-Xa vector by using Xba and EcoRI restriction enzymes, thus yielding to pXMJ19-porB-thr-His and pXMJ19-porC-thr-His plasmids, respectively. All constructs were validated by sequencing (Eurofins MWG operons).
Site-Directed Mutagenesis.
DNA fragment encoding the PorB-S98A single mutant was generated by PCR by using the pAN6-porB-thr-His vector as template and the following primers: porB-forward (see above) and porB-S98A-reverse (5′-tgatgggatccacgcggcaccagggaagcgaagttggaggacagc-3′). The amplified fragment was cloned in the pAN6-porB-thr-His vector by using NdeI, BamHI, and SacI restriction enzymes. By using SacI, WT porB was cleaved off from the pAN6-porB-thr-His vector used as template, and the pAN6-porB-S98A-thr-His vector was obtained. The single mutant PorB-S7A was generated by using the primer extension PCR technique involving the primers S7A-forward and S7A-reverse that have complementary sequence and contain the desire mutation. First, PCR was performed with pAN6-porB-thr-His vector as template and the primers PorB-forward (see above) and PorB-S7A-reverse (ctttgttggtggaggctaggtttgcgaag) to obtain product 1. Second, PCR was achieved with pAN6-porB-thr-His vector as template and the primers PorB-S7A-forward (cttcgcaaacctagcctccaccaacaaag) and PorB-reverse (see above) to obtain the product 2. DNA amplification was generated by PCR by mixing products 1 and 2 and the template pAN6-porB-thr-His and using the primers PorB-forward and PorB-reverse. The amplified DNA fragment encoding for the PorB-S7A mutant protein was inserted into the pAN6-porB-thr-His vector by using NdeI, BamHI, and SacI enzymes as mentioned earlier, and the pAN6-porB-S7A-thr-His was obtained. The plasmid pAN6-porB-S7AS98A-thr-His encoding the double mutant PorB-S7AS98A-thr-His was generated with the same strategy by using pAN6-porB-S98A-thr-His as template and the same primers for all PCR cycles. For PorC-S87A, PorC-S91A, and PorC-S87AS91A mutants, DNA fragments were generated by PCR with the following primers: porC-forward (see above), porC-S87A-reverse (5′-ggtgatgggatccacgcggcaccagagcagtgaagaaggaagaagataggttagaggccagaagctg-3′), porC-S91A-reverse (5′-ggtgatgggatccacgcggcaccagagcagtgaagaaggaagaagctaggttag-3′), and porC-S87AS91A-reverse (5′-ggtgatgggatccacgcggcaccagagcagtgaagaaggaagaagctaggttagaggccagaagctg-3′). Amplified DNA fragments were cloned in pAN6 vector by using pAN6-porB-thr-His as template and NdeI, BamHI, and SacI enzymes. Three additional plasmids were obtained: pAN6-porC-S87A-thr-His, pAN6-porC-S91A-thr-His, and pAN6-porC-S87AS91A-thr-His. All mutants were validated by sequencing (Eurofins MWG operons).
Cell Fractionation.
The extracellular medium was isolated by centrifugation of the bacterial cultures (4,000 × g for 15 min at 4 °C), then subjected to ultracentrifugation (200,000 × g for 30 min at 4 °C) to remove cell debris, and finally, treated with trichloroacetic acid (7% TCA for 1 h at 4 °C) to precipitate secreted macromolecules. The TCA precipitate was harvested by centrifugation (20,000 × g for 30 min at 4 °C) and resuspended in 1 M Tris⋅HCl, pH 8.0. Insoluble material was removed by centrifugation (4,000 × g for 10 min at 4 °C), and the supernatant was dialyzed in 50 mM Tris⋅HCl buffer and 150 mM NaCl, pH 8.0 for 18 h at 4 °C using a membrane (SpectraPor) with a molecular cutoff of 3.5 kDa. For cell envelope (CE) isolation, bacteria were washed in PBS (pH 7.6; Euromedex), resuspended in a lysis buffer (10 mL buffer per 1 g wet cells) consisting of 20 mM Tris⋅HCl, pH 8 containing 0.1 mM PMSF and Benzonase Nuclease (Millipore), and incubated for 60 min on ice. Cells were disrupted with a French press cell (four cycles of 20,000 lb/in2 at 4 °C). Unbroken cells were removed by low-speed centrifugation (2,500 × g for 15 min at 4 °C), and CEs were harvested by ultracentrifugation (200,000 × g for 20 min at 4 °C) of the cell lysate. The supernatant corresponding to the cytoplasm fraction (CYT) was collected for subsequent analysis. The CE pellet was resuspended in 20 mM Tris⋅HCl, pH 8 buffer at a final concentration of 0.1 mg/mL. The PM and the mAGP complex were separated using a sucrose density gradient centrifugation. Briefly, the CE fraction was loaded onto a multilayer sucrose gradient (made of 1 mL of 56%, 1 mL of 53%, 1 mL of 50%, 1 mL of 47%, 1 mL of 44%, 1 mL of 38%, and 1 mL of 36% sucrose) and centrifuged for 17 h in a Beckman SW41 Ti rotor (4 °C at 210,000 × g). Fractions of 500 μL were collected from the bottom to the top of the gradient. To identify sucrose fractions containing PM-associated molecules, the NADH oxidase activities were immediately assayed spectrophotometrically at 20 °C in 1 mL of 20 mM Tris⋅HCl buffer, pH 7.5 containing 0.2 mM of NADH, 1 mM DTT, and 50 µL of every sucrose fraction. The NADH oxidation was monitored at 340 nm for 1 min, and initial rate was determined and normalized to the highest activity. Normalized NADH oxidation rates in pure PM- and mAGP-containing fractions are typically in the 1–0.5 and 0.2–0 ranges, respectively. Sucrose fractions containing pure mAGP complex and pure PM were isolated accordingly, washed twice in 20 mM Tris⋅HCl, pH 8.0 buffer, and resuspended in the same buffer at a final concentration of 0.1 mg/mL. Unless indicated, cellular preparations (i.e., extracellular medium, CE, mAGP, PM, and CYT fractions) were stored at −20 °C until use.
SDS/PAGE and Immunoblotting.
Proteins were mixed in 2× SDS sample buffer before loading the gel and then separated by 12% Tris⋅Tricine SDS/PAGE under denaturing conditions. The gels were usually stained with InstantBlue (Expedeon) or silver-stained. Immunoblotting was performed with a standard semidry protocol using polyvinylidene fluoride membrane (Biorad) for protein immobilization. Bound primary antibodies (anti-His tag; dilution 1:2,000) were visualized by chemiluminescence (Immobilon Western; Millipore) using a 1:10,000 dilution of anti-mouse IgG conjugated to HRP (Biorad). Membranes were analyzed by using the ChemiDoc MP system (BioRad), and images were visualized and processed by using ImageLab software.
Purification of Recombinant Proteins.
For the purification of mAGP-associated recombinant proteins, IPTG-induced bacteria were washed in PBS (pH 7.6; Euromedex) and resuspended in 0.46% (vol/vol) N,N-dimethyldodecylamine-N-oxyde (LDAO) and 1 mM PMSF (Sigma). The cell suspension was incubated at 16 °C during 20 h under gentle stirring and centrifuged (18,000 × g for 30 min at 4 °C) to remove insoluble material. The LDAO-soluble fraction was collected, and the pH was adjusted to 8.2. The mAGP-associated recombinant proteins solubilized in LDAO micelles were purified at 20 °C on a GE AKTA Liquid Chromatography system (GE healthcare) equipped with a 5-mL Ni-NTA affinity column (Qiagen) that was preequilibrated with buffer A. Recombinant proteins were eluted with a linear gradient of 20–500 mM imidazole in buffer A (30 mL at a flow rate of 1 mL/min). Fractions containing pure recombinant protein were pooled and dialyzed in buffer A using a membrane (SpectraPor) with a molecular cutoff of 3.5 kDa to remove imidazole. Secreted proteins were purified from the extracellular medium fraction (see above) on a GE AKTA Liquid Chromatography system (GE Healthcare) at 4 °C and in two steps. First, the extracellular medium fraction was loaded on a 5-mL Ni-NTA affinity column (Qiagen) that was preequilibrated with buffer A′ consisting of 50 mM Tris⋅HCl and 150 mM NaCl, pH 8.0. Recombinant proteins were eluted with a linear gradient of 20–500 mM imidazole in buffer A′ (30 mL at a flow rate of 1 mL/min). Fractions containing pure recombinant protein were diluted fivefold to decrease imidazole concentration and concentrated on a Vivaspin concentrator (Sartorius; 5-kDa cutoff). Second, the concentrated protein fraction was purified by size exclusion chromatography on a High/load 16/600 Superdex 75 prep grade (GE Healthcare Life Sciences) equilibrated with buffer A′. The purified proteins were checked by SDS/PAGE with InstantBlue staining. The protein concentration was estimated by A280 using a Nanodrop 2000 analyzer (ThermoScientific) or a BCA colorimetric protein assay (ThermoFisher Scientific). When necessary, protein fractions were concentrated on concentrators (Sartorius) with a molecular mass cutoff of 5 kDa. Protein samples used for biochemical studies and MS analysis were stored at –20 °C, whereas solution NMR study was attempted with only freshly prepared proteins.
Top-Down LC-MS and LC-MS/MS.
Nano–LC-MS and MS/MS analyses of cell extracts (extracellular medium fractions) and purified recombinant proteins were performed on a nanoRS UHPLC system (Dionex) coupled to an LTQ-Orbitrap Velos mass spectrometer (ThermoFisher Scientific); a 5-µL sample was loaded on a reverse-phase C4-precolumn (300 µm i.d. × 5 mm; ThermoFisher Scientific) at 20 µL/min in 5% acetonitrile and 0.05% TFA. After 5 min of desalting, the precolumn was switched online with an analytical C4 nanocolumn (75 µm i.d. × 15 cm; in-house packed with C4 Reprosil) equilibrated in 95% solvent A (5% acetonitrile, 0.2% formic acid) and 5% solvent B (0.2% formic acid in acetonitrile). Proteins were eluted using either a short binary gradient increasing from 5% solvent B to 40% solvent B during 0.5 min and then from 40% solvent B to 99% solvent B during 4.5 min or a long binary gradient increasing from 5% solvent B to 40% solvent B during 5 min and then from 40% solvent B to 99% solvent B during 33 min at a flow rate of 300 nL/min. The LTQ-Orbitrap Velos was operated either in single MS or data-dependent acquisition mode with the XCaliburTM software (ThermoFisher Scientific). MS scans were acquired in the 500–2,000 m/z range with the resolution set to a value of 60,000, and spectra were deconvoluted with MagTran software. For top-down experiments, survey scan MS was acquired at 60,000 resolving power. Precursors were selected with an isolation width of 5 m/z and fragmented by CID with a normalized collision energy set at a value of 35%. The resulting fragment ions were analyzed in the Orbitrap at a resolution of 60,000. For recombinant porins, precursor ions were selected from an inclusion list established from previous MS analyses. MS spectra were deconvoluted with the Xtract algorithm, and MS/MS spectra were analyzed manually by fragment comparison of unprocessed raw spectra with theoretical masses generated by ProteinProspector v5.18.0 (prospector.ucsf.edu). For endogenous porins, the three most intense peaks were selected as precursor ions. The raw data were directly analyzed with Proteome Discoverer 2.1 (ThermoScientific) using the ProSight PD Top Down High/High node. Briefly, intact protein spectra were deconvoluted with Xtract (signal to noise ratio threshold of three) and searched against the whole C. glutamicum database generated in ProSight PC 3.0 (ThermoScientific). The search was performed in absolute mass mode (Delta M mode) with a fragment tolerance of 20 ppm and a precursor mass tolerance of 1,100 Da.
Solution NMR Spectroscopy.
Two NMR samples were prepared and consisted of 400 μM nonacylated or 250 μM acylated (U-15N)–labeled PorB-His in 20 mM NaH2PO4 and 100 mM NaCl, pH 6.8 [10% D2O and 11.1 μM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS)]. 2D 15N-1H HSQC and 3D heteronuclear single quantum coherence spectroscopy (HSQC)-total correlated spectroscopy (TOCSY) (mixing time of 80 ms) and HSQC-NOESY (mixing time of 120 ms) experiments were performed at 1H Larmor frequencies of 700 and 600 MHz on AVANCE III HD NMR spectrometers (Bruker BioSpin) equipped with QXI and cryogenic TXI triple-resonance probes, respectively. All data were acquired at a sample temperature of 298 K. 1H chemical shifts were calibrated using methyl resonance of DSS as internal reference. 15N chemical shifts were referenced to 1H chemical shifts using a frequency ratio of 0.10132912. Spectra were processed using TopSpin 3.2 (Bruker BioSpin) and analyzed using CCPNMR Analysis software for protein assignment.
Orthology Detection and Alignment Generation.
To generate alignments for the proteins in the benchmark dataset, we used the series of Corynebacteriales whole genomes (taxid = 85007). For orthologous protein identification, each query sequence in the set was searched using BLAST against the Corynebacteriales proteome at an expectation threshold of e = 10−4. Sequences had to have 30% global similarity to the original query for inclusion in the alignment. Multiple sequence alignments were then generated using Clustal Omega (www.clustal.org/omega/) and visualized using ESPript 3 (ESPript; espript.ibcp.fr/).
Discussion
Protein secretion is an essential determinant of bacterial physiology and virulence, and a variety of mechanisms are responsible for transporting proteins into or across the PM, maintaining them in a soluble form, targeting them to their correct cell envelope locations, and then, folding them into the correct structures. Corynebacteriales have evolved a complex cell envelope containing a PM and a mycomembrane harboring mycolic acids and unusual OMPs. Additionally, an outermost layer may exist in some suborder (17, 18). The biogenesis and the organization of the Corynebacteriales mycomembrane are far less documented than for the LPS-containing counterpart from Gram-negative outer membranes (19–21). Notably, very few mycomembrane proteins have been structurally and functionally characterized (10, 22), and secretion pathways resulting in mycomembrane association remained an enigma.
In this paper, we investigated the biogenesis of OMPs in C. glutamicum and deciphered the role of O-mycoloylation in targeting OMPs to the mycomembrane by using dedicated expression systems, refined cell fractionation procedures, and a combination of NMR and advanced top-down MS approaches (Fig. 5). In contrast to traditional bottom-up proteomics relying on trypsin digestion, the top-down MS approach consists of the analysis of entire proteins, enabling the identification of combinations of PTMs and the direct semiquantification of proteoforms (23–25). This approach is particularly relevant for the analysis of C. glutamicum porins that are rather hydrophobic with the presence of PTMs consisting of C32-C36 mycoloyl residues and that lack arginine and lysine residues for the generation of adequate tryptic peptides. This strategy was developed on both purified recombinant proteins and crude cell extracts and yielded the unambiguous identification of nonacylated, monoacylated, and diacylated proteoforms. Each proteoform was then analyzed and fragmented individually to determine the nature and the position of the PTM sites. In total, we identified 5 proteoforms for PorA (with an N-formylation on M1 associated or not with a phosphorylation on S51 or a mycoloylation of S15), 4 proteoforms for PorH (with an N-formylation on M1 associated or not with a mycoloylation of S56), 9 proteoforms for PorC (with a disulfide bond and a pyroglutamylation that are associated or not with one or two mycoloyl residues on S87 and S91), and 12 proteoforms for PorB (with a disulfide bond associated or not with one or two mycoloyl residues on S7 and S98).
Fig. 5.
Model for the biogenesis of major OMPs in C. glutamicum. After synthesis in the CYT and translocation across the PM through Sec-dependent (PorB and PorC) or –independent (PorA and PorH) pathways, OMPs are transported into the perisplam and acylated with one or several mycolic acids by the cMytC on serine residues located within short linear motifs. Specific posttranslational O-mycoloylations of OMPs are essential for targeting to the mycomembrane and may be involved in the subsequent formation of pore-forming assemblies. Nonacylated proteoforms are released in the extracellular medium (EM), but their function remains to be elucidated. AG, arabinogalactan; PG, peptidoglycan; M, O-mycoloylation; MM, mycomembrane; PM, plasma membrane; SEC, general secretion pathway; TAT, twin arginine translocation pathway.
The analysis of the O-mycoloylation sites reveals conserved short linear motifs, which typically consist of a three- to four-residue stretch of the protein sequence with one or two more wildcard positions [i.e., (F/L)SS and VLSG]. Multiple sequence alignments showed a good conservation of the PTM site composition and localization within the orthologous protein sequences (Fig. S6). More importantly, we identified the O-acylation motif in a larger panel of C. glutamicum proteins involved in the biogenesis of the cell envelope, including mycoloyltransferases, an acyl transferase, and a peptidoglycan hydrolase, or the maintenance of its integrity, such as lipocalin and flotillin. Whereas the two latter proteins are known to be acylated in Escherichia coli via an amide linkage (26–29), other proteins, including the mycoloyltransferases, have only been characterized in their soluble, nonacylated forms (7, 30, 31). Furthermore, we showed a physicochemical similarity in the properties of the O-acylation sites between Corynebacteriales and two human proteins (i.e., the ghrelin and Wnt-3a). This observation suggests a possible similarity in the recognition of these sites by the acylating enzymes. In eukaryotes, posttranslational O-acylation is carried out by dedicated membrane-bound O-acyl transferases (MBOATs). Porcupine, the Wnt MBOAT, appends palmitoleate and shorter cis-unsaturated fatty acids to Wnt. A consensus sequence in Wnt-3a (CXCHGXSXXCXXKXC) surrounding the acylated S209 mediates enzyme binding, fatty acid transfer, and Wnt signaling with G207, K215, and disulfide bonds required for fatty acylation (32). In ghrelin, the octanoate is linked to S3 within the N-terminal GSSFL sequence, with G1 and F4 also contributing to substrate recognition by Ghrelin O-acyltransferase (3, 33).
We showed that the mycoloyl residue is a bona fide signal to direct the modified protein to bind to mycomembrane (Fig. 4). There seems to be a correlation between the hydrophilicity and the amount of mycoloylation (Fig. 4B). Thus, PorB and PorC being the most hydrophilic porins are predominantly doubly mycoloylated. When protein O-acylation is abolished in the bacterium, PorB, PorC, and PorH are released into the extracellular medium, implying that mycolate hydrophobicity is the main driving force for mycomembrane association. However, for PorA, which is much more hydrophobic, a substantial amount of nonacylated protein remained associated with the membranes. Interestingly, PorA is not specifically associated with the mycomembrane when nonmycoloylated, and it is also detected in the PM (Fig. 4A), confirming the essential role of the mycoloylation for specific targeting to the mAGP complex.
The O-ester linkage between a mycoloyl residue and the protein is relatively stable. So far, an esterase/hydrolase activity that could cleave a mycoloyl residue from serine has not been shown. However, the existence of nonacylated soluble proteoforms might suggest the reversibility of the O-mycoloylation and resulting porin trafficking between the (myco)membrane and the extracellular medium. Indeed, we showed that nonacylated PorB, PorC, and PorH were predominantly secreted (Figs. 1 and 4 and Fig. S1). Hence, like Wnt proteins, for which palmitoleylation was recently shown to be reversible (34, 35), fatty acid cleavage by a carboxylesterase may regulate targeting of hydrophilic porins. Indeed, such potential carboxylesterases/hydrolases with a consensus mycoloylation site have been found in C. glutamicum (Table S3). This study has advanced our understanding of membrane biogenesis in Corynebacteriales. Nonetheless, important questions remain. One crucial issue is determining whether the O-mycoloylation is a general process to specifically retain proteins at the mycomembrane in C. glutamicum and other Corynebacteriales or indeed, if it is the only modification capable of doing so. Of equal importance will be determining the molecular mechanisms by which O-acylation affects OMP folding and interaction with the mycomembrane. This research line will generate knowledge in fundamental microbiology and in so doing, could bring to light vulnerabilities that could be exploited for new antibacterial drugs or provide efficient new industrial biotechnology procedures.
Experimental Procedures
Expression of Recombinant Cg Porins.
Plasmid construction, site-directed mutagenesis, and bacterial growth media are described in SI Experimental Procedures. For the expression of C-terminal His-tag fusion proteins, Brain Hearth Infusion or CGXII growth medium was inoculated with freshly transformed C. glutamicum cells at A600 of 0.4. The expression of LacI-regulated genes was induced by adding 1 mM IPTG in the early log phase (i.e., A600 ∼ 3–4) and maintained during 16–18 h at 30 °C. Cell fractionation and protein purification were achieved using procedures described in SI Experimental Procedures.
Top-Down LC-MS and LC-MS/MS.
Nano–LC-MS and MS/MS analyses of cell extracts (extracellular medium fractions) and purified recombinant proteins were performed on a nanoRS UHPLC system (Dionex) coupled to an LTQ-Orbitrap Velos mass spectrometer (ThermoFisher Scientific). Additional information on experimental procedures is in SI Experimental Procedures. Data have been deposited in the Mass Spectrometry Interactive Virtual Environment repository with the dataset identifier MSV000080529 (massive.ucsd.edu).
Acknowledgments
We thank Profs. M. Bott and R. Freudl for providing the empty pAN6 plasmid and Prof. R. Benz for providing pXmj19 plasmids with PorA and PorH genes. This work and the postdoctoral fellowship of C.C. were supported by French National Research Agency “Young Researcher” Grant ANR-13-JSV8-0001-01 (to M.A.M.R.). We acknowledge financial support from Integrated Screening Platform of Toulouse–Genotoul platform of Toulouse, CNRS, Université de Toulouse–Université Paul Sabatier, European Structural Funds (Fonds Européens de Développement Régional), the Midi-Pyrénées region, and Toulouse Metropole for the NMR and MS equipment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Mass Spectrometry Interactive Virtual Environment (MassIVE) database, which is a community resource developed by the NIH-funded Center for Computational Mass Spectrometry to promote the global free exchange of MS data (dataset identifier MSV000080529; massive.ucsd.edu).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617888114/-/DCSupplemental.
References
- 1.Resh MD. Fatty acylation of proteins: The long and the short of it. Prog Lipid Res. 2016;63:120–131. doi: 10.1016/j.plipres.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Nile AH, Hannoush RN. Fatty acylation of Wnt proteins. Nat Chem Biol. 2016;12:60–69. doi: 10.1038/nchembio.2005. [DOI] [PubMed] [Google Scholar]
- 6.Huc E, et al. O-mycoloylated proteins from Corynebacterium: An unprecedented post-translational modification in bacteria. J Biol Chem. 2010;285:21908–21912. doi: 10.1074/jbc.C110.133033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huc E, et al. Identification of a mycoloyl transferase selectively involved in O-acylation of polypeptides in Corynebacteriales. J Bacteriol. 2013;195:4121–4128. doi: 10.1128/JB.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rath P, et al. Functional expression of the PorAH channel from Corynebacterium glutamicum in cell-free expression systems: Implications for the role of the naturally occurring mycolic acid modification. J Biol Chem. 2011;286:32525–32532. doi: 10.1074/jbc.M111.276956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rath P, et al. NMR localization of the O-mycoloylation on PorH, a channel forming peptide from Corynebacterium glutamicum. FEBS Lett. 2013;587:3687–3691. doi: 10.1016/j.febslet.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler K, Benz R, Schulz GE. A putative α-helical porin from Corynebacterium glutamicum. J Mol Biol. 2008;379:482–491. doi: 10.1016/j.jmb.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Costa-Riu N, et al. Identification of an anion-specific channel in the cell wall of the Gram-positive bacterium Corynebacterium glutamicum. Mol Microbiol. 2003;50:1295–1308. doi: 10.1046/j.1365-2958.2003.03754.x. [DOI] [PubMed] [Google Scholar]
- 12.Marchand CH, et al. Biochemical disclosure of the mycolate outer membrane of Corynebacterium glutamicum. J Bacteriol. 2012;194:587–597. doi: 10.1128/JB.06138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalbøge H, Bayne S, Pedersen J. In vivo processing of N-terminal methionine in E. coli. FEBS Lett. 1990;266:1–3. doi: 10.1016/0014-5793(90)90001-b. [DOI] [PubMed] [Google Scholar]
- 14.Carrillo DR, et al. Kinetic and structural characterization of bacterial glutaminyl cyclases from Zymomonas mobilis and Myxococcus xanthus. Biol Chem. 2010;391:1419–1428. doi: 10.1515/BC.2010.130. [DOI] [PubMed] [Google Scholar]
- 15.Chou MF, Schwartz D. Biological sequence motif discovery using motif-x. Curr Protoc Bioinformatics. 2011;13:13.15–13.24. doi: 10.1002/0471250953.bi1315s35. [DOI] [PubMed] [Google Scholar]
- 16.Sorek N, Bloch D, Yalovsky S. Protein lipid modifications in signaling and subcellular targeting. Curr Opin Plant Biol. 2009;12:714–720. doi: 10.1016/j.pbi.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuber B, et al. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol. 2008;190:5672–5680. doi: 10.1128/JB.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Geyter J, et al. Protein folding in the cell envelope of Escherichia coli. Nat Microbiol. 2016;1:16107. doi: 10.1038/nmicrobiol.2016.107. [DOI] [PubMed] [Google Scholar]
- 20.Freudl R. Leaving home ain’t easy: Protein export systems in Gram-positive bacteria. Res Microbiol. 2013;164:664–674. doi: 10.1016/j.resmic.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 21.van der Woude AD, Luirink J, Bitter W. Getting across the cell envelope: Mycobacterial protein secretion. Curr Top Microbiol Immunol. 2013;374:109–134. doi: 10.1007/82_2012_298. [DOI] [PubMed] [Google Scholar]
- 22.Niederweis M. Mycobacterial porins--new channel proteins in unique outer membranes. Mol Microbiol. 2003;49:1167–1177. doi: 10.1046/j.1365-2958.2003.03662.x. [DOI] [PubMed] [Google Scholar]
- 23.Marcoux J, Cianférani S. Towards integrative structural mass spectrometry: Benefits from hybrid approaches. Methods. 2015;89:4–12. doi: 10.1016/j.ymeth.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Tran JC, et al. Mapping intact protein isoforms in discovery mode using top-down proteomics. Nature. 2011;480:254–258. doi: 10.1038/nature10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nat Methods. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop RE, Penfold SS, Frost LS, Höltje JV, Weiner JH. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J Biol Chem. 1995;270:23097–23103. doi: 10.1074/jbc.270.39.23097. [DOI] [PubMed] [Google Scholar]
- 27.Flower DR, North AC, Sansom CE. The lipocalin protein family: Structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 28.Neumann-Giesen C, et al. Membrane and raft association of reggie-1/flotillin-2: Role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem J. 2004;378:509–518. doi: 10.1042/BJ20031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barák I, Muchová K. The role of lipid domains in bacterial cell processes. Int J Mol Sci. 2013;14:4050–4065. doi: 10.3390/ijms14024050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand S, Niehaus K, Pühler A, Kalinowski J. Identification and functional analysis of six mycolyltransferase genes of Corynebacterium glutamicum ATCC 13032: The genes cop1, cmt1, and cmt2 can replace each other in the synthesis of trehalose dicorynomycolate, a component of the mycolic acid layer of the cell envelope. Arch Microbiol. 2003;180:33–44. doi: 10.1007/s00203-003-0556-1. [DOI] [PubMed] [Google Scholar]
- 31.De Sousa-D’Auria C, et al. New insights into the biogenesis of the cell envelope of corynebacteria: Identification and functional characterization of five new mycoloyltransferase genes in Corynebacterium glutamicum. FEMS Microbiol Lett. 2003;224:35–44. doi: 10.1016/S0378-1097(03)00396-3. [DOI] [PubMed] [Google Scholar]
- 32.Rios-Esteves J, Haugen B, Resh MD. Identification of key residues and regions important for porcupine-mediated Wnt acylation. J Biol Chem. 2014;289:17009–17019. doi: 10.1074/jbc.M114.561209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, et al. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell. 2015;32:719–730. doi: 10.1016/j.devcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakugawa S, et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]