Significance
We accept that the environment influences human health, but we know little about how human health affects the environment. However, millions of people around the world rely on natural resources for food and livelihoods and confront a high burden of illness. Experience of illness may change people’s physical capacities, outlook, and planning horizons and shape how they engage with the environment. We analyze these impacts in fishing communities of Lake Victoria, Kenya. Although illness may cause the sickest individuals not to fish, many fishers continue fishing but shift their methods. When sick, fishers use methods that are less physically demanding but illegal and environmentally destructive. Our findings suggest that environmental sustainability may be integrally shaped by the health of resource users.
Keywords: environmental change, fishing livelihoods, Lake Victoria, health and environment, social-ecological systems
Abstract
Understanding feedbacks between human and environmental health is critical for the millions who cope with recurrent illness and rely directly on natural resources for sustenance. Although studies have examined how environmental degradation exacerbates infectious disease, the effects of human health on our use of the environment remains unexplored. Human illness is often tacitly assumed to reduce human impacts on the environment. By this logic, ill people reduce the time and effort that they put into extractive livelihoods and, thereby, their impact on natural resources. We followed 303 households living on Lake Victoria, Kenya over four time points to examine how illness influenced fishing. Using fixed effect conditional logit models to control for individual-level and time-invariant factors, we analyzed the effect of illness on fishing effort and methods. Illness among individuals who listed fishing as their primary occupation affected their participation in fishing. However, among active fishers, we found limited evidence that illness reduced fishing effort. Instead, ill fishers shifted their fishing methods. When ill, fishers were more likely to use methods that were illegal, destructive, and concentrated in inshore areas but required less travel and energy. Ill fishers were also less likely to fish using legal methods that are physically demanding, require travel to deep waters, and are considered more sustainable. By altering the physical capacity and outlook of fishers, human illness shifted their effort, their engagement with natural resources, and the sustainability of their actions. These findings show a previously unexplored pathway through which poor human health may negatively impact the environment.
Environmental degradation is widely recognized as a cause of and contributor to adverse human health outcomes (1). Whether through poor air quality, deforestation, or declining biodiversity, our alteration of the environment has been implicated in a range of health concerns, including the rise of asthma, the spread of malaria, and outbreaks of zoonotic diseases, such as Ebola, Zika, and Lyme (2). Quantifying the impacts of declining environmental health on human health is a large and growing field of study. However, remarkably little research has engaged specifically with how human illness impacts environmental change and the sustainable use of natural resources. This knowledge gap is glaring given the hundreds of millions of people on our planet who depend on natural resources for food and income while also confronting the highest burden of infectious diseases (3).
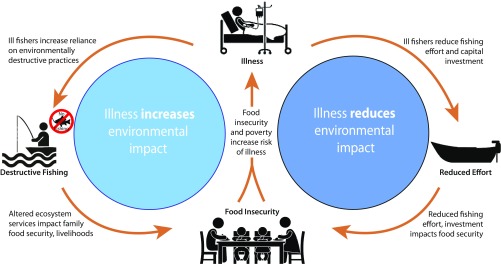
Human illness may alter individual and household use of natural resources in a variety of ways and thereby shape feedback loops between human interaction and environmental sustainability (Fig. 1). The physical effects of illness may force individuals or their families to change the effort or methods with which they engage natural resources. Illness may also alter the outlook of resource users, affecting their livelihood strategies, investment in equipment or labor, and attention to environmental sustainability. Ultimately, characterizing the relative importance of the feedbacks that link human health and environmental sustainability will influence our understanding of social-ecological systems, poverty traps, and natural resource management on a rapidly changing planet.
Fig. 1.
(Right) Traditional and (Left) alternative pathways linking human and environmental health in fishing communities. In the face of illness, households may alter their pressure on environmental resources to increase their reliance on destructive practices or curtail their harvest effort. These feedbacks portend sharply different environmental consequences of human illness, even as outcomes for households remain similar.
The Malthusian hypothesis commonly used to link declining human health to environmental outcomes predicts that illness will reduce human populations or harvest effort, benefitting the environment (Fig. 1, Right) (4). Studies of illness and labor productivity primarily focus on and show this pathway [for example, showing that HIV reduces commercial tea, homestead banana, and subsistence harvests (5–7)]. Such findings have been used to argue that the negative impact of disease, specifically HIV, on household labor has contributed broadly to food shortages across southern Africa (8, 9).
Human illness may also drive changes in natural resource use that are detrimental, rather than beneficial, to environmental sustainability (Fig. 1, Left). A “syndemic” framework has been proposed to understand how disease, specifically HIV in this case, is linked to environmental degradation (10). Where illness reduces physical capital, this shift may lead to a range of household vulnerabilities and alter livelihoods (10). Either illness or environmental changes may constitute a “shock,” with consequent effects on household expenses, planning horizons, and outlook. Illness may also prompt households to turn to natural resources as a “safety net.” Evidence for this mechanism suggests that HIV-affected households may be more reliant on safety net resources, such as bushmeat and wild plants (11, 12). In addition to increasing environmental pressure, harvesting resources that are low-yielding or expose households to legal consequences is a strategy engaged only when shocks constrain options. Conversely, research also demonstrates that the positive health shock of improved medication access may increase conservation agricultural practices in some households (13).
Finally, illness may have longer-term effects on the relationship between people and their ecosystems by altering the outlook of resource harvesters. Over time, shortened lifespans as a result of disease may reduce communities’ environmental knowledge and promote patterns of increasingly exploitive and environmentally unsustainable behaviors (14, 15).
Here, we studied the behavior of fishers around Lake Victoria, Kenya to examine the dynamics through which two dimensions of illness, measured separately as mental and physical health, impact local engagement with the environment. We analyzed fishing effort and methods using panel data from a random sample of 303 households across four time points from December of 2012 to March of 2014. Our analyses provide a quantitative analysis of the pathways through which illness may impact the environment (Fig. 1).
Destructive fishing practices and unsustainable harvest are key concerns throughout global fisheries, including Lake Victoria. To reduce destructive practices, fishing gear and catch regulations are mandated, and the fishery is comanaged by fishers, national ministries, and a lake-wide organization. Despite these safeguards, Nile perch (Lates niloticus), the prevailing fishery, declined by 65% in inshore areas and 30% in deep zones in Lake Victoria from 2002 to 2014 (16), and catch per unit effort dropped 40% in the same time period (17). Fishers in Lake Victoria use a range of fishing techniques spanning different gear types, fish species, and the targeting of near- and off-shore habitats. The legality and destructiveness of fishing methods are tied to their physical demands. Methods that are more sustainable involve traveling into deeper waters, more selective targeting of large fish, higher levels of physical exertion, and fishing at night for dagaa (Rastrineobola argentea), a second targeted species. As is the case throughout small-scale fisheries, fishing livelihoods around Lake Victoria are comprised of diverse roles in and out of the fishery, with fishers switching their participation in fishing, altering the methods that they use throughout the year, and fishing in multiple ways at a single time point (18).
The prevalence of HIV in the Lake Victoria region increased throughout the 1980s and 1990s after extensive migration to the lakeshores by those eager to join an increasingly lucrative international trade in Nile perch (19). The high HIV prevalence around Lake Victoria, reaching 27% among adults in the communities that we studied (20), is mirrored in global fishing communities (21). The fishing methods of Lake Victoria are represented throughout the world’s fisheries. Similar methods are found in coastal, near-shore fisheries and the extensive global inland fisheries, with inland waters the focus of 42% of the fishing boats operating in Africa (22). Although HIV is a particular concern in fishing communities and many resource-dependent settings, myriad health concerns beset rural communities that steward natural resources.
We examined pathways through which fisher illness may impact the sustainability of fishing practices by quantifying (i) fishing effort (e.g., hours fished, catch per unit effort, and investment in labor), (ii) legality of fishing practices, and (iii) fishing methods used (e.g., gillnet and long line). To assess illness, we used the Measured Outcomes Survey–HIV (MOS-HIV), a validated metric of health-related quality of life that provides for physical and mental health summary scores (Methods, Fig. S1, and Tables S1 and S2) (23, 24). Scores were created so that higher scores indicate poorer health and are centered at zero and normalized to 1 SD. We examined pathways from mental and physical health to fishing practices using panel regression models (fishing effort: hours, travel, nights away, and catch per unit effort) and fixed effect conditional logit models (fishing participation, fishing method legality, and fishing methods), which enabled us to compare an individual with himself at different time points. This methodology thus provided a control for individual-level as well as time-invariant factors, so that we could make robust comparisons that aggregate how changes in an individual’s experience of illness are associated with altered fishing behavior.
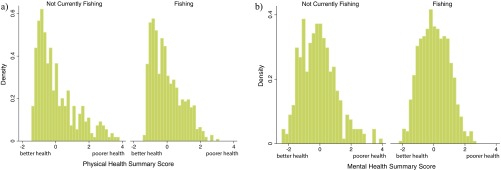
Fig. S1.
Histograms comparing the (A) physical health and (B) mental health scores of individuals who are not currently fishing and are currently fishing. We observe a more skewed distribution of physical than mental health with a relatively long tail, indicating poorer health, particularly among those not currently fishing. Model results of participation in fishing are provided in Table S4.
Table S1.
MOS-HIV subscale scores at baseline using the MOS-HIV
| Variable | Mean | SD | Minimum | Maximum | Cronbach’s α |
| General health | 39.0 | 27.0 | 0 | 100 | 0.71 |
| Physical functioning | 84.0 | 21.5 | 0 | 100 | 0.87 |
| Role functioning | 69.6 | 43.7 | 0 | 100 | 0.90 |
| Social functioning | 81.2 | 26.3 | 0 | 100 | — |
| Cognitive functioning | 71.0 | 20.8 | 0 | 100 | 0.78 |
| Pain | 57.5 | 26.8 | 0 | 100 | 0.85 |
| Mental health | 58.8 | 17.5 | 0 | 100 | 0.72 |
| Energy/fatigue | 56.8 | 17.4 | 0 | 100 | 0.66 |
| Health distress | 70.9 | 23.6 | 0 | 100 | 0.92 |
| Quality of life | 51.1 | 21.5 | 0 | 100 | — |
| Health transition | 56.2 | 22.6 | 0 | 100 | — |
| Physical health summary | 48.1 | 10.6 | 15.1 | 64.4 | |
| Mental health summary | 43.9 | 9.1 | 14.8 | 63.6 |
Table S2.
Correlation matrix of mental health and physical health summary scores calculated using different methodologies
| Reference population | Roche reference | Roche reference, sample Z scores | Ugandan reference |
| Mental health | |||
| Roche reference | 1 | ||
| Roche reference, sample Z scores | 0.9988*** | 1 | |
| Ugandan reference | 0.9106*** | 0.9177*** | 1 |
| Ugandan reference, sample Z scores | 0.9115*** | 0.9171*** | 0.9985*** |
| Physical health | |||
| Roche reference | 1 | ||
| Roche reference, sample Z scores | 0.9987*** | 1 | |
| Ugandan reference | 0.9421*** | 0.9364*** | 1 |
| Ugandan reference, sample Z scores | 0.9612*** | 0.9606*** | 0.9882*** |
The Roche reference population refers to a population of HIV+ patients in a high-income setting, and it is the population described in the MOS-HIV manual and used most widely (44). The Ugandan reference population is a more recently validated sample of Ugandan HIV+ patients who entered the study with low CD4 counts (42). Summary scores are calculated using factor weightings from the reference population and Z scores. We calculated and compared these summary metrics using weights from each reference population (Roche reference, Ugandan reference) and Z scores from our study population (sample Z scores) and the validated reference populations. Our results are highly correlated using all methods, and we elect to use the Roche reference population in all subsequent analyses for its wide reference and the relative similarity of our average scores to the averages for this reference group.
P < 0.001.
We first tested if poor physical and mental health reduced participation in fishing. For individuals who identified their primary occupation as fishing, with poorer physical health fishers were 66% less likely to participate in fishing [odds ratio (OR) = 0.34, SE = 0.19, P < 0.06] (Fig. 2 and Tables S3 and S4). Among individuals not currently fishing (33–37% of all individuals), substantial proportions were engaged in professional (34–37%; e.g., teacher or government official) and other nonfishing occupations (45–50%; e.g., agriculture or carpenter). Among all individuals, even controlling for occupation, there was no effect of illness on whether an individual was fishing at a given time point (Table S4).
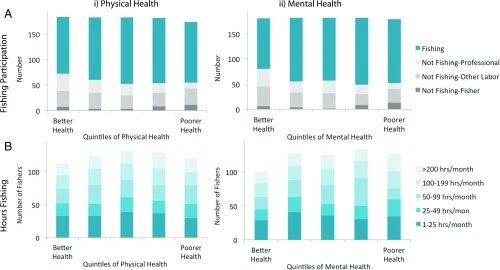
Fig. 2.
For men (n = 253) facing markedly different levels of illness, we observe that (A) fishing participation is widespread; individuals not fishing are largely employed in other sectors, whereas a smaller number of fishers who experience poor health are not fishing. (B) Fishing effort, measured as hours fishing, is relatively stable across experiences of (i) physical health and (ii) mental health. Individual experience of poorer health (moving to the right on each graph) is assessed in multivariate panel regression models. Models show that mental and physical health did not affect participation in fishing, hours fished, nights spent away from home, or income earned per hour and had a modest effect on hours traveled (Tables S4 and S5).
Table S3.
Fishing participation and morbidity across time points
| Variable | By time point | |||||||
| 0 | 3 | 6 | 12 | |||||
| N | Percent | N | Percent | N | Percent | N | Percent | |
| N | 253 | 237 | 218 | 217 | ||||
| Currently fishing | ||||||||
| No | 83 | 33 | 74 | 31 | 74 | 34 | 81 | 37 |
| Yes | 170 | 67 | 163 | 67 | 144 | 66 | 136 | 63 |
| Occupation of individuals not fishing | ||||||||
| Fisher | 7 | 8 | 8 | 11 | 10 | 14 | 7 | 9 |
| Other laborer | 42 | 50 | 34 | 46 | 33 | 45 | 39 | 48 |
| Other professional | 31 | 37 | 25 | 34 | 27 | 36 | 30 | 37 |
| Not reported | 3 | 4 | 7 | 9 | 4 | 5 | 5 | 6 |
| Occupation of individuals currently fishing | ||||||||
| Fisher | 127 | 75 | 109 | 67 | 101 | 70 | 101 | 74 |
| Other laborer | 28 | 16 | 32 | 20 | 26 | 16 | 22 | 16 |
| Other professional | 12 | 7 | 15 | 9 | 11 | 8 | 9 | 7 |
| Not reported | 3 | 2 | 7 | 4 | 6 | 4 | 4 | 3 |
| No. of fishing methods | ||||||||
| 1 | 117 | 46 | 119 | 50 | 108 | 50 | 107 | 50 |
| 2 | 46 | 18 | 40 | 17 | 34 | 16 | 24 | 11 |
| 3+ | 7 | 3 | 4 | 2 | 2 | 1 | 4 | 2 |
| Fishing activities (individuals may use 1+) | ||||||||
| Monofilament | 19 | 11 | 17 | 10 | 16 | 11 | 15 | 11 |
| Beach seine | 68 | 40 | 59 | 36 | 53 | 37 | 48 | 35 |
| Gillnet | 66 | 39 | 60 | 37 | 54 | 38 | 48 | 35 |
| Long line | 15 | 9 | 15 | 9 | 10 | 7 | 14 | 10 |
| Small seine | 35 | 21 | 52 | 32 | 45 | 31 | 38 | 28 |
| Other | 28 | 16 | 9 | 6 | 5 | 3 | 4 | 3 |
| Fishery role | ||||||||
| Laborer | 102 | 60 | 86 | 53 | 79 | 55 | 82 | 61 |
| Self-employed and hired labor | 34 | 20 | 54 | 33 | 48 | 33 | 39 | 29 |
| Hired labor | 34 | 20 | 23 | 14 | 17 | 12 | 14 | 10 |
| Fishing effort | ||||||||
| Fished per mo, h | 76.9 | 117.3 | 65.2 | 97.2 | 69.7 | 105.6 | 74.7 | 123.3 |
| Traveled per mo, h | 27.3 | 55.3 | 17.4 | 23.8 | 21.9 | 52.7 | 15.1 | 24.7 |
| Nights away per mo | 3.2 | 6.1 | 4.4 | 7.9 | 3.8 | 7.0 | 3.4 | 6.8 |
| Income per h | 621.8 | 1,328.8 | 620.2 | 2,031.8 | 319.8 | 411.7 | 571.0 | 1,041.7 |
| Health-related quality of life | ||||||||
| Physical health | 0.22 | 1.05 | 0.06 | 1.00 | −0.09 | 1.00 | −0.23 | 0.88 |
| Mental health | 0.32 | 1.08 | −0.02 | 1.00 | −0.15 | 0.87 | −0.20 | 0.94 |
Table S4.
Conditional fixed effects logit models and random effects logit models with the outcome variable of participation in any type of fishing in the preceding 3 mo
| Variable | Fixed effects | Random effects | ||||
| OR | SE | P value | OR | SE | P value | |
| Fishing participation, fishers only | ||||||
| Observations, groups | 64, 18 | 470, 134 | ||||
| Physical health | 0.34 | 0.19 | 0.06 | 0.39 | 0.16 | 0.02 |
| Mental health | 0.92 | 0.45 | 0.86 | 0.71 | 0.30 | 0.42 |
| Time point (baseline comparison) | ||||||
| 3 | 0.44 | 0.35 | 0.30 | 0.45 | 0.34 | 0.30 |
| 6 | 0.22 | 0.18 | 0.06 | 0.19 | 0.15 | 0.04 |
| 12 | 0.23 | 0.23 | 0.14 | 0.29 | 0.24 | 0.21 |
| Fishing participation | ||||||
| Observations, groups | 214, 57 | 886, 247 | ||||
| Physical health | 0.81 | 0.19 | 0.37 | 0.74 | 0.16 | 0.17 |
| Mental health | 1.10 | 0.26 | 0.66 | 1.10 | 0.24 | 0.67 |
| Occupation (fisher comparison) | ||||||
| Other labor (e.g., farmer) | — | — | — | 0.002 | 0.002 | 0.000 |
| Professional (e.g., teacher) | — | — | — | 0.00 | 0.00 | 0.000 |
| Time point (baseline comparison) | ||||||
| 3 | 1.61 | 0.60 | 0.19 | 1.61 | 0.58 | 0.19 |
| 6 | 0.90 | 0.34 | 0.77 | 0.85 | 0.32 | 0.67 |
| 12 | 0.68 | 0.26 | 0.32 | 0.64 | 0.24 | 0.23 |
Models are conducted among only individuals whose primary occupation is fishing and all individuals in the sample while controlling for occupation. Hausman tests: fishing participation, fishers only, χ2 = 2.62, P = 0.76; fishing participation, χ2 = 0.10, P = 0.99. —, no variation over time.
Contrary to predictions that fishing effort and catch per unit effort would decline as physical capacity was reduced by poor physical and mental health, we found limited evidence for any association between fishing effort and increasing morbidity levels (Fig. 2 and Table S5). Among only individuals currently fishing (63–67% of all individuals and 91–95% of fishers), there was no effect of mental or physical health on the total hours fished per month, nights spent away, or income per hour fishing and a modest effect on the travel time to fish (Table S5). Although high variance in these effort variables may mask a relationship, the magnitude of coefficients associated with the type of fishing suggests that, rather than being associated with illness, the number of hours spent fishing was largely driven by the choice of fishing methods.
Table S5.
Longitudinal fixed and random effects regression models with robust SEs of metrics of fishing effort among individuals who reported fishing in the preceding 3 mo
| Variable | Hours fishing per month | Travel hours per month | Nights spent away per month | Fishing income per hour fishing | ||||||||
| Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | Coefficient | SE | P value | |
| Fixed effects models | ||||||||||||
| Observations, groups | 545, 204 | 545, 204 | 545, 204 | 545, 204 | ||||||||
| Physical health | 12.79 | 7.89 | 0.11 | −3.41 | 2.99 | 0.26 | −0.97 | 0.56 | 0.08 | 62.55 | 89.87 | 0.49 |
| Mental health | 1.45 | 7.91 | 0.18 | 13.80 | 4.60 | 0.00 | 0.78 | 0.65 | 0.22 | −32.30 | 91.26 | 0.72 |
| Laborer: self-employed and hiring labor | 8.55 | 16.17 | 0.60 | 7.73 | 12.01 | 0.52 | 0.88 | 1.39 | 0.53 | 582.02 | 352.76 | 0.10 |
| Comparison: hiring labor | 15.47 | 22.40 | 0.49 | −3.55 | 7.44 | 0.63 | −1.80 | 1.64 | 0.28 | 560.97 | 382.81 | 0.14 |
| Time point (baseline comparison) | ||||||||||||
| 3 | −17.32 | 12.24 | 0.16 | −6.47 | 3.86 | 0.09 | 0.72 | 0.95 | 0.45 | 85.42 | 225.92 | 0.71 |
| 6 | −2.44 | 12.89 | 0.85 | 6.32 | 6.05 | 0.30 | 0.61 | 0.88 | 0.49 | −180.61 | 120.08 | 0.13 |
| 12 | 16.63 | 15.91 | 0.30 | −0.28 | 4.74 | 0.95 | 0.03 | 0.97 | 0.98 | −85.53 | 158.58 | 0.59 |
| Net income (log) | −0.50 | 7.22 | 0.53 | −4.98 | 3.58 | 0.16 | 0.36 | 0.49 | 0.47 | 508.44 | 146.14 | 0.00 |
| Fishing activities | ||||||||||||
| Monofilament | 140.60 | 30.47 | 0.00 | 31.30 | 6.43 | 0.00 | 0.92 | 1.38 | 0.51 | −402.96 | 148.64 | 0.01 |
| Beach seine | 60.35 | 25.98 | 0.02 | 39.23 | 11.06 | 0.00 | −0.48 | 1.10 | 0.66 | −178.99 | 146.86 | 0.22 |
| Gillnet | 143.47 | 21.44 | 0.00 | 60.00 | 16.79 | 0.00 | 1.65 | 1.12 | 0.14 | −506.35 | 105.74 | 0.00 |
| Longline | 98.32 | 30.93 | 0.00 | 47.23 | 11.27 | 0.00 | −1.57 | 2.29 | 0.49 | −525.00 | 200.86 | 0.01 |
| Small seine | 130.42 | 21.13 | 0.00 | 43.71 | 18.65 | 0.02 | 5.34 | 1.75 | 0.00 | −732.41 | 146.86 | 0.00 |
| Other | 141.87 | 21.84 | 0.00 | 56.64 | 12.09 | 0.00 | 1.75 | 1.66 | 0.29 | −471.61 | 1,424.83 | 0.01 |
| Random effects models | ||||||||||||
| Observations, groups | 545, 204 | 545, 204 | 545, 204 | 542, 204 | ||||||||
| Physical health | 3.61 | 5.69 | 0.53 | −3.65 | 2.93 | 0.21 | −0.68 | 0.42 | 0.10 | −51.31 | 89.87 | 0.49 |
| Mental health | −0.56 | 5.26 | 0.92 | 9.29 | 2.98 | 0.00 | 0.91 | 0.46 | 0.05 | −32.30 | 91.26 | 0.72 |
| Laborer: self-employed and hiring labor | −2.54 | 8.93 | 0.78 | 13.13 | 8.70 | 0.13 | 0.24 | 0.86 | 0.78 | 582.02 | 352.76 | 0.10 |
| Comparison: hiring labor | 5.22 | 14.12 | 0.71 | 2.40 | 4.64 | 0.61 | −3.27 | 1.21 | 0.01 | 560.97 | 382.81 | 0.14 |
| Time point (baseline comparison) | ||||||||||||
| 3 | −12.76 | 11.00 | 0.25 | −8.49 | 3.80 | 0.03 | 0.58 | 0.82 | 0.48 | 85.42 | 225.92 | 0.71 |
| 6 | 1.87 | 10.83 | 0.86 | 4.29 | 5.29 | 0.42 | 0.51 | 0.78 | 0.52 | −180.61 | 120.08 | 0.13 |
| 12 | 16.56 | 13.81 | 0.23 | −2.13 | 3.98 | 0.59 | 0.12 | 0.86 | 0.89 | −85.53 | 158.58 | 0.59 |
| Net income (log) | 4.10 | 4.18 | 0.33 | −3.86 | 2.91 | 0.18 | 0.50 | 0.29 | 0.09 | 508.44 | 146.14 | 0.00 |
| Fishing activities | ||||||||||||
| Monofilament | 129.65 | 19.27 | 0.00 | 36.46 | 8.71 | 0.00 | −0.28 | 1.09 | 0.80 | −402.96 | 148.64 | 0.01 |
| Beach seine | 70.51 | 12.64 | 0.00 | 40.24 | 11.31 | 0.00 | −1.96 | 0.93 | 0.04 | −178.99 | 146.86 | 0.22 |
| Gillnet | 144.35 | 14.95 | 0.00 | 53.84 | 12.82 | 0.00 | 1.02 | 1.00 | 0.31 | −506.35 | 105.74 | 0.00 |
| Longline | 119.47 | 19.91 | 0.00 | 50.25 | 10.29 | 0.00 | −2.22 | 1.33 | 0.09 | −525.00 | 200.86 | 0.01 |
| Small seine | 148.58 | 14.14 | 0.00 | 47.03 | 11.98 | 0.00 | 7.30 | 1.06 | 0.00 | −732.41 | 146.86 | 0.00 |
| Other | 154.04 | 19.07 | 0.00 | 68.96 | 15.31 | 0.00 | 2.98 | 1.25 | 0.02 | −471.61 | 1,424.83 | 0.01 |
Both sets of models show that fishing method rather than poorer mental and physical health drives fishing effort.
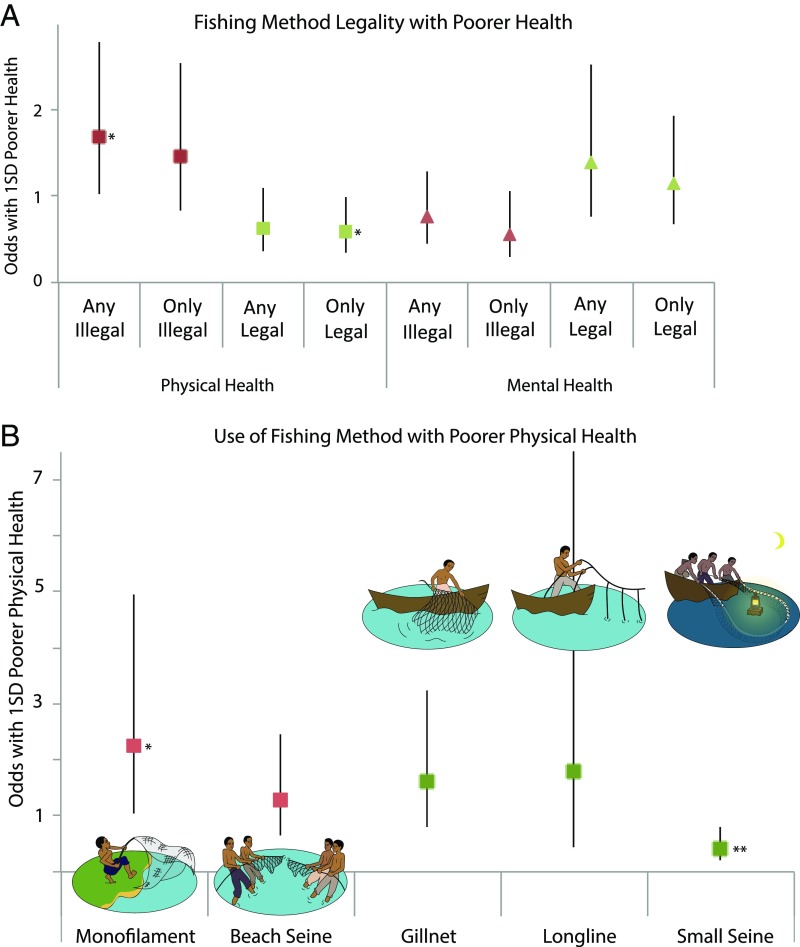
We next tested if fishers responded to poor health by changing their fishing methods. We predicted that, when ill, fishers would select inshore fishing methods that demand less travel and by targeting smaller fish, are physically easier. We grouped methods by their legality and targeted fisheries to compare legal offshore methods and illegal inshore methods. With poorer physical health, fishers were 43% less likely to use only legal methods targeting offshore fisheries (OR = 0.57, SE = 0.15, P < 0.03) and 69% more likely to use any illegal method focused on inshore fishing areas (OR = 1.69, SE = 0.43, P < 0.04) (Fig. 3 and Table S6). Physical health as opposed to mental health or a combination drove this relationship.
Fig. 3.
ORs of (A) fishing method legality and (B) fishing with a particular method. ORs for poorer physical health (A, B) and poorer mental health (A) and 95% confidence intervals (vertical lines) are derived from full conditional fixed effect logit models (Tables S6 and S7) among individuals who report participation in fishing in the preceding 3 mo. In the Lake Victoria fishery, five fishing methods predominate. Three methods—long line, gillnet, and small seine for dagaa—are legal offshore methods (green) and subject to regulations designed to provide for fishery sustainability, including mesh dimensions, hook sizes, and movement patterns. Two methods—beach seine and monofilament—are focused on inshore regions (red), and although outlawed by fishery regulations because they are environmentally destructive, they remain widely practiced (51). Odds are for 1 SD poorer (A, Left) physical health and (A, Right) mental health as well as (B) physical health comparing across individuals who sometimes participated in fishing methods. Models include a full set of time-variant covariates (physical health, mental health, income, fishery role, and time point). Where the 95% confidence intervals cross one, the association is not statistically significant. *P < 0.05, **P < 0.01.
Table S6.
Model results for conditional fixed effect logit and random effects logit models for participation in one or more fishing activities that are only legal, any legal, only illegal, and any illegal
| Variable | Only legal | Any legal | Only illegal | Any illegal | ||||||||
| OR | SE | P value | OR | SE | P value | OR | SE | P value | OR | SE | P value | |
| Fixed effects | ||||||||||||
| Observations, groups | 222, 66 | 189, 59 | 178, 56 | 218, 65 | ||||||||
| Physical health | 0.57 | 0.15 | 0.03 | 0.62 | 0.17 | 0.09 | 1.50 | 0.42 | 0.15 | 1.69 | 0.43 | 0.04 |
| Mental health | 1.16 | 0.31 | 0.57 | 1.38 | 0.51 | 0.29 | 0.61 | 0.19 | 0.11 | 0.78 | 0.20 | 0.35 |
| Fishing income (log) | 1.20 | 0.24 | 0.35 | 1.72 | 0.37 | 0.01 | 0.54 | 0.11 | 0.01 | 0.81 | 0.16 | 0.26 |
| Laborer: self-employed and hiring labor | 0.34 | 0.18 | 0.05 | 0.61 | 0.36 | 0.40 | 1.49 | 0.87 | 0.49 | 2.95 | 1.60 | 0.05 |
| Comparison: hiring labor | 0.51 | 0.36 | 0.33 | 2.68 | 2.22 | 0.23 | 0.41 | 0.35 | 0.30 | 2.53 | 1.79 | 0.19 |
| Time point (baseline comparison) | ||||||||||||
| 3 | 1.70 | 0.65 | 0.16 | 2.08 | 0.93 | 0.10 | 0.56 | 0.26 | 0.22 | 0.71 | 0.27 | 0.37 |
| 6 | 1.22 | 0.49 | 0.62 | 1.89 | 0.87 | 0.16 | 0.64 | 0.30 | 0.34 | 1.00 | 0.40 | 0.99 |
| 12 | 1.11 | 0.45 | 0.79 | 1.06 | 0.46 | 0.90 | 1.34 | 0.60 | 0.52 | 1.22 | 0.51 | 0.63 |
| Random effects | ||||||||||||
| Observations, groups | 545, 204 | 545, 204 | 545, 204 | 545, 204 | ||||||||
| Physical health | 0.64 | 0.13 | 0.03 | 0.69 | 0.15 | 0.08 | 1.46 | 0.33 | 0.09 | 1.54 | 0.32 | 0.04 |
| Mental health | 1.24 | 0.26 | 0.30 | 1.24 | 0.28 | 0.34 | 0.75 | 0.18 | 0.23 | 0.77 | 0.16 | 0.21 |
| Fishing income (log) | 1.37 | 0.21 | 0.04 | 2.11 | 0.35 | 0.00 | 0.44 | 0.08 | 0.00 | 0.70 | 0.11 | 0.02 |
| Laborer: self-employed and hiring labor | 0.69 | 0.28 | 0.36 | 0.87 | 0.37 | 0.74 | 1.21 | 0.53 | 0.67 | 1.54 | 0.62 | 0.28 |
| Comparison: hiring labor | 1.62 | 0.81 | 0.33 | 4.27 | 2.56 | 0.01 | 0.24 | 0.15 | 0.02 | 0.72 | 0.36 | 0.52 |
| Time point (baseline comparison) | ||||||||||||
| 3 | 1.71 | 0.59 | 0.12 | 1.76 | 0.67 | 0.13 | 0.75 | 0.29 | 0.47 | 0.75 | 0.26 | 0.41 |
| 6 | 1.52 | 0.54 | 0.24 | 2.19 | 0.87 | 0.05 | 0.59 | 0.24 | 0.21 | 0.84 | 0.30 | 0.62 |
| 12 | 1.43 | 0.53 | 0.33 | 1.18 | 0.57 | 0.68 | 1.25 | 0.52 | 0.58 | 0.96 | 0.36 | 0.92 |
Results include only individuals who report that they were fishing in the preceding 3 mo. Hausman tests: only legal, χ2 = 9.67, P = 0.29; any legal, χ2 = 15.92, P = 0.04; only illegal, χ2 = 22.46, P = 0.00; any illegal, χ2 = 11.51, P = 0.17.
In analyzing the role of physical health in individual fishing methods (e.g., beach seine or gillnet), we found a pattern of results further indicating that fishers coped with illness through fishing method selection (Fig. 3 and Table S7). The inshore methods chosen by fishers when physically ill require less travel, have relatively lower physical demands, and are outlawed by fishing regulations. In contrast, methods used by physically healthy fishers require fishers to reach deep water or fish overnight to target the more sustainable mature Nile perch and dagaa fisheries. Because of the wide diversity of combinations of methods that fishers use, effects were estimated using multiple models. For relatively less common methods, the number of fishers that switched in and out of a given method was small, and we thus compared fixed effect conditional logit models with random effect models, which yielded similar ORs (Table S7).
Table S7.
Model results for conditional fixed effect logit and random effects logit models for participation in particular fishing activities among individuals who report fishing in the preceding 3 mo
| Variable | Monofilament | Beach seine | Gillnet | Long line | Small seine | ||||||||||
| OR | SE | P value | OR | SE | P value | OR | SE | P value | OR | SE | P value | OR | SE | P value | |
| Fixed effects | |||||||||||||||
| Observations, groups | 120, 37 | 132, 39 | 157, 50 | 55, 17 | 151, 45 | ||||||||||
| Physical health | 2.32 | 0.93 | 0.04 | 1.21 | 0.39 | 0.55 | 1.53 | 0.53 | 0.22 | 1.61 | 1.00 | 0.44 | 0.39 | 0.13 | 0.00 |
| Mental health | 1.01 | 0.41 | 0.97 | 0.79 | 0.26 | 0.48 | 1.18 | 0.35 | 0.57 | 0.98 | 0.56 | 0.98 | 0.93 | 0.36 | 0.86 |
| Fishing income (log) | 1.16 | 0.31 | 0.57 | 0.65 | 0.16 | 0.09 | 0.85 | 0.18 | 0.45 | 1.35 | 0.58 | 0.48 | 1.85 | 0.51 | 0.02 |
| Time point (baseline) | |||||||||||||||
| 3 | 1.20 | 0.074 | 0.77 | 0.48 | 0.25 | 0.16 | 0.80 | 0.35 | 0.60 | 0.87 | 0.71 | 0.86 | 3.67 | 2.16 | 0.03 |
| 6 | 2.50 | 1.64 | 0.16 | 0.49 | 0.27 | 0.19 | 0.98 | 0.44 | 0.97 | 0.44 | 0.36 | 0.32 | 3.69 | 2.05 | 0.02 |
| 12 | 3.44 | 2.38 | 0.08 | 0.56 | 0.31 | 0.29 | 1.08 | 0.52 | 0.88 | 0.58 | 0.45 | 0.48 | 2.03 | 1.17 | 0.22 |
| Laborer (comparison) | |||||||||||||||
| Self-employed and hiring labor | 11.69 | 9.83 | 0.00 | 0.40 | 0.33 | 0.27 | 1.72 | 0.96 | 0.33 | 0.45 | 0.45 | 0.43 | 0.62 | 0.49 | 0.55 |
| Hiring labor | 11.46 | 11.84 | 0.02 | 0.46 | 0.47 | 0.45 | 2.28 | 0.99 | 0.75 | 1.36 | 1.70 | 0.80 | 6.43 | 6.06 | 0.05 |
| Random effects | |||||||||||||||
| Observations, groups | 545, 204 | 545, 204 | 545, 204 | 545, 204 | 545, 204 | ||||||||||
| Physical health | 1.59 | 0.43 | 0.09 | 1.43 | 0.40 | 0.19 | 1.06 | 0.27 | 0.81 | 0.93 | 0.39 | 0.86 | 0.51 | 0.15 | 0.02 |
| Mental health | 0.67 | 0.19 | 0.15 | 0.83 | 0.25 | 0.53 | 1.34 | 0.34 | 0.25 | 1.03 | 0.42 | 0.95 | 0.77 | 0.23 | 0.38 |
| Fishing expenditures (log) | 0.89 | 0.16 | 0.52 | 0.59 | 0.13 | 0.02 | 0.98 | 0.17 | 0.93 | 1.79 | 0.56 | 0.07 | 2.48 | 0.56 | 0.00 |
| Time point (baseline) | |||||||||||||||
| 3 | 0.60 | 0.29 | 0.28 | 0.57 | 0.27 | 0.23 | 0.80 | 0.32 | 0.58 | 0.89 | 0.59 | 0.87 | 3.12 | 1.54 | 0.02 |
| 6 | 0.83 | 0.40 | 0.69 | 0.55 | 0.27 | 0.22 | 1.01 | 0.42 | 0.98 | 0.51 | 0.37 | 0.35 | 3.46 | 1.75 | 0.01 |
| 12 | 0.95 | 0.48 | 0.92 | 0.60 | 0.31 | 0.32 | 1.04 | 0.46 | 0.93 | 0.62 | 0.44 | 0.50 | 1.72 | 0.91 | 0.31 |
| Laborer (comparison) | |||||||||||||||
| Self-employed and hiring labor | 3.22 | 1.62 | 0.02 | 0.46 | 0.28 | 0.21 | 1.74 | 0.84 | 0.25 | 0.62 | 0.47 | 0.43 | 0.56 | 0.32 | 0.31 |
| Hiring labor | 1.44 | 0.94 | 0.58 | 0.25 | 0.19 | 0.07 | 2.55 | 1.55 | 0.13 | 0.92 | 0.93 | 0.92 | 4.58 | 3.24 | 0.03 |
Monofilament and beach seining are illegal, and there are gear restrictions on other fishing methods. Hausman tests: monofilament, χ2 = 12.71, P = 0.12; beach seine, χ2 = 266.85, P = 0.00; gillnet, χ2 = 2.05, P = 0.98; long line, χ2 = 0.97, P = 0.99, small seine, χ2 = 8.59, P = 0.38.
Links between human population growth and pressure on natural resources have shaped assumptions that human illnesses may indirectly benefit the environment by reducing the capacity of harvesters to effectively harvest resources (4). Contrary to findings of previous studies in agricultural systems (5–9), our analyses provided little evidence that illness altered fishing effort to indirectly benefit the environment. We found only limited evidence for the pathway highlighting environmental benefits, and in fact, our results support an alternative conclusion: physical health shifted the strategy and sustainability of fishers’ use of natural resources (Fig. 1). These findings underscore the complexity of the relationship between human health and use of natural resources.
Our findings have implications for the sustainability of the Lake Victoria fisheries. In driving selection for and against particular methods, in this setting, physical health may play a role in concentrating fishers in inshore areas and fostering the uptake of illegal fishing practices widely identified as destructive (25). For example, beach seines have long been implicated in the depletion of inshore Lake Victoria fisheries (26). These methods along with monofilament nets capture a high proportion of juvenile fish and disturb shallow fish breeding habitats (26). The limited duration of our study left us unable to quantify the potential longer-term effects of illness on dynamics of resource use, disentangle the importance of illness in driving illegal fishing relative to other motivations, or measure the ecological implications of fishing practices.
Ultimately, the reliance of ill fishers on illegal harvest practices will be mediated by local regulations and their enforcement. Around Lake Victoria, enforcement of fishery regulations is notoriously weak (25). Critically, our findings suggest that fishing regulations may particularly impact the sick. Although physical rather than mental health drove the impacts that we observed, altered outlooks, planning, and regulation observance may also accompany poor physical health. A community reliant on local enforcement through comanagement may mirror choices made by individuals and may have less community planning for or attention to environmental sustainability. Comanagers may even permit environmentally destructive practices among the sick, a scenario with analogs to tradeoffs made by fishers in coastal Kenyan communities (27).
We measured only one aspect of how illness may alter the environment, although illness may impact not only fisheries and fishers but also, Lake Victoria communities and women. Negative impacts could occur when family members of fishers who die from illness turn to the environment as a safety net or when ecological knowledge of sustainable practices is lost, driving cross-generational changes in environmental engagement. Men are the predominant harvesters of fish, but women play a substantial role in processing and trading fish around Lake Victoria. Women’s fish access, migration, and HIV risk are also affected by reliance on the same natural resource (28–30). In some cases, women may access fish through transactional fish for sex exchanges, which are shaped by fish availability and exacerbate HIV risk (31). Family members’ engagement of alternative livelihoods may also stem from illness, although with uncertain environmental consequences. For example, although reliance on agriculture may relieve pressure on fisheries, deforestation and farming on steep slopes in the region may also have negative environmental and hydrological consequences. Moreover, the long time horizon to harvest for agricultural crops relative to fish may shape the ways illness impacts engagement with natural resources.
Our work was conducted in an HIV-affected community, and much of the literature on illness and labor dynamics focuses on HIV/AIDS. Although time horizons and discounting may differ in individuals who anticipate a shortened lifespan, the physical experience of frequent illness is not unique to individuals living with HIV/AIDS. Social and economic consequences of illnesses ranging from malaria to tuberculosis are substantial in many regions, especially sub-Saharan Africa (32). The extent of all-cause morbidity in adults in developing countries is relatively unknown; although contemporaneous information is unavailable, a study of self-reported morbidity found that, staggeringly, Kenyan men, nonpregnant women, and pregnant women were ill 21, 38, and 41% of days, respectively (33). Although the particular etiology, associated stigma, and psychological effects of HIV are relevant to the impact of illness on the environment, we contend that the chronic experience of illness faced by many communities may have environmental implications independent of a specific disease.
Meaningfully addressing the health of people, particularly adults, who rely on natural resources may be important for ensuring environmental sustainability. Coupled models that integrate ecological processes with infectious diseases and economic development provide promising approaches to extend understanding in these systems (34). Meeting basic needs of healthcare and medications will also shape the physical effort and long-term investment that people bring to their livelihoods in ways that do not only increase the pressure that they exert on the environment. As such, institutions and organizations focused on protecting the environment may need to more deeply consider the health of communities.
Our study emphasizes the importance of considering health, governance, and ecosystems through an integrative lens. We focus on links between health and environmental use, and the integration with governance is equally important. Improving environmental governance remains a key challenge for protected areas, forests, fisheries, and other resources (1), and implementing sustainable harvest practices, conservation efforts, and community-based resource management often falls to communities dependent on natural resources for food and income. The loss of natural resources invariably affects these communities first and most severely. Although promising governance regimes may incentivize resource stewardship and adjust property rights, where these mechanisms fail to address the vulnerabilities that resource users face, these strategies are likely insufficient (35). Fisher vulnerability—and thereby resource vulnerability—may be closely related to the health of resource users. This finding is potentially relevant to temporally similar forms of resource harvest, including hunting, harvesting forest products, and burning charcoal, and may have additional analogs to agriculture.
Our findings on human–environment health linkages in East Africa highlight the need for critical reevaluation of the longstanding assumption that disease provides a natural check to human overexploitation of the environment. Although evidence from agricultural systems suggests that widespread illness may reduce food production (8, 9), we find that illness may also alter resource use and the sustainability of harvest practices. We must disentangle these complex feedback systems to better address the linked fates of human health and environmental sustainability.
Methods
Study Site.
We conducted this study on Mfangano Island located within Homa Bay County in Nyanza Province, Kenya. A continental island in Lake Victoria, Mfangano Island is ∼65 km2 and home to 21,000 people (36). Fishery involvement for trade and subsistence is widespread, and Mfangano Island has limited health infrastructure and electricity, and no running water or paved roads. Food insecurity is common throughout Mfangano Island and ubiquitous among the 27% of adults living with HIV/AIDS (20, 37–39).
Survey Methods.
From December of 2012 to March of 2014, we collected data at four time points: baseline and 3, 6, and 12 mo. Data are from 303 participant households randomly selected from an enumerated sampling list of all households with a child <2 y of age and living on Mfangano Island, Kenya. We present data from adult male respondents, who were initially present in 253 (83%) households. Local enumerators conducted surveys in Dholuo, the local language. We obtained written consent from study participants before enrollment. The study protocol was reviewed and approved by the University of California, Berkeley Committee on Human Research and the Ethical Review Committee of the Kenya Medical Research Institute.
We created surveys through a compilation of validated measures and adaptations. Fishing activities surveys were created based on Lake Victoria Frame Surveys documenting regional fishing methods (40). Fishers reported fishing methods, role, region, and income over the preceding 3 mo. Although it is possible that illegal and destructive fishing methods were underreported, we aimed to minimize bias by conducting interviews in private and away from fish landing sites; the relative commonality of illegal methods in practice and our results suggest that underreporting is not common.
The MOS-HIV was used to assess health-related quality of life (23, 24). The MOS-HIV has been used in hundreds of studies around the world, adapted for use in East Africa (41, 42), and used to assess health in HIV-affected populations (43). The MOS-HIV has also been validated against metrics of disease progression (e.g., WHO stage and treatment adherence), mental health (e.g., Center for Epidemiological Depression Scale), and physical health [e.g., CD4 count and Karnofsky Performance Status (23, 44)] and is highly correlated to other health questionnaires [e.g., Quality of Well-Being Scale and Short Form Health Survey-12 (45, 46)]. We used a modified version of the scale designed for use in East Africa (41, 42). The MOS-HIV is a 35-question scale measuring self-reported health-related quality of life designed to assess 11 dimensions of health: general health perceptions, pain, physical functioning, social functioning, role functioning, mental health, energy/fatigue, cognitive function, health distress, quality of life, and health transition. Raw scores were tallied and transformed to a 0–100 scale (Table S1). Previous analyses identified a two-factor structure that provides for mental and physical health summary scores (44). Mental and physical health subscales have been shown to be reliable and valid in a high-income reference population [Roche reference (44)] as well as with an East African reference population (41). We calculated mental and physical health summary scores using each of these reference populations as well as with Z scores derived from the reference and our population; resulting scores are all highly correlated (Table S2), and we used the Roche reference weights and Z scores in subsequent analyses because of relative similarities in subscale averages with our population and the potential for broad comparison based on their wider use. Importantly, mental and physical health summary scores are independent of disease status; for example, an individual with HIV that is well-controlled by antiretroviral therapy may register low morbidity.
We did not find evidence of differences in participation in or time spent fishing related to mental and physical health, although there may be a small survival bias (n = 4 fishers died during our study; 1.5%). However, fishing incomes remained relatively high, and migration to the lakeshore was substantial, with fishing-crew populations fluctuating up to 50% (47, 48). Thus, individual deaths may not have an aggregate effect on fishing pressure when fluctuation in fisher populations is substantial relative to even the region’s high death rate. Similar conclusions about the unlikeliness of fisher deaths reducing fishing pressure have been reached in research considering the impact of HIV on fisheries across Africa (14).
Statistical Methods.
We examined the effects of mental and physical health on whether individuals are fishing, legality of fishing methods, and specific types of fishing activities using conditional fixed effects logit models (49, 50). Our regression model was as follows:
| [1] |
where cit is a binary variable equal to one if participant i is engaged in that particular kind of fishing at time t and zero if participant i is not engaged in that particular kind of fishing at time t; αi is an individual-specific effect that accounts for all characteristics specific to individual i, and δt is a time effect that accounts for all variation within a given time t. Mit denotes our morbidity measure, and the variable is centered at 0 and normalized, such that 1 and −1 are 1 SD away from the mean (Fig. S1). Xit is a vector of control variables, and εit is an error term.
The model thus estimated within-individual differences across time points by using the individual as a control for himself in estimating the effect of the predictor variables when the outcome was present compared with the time points when the outcome was not present. The approach controlled for individuals’ time-invariant characteristics (e.g., sex, ethnicity, fishing preferences, and history with particular methods, etc.) both observed and unobserved. In comparing the relative difference in a participant’s responses to the MOS-HIV questionnaire, we thus also accounted for individual differences in reporting on the scale. Moreover, comparisons of within-individual differences minimized the potential effects of survival bias, because individuals who die are still included during the time points in which they are surveyed. We transformed the estimated coefficients to ORs and similarly transformed SEs and 95% confidence intervals. All models also included time-variant covariates that may alter selection of fishing methods: fishing net income, fishery role (e.g., laborer or gear owner), and season.
Fishing methods were not exclusive, meaning that fishers often participated in more than one type of fishing and switched fishing methods often. Because there was substantial variation in methods that were paired, we were unable to concurrently estimate engagement in fishing activities with a multinomial model. Our conditional fixed effect logit models included individuals who sometimes used a particular fishing method at any of four time points to estimate within-individual differences. The models did not include individuals who always or never used a particular method, because differences in behavior are necessary to estimate ORs. We also conducted less restrictive random effects logit models that did not exclude individuals who never or always participated in fishing or a particular fishing method and compared results using Hausman tests (Tables S4–S7). Although only some of the Hausman tests rejected the random effects model at the 10% level, they showed relatively similar effect sizes. We present results from conditional fixed effect models in the text and figures.
We used longitudinal regression models to examine fishing effort represented using continuous measures of hours that fishers fished per month, income per hour fishing, nights spent away, and hours traveled to fish and similarly compared fixed and random effects models (Table S5). We divided our physical and mental health summary scores into quintiles to calculate the hours and days spent fishing by individuals experiencing different levels of illness to visualize changing effort across morbidity quintiles (Fig. 2), although we used the aforementioned controlled regression models to assess associations between these continuous variables.
Acknowledgments
We thank C. Barrett, H. Bodwitch, K. Gaynor, and the Barrett group for comments on earlier versions of this manuscript and M. Potts and L. Fortmann for feedback in shaping this study. We also thank the Ekialo Kiona Center, Organic Health Response, Kenya Ministry of Fisheries Development, Kenya Medical Research Institute, and the Mfangano Island community. National Science Foundation Graduate Research Fellowship Program, National Science Foundation Doctoral Dissertation Research Improvement grant, Cornell’s Atkinson Center (K.J.F.), and National Science Foundation Coupled Human and Natural Systems Grant 115057 (to J.S.B.) supported this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613260114/-/DCSupplemental.
References
- 1.IPCC . In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Field CB, et al., editors. Cambridge Univ Press; Cambridge, UK and New York: 2014. [Google Scholar]
- 2.Myers SS, et al. Human health impacts of ecosystem alteration. Proc Natl Acad Sci USA. 2013;110(47):18753–18760. doi: 10.1073/pnas.1218656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P. Human Well-Being and the Natural Environment. Oxford Univ Press; New York: 2001. [Google Scholar]
- 4.Malthus TR. An Essay on the Principle of Population. J. Johnson; London: 1798. [Google Scholar]
- 5.Fox MP, et al. The impact of HIV/AIDS on labour productivity in Kenya. Trop Med Int Health. 2004;9(3):318–324. doi: 10.1111/j.1365-3156.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- 6.Byrne R. AIDS in Zambia, the effect on subsistence farmers. Geogr Rev. 2002;15:29–31. [Google Scholar]
- 7.Nguthi FN, Niehof A. Effects of HIV/AIDS on the livelihood of banana-farming households in Central Kenya. NJAS Wagening J Life Sci. 2008;56(3):179–190. [Google Scholar]
- 8.de Waal A, Whiteside A. New variant famine: AIDS and food crisis in southern Africa. Lancet. 2003;362(9391):1234–1237. doi: 10.1016/S0140-6736(03)14548-5. [DOI] [PubMed] [Google Scholar]
- 9.Mason NM, Jayne TS, Chapoto A, Myers RJ. A test of the new variant famine hypothesis: Panel survey evidence from Zambia. World Dev. 2010;38(3):356–368. [Google Scholar]
- 10.Talman A, Bolton S, Walson JL. Interactions between HIV/AIDS and the environment: Toward a syndemic framework. Am J Public Health. 2013;103(2):253–261. doi: 10.2105/AJPH.2012.300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter LM, Twine W, Patterson L. “Locusts are now our beef”: Adult mortality and household dietary use of local environmental resources in rural South Africa. Scand J Public Health Suppl. 2007;69:165–174. doi: 10.1080/14034950701356385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challe JFX, Struik PC. The impact on orchid species abundance of gathering their edible tubers by HIV/AIDS orphans: A case of three villages in the Southern Highlands of Tanzania. NJAS Wagening J Life Sci. 2008;56(3):261–279. [Google Scholar]
- 13.Damon M, Zivin JG, Thirumurthy H. Health shocks and natural resource management: Evidence from Western Kenya. J Environ Econ Manage. 2015;69:36–52. doi: 10.1016/j.jeem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison EH, Seeley JA. HIV and AIDS among fisherfolk: A threat to ‘responsible fisheries’? Fish Fish (Oxf) 2004;5:214–234. [Google Scholar]
- 15.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211(4489):1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 16.Taabu-Munyaho A, et al. Spatial and temporal variation in the distribution and density of pelagic fish species in Lake Victoria, East Africa. Aquat Ecosyst Health Manag. 2014;17(1):52–61. [Google Scholar]
- 17.Omwoma S, et al. Declining commercial fish catches in Lake Victoria’s Winam Gulf: The importance of restructuring Kenya’s aquaculture programme. Lakes Reservoirs: Res Manage. 2014;19(3):206–210. [Google Scholar]
- 18.Geheb K, Binns T. ‘Fishing farmers’ or ‘farming fishermen’? The quest for household income and nutritional security on the Kenyan shores of Lake Victoria. Afr Aff (Lond) 1997;96(382):73–93. [Google Scholar]
- 19.Balirwa JS. Ecological, environmental and socioeconomic aspects of the Lake Victoria’s introduced Nile perch fishery in relation to the native fisheries and the species culture potential: Lessons to learn. Afr J Ecol. 2007;45(2):120–129. [Google Scholar]
- 20.Kenya Ministry of Health . Kenya County HIV Service Delivery Profiles. Ministry of Health; Nairobi, Republic of Kenya: 2013. [Google Scholar]
- 21.Allison EH, Seeley JA. Another group at high risk for HIV. Science. 2004;305(5687):1104. doi: 10.1126/science.305.5687.1104b. [DOI] [PubMed] [Google Scholar]
- 22.FAO 2012. The State of World Fisheries and Aquaculture, ed Fisheries and Aquaculture Department (Food and Agriculture Organization of the United Nations, Rome)
- 23.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6(6):481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 24.Wu AW, Hays RD, Kelly S, Malitz F, Bozzette SA. Applications of the Medical Outcomes Study health-related quality of life measures in HIV/AIDS. Qual Life Res. 1997;6(6):531–554. doi: 10.1023/a:1018460132567. [DOI] [PubMed] [Google Scholar]
- 25.Njiru M, Kazungu J, Ngugi CC, Gichuki J, Muhoozi L. An overview of the current status of Lake Victoria fishery: Opportunities, challenges and management strategies. Lakes Reservoirs: Res Manage. 2008;13:1–12. [Google Scholar]
- 26.Lake Victoria Fisheries Organization . State of Fish Stocks. Lake Victoria Fisheries Organization; Jinja, Uganda: 2012. [Google Scholar]
- 27.Daw TM, et al. Evaluating taboo trade-offs in ecosystems services and human well-being. Proc Natl Acad Sci USA. 2015;112(22):6949–6954. doi: 10.1073/pnas.1414900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camlin CS, Kwena ZA, Dworkin SL, Cohen CR, Bukusi EA. “She mixes her business”: HIV transmission and acquisition risks among female migrants in western Kenya. Soc Sci Med. 2014;102:146–156. doi: 10.1016/j.socscimed.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camlin CS, Kwena ZA, Dworkin SL. Jaboya vs. jakambi: Status, negotiation, and HIV risks among female migrants in the “sex for fish” economy in Nyanza Province, Kenya. AIDS Educ Prev. 2013;25(3):216–231. doi: 10.1521/aeap.2013.25.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mojola SA. Fishing in dangerous waters: Ecology, gender and economy in HIV risk. Soc Sci Med. 2011;72(2):149–156. doi: 10.1016/j.socscimed.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorella KJ, et al. Transactional fish-for-sex relationships amid declining fish access in Kenya. World Dev. 2015;74:323–332. [Google Scholar]
- 32.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray CJL, Feachem RGA, Phillips MA, Willis C. 1992. Adult morbidity: limited data and methodological uncertainty. The Health of Adults in the Developing World, World Bank Publication Series, eds Feachem RGA, Phillips MA, Kjellstrom T, Murray C, Over M (Oxford Univ Press, Oxford), pp 113–160.
- 34.Ngonghala CN, et al. Poverty, disease, and the ecology of complex systems. PLoS Biol. 2014;12(4):e1001827. doi: 10.1371/journal.pbio.1001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison EH, et al. Rights-based fisheries governance: From fishing rights to human rights. Fish Fish (Oxf) 2012;13:14–29. [Google Scholar]
- 36.Mbita Division . Population Projection. Nairobi, Government of Kenya; Kenya: 2009. [Google Scholar]
- 37.Fiorella KJ, et al. Fishing for food? Analyzing links between fishing livelihoods and food security around Lake Victoria, Kenya. Food Secur. 2014;6:1–10. doi: 10.1007/s12571-014-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata JM, et al. Socio-demographic and health associations with body mass index at the time of enrollment in HIV care in Nyanza Province, Kenya. AIDS Care. 2013;25(12):1491–1498. doi: 10.1080/09540121.2013.775399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata JM, et al. Around the table: Food insecurity, socio-economic status, and instrumental social support among women living in a rural Kenyan island community. Ecol Food Nutr. 2015;54(4):358–369. doi: 10.1080/03670244.2014.995790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lake Victoria Fisheries Organization . Regional Status Report on Lake Victoria Biennial Frame Surveys Between 2000 and 2012. Lake Victoria Fisheries Organization; Jinja, Uganda: 2013. Regional frame survey report; pp. 1–64. [Google Scholar]
- 41.Mast TC, et al. Measuring quality of life among HIV-infected women using a culturally adapted questionnaire in Rakai district, Uganda. AIDS Care. 2004;16(1):81–94. doi: 10.1080/09540120310001633994. [DOI] [PubMed] [Google Scholar]
- 42.Stangl AL, Bunnell R, Wamai N, Masaba H, Mermin J. Measuring quality of life in rural Uganda: Reliability and validity of summary scores from the medical outcomes study HIV health survey (MOS-HIV) Qual Life Res. 2012;21(9):1655–1663. doi: 10.1007/s11136-011-0075-5. [DOI] [PubMed] [Google Scholar]
- 43.McDonnell KA, Gielen AC, O’Campo P, Burke JG. Abuse, HIV status and health-related quality of life among a sample of HIV positive and HIV negative low income women. Qual Life Res. 2005;14(4):945–957. doi: 10.1007/s11136-004-3709-z. [DOI] [PubMed] [Google Scholar]
- 44.Revicki DA, Sorensen S, Wu AW. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36(2):126–137. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Copfer AE, et al. The use of two measures of health-related quality of life in HIV-infected individuals: A cross-sectional comparison. Qual Life Res. 1996;5(2):281–286. doi: 10.1007/BF00434750. [DOI] [PubMed] [Google Scholar]
- 46.Chariyalertsak S, et al. Reliability and validity of Thai versions of the MOS-HIV and SF-12 quality of life questionnaires in people living with HIV/AIDS. Health Qual Life Outcomes. 2011;9:15. doi: 10.1186/1477-7525-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunan F, et al. Finding space for participation: Fisherfolk mobility and co-management of Lake Victoria fisheries. Environ Manage. 2012;50(2):204–216. doi: 10.1007/s00267-012-9881-y. [DOI] [PubMed] [Google Scholar]
- 48.Nunan F. Mobility and fisherfolk livelihoods on Lake Victoria: Implications for vulnerability and risk. Geoforum. 2010;41:776–785. [Google Scholar]
- 49.Chamberlain G. Analysis of covariance with qualitative data. Rev Econ Stud. 1980;47:225–238. [Google Scholar]
- 50.Allison PD. Fixed Effects Regression Methods for Longitudinal Data. SAS Institute Inc.; Cary, NC: 2005. [Google Scholar]
- 51.Government of Kenya 2012. Fisheries (General) Regulations. In Cap. 378.