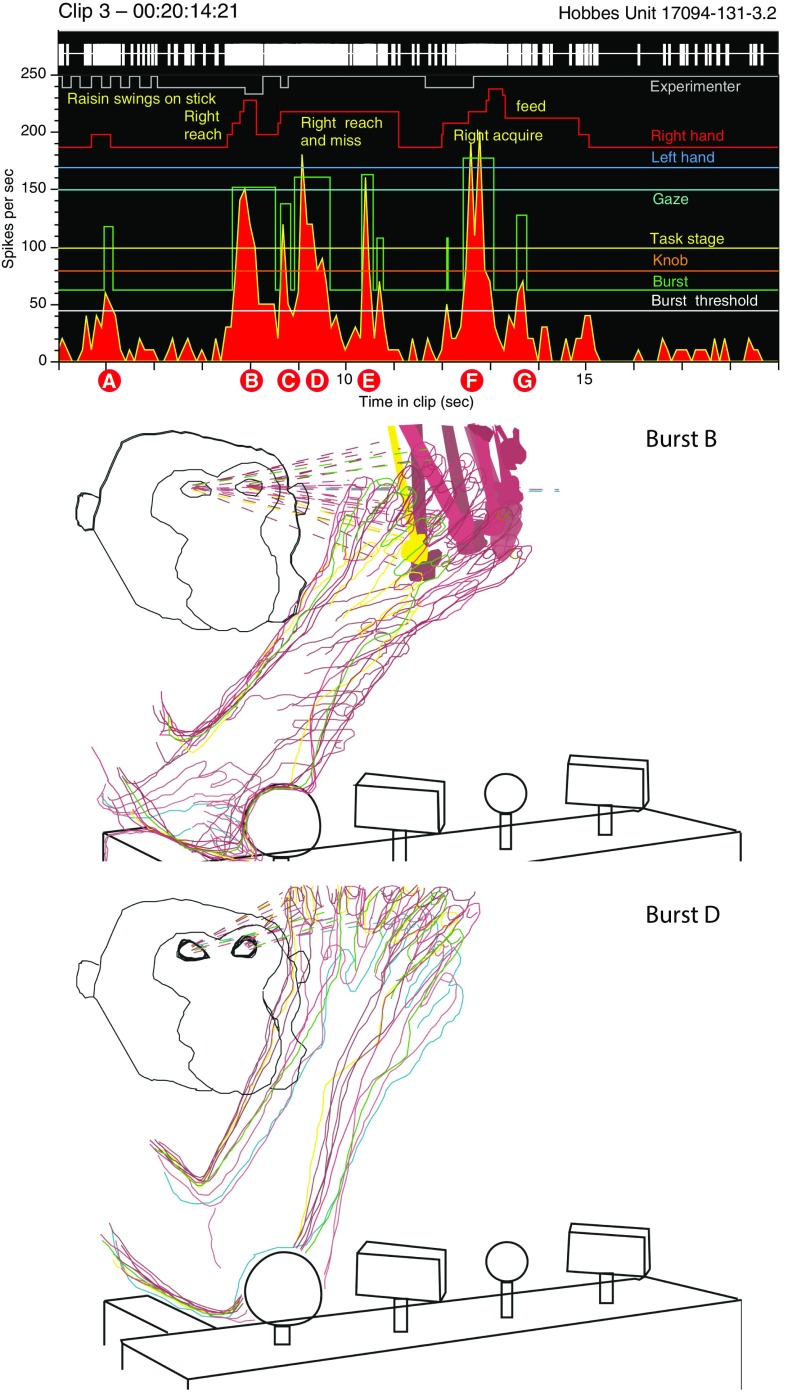
A piece of fruit—a raisin—swings on a stick in front of a monkey (Fig. 1). He likes raisins; he wants this one. He stretches out his arm, opens his hand with the fingers spread wide apart, and tries to capture it; he misses (bursts B and D). He tries again, this time successfully (burst F). He grabs the raisin, pulls it off the stick, brings it to his mouth, and eats it. Delicious! What happens in the brain when the animal performs these actions? The chart above the images in Fig. 1 tracks responses of a neuron recorded in lateral area 5 bordering the intraparietal sulcus, a subregion of the posterior parietal cortex (PPC) originally studied by Mountcastle et al. (1, 2), and described as a hand-manipulation “command neuron” engaged in purposeful actions of the hand: reaching and grasping an object of behavioral interest.
Fig. 1.
(Upper) Burst analysis of neural responses to spontaneous reaches and attempted grasps of a raisin on a stick moving through the workspace. The green burst trace marks intervals when firing rates are one SD greater than the mean during the 3-min analysis period. Firing rates are highest as the animal reaches toward the raisin and attempts to grasp it (bursts B, D, and F), and decay when the hand withdraws to a rest position. (Lower) Frame-by-frame tracings of the hand kinematics, gaze direction, and target location in digital video clips recorded simultaneously with neural responses. Same neuron as in figures 4–7 of Gardner et al. (30).
Mountcastle et al. (1) proposed that these regions of the PPC,
…receive afferent signals descriptive of the position and movement of the body in space, and contain a command apparatus for operation of the limbs, hands, and eyes within immediate extrapersonal space. This general command function is exercised in a holistic fashion. It relates to acts aimed at certain behavioral goals and not to the details of muscular contraction during execution. These details are, on this hypothesis, made precise by the motor system, for which it is well suited by virtue of its powerful mechanisms for specifying movement exactly.
However, no anatomical substrate was defined in that report beyond “the motor system.”
In PNAS, Rathelot et al. (3) use a state-of-the-art neuroanatomical pathway tracing to demonstrate a direct pathway from lateral area 5 to interneurons of the spinal cord, providing an efficient, rapid route for modulating hand and arm movements during goal-directed behaviors. These authors harnessed bacterial toxins and viruses to label the biological circuits that implement the command functions originating from the PPC, providing a wiring diagram that complements the traditional voluntary movement pathways from area 5 to corticomotoneuronal fiber tracts to motoneurons (4–13).
Rathelot et al. (3) take the reader, step-by-step, through the pathway from the PPC to action. First, they placed microelectrodes into lateral area 5, where Mountcastle et al. (1) had recorded “command” neurons, and evoked grasping type movements of the thumb and fingers following trains of electrical pulses delivered by the electrodes. Grasping movements were evoked from both the anterior bank of the intraparietal sulcus (area PEip), as well as the adjacent cortical surface caudal to the S1 hand area (area PE).
To determine the anatomical projection targets of these physiologically identified zones, Rathelot et al. (3) injected lateral area 5 with cholera-toxin subunit B (CTb), a highly sensitive anterograde tracer molecule. CTb uptake into neurons is mediated by absorptive endocytosis when it binds to monosialoglycoside GM1 in neuronal membranes, and is actively transported bidirectionally within the neuron, retrograde (back toward the cell soma and dendrites) and anterograde (forward along the axon to the synaptic terminals) (14, 15). Rathelot et al. (3) demonstrate that anterograde transport of CTb produces dense labeling of axon terminals in the medial dorsal horn of the spinal cord from C2 to T2. This region is populated by spinal interneurons that receive somatosensory input from mechanoreceptors in the hand (16); many of them also participate in reflex pathways to hand motoneurons. The descending corticospinal projection from area 5 is contralateral and excludes the ventral horn where motoneurons reside. The pathway from area 5 is disynaptic and does not excite motoneurons directly.
In a second series of experiments, Rathelot et al. (3) injected rabies virus into specific hand muscles to delineate the circuitry of anatomical projections to their spinal motoneurons. In previous studies, these authors and others demonstrated retrograde transneuronal transport of rabies virus from hand muscles to motoneuron pools of the spinal cord, and subsequently to corticomotoneuronal cells of the primary motor cortex (13, 14, 17–19). Multiple levels of retrograde transneuronal transport depend upon the survival time after viral injection. Rathelot et al. (3) demonstrate that short survival times labeled the cell somas of specific motoneurons as well as those of interneurons in laminae IV–VIII. Moreover, there was much overlap between the retrogradely virus-labeled interneurons projecting to specific hand motoneurons and the anterogradely labeled nerve terminals from the CTb injections from area 5. These data indicate that descending projections from area 5 modulate activity of motoneurons innervating hand muscles through a disynaptic pathway mediated by last-order interneurons in the medial dorsal horn.
Longer survival times labeled third-order transneuronal projections from the cerebral cortex to the virus-injected hand muscles. These regions include the primary motor cortex (M1), several regions of the premotor cortex, and importantly, layer V of lateral area 5. The retrograde label in areas PE and PEip overlapped the anterograde projection sites demonstrated in the CTb and microstimulation experiments. Moreover, the density of transsynaptically labeled neurons in the PPC was substantially greater when rabies virus was injected into distal muscles of the hand, than into proximal muscles of the elbow and shoulder. Retrogradely labeled corticospinal neurons from lateral area 5 were only about half as frequent as those in M1, but nearly 1.5 times more numerous than those in the dorsal premotor cortex.
Rathelot et al. (3) conclude that the “lateral region within area 5 has corticospinal neurons that are directly linked to the control of hand movements” and that “a localized region within the posterior parietal cortex has” a more direct “route to access motor output” than pathways from premotor areas. Their data clearly fit Mountcastle’s view of a command function for the PPC (1, 2).
It is significant that the descending area 5 projections demonstrated by Rathelot et al. (3) terminate on interneurons, not motoneurons, thus modulating the excitability of motoneuron pools. Terminating on motoneurons would simply confer a direct motor role on the PPC. Instead, by terminating on interneurons that activate specific motor pools, PPC cells may rev up the engine or slow it down, enabling facilitation or inhibition of specific actions. Additionally, by terminating on interneurons in a somatosensory projection zone, as demonstrated by Rathelot et al., PPC neurons may enhance relevant sensory inputs, and suppress or gate irrelevant, distracting signals when subjects engage in purposeful acts.
The 1975 Mountcastle et al. paper (1) was a game-changer, a paradigm shift in neuroscience, because it demonstrated that it was possible to study higher functions of the brain in nonhuman primates (topics such as intention, attention to sensory events, motor planning, and decision-making), and to analyze spontaneous complex behaviors under subjective control of the animal. The report also debunked the idea that the PPC was a higher-order sensory-association area in which several modalities converged on individual neurons. The actions described in the 1975 report and illustrated in Fig. 1 combine visual tracking of an object and proprioception of hand and arm actions during reach and grasp, with motor behaviors needed to accomplish the goal of acquisition of an unpredictable target swinging in space.
Is the role of the PPC sensory, motor, or a combination of these functions? When an animal explores its environment with its hands or eyes, it is seeking some information or trying to acquire a desired object, such as food. These actions persist until the goal is achieved. Sensory feedback confirms subjective expectations or alters actions to achieve that goal.
Later studies from other neurophysiologists using trained tasks clearly established that PPC neurons in areas 5 and 7 are engaged in planning goal-directed movements of the hand, arm, and eyes to obtain a reward, and provide relevant sensory feedback concerning achievement of task goals (20). These functions are anatomically segregated into specific subregions of the PPC. Medial areas, such as the medial intraparietal area and the “parietal reach region,” are engaged by reaching to cued targets in the workspace (21–26); lateral cortical regions surrounding the intraparietal sulcus (area 5 and the anterior interparietal area) signal the hand postures used to grasp specific objects (27–31); and areas of the medial wall of the hemisphere integrate arm and hand movements with visual signals (32, 33). Motor plans and sensory attention are transformed into action by parietal interconnections with relevant premotor areas of the frontal lobe. The Rathelot et al. report (3) demonstrates a new pathway: direct parietal connections to spinal interneuron pools, enabling modulation of spinal circuits for goal-directed actions of the hand and arm, supplementing the more traditional direct frontal motor pathways.
Footnotes
The author declares no conflict of interest.
See companion article on page 4255.
References
- 1.Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: Command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- 2.Mountcastle VB. The parietal system and some higher brain functions. Cereb Cortex. 1995;5:377–390. doi: 10.1093/cercor/5.5.377. [DOI] [PubMed] [Google Scholar]
- 3.Rathelot J-A, Dum RP, Strick PL. Posterior parietal cortex contains a command apparatus for hand movements. Proc Natl Acad Sci USA. 2017;114:4255–4260. doi: 10.1073/pnas.1608132114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strick PL, Kim CC. Input to primate motor cortex from posterior parietal cortex (area 5). I. Demonstration by retrograde transport. Brain Res. 1978;157:325–330. doi: 10.1016/0006-8993(78)90035-5. [DOI] [PubMed] [Google Scholar]
- 5.Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- 6.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 7.Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- 8.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 11.Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Rathelot J-A, Strick PL. Muscle representation in the macaque motor cortex: An anatomical perspective. Proc Natl Acad Sci USA. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathelot J-A, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trojanowski JQ. Native and derivatized lectins for in vivo studies of neuronal connectivity and neuronal cell biology. J Neurosci Methods. 1983;9:185–204. doi: 10.1016/0165-0270(83)90082-1. [DOI] [PubMed] [Google Scholar]
- 15.Sawchenko PE, Gerfen CR. Plant lectins and bacterial toxins as tools for tracing neuronal connections. Trends Neurosci. 1985;8:378–384. [Google Scholar]
- 16.Abraira VE, et al. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell. 2017;168:295–310.e19. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- 18.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dum RP, Strick PL. Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr Opin Neurobiol. 2013;23:245–249. doi: 10.1016/j.conb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: The cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- 21.Kalaska JF, Caminiti R, Georgopoulos AP. Cortical mechanisms related to the direction of two-dimensional arm movements: Relations in parietal area 5 and comparison with motor cortex. Exp Brain Res. 1983;51:247–260. doi: 10.1007/BF00237200. [DOI] [PubMed] [Google Scholar]
- 22.Crammond DJ, Kalaska JF. Neuronal activity in primate parietal cortex area 5 varies with intended movement direction during an instructed-delay period. Exp Brain Res. 1989;76:458–462. doi: 10.1007/BF00247902. [DOI] [PubMed] [Google Scholar]
- 23.Lacquaniti F, Guigon E, Bianchi L, Ferraina S, Caminiti R. Representing spatial information for limb movement: Role of area 5 in the monkey. Cereb Cortex. 1995;5:391–409. doi: 10.1093/cercor/5.5.391. [DOI] [PubMed] [Google Scholar]
- 24.Andersen RA. Encoding of intention and spatial location in the posterior parietal cortex. Cereb Cortex. 1995;5:457–469. doi: 10.1093/cercor/5.5.457. [DOI] [PubMed] [Google Scholar]
- 25.Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol. 1997;7:849–859. doi: 10.1016/s0959-4388(97)80146-8. [DOI] [PubMed] [Google Scholar]
- 26.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 27.Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- 28.Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- 29.Debowy DJ, Ghosh S, Ro JY, Gardner EP. Comparison of neuronal firing rates in somatosensory and posterior parietal cortex during prehension. Exp Brain Res. 2001;137:269–291. doi: 10.1007/s002210000660. [DOI] [PubMed] [Google Scholar]
- 30.Gardner EP, et al. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol. 2007;97:387–406. doi: 10.1152/jn.00558.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaffelhofer S, Scherberger H. Object vision to hand action in macaque parietal, premotor, and motor cortices. Elife. 2016;5:e15278. doi: 10.7554/eLife.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P. Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res. 2003;153:158–170. doi: 10.1007/s00221-003-1589-z. [DOI] [PubMed] [Google Scholar]
- 33.Fattori P, Breveglieri R, Amoroso K, Galletti C. Evidence for both reaching and grasping activity in the medial parieto-occipital cortex of the macaque. Eur J Neurosci. 2004;20:2457–2466. doi: 10.1111/j.1460-9568.2004.03697.x. [DOI] [PubMed] [Google Scholar]