Significance
The First World War was a historical experiment for early-life stress. Fathers of hundreds of thousands of children of all ages were killed during the conflict. The Developmental Origins of Health and Disease hypothesis predicts long-term effects of early-life stress. We collected historical data on French orphans born 1914–1916 and their fathers’ military records and compared the orphans’ mortality in adulthood (age 31–99 y) with that of matched nonorphans. We found a strong decrease in lifespan, reflecting increased mortality before age 65 y, in persons whose fathers died before, not after, their birth. These results support the notion that maternal psychological stress in pregnancy decreases adult longevity in offspring.
Keywords: intrauterine programming, maternal bereavement, adult mortality, historical cohort, World War One
Abstract
Although early-life stress is known to alter health, its long-term consequences on mortality remain largely unknown. Thanks to unique French legislation established in 1917 for war orphans and children of disabled soldiers, we were able to study the adult mortality of individuals born in 1914–1916 whose fathers were killed during World War 1. Vital information and socio-demographic characteristics were extracted manually from historical civil registers for 5,671 children born between 1 August 1914 and 31 December 1916 who were granted the status of “pupille de la Nation” (orphan of the Nation). We used a database comprising 1.4 million deceased soldiers to identify war orphans and collect information on their fathers and then paired each orphan with a nonorphan from the same birth register matched for date of birth, sex, and mother’s age at the infant’s birth. Mortality between ages 31 and 99 y was analyzed for 2,365 orphan/nonorphan pairs. The mean loss of adult lifespan of orphans who had lost their father before birth was 2.4 y (95% CI: 0.7, 3.9 y) and was the result of increased mortality before age 65 y. Adult lifespan was not reduced when the father’s death occurred after the infant’s birth. These results support the notion that intrauterine exposure to a major psychological maternal stress can affect human longevity.
Multiple lines of evidence indicate that exposure to adverse environmental cues in the early stages of development may have durable effects on biological vulnerability at older ages (1, 2). Following D. J. Barker’s seminal observations (“the womb may be more important than the home”) in children born during 1911–1930 (3), the Fetal Origins of Adult Disease hypothesis (4) and the Developmental Origin of Health and Disease (DOHaD) hypothesis (5) have developed. Two main kinds of early-life adversities, undernutrition and psychic maternal stress, have been studied in humans. Indeed, exposure to famine during pregnancy can have long-range consequences on offspring’s morbidities including cardiovascular, metabolic, and mental diseases (6–8). Psychic suffering of pregnant mothers has been associated with functional alteration in the offspring’s hypothalamo–pituitary–adrenal (HPA) axis (9, 10) that may pave the way for psychic vulnerabilities (11), obesity (12), diabetes (13), and cardiovascular diseases (14).
Stressors in critical periods of development induce both immediate, reversible homeostatic mechanisms and whole-life modifications of the response to environmental challenges (15). The mechanisms implicated in early programming of adult disorders remain largely unknown, although epigenetics is likely involved (16, 17). The intrauterine and early postnatal periods are characterized by high epigenetic plasticity (18); thus, as shown in laboratory rodents, early-life stress can affect development by durably imprinting specific brain regions and other tissues through epigenetic modifications (19). Restraint stress (20) or repeated exposure of pregnant dams to an aggressive congener (21), poor maternal care (22), or separation of recently born pups from the mother (23, 24) were shown to result in tissue-specific changes in DNA methylation and in the activity of genes involved in the stress response (22–25); some of these effects are sexually dimorphic (26). Epigenetics, however, is only one of the many effectors of early-life stress that can act on brain development (19); for example, prenatal stress in rats induces a reduction of neurogenesis in specific brain regions (27).
Few studies have investigated the mortality consequences of early-life conditions, including caloric deprivation during gestation (28, 29), economic conditions at birth (30), and season of birth (31). For instance, males born at the height of the Finnish famine lost ∼1 y of life expectancy at age 40 y (29). To our knowledge, only two studies have related early psychological stress and adult mortality (32, 33). No increased mortality between age 27 and 69 y was found in the 1,726 members of the Helsinki Birth Cohort (born 1934–1944 and followed from 1971–2003) who were separated as children from their parents during the Second World War (32). In the 1958 British birth cohort, increased all-cause mortality before age 50 y was found for the 4,543 individuals who experienced events such as parental divorce or bereavement in early life (33).
World War I (WWI) has been described as a “vast human experiment in stress” (34). Based on universal conscription, the French army suffered heavy losses (∼1.4 million deaths). The great majority of casualties were men aged 18–35 years old in 1914 and were caused mainly by artillery bombardments (35). Every day, about 350 French women lost their husbands, an event found to top a list of 43 stressful life events (36).
We studied the adult mortality of individuals whose fathers were killed during WWI. Our working hypothesis was that the father’s death, known to be associated with maternal stress of extreme psychic intensity, could program orphans for various disorders, leading to an increased mortality in adulthood. Further, we hypothesized that if specific in utero programming did take place, it could be revealed by a decreased lifespan observed only in orphans whose fathers died before they were born (prenatal orphans). Depending on the mechanisms involved, male and female offspring might not have been equally affected.
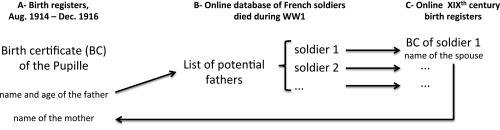
We chose the design of a historical (non-concurrent) cohort study to test the relation between the loss of father and the mortality outcome. We took advantage of a specific legal status created in July 1917 and granted upon request to war orphans from 1918 onwards (37) that enabled state assistance and financial support if needed. The children, named “pupilles de la Nation” (orphans of the Nation), were said to be “adopted by the Nation,” an event noted on their birth certificate (Fig. 1A). By law, adoption, marriage, divorce, and, since 1945, death also were systematically inscribed on birth certificates, no matter where they occurred. Any French city hall continuously updates its birth registers, receiving systematic notifications from the ∼36,000 other city halls covering France and consulates abroad (death and marriage notifications) and from civil tribunals and law firms (adoptions and divorces).
Fig. 1.
Historical material used: Examples of an orphan’s birth certificate (A) and the military record of the orphan’s father (B). (A) Birth certificate of a pupille de la Nation, André L., born of Gabriel L. on 31 August, 1914, in Bordeaux. The tribunal of the city of Versailles granted him pupille de la Nation status in 1923. That information was automatically transmitted by the Versailles tribunal to the city hall of Bordeaux and was transcribed by a civil servant in the upper left margin of the birth certificate. Similarly, notification of André L.’s marriage (in 1945) and death (in 1974) were transmitted (both by the city hall of the 16th district of Paris) to the city hall of Bordeaux, which noted these life events on the left margin of the birth certificate a few days after they occurred (© archives Bordeaux métropole – Bordeaux 1 E 427). (B) We searched for the father of André L., Gabriel L., by name and age in the database of French soldiers who died during WW1 (available at www.memoiredeshommes.sga.defense.gouv.fr/en/article.php?larub=80). The database yielded the image of the father’s record (selected sections are shown). Gabriel L. was killed in action on 9 June 1915.
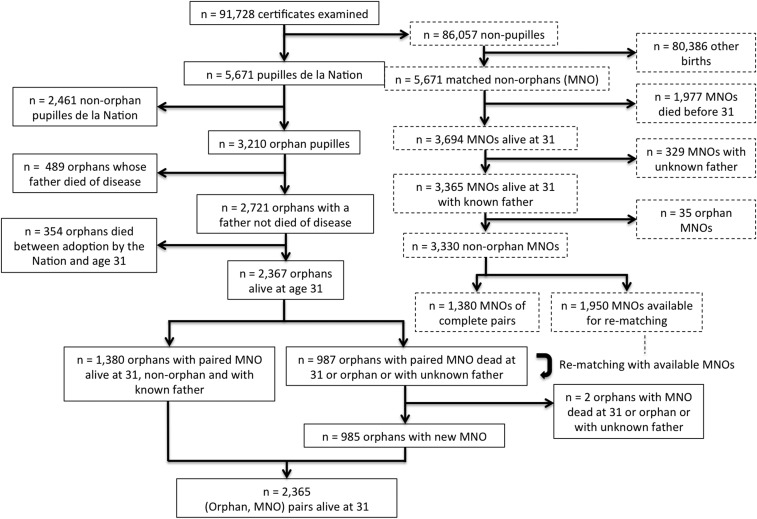
We identified and computerized the birth certificates of 5,671 pupilles de la Nation born between 1914 and 1916 in two large cities, Paris and Bordeaux. The dates of death of pupilles who died after 1945 were known by the notifications on their birth certificates. For the 3,210 pupilles identified as war orphans, we retrieved the dates and causes of paternal death in the publicly available database of all military deaths that occurred between 3 August 1914 and 1 June 1919 (Fig. 1B). After we excluded those whose father died from disease, we compared the adult lifespan of orphans with that of non-pupille individuals (i.e., matched nonorphans, MNOs) drawn from the same birth registers. An orphan and an MNO were matched by sex, mother’s age (± 2 y), district, and date of birth. The flowchart of the study is shown in Fig. S1. The adult lifespan of 2,365 orphan/MNO pairs alive at age 31 y was analyzed. Lifespan comparisons with MNOs were performed separately for prenatal and postnatal orphans.
Fig. S1.
Flowchart showing the definition of the cohorts of war orphans and MNOs. Pupilles were by definition children who survived from birth to their being adopted by the Nation at some point in childhood (on average at ∼ age 5 y). Their probability of death before age 31 y is conditional on having being adopted (354/2721 = 13%) and therefore was expected to be lower than the unconditional probability of death before age 31 y measured in MNOs (1,977/5,671 = 34.9%).
Results
Baseline Characteristics of Subjects.
Table 1 shows the characteristics of the 2,365 pairs (Table S1 for detailed characteristics); 27.7% (95% CI: 25.9, 29.6%) of orphans were prenatal orphans, and 72.3% (95% CI: 70.4, 74.1%) were postnatal orphans.
Table 1.
Baseline characteristics of the 2,365 studied orphan/MNO pairs
| Variable | Prenatal orphans (n = 656) | MNOs (n = 656) | Postnatal orphans (n = 1,709) | MNOs (n = 1,709) |
| Date of birth, mean | 28 April 1915 | 27 April 1915 | 1 April 1915 | 2 April 1915 |
| Sex, n (%) | ||||
| Female | 319 (48.6) | 319 (48.6) | 814 (47.6) | 814 (47.6) |
| Male | 337 (51.4) | 337 (51.4) | 895 (52.4) | 895 (52.4) |
| Legitimacy, n (%) | ||||
| Legitimate | 630 (96.0) | 552 (84.1) | 1,509 (88.3) | 1,413 (82.7) |
| Illegitimate | 26 (4.0) | 104 (15.9) | 200 (11.7) | 296 (17.3) |
| Paternal OS (95% CI) | 2.60 (2.46, 2.74) | 2.70 (2.58, 2.82) | 2.62 (2.55, 2.69) | 2.75 (2.68, 2.83) |
| Maternal OS (95% CI) | 4.42 (4.26, 4.59) | 4.70 (4.53, 4.86) | 4.44 (4.34, 4.54) | 4.62 (4.52, 4.72) |
| Maternal age, mean (SD) | 25.8 (4.3) | 26.2 (4.5) | 25.6 (4.9) | 26.4 (5.0) |
| Paternal age, mean (SD) | 28.7 (4.3) | 30.4 (5.7) | 28.5 (4.9) | 30.6 (6.2) |
| Status at age 99, n (%) | ||||
| Dead | 630 (96.0) | 637 (97.1) | 1,645 (96.3) | 1,632 (95.5) |
| Alive | 26 (4.0) | 19 (2.9) | 64 (3.7) | 77 (4.5) |
OS, occupation score (Methods).
Table S1.
Detailed characteristics of the studied 2,365 orphan/MNO pairs
| Variable | Prenatal orphans (n = 656) | MNOs (n = 656) | Postnatal orphans (n = 1,709) | MNOs (n = 1,709) |
| Date of birth, mean | 28 April 1915 | 27 April 1915 | 1 April 1915 | 2 April 1915 |
| Sex, n (%) | ||||
| Female | 319 (48.6) | 319 (48.6) | 814 (47.6) | 814 (47.6) |
| Male | 337 (51.4) | 337 (51.4) | 895 (52.4) | 895 (52.4) |
| Legitimacy, n (%) | ||||
| Legitimate | 630 (96.0) | 552 (84.1) | 1509 (88.3) | 1413 (82.7) |
| Illegitimate | 26 (4.0) | 104 (15.9) | 200 (11.7) | 296 (17.3) |
| Paternal occupation, n (%) | ||||
| Worker | 190 (29.0) | 168 (25.6) | 493 (28.9) | 456 (26.7) |
| Craftsman | 117 (17.9) | 151 (23.0) | 329 (19.3) | 367 (21.5) |
| Employee | 224 (34.1) | 190 (28.9) | 535 (31.3) | 455 (26.6) |
| Shopkeeper | 55 (8.3) | 54 (8.2) | 153 (8.9) | 165 (9.7) |
| Middle class | 30 (4.6) | 46 (7.0) | 81 (4.8) | 110 (6.5) |
| Upper class | 41 (6.2) | 48 (7.4) | 118 (6.9) | 156 (9.1) |
| Paternal occupation score (95% CI) | 2.60 (2.46, 2.74) | 2.70 (2.58, 2.82) | 2.62 (2.55, 2.69) | 2.75 (2.68, 2.83) |
| Maternal occupation, n (%) | ||||
| Servant | 57 (8.7) | 61 (9.3) | 164 (9.6) | 158 (9.2) |
| Worker | 87 (13.2) | 60 (9.2) | 212 (12.4) | 190 (11.1) |
| Craftswoman | 122 (18.6) | 109 (16.7) | 318 (18.6) | 284 (16.6) |
| Employee | 68 (10.3) | 50 (7.7) | 146 (8.5) | 121 (7.1) |
| Housekeeper | 100 (15.2) | 115 (17.6) | 284 (16.6) | 297 (17.4) |
| Shopkeeper | 35 (5.4) | 37 (5.6) | 92 (5.4) | 101 (5.9) |
| Housewife | 178 (27.1) | 212 (32.3) | 448 (26.2) | 526 (30.8) |
| Middle and upper class | 10 (1.5) | 11 (1.7) | 44 (2.6) | 31 (1.8) |
| Maternal occupation score (95% CI) | 4.42 (4.26, 4.59) | 4.70 (4.53, 4.86) | 4.44 (4.34, 4.54) | 4.62 (4.52, 4.72) |
| Age of the mother, mean (SD) | 25.8 (4.3) | 26.2 (4.5) | 25.6 (4.9) | 26.4 (5.0) |
| Age of the father, mean (SD) | 28.7 (4.3) | 30.4 (5.7) | 28.5 (4.9) | 30.6 (6.2) |
| Status at age 99 y, n (%) | ||||
| Dead | 630 (96.0) | 637 (97.1) | 1,645 (96.3) | 1,632 (95.5) |
| Alive | 26 (4.0) | 19 (2.9) | 64 (3.7) | 77 (4.5) |
Paternal occupations and age at birth for illegitimate births were multiply imputed using MICE (Methods). Ten completed datasets were created. Figures by paternal occupation are averaged over the 10 completed datasets and are rounded to the nearest integer.
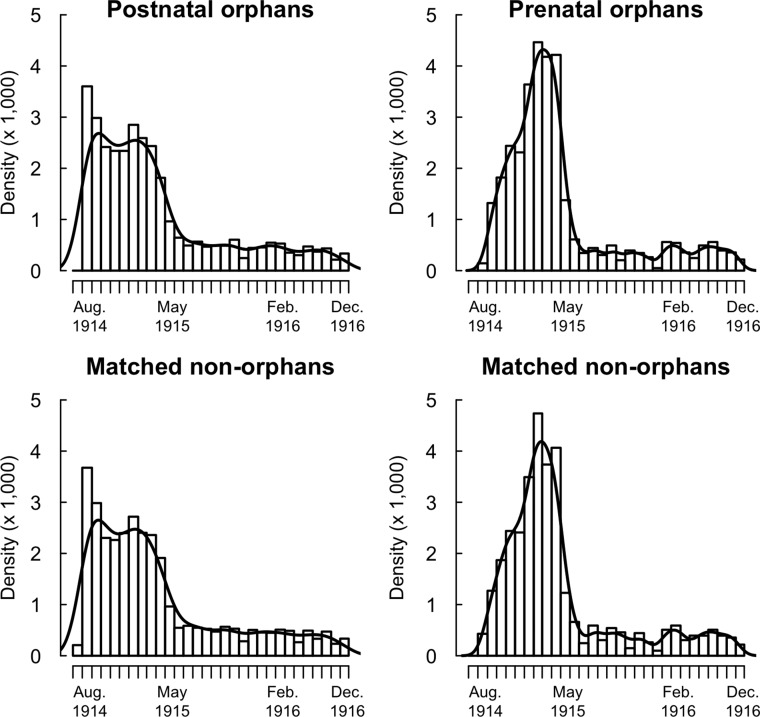
Dates of birth of MNOs matched those of orphans. The distribution of dates of birth of prenatal and postnatal orphans showed differences (Fig. S2). The monthly number of orphans fell in May 1915. Although the dates of birth of postnatal orphans were uniformly distributed before May 1915, a peak in prenatal orphans occurred in February–April 1915 (see legend of Fig. S2).
Fig. S2.
Birth dates of prenatal and postnatal orphans and MNOs. The majority of orphans were conceived before August 1914 (the beginning of the war) and thus were born before May 1915. The observed abrupt decline in the monthly number of orphan births in May 1915 paralleled the decline observed at the country level (66). The distributions of dates of birth were different in prenatal and postnatal orphans. By design, the distribution of dates of birth is identical in orphans and MNOs for both types of orphans. The number of prenatal orphans born in a given month was determined by both the number of conceptions 9 mo before and the father’s risk of death during pregnancy. Men who impregnated women just before the war began had a prewar fertility and were at risk during the woman’s entire pregnancy. The observed peak in the birth of prenatal orphans in February–April 1915 therefore was expected. In contrast with prenatal orphans, the monthly number of postnatal orphans was approximately constant before May 1915. MNOs are matched to orphans by district and date of birth, so any cofounding associated with the place and date of birth (e.g., changing disease environment) is removed in the comparison of orphans and MNOs. The solid line shows the Gaussian kernel density estimate.
The paternal occupation score (range: 1–6) was 2.60 (95% CI: 2.46, 2.74) in prenatal orphans and 2.70 (95% CI: 2.58, 2.82) in their MNOs (median P of the χ2 test over the 10 datasets obtained by multiple imputation = 0.02). This orphan/MNO difference was similar in postnatal orphan/MNO pairs [postnatal orphans: 2.62 (95% CI: 2.55, 2.69); MNOs: 2.75 (95% CI: 2.68, 2.83)]. The illegitimacy rate was lower in orphans than in MNOs (Table 1): 4.0% (95% CI: 2.7, 5.8%) in prenatal orphans, 11.7% (95% CI: 10.2, 13.3%) in postnatal orphans, and 15.9% (95% CI:13.2. 18.9%) and 17.3% (95% CI:15.6, 19.2%) in their respective MNOs.
Difference in Adult Lifespan Between Orphans and MNOs.
Adult lifespan was different in prenatal orphans and their MNOs (Wilcoxon test: P = 8.1 × 10−3). No difference was found between postnatal orphans and their MNOs (Wilcoxon test: P = 0.99). Prenatal and postnatal orphans had different losses of lifespan (permutation test: P = 0.03). Mean lifespan was 75.9 y (95% CI: 74.6, 77.1 y) in prenatal orphans vs. 78.2 y (95% CI: 77.1, 79.3 y) in their MNOs and was 76.7 y (95% CI: 76.0, 77.4 y) in postnatal orphans vs. 77.0 y (95% CI: 76.3, 77.7 y) in their MNOs. The mean lifespan of prenatal orphans was 2.4 y (95% CI: 0.7, 3.9 y) less than that of their MNOs. The difference was 0.3 y (95% CI: −0.8, 1.3 y) in postnatal orphans. Using a Generalized Additive Model (GAM) to control for legitimacy, parental occupation, and age, the adjusted difference between orphans and MNOs was 2.0 y (95% CI: 0.1, 4.0 y) higher in prenatal than in postnatal pairs.
The observed difference lifespan in orphan/MNO prenatal pairs was stronger in males [males: 3.1 y (95% CI: 0.8, 5.4 y); females: 1.3 y (95% CI: −1.1, 3.5 y)]. However, the male/female difference in effects did not achieve significance (permutation test: P = 0.29).
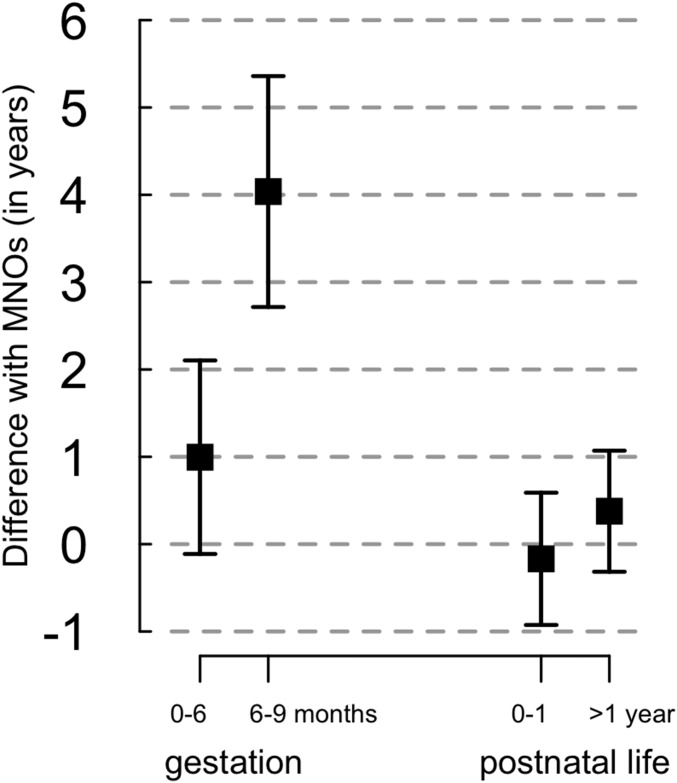
We examined the possibility of an increased effect of father’s death in early pregnancy (first trimester vs. pooled second and third trimesters) or late pregnancy (third trimester vs. pooled first and second trimesters). No difference in orphan/MNO lifespan was found when the father’s death occurred during early pregnancy (permutation test: P = 0.80), but the orphan/MNO difference reached 4.1 y (95% CI: 1.5, 6.7 y) when the father’s death occurred during the third trimester vs. 1.2 y (95% CI: −0.8, 3.2 y) when the father’s death occurred during the first/second trimester (permutation test: P = 0.07) (Fig. 2). For postnatal orphans, the mean difference between the orphans and the MNOs was similar when the father’s death occurred in the first year of postnatal life or after age 1 y (permutation test: P = 0.59) (Fig. 2) and when the father’s death occurred before or after 6 mo of age (permutation test: P = 0.94).
Fig. 2.
Mean difference in lifespan between war orphans and MNOs according to age at father’s death. Data are shown as mean ± SE.
Evolution of Differences in Lifespan with Age.
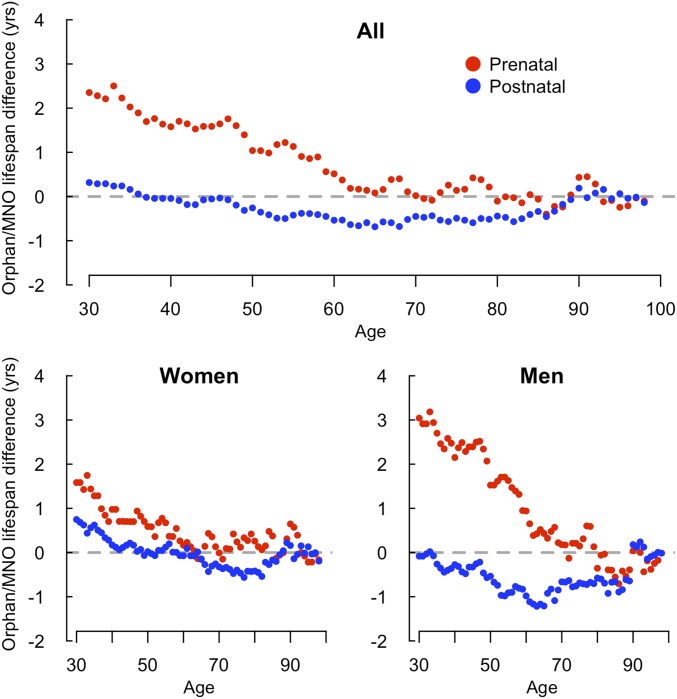
A larger proportion of prenatal orphans (150/656) than their MNOs (107/656) died before age 65 y (P = 3.5 × 10−3). No difference was found in remaining lifespan for prenatal orphans and MNOs alive at age 65 y (Fig. 3), indicating that the 2.4-y difference in remaining life expectancy at age 31 y reflected increased mortality between the ages of 31 and 65 y.
Fig. 3.
Variation with age of the difference between orphan and MNO remaining lifespans, by sex.
Sensitivity Analyses.
Because of the difference in the proportion of legitimate children in orphans and MNOs, the analyses were performed on the subset of orphan/MNO pairs in which both members were legitimate. The difference between prenatal and postnatal pairs was maintained: The mean loss of lifespan of orphans was 2.7 y (95% CI: 0.9, 4.4 y) in prenatal legitimate pairs and was 0.2 y (95% CI: −0.9, 1.4 y) in postnatal legitimate pairs (permutation test: P = 0.03). In the multivariate analysis restricted to legitimate pairs, the difference with MNOs was 2.6 y (95% CI: 0.4, 4.7 y) greater in prenatal than in postnatal orphans.
Results remained consistent when rematched pairs were removed (Methods), when the analysis was restricted to Paris, when median, not mean, differences were analyzed, when only pairs born before May 1915 (i.e., conceived before the beginning of the war) were included, or when the longevity of those still alive at age 99 y was imputed and included in the analyses.
Discussion
All persons in the current study were born in 1914–1916, at a time when most mothers faced uncertainty about safety and nutrition and an increased workload. Those whose spouse survived may have lost a brother or friend. Those who lost their spouse were not always informed immediately (SI Text). Our main finding was a large difference of 2.4 y in adult lifespan between prenatal orphans and MNOs because of increased mortality before age 65 y. In contrast, postnatal orphans and their MNOs had quasi-identical lifespans. Both findings are of interest in the context of the DOHaD hypothesis.
It is known that early mortality selection may mask the negative long-term effects of an early-life adversity by enriching the proportion of more robust individuals in the studied population (30). A striking example is the Finnish famine, when selection induced by the immediate threefold increase in mortality was strong enough to mask a 1-y decrease in adult life expectancy (28, 29). Had preferential mortality been present among WWI orphans before they reached age 31 y, a more robust proportion of them would have been selected, strengthening our finding of a loss of lifespan in prenatal orphans.
In fact, orphans and MNOs followed a similar path of adversities except for the father’s death. Because adoptions by the Nation started in 1918, only orphans alive in 1918 could become pupilles, precluding a direct study of infant and child mortality. However, there is no evidence for any selective pressure comparable to a famine in the early life of WWI orphans. When the Spanish influenza pandemic started in France in August 1918 (38), the studied orphans and MNOs were 1.5–4 y old. We see no reason to postulate that the Spanish flu struck significantly more war orphans than MNOs. Indirect evidence against a significant early-mortality selection also comes from the examination of paternal occupations. The odds ratio of survival in the Finnish famine was ∼2.5 between rich and poor families (39). In contrast, we observed only minimal differences in the occupations of the fathers of the war orphans who survived to adulthood and the fathers of their MNOs (Table 1 and Table S1).
Our rule for recruiting MNOs aimed at limiting confounding factors. An orphan and the MNO were born in the same district. In 87.8% of the orphan/MNO pairs, the difference in dates of birth was less than 2 wk, guaranteeing that an orphan and the MNO faced a comparable external environment and food supply at the earliest stages of development. Matching of date and district of birth also was critical because of the migrations induced by the war. In particular, refugees from the invaded regions of the northeast, including pregnant women, progressively settled in Paris (40), thereby modifying the area of recruitment (Fig. S3C).
Fig. S3.
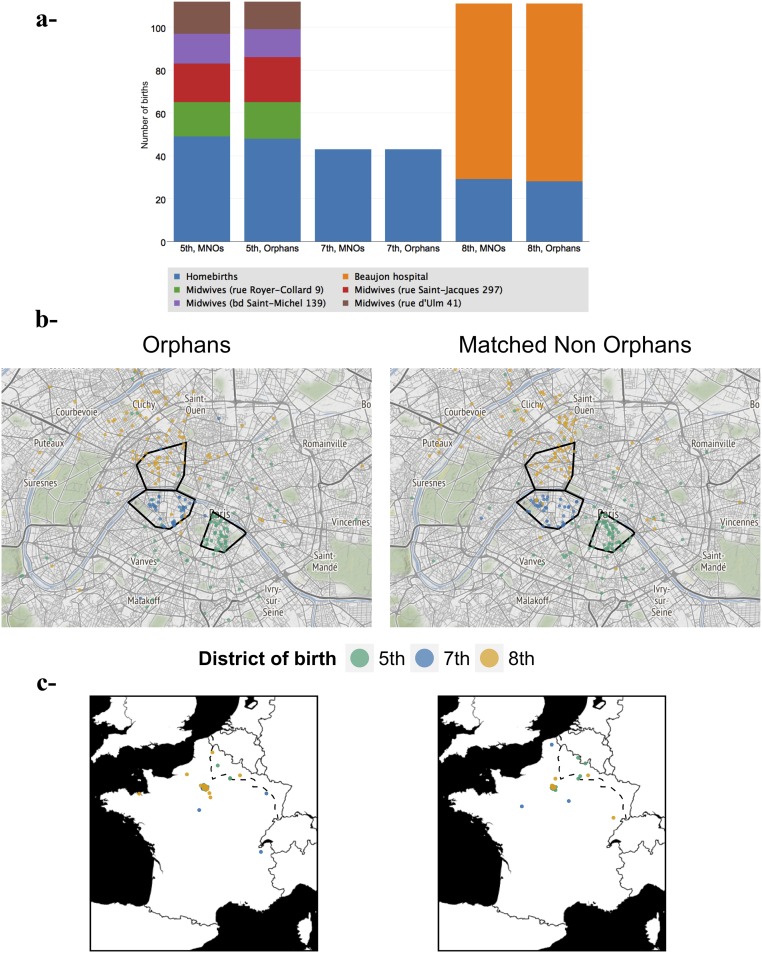
Type of birth and home address of parents for orphans and MNOs born in three contrasted districts of Paris. For orphan/MNO pairs born in three districts of Paris (fifth, n = 112; seventh, n = 43; and eighth, n = 111) with contrasted delivery modes (hospital, midwife’s house, at-home births), the home address of the parents was computerized (n = 532). Addresses were geocoded using Google Maps API, called from R with the geocode function of the ggmap package (67). The addresses for which this geocoding failed were examined manually (n = 52): 33 streets whose name had changed since 1914 were identified by comparing 1914 and modern-day maps, and the Google Map geocoding was relaunched accordingly. Addresses in streets that had disappeared were geocoded manually (n = 7). Finally, addresses in streets that we failed to identify on 1914 maps were set at the district/town center (n = 12). (A) Number of hospital births, homebirths, and births at midwives’ houses for orphans and MNOs. (B) Districts with hospitals or midwives’ houses (i.e. the fifth and eighth districts) recruited women living in neighboring districts and suburban towns. In the seventh district all births were homebirths. The three selected districts are outlined in black. (C) Among parents domiciled outside Paris were war refugees who had settled in Paris but were still domiciled in their region of origin. (The dotted line shows the stabilized frontline from October 1914.)
We searched for variables that might have influenced both the offspring’s lifespan and the paternal risk of death at war. The risk of death affected all strata of society because of universal conscription and depended on three key factors: age, health, and occupation. Men aged ≥35 y in 1914 were assigned to less exposed units (territorial regiments) (35). Because paternal age was not always available on birth certificates, we matched MNOs and orphans by maternal age, with which paternal age is associated. This matching ensured that mean paternal age was comparable in MNOs and orphans (Table 1). After thorough medical examination, French men in poor health were declared unfit for military service or were assigned to units not involved in combat. Thus fathers of orphans were men in good health. Another source of confounding might be that specific occupations at all levels of the social ladder protected a fraction of citizens from combat (e.g., railroad workers, policemen, physicians). Our detailed classification of paternal occupations in six socioeconomic categories showed only minimal differences between fathers of orphans and of MNOs (Table 1). Heterogeneity that might remain within categories is unlikely to be different in prenatal and postnatal pairs, so it could not account for the observed difference in loss of adult lifespan.
Because the pupille de la Nation status was granted upon request, we checked to confirm that the non-pupille individuals initially drawn to serve as MNOs were not war orphans (Fig. S1). We found that only 1% of the MNOs alive at age 31 y actually were war orphans who did not become pupilles (Table S2). There was also the question of legitimacy. A strikingly low illegitimacy rate (4.0%) was observed in prenatal orphans (Table 1), perhaps because, for unmarried couples, the early death of the partner made recognition of the child impossible. For mothers who had no definite proof of paternity (letters or other evidence), the lack of recognition made application to the pupille status impossible. We found that legitimacy cannot explain the difference in lifespan, because this difference also was observed in the analysis performed on legitimate children only.
Table S2.
Paternal occupations in the 35 prospective MNOs that were identified as war orphans compared with pupille orphans
| Group | Worker | Craftsman | Employee | Shopkeeper | Middle class | Upper class |
| MNOs, n = 35 (%) | 6 (17) | 3 (8) | 12 (34) | 2 (5) | 2 (6) | 11 (30) |
| Pupille orphans, n = 2,791 (%) | 828 (30) | 521 (19) | 879 (32) | 244 (9) | 130 (5) | 190 (7) |
Initially 5,671 non-pupille births were drawn from birth registers to serve as MNOs. Among the 3,365 MNOs with a known father that were alive at 31 y, 35 (1.0%) identified as war orphans were subsequently removed from the analysis. Paternal occupations of orphan MNOs were different from those of orphan pupilles alive at 31 y (median P value of Fisher’s exact test over the 10 completed datasets: 2.5 × 10−4). No difference was found between MNOs and orphan pupilles after the removal of those born of an upper class father (median P = 0.25). The excess upper class paternal occupation in orphan MNOs suggests a lower propensity of upper class families to apply for the pupille de la Nation status. Figures by occupation are averaged over the 10 completed datasets and are rounded to the nearest integer.
Our main argument for our belief that potential confounding factors were unlikely to explain our observation is that we observed no association between postnatal father’s death and reduced lifespan. Similarly, the decreased lifespan found in prenatal orphans could not be explained by degraded familial socioeconomic status, because we observed no loss of lifespan in the postnatal orphans, who faced comparable conditions in childhood and adolescence.
Finally, maternal stress during pregnancy appears the most plausible cause for the reduced lifespan of prenatal orphans. Glucocorticoids are a major candidate for programming the womb and the fetal HPA axis during prenatal stress (41). The maternal HPA axis undergoes dramatic changes during pregnancy. Although cortisol levels rise threefold by the third trimester, placental 11β-hydroxysteroid dehydrogenase type 2 (11β‐HSD2) maintains low concentrations of cortisol in the fetal circulation until late in gestation (42). This hypocortisolic fetal milieu seems crucial for fetal HPA axis maturation and regulation of steroidogenesis (43). Placental 11β‐HSD2 is more active in females (44) and is sensitive to maternal stress, which causes a greater transfer of glucocorticoids from mother to fetus. A reduction in placental levels of 11β‐HSD2 occurs during late gestation, allowing an increased transfer of maternal glucocorticoids to the fetus and exacerbation of the effects of maternal stress on the fetus (45, 46). Because maternal cortisol levels are much higher than fetal levels, even moderate changes in placental 11β‐HSD2 can significantly modify the glucocorticoid exposure of the fetus. The increase in fetal glucocorticoid levels can lead to fundamental changes in gene regulation and fetal development in many developing organs and in the HPA axis, in particular (47, 48).
Some of these changes may relate to the powerful effects of glucocorticoids on the epigenome (49), which can imprint male and female fetuses for life. Indeed, durable epigenetic imprints involving genes expressed in regions of the HPA axis may have a long-lasting influence on the expression of these genes and cause an altered cortisol reactivity to future stress in later life (50). Human studies have shown that maternal psychological trauma during pregnancy can influence the offspring’s epigenome, notably the human glucocorticoid receptor gene (NR3C1) that mediates the effects of fetal cortisol on HPA-axis development. Precisely, the NR3C1 locus was found to be hypermethylated in children whose mothers suffered from depression and anxiety during the third trimester of their pregnancy (51). Women’s experience of intimate partner violence during pregnancy also has been associated with increased NR3C1 methylation in their adolescent offspring (52). When women were exposed to the Tutsi genocide during pregnancy, their children had higher methylation of the NR3C1 exon 1F than offspring from nonexposed women (53). NR3C1 is not the only locus where DNA methylation is modified by maternal stress. Other methylation changes in offspring exposed to maternal depression were detectable in the immune system at birth and persisted until adulthood in the hippocampus, a known regulator of HPA activity (54).
Remarkably, the methylation status of the NR3C1 promoter was found to be more sensitive to maternal depression in late pregnancy (55). This finding is consistent with our observation of a trend for a greater increase in adult mortality when the father’s death occurred during the third trimester of intrauterine life. Trimester-specific early-life adversities may have differential effects on particular systems. In this respect, psychic maternal stress is unlikely to have the same effects on offspring health as famine, which is known to favor late development of diseases if it occurs during early gestation. It is important to note that programming mechanisms remain out of reach in humans, notably with regard to the tissue‐specific nature of epigenetic modifications, because the tissues of interest, such as the hippocampus, hypothalamus, or pituitary, escape investigation. Nevertheless epigenetic imprints probably represent a critical component of the programming process and could be partly responsible for the long-range effects of antenatal glucocorticoid exposure on neurologic, cardiovascular, and metabolic function through their persistent effects on the HPA axis (56). Epigenetic programming of neuroendocrine and behavioral phenotypes has some sex specificity (26). It is conceivable that imprints in the HPA axis following the father’s death could be perpetuated in subsequent generations through the female lineage and pregnancies.
In conclusion, our study of the consequences of maternal bereavement during WWI, a single stress of extreme psychic intensity, allowed us to study the effects of the loss of the father at specific moments of child development and to compare the whole-life adult mortality of persons whose mothers had experienced this stress with that of matched individuals born at the same time and place to mothers who had not experienced this particular stress. Although we were not able to dissect the causes of the increased mortality in orphans, our study contributes to the assessment of the health impact of severe maternal psychic stress during pregnancy.
SI Text
Identification of Pupilles de la Nation on the Birth Registers.
Both Paris and Bordeaux remained protected from the frontline during WWI. Paris’ city halls were readily accessible. Bordeaux is one of the few French cities for which images of its 1914 and 1915 birth registers are already online (available at archives.bordeaux-metropole.fr). The distribution of births by district reflected the distribution of hospitals and midwives’ houses (Fig. S3 and Table S1). A total of 91,728 certificates were manually examined for the presence of the “Adopted by the Nation” notification, and 5,671 pupilles were identified thereby. Information collected and computerized from the handwritten birth registers were date of birth, sex, parental age (level of precision: year) and occupation at the time of the birth, legitimacy status (i.e., whether the parents were married at the time of the birth), date of death, and date of other life events (marriages, divorces, guardianships) noted in the margin of birth certificates (Fig. 1A). The presence of possible new life events was rechecked at the end of the data-collection process (8 October 2015) on the birth certificates of all included individuals (pupilles de la Nation and MNOs).
Longevity Information Available on Birth Certificates.
Included individuals were born between 1 August 1914 and 31 December 1916. Dates of death have been recorded on birth certificates since 29 March 1945, so that 31–99 y is the range for which the age at death is known for all included individuals. The individuals fall into three categories: those who died at an unknown age before age 31 y, those who died between age 31 and 99 y (with known age at death), and those alive at age 99 y.
An individual with no life event recorded on the birth certificate after 29 March 1945 (i.e., with no notification of marriage, divorce, guardianship, or death after this date) was considered to have died before age 31 y. Conversely, an individual without the notification of death but at least one other life event recorded after 29 March 1945 was considered to be alive at age 99 y.
Identification of War Orphans Among Pupilles de la Nation.
The “Morts pour la France” database (available at www.memoiredeshommes.sga.defense.gouv.fr/en/article.php?larub=80) is searchable by name. To each soldier is attached an image of his record (Fig. 1B for an example). We computerized the necessary available information on the image, e.g., date and type of death (killed in action, missing in action, died of wounds, disease, other). To ensure that a given soldier was actually the father, not a homonymous person, positive identification was achieved as described in Fig. S4. This search yielded 3,210 orphans (56.6% of the 5,671 pupilles). The 489 orphans whose father had died of disease were removed from the analysis.
Fig. S4.
Process for the identification of orphans’ fathers. We manually searched for the father of each of the 5,671 included pupilles de la Nation (A) in the online database of soldiers who died during WW1 (B) (available at www.memoiredeshommes.sga.defense.gouv.fr), based on the father’s name and age, to determine whether the pupille was an orphan and to retrieve the father’s date and cause of death. The record of each soldier also contains his date and place of birth. In the cases where the search yielded several candidates, or when uncertainty remained as to whether the soldier found was indeed the father of the pupille, we took advantage of the fact that the birth certificates of candidate fathers found in the database (commonly born 1880–1895) (C) are available on the websites of the local archives (“Archives départementales”) of their place of birth. We manually examined the birth certificate of each candidate to check whether his wife (whose name is given on the marriage notification on the birth certificate) was named as the mother of the pupille on the pupille’s birth certificate. This method yielded certain identification for all pupilles de la Nation whose parents married, whether before or after birth. Because of a recent digitization program, images of >90% of birth registers before 1900 (C) are now available online (current completion by region may be seen at www.archivesdefrance.culture.gouv.fr, from which local websites may be reached). Marriages began to be noted on the birth certificates of those marrying in 1897, thus providing a simple, systematic, positive method of verifying the linkage between birth registers and the WWI military deaths database. For a pupille de la Nation whose parents never married but who was recognized by his father, the act of recognition (which mentions the date and place of birth of the father and thus yields positive identification) was examined, provided that the recognition took place in one of the districts included in the study.
Statistical Analysis: Trimester-Specific Effects.
To investigate specific effects of paternal death early (first trimester) or late (third trimester) in pregnancy, we compared (with the same procedure used for the prenatal/postnatal orphans comparison) those orphans whose father died in the first trimester with those whose father died later in the pregnancy (second and third trimester loss) and compared orphans whose father died in the third trimester with those whose father died earlier in the pregnancy (first and second trimester loss). Similarly, among postnatal orphans we compared those who had experienced loss of father before and after age 1 y and those who had lost their father before and after age 6 mo.
Statistical Analysis: Sensitivity Analyses.
Six sensitivity analyses were performed. We compared median lifespans instead of mean lifespans. We restricted the analysis to legitimate children. We restricted the analysis to the Paris districts (Bordeaux was excluded). Also, rematched pairs were removed from the analysis. Only orphan/MNO pairs born before May 1915 (conceived before August 1914) were analyzed. Finally, we conducted the analysis after imputation of the lifespan of those alive at age 99 y; their lifespans were set to 99 + er, where er is the life expectancy remaining at age 99 y for the French 1915 cohort (2.10 y for men and 2.52 y for women) given by the Human Mortality Database (64).
Announcement of Father's Death to the Mother.
The official notification of a soldier’s death to his family could take weeks, sometimes months. A frequent channel of information to the mothers was a direct letter from the comrades of the dead soldier to the spouse. Because of its very nature, evidence for this second channel of information is qualitative (65).
To check that the women who lost their husbands during pregnancy were actually informed of their husbands’ deaths before delivery, we analyzed the transmission of the father’s first name. Among the 337 male orphans who lost their father before birth, 40.7% were given their father’s first name, compared with 17.2% of the 895 male orphans who lost their father after birth (P = 1.1 × 10−17) and 12.4% of the 1,232 male MNOs (P = 6.8 × 10−32).
Methods
Identification of Pupilles de la Nation on the Birth Registers.
All pupilles de la Nation born in 17 districts of Paris between 1 August 1914 and 31 December 1916 and in the four districts of Bordeaux from 1 August 1914 to 31 December 1915 (Table S3) were identified by the “adopted by the Nation” notification that was systematically inscribed on their birth certificates following adoption. Vital information available on birth certificates was computerized; SI Text for details. The Commission Nationale de l’Informatique et des Libertés (57) authorized us to access and analyze those data (registration number 915774).
Table S3.
Districts included in the study and period covered
| Civil registration service | Region | Period covered | Orphan/MNO pairs, n = 2,365 |
| 1st arrondissement | Paris | 1 August 1914–31 December 1916 | 17 |
| 2nd arrondissement | Paris | 1 August 1914–31 December 1916 | 18 |
| 3rd arrondissement | Paris | 1 August 1914–31 December 1916 | 26 |
| 5th arrondissement | Paris | 1 August 1914–31 December 1916 | 112 |
| 6th arrondissement | Paris | 1 August 1914–31 December 1916 | 170 |
| 7th arrondissement | Paris | 1 August 1914–31 December 1916 | 43 |
| 8th arrondissement | Paris | 1 August 1914–31 December 1916 | 111 |
| 9th arrondissement | Paris | 1 August 1914–31 December 1916 | 35 |
| 12th arrondissement | Paris | 1 August 1914–31 December 1916 | 180 |
| 13th arrondissement | Paris | 1 August 1914–31 December 1916 | 239 |
| 14th arrondissement | Paris | 1 August 1914–31 December 1916 | 708 |
| 15th arrondissement | Paris | 1 August 1914–31 December 1916 | 184 |
| 16th arrondissement | Paris | 1 August 1914–31 December 1916 | 36 |
| 17th arrondissement | Paris | 1 August 1914–31 December 1916 | 99 |
| 18th arrondissement | Paris | 1 August 1914–31 December 1916 | 195 |
| Le Kremlin Bicêtre | Paris | 1 August 1914–31 December 1916 | 5 |
| Neuilly-sur-Seine | Paris | 1 August 1914–31 December 1916 | 23 |
| 1st section | Bordeaux | 1 August 1914–31 December 1915 | 56 |
| 2nd section | Bordeaux | 1 August 1914–31 December 1915 | 41 |
| 3rd section | Bordeaux | 1 August 1914–31 December 1915 | 37 |
| 4th section | Bordeaux | 1 August 1914–31 December 1915 | 30 |
Choice of MNOs.
MNOs were used for comparison of orphans’ lifespans. Because MNOs were selected before the identification of war orphans among pupilles (see below), an MNO was paired to each pupille, whether the pupille was an orphan or the child of a disabled soldier. The MNO of a pupille was selected from the same birth register and was the closest (going upward in the birth register, from the pupille’s certificate) non-pupille same-sex birth with an age difference ≤2 y between the MNO’s mother and the pupille’s mother.
Claiming the status of pupille de la Nation required a proof of dead soldier’s paternity of the child. Therefore, a child whose father’s identity was unknown could not become a pupille, whatever his father’s war experience. Pupilles were legitimate children or children who were born illegitimate but later were recognized by their father or by judgment (in cases in which the father died before recognition but where definite proof of paternity existed). MNOs with an unknown father therefore were excluded from the analysis.
Longevity Information Available on Birth Certificates.
The date of death has been systematically recorded on French birth certificates since 29 March 1945. Wherever and whenever death occurs, the civil registration service at the place of death notifies the civil registration service at the place of birth, so that the birth certificate is updated within a few weeks, at most, after death. For individuals who die abroad, the information is transmitted to the place of birth by the local French consulate. Thus 31–99 y is the range within which the age at death is known for all included individuals, from the youngest (born December 1916) to the oldest (born August 1914) (SI Text for details).
Identification of War Orphans Among Pupilles de la Nation.
A pupille de la Nation is the child of a soldier who died or was disabled (injured or ill) during the war. To identify orphans, we searched for the father of each pupille de la Nation in an online database (58) (available at www.memoiredeshommes.sga.defense.gouv.fr/en/article.php?larub=80), which records all French soldiers who died during WWI (SI Text and Fig. S4). We similarly searched for the father of each MNO in the database of French soldiers who died during WWI. The 35 MNOs alive at age 31 y who were identified as war orphans were excluded from the analysis (Fig. S1).
Rematching.
A total of 987 orphans alive at age 31 y were rematched to an available MNO of same sex and same district of birth (Fig. S1) because the initial MNO had died before age 31 y, was identified as a war orphan, or had an unknown father. When several MNOs were available for rematching, we selected the one with the date of birth closest to that of the orphan. Each available MNO was used only once in rematching. Rematching was performed after a random permutation of the 987 orphans. Rematching was successful for 985 of the 987 orphans, yielding 2,365 orphan/MNO pairs in the final dataset analyzed.
Classification of Parental Occupations.
We computed an ordinal score of paternal occupations based on the following six categories: i) worker; ii) craftsman; iii) employee; iv) shopkeeper; v) middle class, and vi) upper class. Similarly, we defined eight categories of maternal occupations: i) servant; ii) worker; iii), craftswoman; iv) employee; v) housekeeper; vi) shopkeeper; vii) housewife, and viii) middle and upper class. The maternal occupation at the time of birth was available on 99.4% of birth certificates. The paternal occupation was available for 97.2% of legitimate children.
Statistical Analysis.
To impute the unavailable paternal and maternal occupations and those we could not classify, we performed multivariate imputation by chained equations (MICE) (59) using district and date of birth, sex, status (alive/dead) at age 31 y, parental occupations, legitimacy, and maternal age. We created 10 completed datasets. The parental occupation scores and regression coefficients obtained on the 10 datasets then were combined following Rubin’s rules (60).
The main outcome analyzed was the lifespan of those died between the ages of 31 and 99 y. We tested for differences in the distribution of lifespans between orphans and MNOs using paired Wilcoxon signed-ranked tests on pairs in which the lifespan of both members was measured (i.e., both died between the ages of 31 and 99 y). The presence of differences was assessed separately for prenatal orphan/MNO and postnatal orphan/MNO pairs. We computed the mean lifespan of each group: prenatal orphans, MNOs of prenatal orphans, postnatal orphans, and MNOs of postnatal orphans. Bootstrapped 95% CIs were based on B = 1,000 replicates. To test the hypothesis that mean losses of lifespan were different in prenatal orphans and postnatal orphans, permutation tests were performed on prenatal and postnatal pairs. For each orphan/MNO pair, prenatal or postnatal, in which both members died between the ages of 31 and 99 y, an orphan/MNO difference in lifespan, δ, was determined. We ran P = 10,000 permutations on the prenatal/postnatal variable to estimate the null distribution of differences of means between prenatal and postnatal pairs.
Finally, the δs were regressed in a GAM (61) on status (postnatal/prenatal pair) with control for six categorical variables (paternal occupation, maternal occupations, legitimacy) and paternal and maternal ages (modeled by cubic regression splines with 5 df and evenly spaced knots; smoothing parameters determining the effective df were selected to minimize the unbiased risk estimator score).
Analyses were repeated for prenatal orphans according to the prenatal trimester of the father’s death, and six sensitivity analyses were performed (SI Text).
All analyses were performed in R v3.0.3, with the package mice for multiple imputation (62) and the package mgcv for GAM regressions (63).
Acknowledgments
We thank S. Le Fur and F. Balazard for hours of discussion, A. Breteau, G. de Saint-Léger, and T. Voïta, who helped us collect data, the staff of the civil registration services who managed the birth registers used in the present study, and P. de Villiers for personal help. P.B. thanks Eva Jablonka for introducing him to epigenetics. This work was supported by the Groupe d’Etudes de Thérapeutique de la Croissance, INSERM, the Université Pierre-et-Marie Curie, and Paris Saclay University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617911114/-/DCSupplemental.
References
- 1.Almond D, Currie J. Killing me softly: The fetal origins hypothesis. J Econ Perspect. 2011;25:153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJP, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman P, Hanson M, editors. Developmental Origins of Health and Disease. Cambridge Univ Press; Cambridge,UK: 2006. [Google Scholar]
- 6.Lumey LH, et al. Cohort profile: The Dutch Hunger Winter families study. Int J Epidemiol. 2007;36:1196–1204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 7.Lumey LH, van Poppel FWA. The Dutch Famine of 1944–45 as a human laboratory: Changes in the early life environment and adult health. In: Lumey LH, Vaiserman A, editors. Early Life Nutrition and Adult Health and Development. Nova Publishers; New York: 2013. pp. 59–76. [Google Scholar]
- 8.St Clair D, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor TG, et al. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Oberlander TF, et al. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talge NM, Neal C, Glover V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohwü L, Li J, Olsen J, Sørensen TIA, Obel C. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: A Danish National Cohort Study. PLoS One. 2014;9:e97490. doi: 10.1371/journal.pone.0097490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virk J, et al. Prenatal exposure to bereavement and type-2 diabetes: A Danish longitudinal population based study. PLoS One. 2012;7:e43508. doi: 10.1371/journal.pone.0043508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plana-Ripoll O, et al. Prenatal exposure to maternal stress following bereavement and cardiovascular disease: A nationwide population-based and sibling-matched cohort study. Eur J Prev Cardiol. 2016;23:1018–1028. doi: 10.1177/2047487315585294. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman PD, et al. Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet. 2009;373:1654–1657. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 16.Raabe F, Spengler D. Epigenetic risk factors in PTSD and depression. Front Psychiatry. 2013;4:80. doi: 10.3389/fpsyt.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaiserman A. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: A potential link to disease susceptibility? Clin Epigenetics. 2015;7:96. doi: 10.1186/s13148-015-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szyf M, Bick J. DNA methylation: A mechanism for embedding early life experiences in the genome. Child Dev. 2013;84:49–57. doi: 10.1111/j.1467-8624.2012.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 20.Jensen Peña C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS One. 2012;7:e39791. doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: Sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 22.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 23.Murgatroyd C, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Patchev AV, Daniel G, Almeida OFX, Spengler D. Early-life stress reduces DNA methylation of the Pomc gene in male mice. Endocrinology. 2014;155:1751–1762. doi: 10.1210/en.2013-1868. [DOI] [PubMed] [Google Scholar]
- 25.Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: Neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- 26.Menger Y, Bettscheider M, Murgatroyd C, Spengler D. Sex differences in brain epigenetics. Epigenomics. 2010;2:807–821. doi: 10.2217/epi.10.60. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannisto V, Christensen K, Vaupel JW. No increased mortality in later life for cohorts born during famine. Am J Epidemiol. 1997;145:987–994. doi: 10.1093/oxfordjournals.aje.a009067. [DOI] [PubMed] [Google Scholar]
- 29.Doblhammer G, van den Berg GJ, Lumey LH. A re-analysis of the long-term effects on life expectancy of the Great Finnish Famine of 1866-68. Popul Stud (Camb) 2013;67:309–322. doi: 10.1080/00324728.2013.809140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Berg GJ, Lindeboom M, Portrait F. Economic conditions early in life and individual mortality. Am Econ Rev. 2006;96:290–302. doi: 10.1257/000282806776157740. [DOI] [PubMed] [Google Scholar]
- 31.Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci USA. 2001;98:2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alastalo H, et al. Cardiovascular morbidity and mortality in Finnish men and women separated temporarily from their parents in childhood–a life course study. Psychosom Med. 2012;74:583–587. doi: 10.1097/PSY.0b013e31825b3d76. [DOI] [PubMed] [Google Scholar]
- 33.Kelly-Irving M, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol. 2013;28:721–734. doi: 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones E, Wessely S. Battle for the mind: World War 1 and the birth of military psychiatry. Lancet. 2014;384:1708–1714. doi: 10.1016/S0140-6736(14)61260-5. [DOI] [PubMed] [Google Scholar]
- 35.Pedroncini G, editor. 1992. Histoire Militaire de la France. (Presses Universitaires de France, Paris) Vol. 3, De 1871 à 1940. French.
- 36.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 37.Faron O. 2001. Les enfants du deuil: Orphelins et pupilles de la nation de la Première Guerre mondiale (1914-1941) (La Découverte, Paris). French.
- 38.Ansart S, et al. Mortality burden of the 1918-1919 influenza pandemic in Europe. Influenza Other Respi Viruses. 2009;3:99–106. doi: 10.1111/j.1750-2659.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayward AD, Rigby FL, Lummaa V. Early-life disease exposure and associations with adult survival, cause of death, and reproductive success in preindustrial humans. Proc Natl Acad Sci USA. 2016;113:8951–8956. doi: 10.1073/pnas.1519820113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinard A. De la protection de l’enfance pendant la troisième année de guerre dans le camp retranché de Paris. Bull Acad Natl Med. 1917;78:751–791. French. [Google Scholar]
- 41.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 42.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 43.Stewart PM, Rogerson FM, Mason JI. Type 2 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: Its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J Clin Endocrinol Metab. 1995;80:885–890. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- 44.Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R510–R514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- 45.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 46.Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98:106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 49.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 2: Mechanisms. Nat Rev Endocrinol. 2014;10:403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 50.Murgatroyd C, Spengler D. Epigenetics of early child development. Front Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10:408–417. doi: 10.1080/15592294.2015.1039221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radtke KM, et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perroud N, et al. The Tutsi genocide and transgenerational transmission of maternal stress: Epigenetics and biology of the HPA axis. World J Biol Psychiatry. 2014;15:334–345. doi: 10.3109/15622975.2013.866693. [DOI] [PubMed] [Google Scholar]
- 54.Nemoda Z, et al. Maternal depression is associated with DNA methylation changes in cord blood T lymphocytes and adult hippocampi. Transl Psychiatry. 2015;5:e545. doi: 10.1038/tp.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberlander TF, et al. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 56.Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1Outcomes. Nat Rev Endocrinol. 2014;10:391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 57.Commission Nationale de l'Informatique et des Libertés 2016 Available at https://www.cnil.fr. Accessed October 25, 2016.
- 58.Service historique de la Défense 2016 Database of those who died for France in the First World War. (Paris). Available at www.memoiredeshommes.sga.defense.gouv.fr.
- 59.van Buuren S. Flexible Imputation of Missing Data. Chapman & Hall/CRC; Boca Raton: 2012. [Google Scholar]
- 60.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons; New York: 1987. [Google Scholar]
- 61.Wood S. Generalized Additive Models: An Introduction with R. Chapman & Hall; Boca Raton: 2006. [Google Scholar]
- 62.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 63. Wood S Package ‘mgcv’. Available at ftp://cran.rproject.org/pub/R/web/packages/mgcv/mgcv.pdf. Accessed June 6, 2016.
- 64. University of California, Berkeley and Max Planck Institute for Demographic Research Human Mortality Database. Available at www.mortality.org. Accessed February 2, 2015.
- 65.Bette P. 2012 Veuves Française de la Première Guerre Mondiale: Statuts, Itinéraires et Combats. Thesis (Université Lumière Lyon 2, Lyon, France). Available at theses.univ-lyon2.fr/. Accessed December 29, 2014. French.
- 66.Festy P. Effets et répercussions de la première guerre mondiale sur la fécondité française. Population (Paris) 1984;39:977–1010.French. [PubMed] [Google Scholar]
- 67.Kahle D, Wickham H. ggmap: Spatial visualization with ggplot2. R J. 2013;5:144–161. [Google Scholar]