Significance
Our study reveals that prolonged sleep deprivation is accompanied by an A1 adenosine receptor (A1AR) upregulation in the human brain. Recovery sleep quickly restores A1AR availability to control levels. High individual A1AR availability is related to a low sleep pressure and good cognitive performance. Sleep deprivation is an efficient but short-lasting therapeutic strategy in depression. A causal sleep–wake dysregulation has been proposed, possibly mediated by cerebral adenosine and its A1AR. The restoration of the A1AR availability after recovery from sleep deprivation mimics the rapid relapse following the end of therapeutic sleep deprivation. Understanding the adenosine regulation under sleep restriction, especially regarding individual characteristics, might improve the rationale for the individual indication and design of therapeutic sleep modulation in depression.
Keywords: sleep deprivation, cognitive performance, interindividual differences, depression, sleep homeostasis
Abstract
Adenosine and functional A1 adenosine receptor (A1AR) availability are supposed to mediate sleep–wake regulation and cognitive performance. We hypothesized that cerebral A1AR availability after an extended wake period decreases to a well-rested state after recovery sleep. [18F]CPFPX positron emission tomography was used to quantify A1AR availability in 15 healthy male adults after 52 h of sleep deprivation and following 14 h of recovery sleep. Data were additionally compared with A1AR values after 8 h of baseline sleep from an earlier dataset. Polysomnography, cognitive performance, and sleepiness were monitored. Recovery from sleep deprivation was associated with a decrease in A1AR availability in several brain regions, ranging from 11% (insula) to 14% (striatum). A1AR availabilities after recovery did not differ from baseline sleep in the control group. The degree of performance impairment, sleepiness, and homeostatic sleep-pressure response to sleep deprivation correlated negatively with the decrease in A1AR availability. Sleep deprivation resulted in a higher A1AR availability in the human brain. The increase that was observed after 52 h of wakefulness was restored to control levels during a 14-h recovery sleep episode. Individuals with a large increase in A1AR availability were more resilient to sleep-loss effects than those with a subtle increase. This pattern implies that differences in endogenous adenosine and A1AR availability might be causal for individual responses to sleep loss.
Sleep loss is known to impair almost every aspect of cognition, such as learning (1), long-term memory consolidation (2), attention and psychomotor vigilance (PVT) (3), and executive functions (4), including decision making (5) and emotional control (6). Sleep deprivation further typically alters the frequency distribution of the waking electroencephalogram (EEG) as an indicator of alertness corresponding to cognitive performance (7). However, large interindividual differences exist in the degree of cognitive performance decline during sleep deprivation (3). In a trait-like process, some individuals keep high-level performance during sustained wakefulness, whereas others suffer from severe performance loss (3). The neuro-molecular mechanisms in the brain responsible for these different vulnerabilities are still largely unknown. Caffeine, commonly consumed for fighting fatigue, promotes wakefulness via adenosine receptor antagonism. It seems likely that the adenosinergic system is a neurochemical link between performance and sleep (8). Adenosine is contributing to the homeostatic process of sleep–wake regulation (for review, see refs. 9–12). As has been shown in cats and rats, extracellular adenosine concentration fluctuates rhythmically in many brain regions, such as the basal forebrain, increasing during wakefulness and decreasing during sleep: it thereby induces sleep after wake extension and is in turn restored to baseline levels after recovery sleep (13). For additional information on adenosine, SI Text.
According to the two-process model of sleep–wake regulation (14), homeostatic sleep pressure increases with time awake according to a saturating exponential function, and declines exponentially during sleep. It has been proposed that the development of depressive symptoms is associated with a dysfunction in this homeostatic sleep drive (15). Recently a synaptic plasticity model of therapeutic sleep deprivation in major depression has been proposed (16). The model integrates the synaptic plasticity hypothesis of depression (17) and the synaptic homeostasis hypothesis (18). According to this model, therapeutic sleep deprivation strengthens synapses, thereby shifting the deficient long-term potentiation in patients with major depressive disorder in a more favorable range of associative plasticity. Sleep deprivation and sleep restriction are effective but short-lasting treatments (19) in depression. In contrast, healthy individuals show negative effects concerning mood, alertness, and cognition. Adenosine-related interactions are also crucial in astrocyte–neuron communication, which underlies both cortical sleep (20) and also antidepressive effects of sleep deprivation (21). Apart from extracellular adenosine itself, evidence exists for the mediating subtype of adenosine receptors to regulate sleep–wake rhythmicity. In the central nervous system, the A1 subtype shows the widest distribution among adenosine receptors, with particularly high densities in various areas of the cortex, striatum, and thalamus (22). The neurophysiological and behavioral effects of sleep deprivation in cats were mimicked by increasing the adenosine concentration experimentally (13, 23). Several studies in cats and rodents revealed that activation of the A1 adenosine receptor (A1AR) by an agonist and blockage by an antagonist up- and down-regulated sleep propensity (24, 25). Moreover, A1AR mRNA was shown to increase in the basal forebrain under sleep restriction (11). Inhibiting the A1AR mRNA translation in rats decreased nonrapid eye-movement sleep and increased wakefulness (26). An up-regulation of A1AR density in the human and in the rat brain in response to acute sleep loss (10, 27, 28) has been shown. Neither adenosine nor adenosine receptors can easily be studied in vivo in the human brain. However, positron emission tomography (PET) is a tool that allows for exploring adenosine receptors in vivo. In earlier experiments, we already found evidence that A1AR availability is stable after repeated 8-h sleep episodes (29) and increased after 24 h of sleep deprivation (27). In the present study, we intended to increase sleep pressure even further to examine if A1AR availability is satiating, as predicted by the two-process model, and whether the exponential discharge of sleep pressure during recovery sleep is reflected in A1AR availability.
The aims of this study were therefore to determine in healthy volunteers: (i) to what extent 14 h of recovery sleep reduces cerebral A1AR availability as measured following 52 h of sleep deprivation (primary outcome parameter); (ii) if such recovery sleep restores A1AR availability to the rested levels found in an independent control group after an 8-h sleep episode without preceding sleep deprivation; and (iii) if impairment of cognitive performance under sleep deprivation compared with following recovery sleep is correlated with a higher cerebral A1AR availability (exploratory analyses). A1AR availability was measured in 14 participants using PET after 52 h (SD52) of sustained wakefulness, followed by 14 h of recovery sleep (REC14), and compared with A1AR availability after an 8-h sleep episode in a control group of 20 participants.
For reasons of radiation protection, it was not possible to investigate each participant more than twice. Instead of measuring baseline A1AR availability after an 8-h sleep episode, we performed a scan after sleep deprivation and after recovery sleep. As shown previously, there is a high test–retest reliability of A1AR availability after an 8-h sleep episode (29), which is also comparable between groups of the same age (30). Receptor binding data of earlier experiments after 8 h of sleep at night (27, 29) were therefore integrated into the present analyses as independent control group values (Table 1).
Table 1.
Regional A1AR distribution volumes [VT (mL/mL)] in two groups after 8-h control sleep, 52 h of sleep deprivation, and 14-h recovery sleep
| Region | Receptor binding VT | ANOVA | Unpaired | Unpaired | Paired t test | ||
| CTR | SD52 | REC14 | CTR vs. SD52 | CTR vs. REC14 | SD52 vs. REC14 | ||
| Anterior cingulate cortex | 0.77 ± 0.11 | 0.78 ± 0.11 | 0.69 ± 0.12 | 0.0094 | 0.7129 | 0.0490 | 0.0057 |
| Insula | 0.80 ± 0.11 | 0.86 ± 0.12 | 0.76 ± 0.13 | 0.0171 | 0.1484 | 0.3634 | 0.0083 |
| Amygdala | 0.75 ± 0.10 | 0.78 ± 0.11 | 0.67 ± 0.10 | 0.0162 | 0.3789 | 0.0397 | 0.0103 |
| Frontal cortex | 0.78 ± 0.13 | 0.91 ± 0.12 | 0.80 ± 0.11 | 0.0082 | 0.0101 | 0.5659 | 0.0063 |
| Orbitofrontal cortex | 0.73 ± 0.12 | 0.81 ± 0.12 | 0.71 ± 0.13 | 0.0031 | 0.0460 | 0.9001 | 0.0020 |
| Occipital cortex | 0.80 ± 0.14 | 0.92 ± 0.13 | 0.81 ± 0.13 | 0.0058 | 0.0144 | 0.6582 | 0.0042 |
| Parietal cortex | 0.77 ± 0.13 | 0.91 ± 0.13 | 0.80 ± 0.13 | 0.0061 | 0.0059 | 0.5323 | 0.0074 |
| Temporal cortex | 0.75 ± 0.12 | 0.85 ± 0.12 | 0.76 ± 0.12 | 0.0130 | 0.0272 | 0.7261 | 0.0089 |
| Thalamus | 0.78 ± 0.13 | 0.88 ± 0.14 | 0.75 ± 0.12 | 0.0025 | 0.0328 | 0.8381 | 0.0015 |
| Striatum | 0.79 ± 0.15 | 0.88 ± 0.13 | 0.75 ± 0.12 | 0.0050 | 0.0599 | 0.4405 | 0.0020 |
Values are given as mean ± SD; ANOVA P is the probability value of a mixed one-way ANOVA with subject as random; statistical comparisons that exceed the multiple-comparison–adjusted threshold (FDR, Benjamini and Hochberg method, P < 0.022, n = 30 t tests) are in boldface. Abbreviations: CTR, 8-h control sleep (n = 20); REC, 14-h recovery sleep; SD52, 52 h sleep deprivation (n = 14); VT, A1AR distribution volume.
SI Text
Adenosine is an important neuromodulator in the central nervous system and a key substance for the energetic homeostasis in cells (10). In times of high energy demand, adenosine triphosphate (ATP) as a ubiquitous energy storage is dephosphorylated and adenosine is generated via adenosine di- and monophosphate by ATPase, ADPase, and 5′-nucleotidase. Adenosine is not stored in vesicles, but atypically released into the extracellular space via transporters. These bidirectional nucleoside transporters ensure adenosinergic equilibrium between the extra- and intracellular space via concentration-driven passive replacement. Adenosine is rapidly converted to inosine by adenosine deaminase or to AMP by adenosine kinase, both intra- and extracellularly. Additionally, the extracellular adenosine level is regulated via SNARE-dependent gliotransmission of ATP from astrocytes (53). ATP is rapidly metabolized by ectonucleotidases into adenosine, which in turn depresses synaptic activity on local neurons (54).
In general, adenosine decreases the activity of wakefulness/vigilance-promoting neurons in several brain regions, including the basal forebrain and brainstem. Additionally, adenosine might account for general cortical inhibition by attenuating input from ascending excitatory cholinergic and monoaminergic pathways. Adenosinergic action in the basal forebrain might serve as the link to neurocognitive implications during sleep loss, because this region has been shown to be involved in the control of sustained attention. Thus, the cortical sustained attention network, which is closely linked to PVT performance in humans, receives input from basal forebrain neurons and is likely modulated by them (for review, see ref. 55).
Results
Group Characteristics.
Table S1 provides an overview of participants’ demographic data and scanning parameters. Both groups were not significantly different in these aspects.
Table S1.
Study participants’ demographic and experimental parameters
| Demographic | Control group | SD52/REC14 group | P value |
| n | 20 | 14 | NA |
| Age (y) | 26.2 ± 1.1 | 27.7 ± 5.4 | 0.4 |
| BMI (kg/m2) | 22.5 ± 3.6 | 24.5 ± 3.5 | 0.9 |
| Chronic caffeine consumption (in 0.15 L cups of coffee) | 1.3 ± 1.4 | 1.2 ± 1.7 | 0.87 |
| Clock time of scanning | 11:38 AM | 11:41 AM | 0.87 |
| Injected radioactivity (MBq) | 260.1 ± 6.2 | 277.1 ± 6.9 | <0.001 |
| Injected mass (nmol) | 4.5 ± 2.2 | 3.1 ± 1.8 | 0.06 |
Values are given as mean ± SD. Unpaired t test. BMI, body mass index; NA, not applicable.
Imaging Quantification.
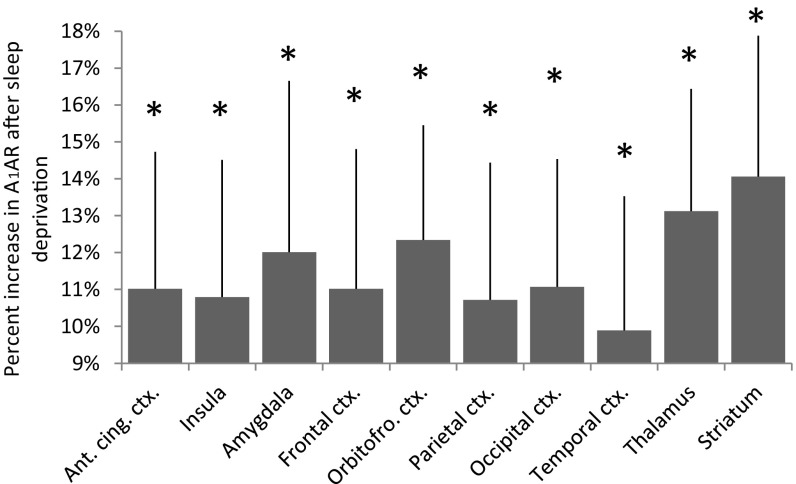
Regionalized A1AR availability values and statistics are presented in Table 1. Under sleep deprivation, the time spent awake before the scans was 52:26 h:min ± 1:45 h:min. A1AR availability was significantly higher after 52 h of sleep deprivation in all examined brain regions compared with the scan after recovery sleep and in some regions compared with the control group. A1AR availability did not differ between recovery and the control group. Fig. 1 displays the average parametric images of A1AR availability for both conditions. A higher cortical binding after sleep deprivation in comparison with recovery sleep is apparent in various regions. The average regional decrease of A1AR availability after recovery from sleep deprivation (i.e., SD52 − REC14) ranged from 14% (striatum) to 10% (temporal cortex). Fig. 2 shows the distribution of the relative difference between both days [(SD52 – REC14)/SD52] of the examined regions of interest (ROI).
Fig. 1.
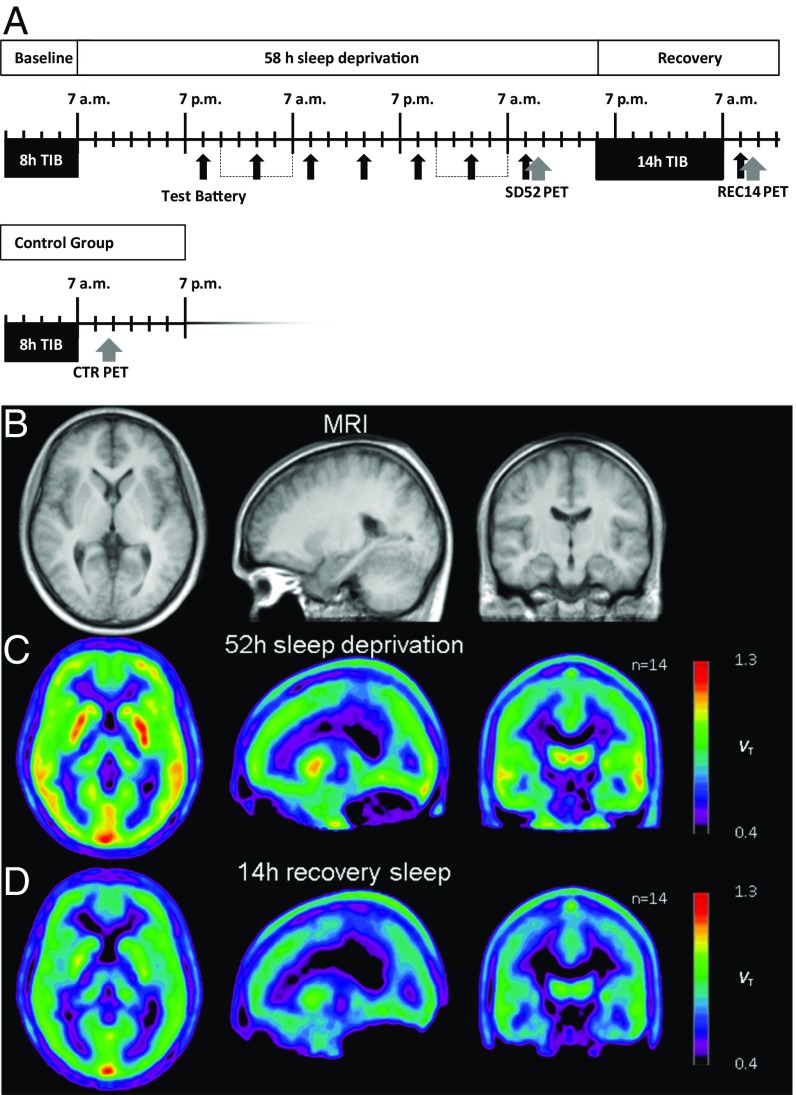
(A) Study design. Black arrows indicate times of the six hourly neuropsychological testings. Gray arrows indicate time points of PET scans. TIB, time in bed. Average images of anatomy (B, MRI) and A1AR availability (C and D, PET) after spatial normalization. (Left) Axial, (Middle) sagittal, (Right) coronal views; coordinates according to the Montreal Neurological Institute Brain Atlas were 22, −17, 0 (x, y, z); n = 14.
Fig. 2.
Average relative differences [(SD52 – REC14)/SD52] of A1AR availability (distribution volume VT) in brain regions. Error bars indicate SEMs. The asterisks represent significant differences between sleep deprivation and recovery (paired t test, *P < 0.022) (Table 1), n = 14. Ant. cing. ctx., anterior cingulate cortex; Orbitofro., orbitofrontal.
Cognitive Performance, Sleep, and Sleepiness.
Mixed linear regression showed that performance in PVT and N-back declined, and sleepiness increased significantly with time awake [PVT: response speed and lapses P < 0.0001, N-back: correct response (sum 1-, 2-, and 3-back) P < 0.0001, Karolinska sleepiness scale (KSS) P < 0.0001]. The 14-h recovery sleep period restored performance and sleepiness (Table S2).
Table S2.
Effect of recovery sleep after prolonged sleep deprivation on cognition
| Measurement | SD52 | REC14 | P |
| Karolinska Sleepiness Scale | 8.3 ± 0.8 | 2.0 ± 0.9 | <0.0001 |
| Fatigue checklist (0–20) | 15.1 ± 2.4 | 5.4 ± 2.5 | <0.0001 |
| N-back task: Correct responses | |||
| 3-back | 8.4 ± 5.4 | 12.9 ± 5.5 | 0.0309 |
| Sum (1- to 3-back) | 36.8 ± 11.3 | 48.9 ± 10.9 | 0.0083 |
| PVT | |||
| Mean reaction speed (1/s) | 3.91 ± 0.61 | 4.74 ± 0.69 | 0.0001 |
| Lapses | 4.23 ± 5.1 | 0.38 ± 0.77 | 0.0213 |
P values represent two-tailed paired t test. Values are given as mean ± SD. n = 14 (except for PVT, n = 13).
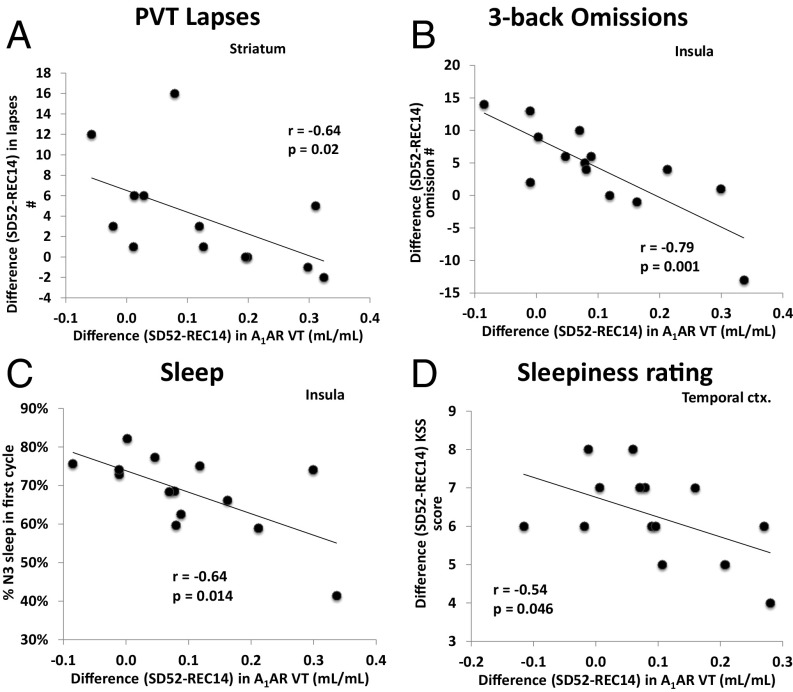
A1AR availability and cognitive performance in response to sleep deprivation varied considerably among individuals. In contrast to our third hypothesis, Fig. 3 illustrates that across individuals larger decreases in A1AR availability (SD52 − REC14) were correlated with smaller decrements in PVT and N-back task performance, as well as with less sleepiness (significant correlations for other brain regions can be found in Table S3). Furthermore, A1AR availability also correlated negatively with the percent time spent in slow-wave sleep (i.e., stage N3) during the first sleep cycle of recovery sleep.
Fig. 3.
Significant correlations (Spearman) and regressions between the difference of A1AR distribution volumes (VT) after sleep deprivation and recovery sleep and (A) difference in PVT performance (number of lapses of attention) in the striatum, (B) difference in 3-back performance (number of omissions) in the insula, (C) fraction of slow-wave sleep in the first sleep cycle in the insula, (D) subjective sleepiness rating in the temporal cortex (ctx.).
Table S3.
Correlations (Spearman) between the difference of A1AR distribution volume (VT) after sleep deprivation and recovery sleep
| Δ VT | Performance PVT Δ Lapses # | Performance 3-back Δ Omissions # | Sleep % N3 in first cycle | Sleepiness rating Δ KSS |
| Anterior cingulate cortex | r = −0.65; P = 0.016 | r = −0.58; P = 0.029 | r = −0.60; P = 0.023 | r = −0.44; P = 0.120 |
| Insula | r = −0.30; P = 0.327 | r = −0.79; P = 0.001 | r = −0.64; P = 0.014 | r = −0.32; P = 0.273 |
| Amygdala | r = −0.46; P = 0.110 | r = −0.60; P = 0.022 | r = −0.49; P = 0.078 | r = −0.47; P = 0.087 |
| Frontal cortex | r = −0.58; P = 0.040 | r = −0.64; P = 0.014 | r = −0.53; P = 0.054 | r = −0.49; P = 0.078 |
| Orbitofrontal ocrtex | r = −0.01; P = 0.986 | r = −0.55; P = 0.040 | r = −0.41; P = 0.149 | r = −0.53; P = 0.052 |
| Parietal cortex | r = −0.46; P = 0.117 | r = −0.77; P = 0.001 | r = −0.60; P = 0.022 | r = −0.33; P = 0.248 |
| Occipital cortex | r = −0.39; P = 0.192 | r = −0.74; P = 0.002 | r = −0.48; P = 0.085 | r = −0.50; P = 0.068 |
| Temporal cortex | r = −0.45; P = 0.125 | r = −0.67; P = 0.009 | r = −0.44; P = 0.114 | r = −0.54; P = 0.046 |
| Thalamus | r = −0.54; P = 0.055 | r = −0.62; P = 0.017 | r = −0.51; P = 0.061 | r = −0.53; P = 0.053 |
| Striatum | r = −0.64; P = 0.020 | r = −0.57; P = 0.035 | r = −0.60; P = 0.023 | r = −0.42; P = 0.140 |
Correlations (Spearman) between the difference of A1AR distribution volume (VT) after sleep deprivation and recovery sleep and: (i) the difference in psychomotor vigilance task performance (number of lapses of attention), (ii) the difference in 3-back performance (number of omissions), (iii) the fraction of slow wave sleep in the first sleep cycle, and (iv) the difference in subjective sleepiness rating. Significant correlations are in boldface.
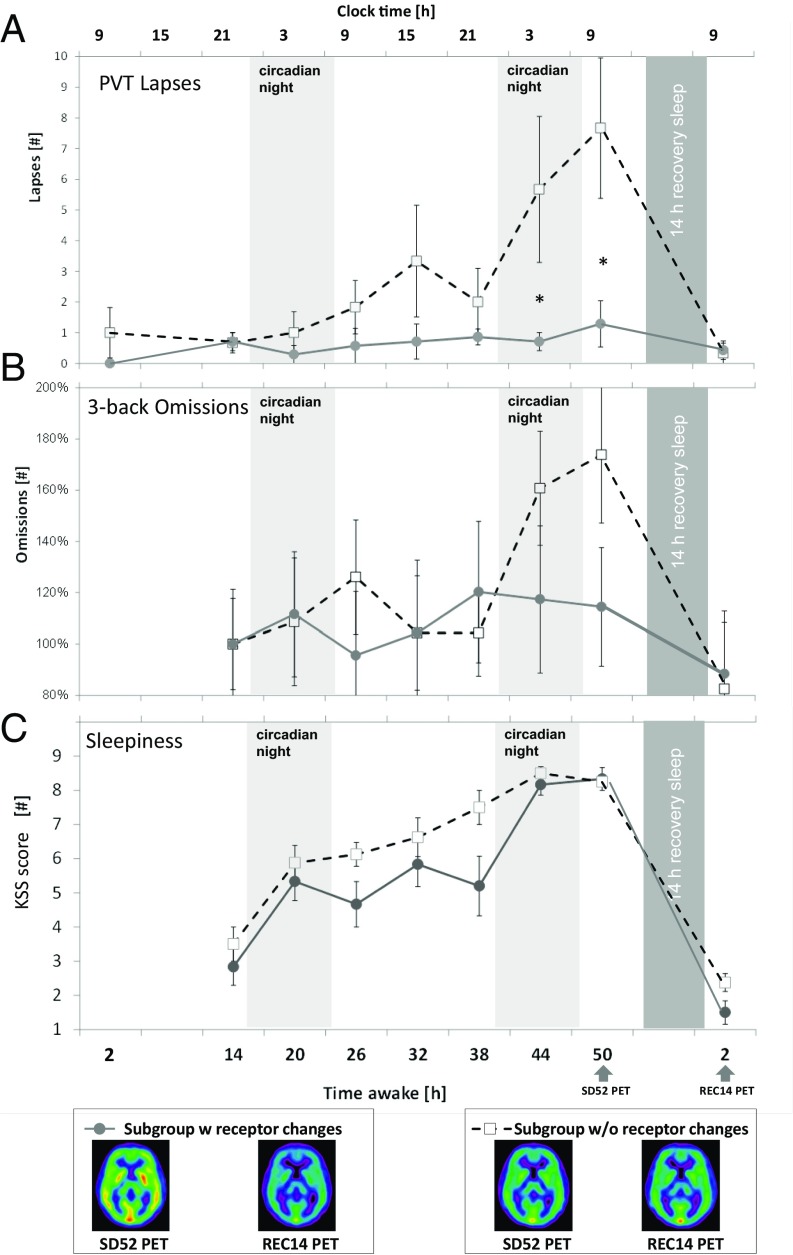
Two subgroups were identified based on interindividual differences in A1AR decrease between 52 h of sleep deprivation and the recovery condition. A PET–A1AR availability test–retest evaluation study revealed that in the striatal region the average of the absolute difference between scans was 0.1 mL/mL (29). This value was used as cut-off criterion to divide subjects into two groups, one group (n = 8) with a large difference (>0.1 mL/mL) in A1AR availability between sleep deprivation and recovery, and one group (n = 6) with small difference (≤0.1 mL/mL). Individuals with large differences in A1AR availability proved resilient to the effects of sleep loss on performance, whereas individuals with minor differences in A1AR availability showed strong degradations in performance (Figs. 3 and 4). Such group differences were not found for sleepiness.
Fig. 4.
Time course of (A) PVT, (B) 3-back omissions, and (C) KSS-sleepiness during 58 h of sleep deprivation and after 14 h of recovery sleep. Based on high or low A1AR availability, the subjects were divided into two subgroups. The absolute difference of the test–retest evaluation revealed that in the striatal region the average of the absolute difference between scans was 0.1 (29). This variance was selected as cut-off criterion for selecting groups with high and low receptor availability. The small insets indicate average parametric receptor maps of subgroup high or low A1AR availability at corresponding time points. Error bars indicate SEM. An asterisk represents significant differences in unpaired t tests between subgroups at corresponding time points. For visualization purposes N-back time courses have been normalized to the respective baseline values at 14 h awake.
The comparison of the subjects with minor and predominant A1AR decrease from SD52 to REC14 did not reveal any significant difference in receptor binding at SD52 after correction for multiple testing (Benjamini and Hochberg method, n = 10). On the other hand, when comparing the changes within the subjects with minor or predominant A1AR availability decrease, all brain regions (except the orbitofrontal and temporal cortex) are significantly different between SD52 and REC14 for the group that showed predominant A1AR availability decreases (Benjamini and Hochberg method, P < 0.02, n = 20 t tests) (Table S4).
Table S4.
Subgroup analysis
| Region | Receptor binding VT | Student’s t test P value | |||||
| n = 6 SD52 Low diff | n = 8 SD52 High diff | n = 6 REC14 Low diff | n = 8 REC14 High diff | Unpaired | Paired | Paired | |
| SD52 low diff vs. SD52 high diff | SD52 low diff vs. REC14 low diff | SD52 high diff vs.REC14 high diff | |||||
| Anterior cingulate cortex | 0.73 | 0.83 | 0.71 | 0.67 | 0.119 | 0.849 | 0.010 |
| Insula | 0.79 | 0.91 | 0.78 | 0.74 | 0.090 | 0.846 | 0.011 |
| Amygdala | 0.69 | 0.87 | 0.70 | 0.67 | 0.007 | 0.863 | 0.007 |
| Hippocampus | 0.71 | 0.82 | 0.70 | 0.66 | 0.040 | 0.890 | 0.005 |
| Frontal cotex | 0.84 | 0.95 | 0.83 | 0.77 | 0.076 | 0.936 | 0.003 |
| Orbitofrontal cortex | 0.76 | 0.86 | 0.72 | 0.72 | 0.152 | 0.606 | 0.039 |
| Occipital cortex | 0.86 | 0.97 | 0.84 | 0.79 | 0.131 | 0.782 | 0.012 |
| Temporal cortex | 0.79 | 0.92 | 0.78 | 0.75 | 0.072 | 0.972 | 0.023 |
| Thalamus | 0.81 | 0.93 | 0.78 | 0.74 | 0.105 | 0.704 | 0.007 |
| Striatum | 0.80 | 0.92 | 0.79 | 0.70 | 0.058 | 0.911 | 0.0003 |
Regional A1AR distribution volumes [VT; (mL/mL)] in two groups after 8-h control sleep, 52 h of sleep deprivation, and 14-h recovery sleep and subgroups with minor and predominant changes. Values are given as mean; statistical comparisons that exceed the multiple-comparison–adjusted threshold (FDR, Benjamini and Hochberg method) are in boldface. diff, difference.
Discussion
This study reveals that 14 h of recovery sleep after 52-h sleep deprivation decreases elevated A1AR availability in the human brain. This decrease in A1AR was predominant in the striatum and thalamus, but also evident in other brain regions, including the orbitofrontal cortex, amygdala, occipital cortex, frontal cortex, anterior cingulate cortex, and insula, parietal, and temporal cortex (in descending order). In comparison with a well-rested independent control group, we observed an increase in A1AR availability after 52-h sleep deprivation that was significant in the frontal, occipital, and parietal cortices. Our human data confirm earlier autoradiography experiments in rats that were kept under 48 h of sleep deprivation (31). The authors of that study observed an up-regulation in A1AR availability of up to 23% in the striatum and 13% in the cortex. Our own studies in rats that were sleep-deprived for 12 or 24 h also showed an increase in A1AR availability in the basal forebrain and in cortical areas (28). Nevertheless it should be kept in mind that the impact of prolonged sleep deprivation (∼50 h) cannot easily be compared between rats and humans, given the sizable differences in the kinetics (i.e., time constants, triggers, metabolic processes) of the homeostatic build-up and continuity of sleep between the species. Moreover, there are large differences in the procedure to apply sleep deprivation in humans and rats that may impact the results as well. Common methods for sleep deprivation in rodents impose varying levels of stimulation, physical activity, or stress on the animals that is fundamentally different from voluntary wakefulness in human subjects or patients.
Compared with a previous human study in which we investigated A1AR availability after 28 h of sleep deprivation (27), there was no significant additional increase in receptor availability in the present study with 52 h of sleep deprivation, although the sample sizes might have been too small to detect subtle differences. However, the results appear to be consistent with the two-process model, which because of the saturating kinetics of sleep pressure, only predicts a small additional increase between 28 and 52 h of wakefulness. A single night of 14-h recovery sleep was sufficient to restore A1AR availability to levels that were observed in the well-rested control group, consistent with the rapid exponential discharge of sleep pressure during sleep. Taken together, the data support the assumption that the sleep–wake-dependent fluctuations of homeostatic sleep pressure are mediated, at least in part, by the amount of A1AR available.
Another key but counterintuitive finding of the present study was that the decrease in A1AR availability was highly, but negatively correlated with: (i) the degradation of cognitive performance in the PVT and in the N-back task; (ii) the rise in subjective sleepiness during prolonged wakefulness; as well as (iii) sleep pressure reflected in the amount of N3 in the first sleep cycle of recovery sleep. The brain regions in which we observed decreases in receptor availability have previously been identified as highly relevant for cognitive performance. In functional neuroimaging studies, a widespread pattern of frontal and parietal cortical, as well as thalamic, brain areas was found to be active during “good” cognitive performance (i.e., in the absence of lapses of attention), in contrast to lower activity in these areas during poor performance in a visual, selective attention task (not PVT) (32). In an investigation of the PVT, frontal and parietal activations were required to assure a good task performance after sleep deprivation versus baseline (33). From cognitive performance under sleep-deprivation conditions, it is known that the degree of impairment varies highly among individuals (34). Differences in caffeine effects have been linked to sleep-loss–induced performance impairments (35) and to genetic variants of the adenosinergic system (36, 37). Along these lines, we defined two subgroups based on the A1AR availability decrease, of which one group showed a strong decline in receptor availability, whereas the other revealed only a minor decline. The group with predominant decreases in A1AR availability proved resilient to the effects of sleep deprivation on cognitive performance. Participants with a minor A1AR availability decrease, however, were vulnerable and reacted with performance decline to sleep deprivation. These observations seem paradoxical at first glance. Although speculative, the observations might be explained by individual differences in the interplay of both a sleep-loss–dependent increase in endogenous adenosine levels and a sleep-loss–dependent up-regulation of adenosine receptors, which both have been shown in animal experiments. If both groups experienced A1AR up-regulation in response to the prolonged time awake, but in the vulnerable group this up-regulation was accompanied by a considerable increase in endogenous adenosine levels, increased receptor activation could have mediated the large performance impairing effects. In contrast, in the resilient group the increase in adenosine levels may have been less pronounced, thus mediating smaller performance impairments, but leaving more A1AR available for binding with the PET receptor ligand [18F]CPFPX. This interpretation is supported by several observations from animal experiments. First, adenosine concentrations were found to be increased in specific brain sites with prolonged wake-time (13). In vitro, we found evidence, that adenosine competes with CPFPX binding at the A1AR (38). However, so far it has not been shown in humans that adenosine levels increase with prolonged wake-time. In contrast, in medicated epilepsy patients with pharmacologically refractory seizures, no significant increase was detected with microdialysis in preparation for surgical resections in the amygdala (n = 7), hippocampus (n = 1), or motor cortex (n = 1) (39). Second, after an initial internalization of receptors, long-term agonist stimulation led to an increase in receptor mRNA and higher receptor availability (40). These findings imply that different from other downscaled G protein-coupled receptors (41), A1AR are up-regulated during prolonged wakefulness. This effect seems to enable sustained responsiveness of the system and to amplify the sleep-inducing function of adenosine.
In the current dataset, we further tried to link the differences in adenosine receptor availability to genetic polymorphisms that have been reported to explain resiliency to sleep deprivation and caffeine effects on sleep and performance [ADORA2A SNP rs5751876, ADORA2A haplotype 4 (42)] and anxiety (43) or sleep [adenosine deaminase SNP rs73598374 (44)]. ADORA2A SNPs might be relevant, as we previously found an association between A1AR availability under baseline conditions and ADORA2A SNP (rs5751876 and rs2236624) in another population (43). However, presumably because of the rather small sample size, we could not detect a significant association here. There was also no relationship between adenosine receptor availability and subjective caffeine sensitivity based on a previously evaluated questionnaire (35). Furthermore, no association was found between the caffeine sensitivity subtype and cognitive performance or sleep parameters. Interestingly, subjective sleepiness (SD52 − REC14) differed between the two subgroups, indicating that caffeine-sensitive subjects felt sleepier (Mann–Whitney u test: P = 0.008, KSS median difference 6) than insensitive ones (KSS median difference 7).
At the time of the PET scans, the subjects were off caffeine for at least 5 d, but duration of withdrawal might be up to 9 d (45). Saliva samples at the beginning of the study proved caffeine abstinence. None of the subjects reported withdrawal-related symptoms, like headache, during the study period.
The negative correlation between the sleep-loss–dependent decreases in A1AR availability and increase in N3 in the first sleep cycle of recovery sleep seemingly contradicts animal findings on the involvement of the adenosinergic system in the homeostatic regulation of sleep (24–26, 46). This negative correlation—similar to the correlations for the cognitive performance impairments and sleepiness—is most likely because of a wake-dependent increase of adenosine release/concentration, outweighing the homeostatic up-regulation of A1AR, and thus leaving fewer sites available for binding with the PET receptor ligand. In our human dataset the recovery night did not only restore A1AR availability to control levels, it also recovered cognitive performance and sleepiness ratings. Our findings are consistent with the concept of activity-dependent local sleep of groups or single neurons (47, 48) that integrates the synaptic homeostasis theory and metabolic theories, based on the occurrence of local neuromodulators, like ATP and adenosine.
It is a robust finding that sleep deprivation improves depressive symptoms in a large proportion of human patients (49). Shortly after the first description of the two-process model of sleep–wake regulation, it was hypothesized that in depressed patients the homeostatic regulation might be deficient as reflected in a lower build-up of sleep pressure during wakefulness (15). A key candidate mediating both the homeostatic process and the antidepressant effect is adenosine. Interestingly, S-adenosylmethionine, a precursor of adenosine, is a widely used over-the-counter medication of major depression (50). More directly, it was recently shown in a readout model of antidepressive effects (forced swim test in mice) that astrocytic adenosine signaling to A1AR during sleep deprivation is necessary to reduce depressive-like behaviors (21). Up-regulating A1AR in a transgenic mouse model of conditionally enhanced forebrain A1AR expression promoted resilience against depression-resembling reactions in various behavioral tests (51). Conversely, A1AR knockout mice had an increased depressive-like behavior and lacked the antidepressant effects of sleep deprivation. The rapid relapse following the end of therapeutic total sleep deprivation is in line with our findings of a normalization of A1AR availability following a single episode of recovery sleep. It is tempting to speculate that potential interventions that induce a chronic up-regulation of A1AR may have a longer-lasting therapeutic effect. In fact, continued sleep restriction following total sleep deprivation was reported to extend the antidepressant effect in some patients (52). Our findings therefore have potential clinical implications. The subtle differences in the settings of the adenosine receptor system that we found to be associated with different behavioral responses (vulnerable or resilient against sleep loss) might also serve as indicators for the outcome of therapeutic sleep deprivation in patients with major depression. Even more, there might be a specific “depression-type” of cerebral receptor/enzyme settings, which accounts for differential therapy efficacy, but also represents a primary neurochemical basis for depression-associated patterns of sleep disturbances and disease-associated behavioral phenotypes. In line with this assumption, the beneficial therapeutic effects of sleep deprivation in major depression could arise from an adjustment of the pathological receptor/enzyme setting.
In conclusion, we found that sleep deprivation resulted in a higher A1AR availability in the human brain. The increase that was observed over 52 h of wakefulness was restored to control levels during a 14-h sleep episode. Individuals with a large increase in A1AR availability were more resilient to sleep-loss effects than those with a subtle increase. This pattern implies that differences in the endogenous adenosine and A1AR availability might be causal for individual responses to sleep loss. We therefore propose that endogenous adenosine and its receptors are key players in the individual regulation of sleep–wake behavior and cognitive performance. Understanding the mechanistic link between mood and adenosine regulation under sleep restriction, especially in the light of individual characteristics, might improve the rationale for the individual indication and design of therapeutic sleep modulation in depression.
Methods
Participants.
The study was approved by the Ethics Committee of the Medical Faculty of the University of Duesseldorf and the German Federal Office for Radiation Protection. Fifteen healthy, male volunteers gave written informed consent of which 14 (mean age 27.7 ± 5.4 y) were included in the analyses. For details on participant selection, SI Methods.
Study Design.
One week before the arrival in the laboratory, subjects maintained a sleep log and routine (bed time 11:00 PM to 7:00 AM). Four days before the arrival, subjects abstained from caffeine, which was checked with saliva samples upon arrival and by plasma samples at the time of PET scans. The last 3 d before the laboratory stay, subjects wore an actigraph to check compliance. After an adaptation night (11:00 PM–7:00 AM), polysomnographic measurements were recorded during one baseline night (11:00 PM–7:00 AM). From Monday morning until Wednesday afternoon (5:00 PM), two participants at a time were sleep-deprived. The two participants completed the neuropsychological test batteries 1-h apart of each other. Starting at 9:00 PM on Monday, participants completed the test battery and a 3-min recording of waking EEG at 6-h intervals. Out of testing sessions, subjects were allowed to do nonvigorous activities. Subjects were continuously monitored by at least one study staff member to ensure wakefulness and adherence to the protocol. After SD52 and REC14 (5:00 PM–7:00 AM), participants were scanned with the two scans scheduled 24-h apart. The first subject was scanned at 10:00 AM and the second one at 12:00 AM (mean scanning clock times: 11:42 AM ± 1:16 h). The control group underwent the same scanning protocol but was allowed to sleep for 8 h during the night before the scan (baseline) but without neuropsychological testing. The study design is further presented in Fig. 1 and SI Methods.
Polysomnography and Neurobehavioral Testing.
For polysomnography and neurobehavioral testing, SI Methods.
PET.
[18F]CPFPX PET were performed as previously reported (27, 29); SI Methods and Fig. S1.
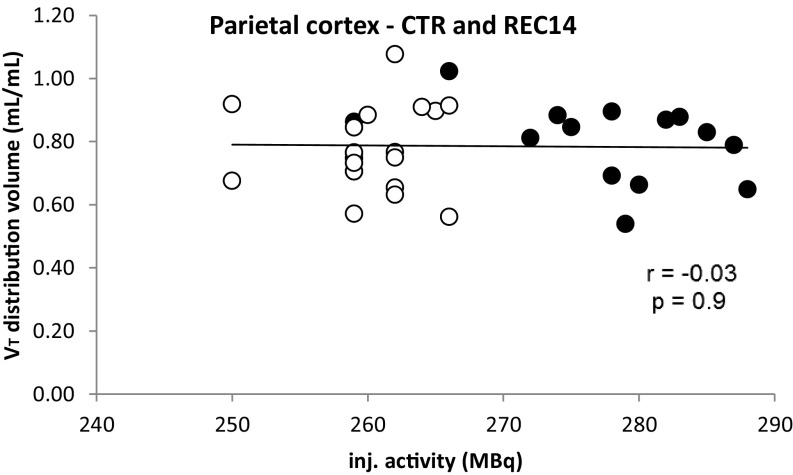
Fig. S1.
Distribution volume in relation to the amount of injected (inj.) activity (open circles denote the control group and the filled circles the experimental group).
Statistical Analyses.
The sleep-loss response in the PET [regional A1AR distribution volumes (VT; mL/mL) was quantified in reference to: (i) control and (ii) recovery condition with a one-way mixed ANOVA with subject as random factor (P < 0.05). Post hoc t tests were used for pairwise comparisons with a false-discovery rate (FDR)-corrected significance level. Spearman rank correlation and regression analyses were used to evaluate associations between adenosine receptor availability and: (i) performance measurements, (ii) self-ratings of sleepiness, and (iii) sleep parameters. The effect of recovery sleep on performance measures and sleepiness ratings was evaluated with two-tailed paired t tests. Average values are reported as mean ± SD. For all analyses, significance was assumed at P < 0.05 if not stated differently.
SI Methods
Participants.
Detailed interviews ensured that volunteers did not have a history of neurological and psychiatric diseases, sleep disorders, shift work, jet-lag, night work, head injury, and alcohol or substance abuse. Only nonsmoking subjects without any medication were included. Caffeine intake was restricted 84 h before the arrival at the laboratory, which was controlled by saliva samples upon arrival at the laboratory and by plasma samples at the time of the PET scans (below detection limits in all subjects: <0.5 mg/L). One subject suffered from apneas and was excluded from all analyses. According to the caffeine-sensitivity questionnaire (35), six participants reported sleep problems after caffeine intake in the afternoon, indicating sensitivity to caffeine; six participants had no sleep impairment, indicating insensitivity to caffeine; and two participants did not consume caffeine at all. The average Becks depression inventory (BDI II) score sum of the subjects was 0.43 ± 0.94 at the beginning of the in-patient phase of the study.
During the beginning of the study, participants filled out a chronotype questionnaire (D-MEQ) (56). Based on the questionnaire, participants were classified as morningness, eveningness, or intermediate-type. Our evaluation shows that most of our sample belongs to the intermediate chronotype. Only two participants were classified as evening-type and one as morning-type.
Protocol.
The subjects were asked to maintain a sleep log and adhere to a constant sleep–wake routine (bed time 11:00 PM to 7:00 AM) in the week before the arrival at the laboratory. For the 3 d before the arrival, the subjects wore an actigraph on their wrists, allowing for checking compliance.
Between the adaptation night and the baseline night, participants were allowed to leave the laboratory during the day (between 9:15 AM and 7:30 PM) with an actigraph attached to the nondominant wrist. Upon arrival in the evening, saliva was tested for caffeine and actigraphs were checked to exclude daytime napping. Apart from the previously described daytime, participants spent the scheduled time awake, including the testing sessions, in the research facilities of the Forschungszentrum Jülich and in the sleep laboratory of the German Aerospace Center. In between the testing sessions, nonvigorous activities (e.g., talking with each other, watching television, playing calm games, reading, surfing the internet) were allowed. Under constant monitoring of at least one study staff member, two participants at a time were sleep-deprived. Participants weren’t allowed to close their eyes to ensure wakefulness. Whenever subjects closed their eyes, they were addressed by the experimenter and encouraged to stay awake.
Polysomnography and Neurobehavioral Testing.
During baseline and recovery sleep EEG (F4/A1, C4/A1, O2/A1), electrooculography (EOG), electromyography, electrocardiography, finger pulse, and breathing were recorded. Recordings were scored according to conventional criteria (57).
Every 6 h during wakefulness, cognitive performance was assessed with a 3-min version of the PVT (58) on a portable, handheld computer. Reaction times in response to the lighting up of the battery lamp as trigger signal were recorded. Lapses in attention were defined as reactions longer than 500 ms.
Working-memory capacity was tested with an N-back task in which the spatial position of a circle had to be remembered for one, two, and three positions back (59).
Sleepiness was measured on the KSS (60) and fatigue with the Samn and Perelli fatigue checklist.
PET.
A Siemens ECAT EXACT HR+ scanner (Siemens-CTI) was used for 3D PET data acquisition. The radiotracer was injected as a bolus followed by a constant infusion with a Kbol value of 55 min. Scan duration was 100 min (for start time, Table S1). Arterialized venous blood sampling took place at minute timepoints 1, 5, and 10, and every 10 min subsequently. The total distribution volume VT in the equilibrium (between 50 and 100 min) can be expressed as VT = TAC/Cp, with TAC being the tissue activity concentration and Cp the plasma activity (27).
Realignment, coregistration, segmentation, and normalization of PET data and corresponding MRI (acquired on a 3T Siemens Magnetom Trio with MPRAGE sequence) were done with PMOD (v3.305, PMOD Group).
To ensure comparability with our own previous data and other neuroreceptor imaging studies of sleep–wake regulation, the selection of ROI was done as described in Elmenhorst et al. (27) (individually drawn ROI) and Hefti et al. (61) (standard ROI from the Montreal Neurological Institute Brain Atlas, anterior cingulate cortex, insula, and amygdala). In detail, the ROI that previously showed a significant increase in A1AR after 28 h of sleep deprivation compared with 4 h of wakefulness were selected for the current analysis.
Activity data from PET voxels, which were classified as gray matter (probability higher than 10%) based on the anatomical MRI, were corrected for the contribution (5%) of activity from blood within the tissue and used for further analysis.
The A1AR availability that we refer to in the following is directly proportional to the equilibrium total distribution volume. VT in the equilibrium (between 50 and 100 min) can be expressed as VT = TAC/Cp, with TAC being the tissue activity concentration and Cp the plasma activity (27).
Participants’ constant wakefulness during PET scans was checked by video monitoring of subjects’ eye blink behavior. Two participants were examined per day with a 2-h delay. The time slot of the scan (i.e., first or second scan of the day) did not have any significant effect on the outcome parameters (P > 0.47). Vigilance during the PET scanning was determined with simultaneous EEG and EOG. At signs of drowsiness or sleep-like EEG patterns, the subjects were requested to open their eyes.
The quantification of the receptor availability is based on an equilibrium pharmacokinetic model. Under steady-state conditions, the diffusion of the radioligand between the blood plasma compartment (input from the computerized infusion pump) and the brain tissue compartment (tissue “behind” the blood–brain barrier) results in a (temporally constant) ratio of these compartments, which reflects the distribution volume which is directly proportional to the receptor density. Therefore, the applied model is independent of changes in the amount of injected radioactivity. The difference of injected radioactivity (Table S1) between both studies is about 6%, but the injected dose is slightly—albeit not significantly—lower. In both studies tracer conditions—for example, conditions excluding any pharmacological effects—were guaranteed. We have previously shown that at these conditions the mass of injected CPFPX had no effect on the binding parameters (62).
Fig. S1 shows the distribution volume in relation to the amount of injected activity (open circles denote the baseline group and the filled circles the experimental group) for the parietal cortex.
Acknowledgments
We thank the teams of the Institute of Neuroscience and Medicine (INM)-2, INM-4, and INM-5 of the Forschungszentrum Jülich for excellent technical assistance and radioligand supply; colleagues from the German Aerospace Center Division of Flight Physiology for excellent support in study conductance; and Svenja Caspers for her help with programming the N-back task.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614677114/-/DCSupplemental.
References
- 1.Drummond SP, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 4.Nilsson JP, et al. Less effective executive functioning after one night’s sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 5.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—A prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 8.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–190. doi: 10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 14.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 15.Borbély AA. The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry. 1987;20:23–29. doi: 10.1055/s-2007-1017069. [DOI] [PubMed] [Google Scholar]
- 16.Wolf E, et al. Synaptic plasticity model of therapeutic sleep deprivation in major depression. Sleep Med Rev. 2016;30:53–62. doi: 10.1016/j.smrv.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013;70:983–989. doi: 10.1001/jamapsychiatry.2013.1. [DOI] [PubMed] [Google Scholar]
- 18.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: Mechanisms of action. Int J Neuropsychopharmacol. 2012;15:695–713. doi: 10.1017/S1461145711000927. [DOI] [PubMed] [Google Scholar]
- 20.Blutstein T, Haydon PG. The importance of astrocyte-derived purines in the modulation of sleep. Glia. 2013;61:129–139. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry. 2013;3:e212. doi: 10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredholm BB. Purinoceptors in the nervous system. Pharmacol Toxicol. 1995;76:228–239. doi: 10.1111/j.1600-0773.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 23.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: A microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 24.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 25.Schwierin B, Borbély AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 26.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: Effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmenhorst D, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: A positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmenhorst D, et al. Test-retest stability of cerebral A1 adenosine receptor quantification using [18F]CPFPX and PET. Eur J Nucl Med Mol Imaging. 2007;34:1061–1070. doi: 10.1007/s00259-006-0309-x. [DOI] [PubMed] [Google Scholar]
- 30.Meyer PT, et al. Effect of aging on cerebral A1 adenosine receptors: A [18F]CPFPX PET study in humans. Neurobiol Aging. 2007;28:1914–1924. doi: 10.1016/j.neurobiolaging.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Yanik G, Radulovacki M. REM sleep deprivation up-regulates adenosine A1 receptors. Brain Res. 1987;402:362–364. doi: 10.1016/0006-8993(87)90046-1. [DOI] [PubMed] [Google Scholar]
- 32.Chee MWL, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond SP, et al. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- 34.Van Dongen HP, Belenky G. Individual differences in vulnerability to sleep loss in the work environment. Ind Health. 2009;47:518–526. doi: 10.2486/indhealth.47.518. [DOI] [PubMed] [Google Scholar]
- 35.Rétey JV, et al. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–10479. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rétey JV, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 37.Byrne EM, et al. A genome-wide association study of caffeine-related sleep disturbance: Confirmation of a role for a common variant in the adenosine receptor. Sleep. 2012;35:967–975. doi: 10.5665/sleep.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmenhorst D, Garibotto V, Prescher A, Bauer A. Adenosine A(1) receptors in human brain and transfected CHO cells: Inhibition of [(3)H]CPFPX binding by adenosine and caffeine. Neurosci Lett. 2011;487:415–420. doi: 10.1016/j.neulet.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 39.Zeitzer JM, et al. Extracellular adenosine in the human brain during sleep and sleep deprivation: An in vivo microdialysis study. Sleep. 2006;29:455–461. doi: 10.1093/sleep/29.4.455. [DOI] [PubMed] [Google Scholar]
- 40.Souazé F. Maintaining cell sensitivity to G-protein coupled receptor agonists: Neurotensin and the role of receptor gene activation. J Neuroendocrinol. 2001;13:473–479. doi: 10.1046/j.1365-2826.2001.00658.x. [DOI] [PubMed] [Google Scholar]
- 41.Böhm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rupp TL, Wesensten NJ, Newman R, Balkin TJ. PER3 and ADORA2A polymorphisms impact neurobehavioral performance during sleep restriction. J Sleep Res. 2013;22:160–165. doi: 10.1111/j.1365-2869.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 43.Hohoff C, et al. Association of adenosine receptor gene polymorphisms and in vivo adenosine A1 receptor binding in the human brain. Neuropsychopharmacology. 2014;39:2989–2999. doi: 10.1038/npp.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichert CF, et al. Insights into behavioral vulnerability to differential sleep pressure and circadian phase from a functional ADA polymorphism. J Biol Rhythms. 2014;29:119–130. doi: 10.1177/0748730414524898. [DOI] [PubMed] [Google Scholar]
- 45.Juliano LM, Griffiths RR. A critical review of caffeine withdrawal: Empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology (Berl) 2004;176:1–29. doi: 10.1007/s00213-004-2000-x. [DOI] [PubMed] [Google Scholar]
- 46.Bjorness TE, et al. An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J Neurosci. 2016;36:3709–3721. doi: 10.1523/JNEUROSCI.3906-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyazovskiy VV, Harris KD. Sleep and the single neuron: The role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem. 2011;11:2490–2492. doi: 10.2174/156802611797470330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemmeter UM, Hemmeter-Spernal J, Krieg JC. Sleep deprivation in depression. Expert Rev Neurother. 2010;10:1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter DJ. St. John’s wort and S-adenosyl methionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: What is the evidence for clinically relevant benefit? Altern Med Rev. 2011;16:17–39. [PubMed] [Google Scholar]
- 51.Serchov T, et al. Increased signaling via adenosine A(1) receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of homer1a. Neuron. 2015;87:549–562. doi: 10.1016/j.neuron.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riemann D, et al. How to preserve the antidepressive effect of sleep deprivation: A comparison of sleep phase advance and sleep phase delay. Eur Arch Psychiatry Clin Neurosci. 1999;249:231–237. doi: 10.1007/s004060050092. [DOI] [PubMed] [Google Scholar]
- 53.Halassa MM, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones BE. Glia, adenosine, and sleep. Neuron. 2009;61:156–157. doi: 10.1016/j.neuron.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Urry E, Landolt HP. Adenosine, caffeine, and performance: From cognitive neuroscience of sleep to sleep pharmacogenetics. Curr Top Behav Neurosci. 2015;25:331–366. doi: 10.1007/7854_2014_274. [DOI] [PubMed] [Google Scholar]
- 56.Griefahn B, Künemund C, Bröde P, Mehnert P. Zur Validität der deutschen Übersetzung des Morningness-Eveningness-Questionnaires von Horne und Östberg. Somnologie (Berl) 2001;5:71–80. [Google Scholar]
- 57.Silber MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–131. [PubMed] [Google Scholar]
- 58.Elmenhorst EM, Rooney D, Pennig S, Vejvoda M, Wenzel J. Validating a 3-min psychomotor vigilance task for sleep loss-induced performance impairments. J Sleep Res. 2012;21:115. [Google Scholar]
- 59.Groeger JA, et al. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 60.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 61.Hefti K, et al. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73:161–168. doi: 10.1016/j.biopsych.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 62.Meyer PT, et al. Quantification of cerebral A1 adenosine receptors in humans using [18F]CPFPX and PET. J Cereb Blood Flow Metab. 2004;24:323–333. doi: 10.1097/01.WCB.0000110531.48786.9D. [DOI] [PubMed] [Google Scholar]