Significance
The study of molecules and materials is of great significance to both science and human welfare. The noninvasive techniques of NMR and MRI reflect two of the most important methods to study them. However, both of these approaches are insensitive, and hyperpolarization methods to improve sensitivity are needed to access new applications. The hyperpolarization approach signal amplification by reversible exchange is used to produce a signal that is 100,000 times larger than that which would be seen on a routine clinical MRI scanner under Boltzmann equilibrium conditions. By revealing the broad scope of this approach we demonstrate its potential for the future diagnostic detection of metabolites, drugs, and many other small molecules.
Keywords: hyperpolarization, SABRE, catalysis, NMR, MRI
Abstract
Hyperpolarization turns typically weak NMR and MRI responses into strong signals so that ordinarily impractical measurements become possible. The potential to revolutionize analytical NMR and clinical diagnosis through this approach reflect this area's most compelling outcomes. Methods to optimize the low-cost parahydrogen-based approach signal amplification by reversible exchange with studies on a series of biologically relevant nicotinamides and methyl nicotinates are detailed. These procedures involve specific 2H labeling in both the agent and catalyst and achieve polarization lifetimes of ca. 2 min with 50% polarization in the case of methyl-4,6-d2-nicotinate. Because a 1.5-T hospital scanner has an effective 1H polarization level of just 0.0005% this strategy should result in compressed detection times for chemically discerning measurements that probe disease. To demonstrate this technique’s generality, we exemplify further studies on a range of pyridazine, pyrimidine, pyrazine, and isonicotinamide analogs that feature as building blocks in biochemistry and many disease-treating drugs.
NMR is one of the most powerful methods available to study materials, and MRI is used widely in clinical diagnosis (1). One route to improve the diagnostic capabilities of MRI involves monitoring in vivo changes in metabolite flux to noninvasively probe human physiology (2). However, for reasons of low sensitivity, MRI detection needs to be coupled with approaches to increase signal strength such as hyperpolarization (3). This process has been exemplified by studies on pyruvate that harness dissolution dynamic nuclear polarization (DNP) (4) to produce the signal strength necessary to track its metabolism (5). Additionally, the related method of optical pumping noble gases, such as 129Xe (6), has provided the sensitivity needed to produce lung MR images that probe pulmonary disease (5, 7). A recent report using γ-rays in conjunction with hyperpolarized 131Xe has been used to image the distribution of a radioactive tracer at high sensitivity (8).
Parahydrogen (p-H2) induced polarization (9) is another hyperpolarization method that is showing great promise (10, 11). p-H2 is a unique hyperpolarized feedstock that has no magnetic moment and is not observable itself. Instead, a hyperpolarized agent is obtained when it adds to an unsaturated center as a consequence of this chemical change (9). The resulting products’ intrinsic magnetization, more commonly referred to as polarization, can lie so far from thermodynamic equilibrium that measurements that normally take months become possible in seconds (12). However, this is only possible if the agent is detected before signal relaxation. Moreover, because the hyperpolarized magnetization is not replenished after relaxation the signal detection strategy needs careful consideration. Hence, imaging of hyperpolarized agents must be rapid compared with their relaxation rates. The development of this technique is, therefore, fraught with experimental challenges because fresh samples are needed for each study. Furthermore, the use of NMR spectroscopy as an analytical tool would benefit substantially if materials could be rapidly characterized at lower concentrations than is currently possible (13–15).
In this study, we illustrate a number of developments to improve the p-H2–based method signal amplification by reversible exchange (SABRE) (16). This route shows great potential for enhancing both analytical NMR and clinical diagnosis in the future (17–22). SABRE does not change the chemical identity of the agent it hyperpolarizes but equilibrates polarization between p-H2 and the selected agent through binding to an iridium center (23). This simple process takes just a few seconds and is illustrated in Fig. 1 (24). Furthermore, the agents’ hyperpolarization level can be maintained in low field when under p-H2 because it can be continually repolarized (24, 25). Consequently we predict that the future optimization of this method will be easier than that of many other approaches if we can deliver agents with high signal strengths and appropriately long-lived magnetization.
Fig. 1.
Schematic representation of the SABRE effect.
We present here a series of advances in substrate and catalyst design to meet these two challenges by reference to the substrates nicotinamide (1) and methyl nicotinate (2) before demonstrating the approach’s wider applicability. These agents were selected because they exhibit low toxicity and play important roles in cellular biology (26). The relaxation times and hyperpolarization levels of these agents are increased by the use of spin dilution through 2H labeling, a strategy already used with DNP (27). We also improve the lifetime of the agents’ magnetization when bound to iridium through 2H labeling of the SABRE catalyst (28). The hyperpolarization levels are increased still further with high p-H2 pressures, and when a fully 2H-labeled coligand is used, 50% hyperpolarization is achieved in conjunction with magnetic-state lifetimes that approach 100 s in a 2H-labeled nicotinate. By expanding the scope of this strategy to other target agents we show that a universal improvement in 1H-hyperpolarization level and relaxation time is achieved. Single-scan MRI detection is then used to demonstrate that these improvements facilitate the acquisition of images whose intensity and contrast are vastly superior to those without hyperpolarization. Hence, we are confident that the presented results reflect a series of key steps toward successful in vivo SABRE while serving to improve the method’s potential as an analytical tool for NMR.
Results and Discussion
The SABRE method starts by dissolving an agent (substrate) in a solvent that contains a metal-based polarization transfer catalyst and then exposing it to p-H2 for a few seconds in low magnetic field. The hyperpolarized agent can then be detected in a second step by NMR or MRI methods. Protio-nicotinamide (1) was one of the first substrates to be polarized by SABRE (16) and aspects of its 1H, 13C, and 15N signal enhancement have been previously communicated (29–33).
In this work we undertake the SABRE process in the solvents methanol-d4 or ethanol-d6 at a field of 65 G unless otherwise stated and use a 400-MHz or 9.4-T field for the final measurement step. When 1 is hyperpolarized in this way through SABRE (30) in methanol-d4 solution, as detailed in SI Appendix, the signal for H-2 is ca. 650 times larger than that observed in a control measurement under Boltzmann conditions at a 9.4-T field. In contrast, the corresponding signals for H-4, H-5, and H-6 are 620, 190, and 590 times larger, respectively, than those of the H-2 control signal. Fig. 2, Left illustrates a typical measurement. This signal intensity corresponds to detecting 2.1% H-2 polarization, created after a combination of just a 7-s exposure to 3 bar of p-H2 and 3 s for subsequent transfer into the measurement field. The corresponding polarization values in ethanol-d6 range from 1.1 to 0.1% as detailed in Table 1. It is therefore straightforward to conclude that 1 is highly amenable to SABRE. Furthermore, according to the laws of signal averaging, if 31 s were needed to repeat each control measurement, it would take ca. 150 d of data collection to reproduce the previously detailed hyperpolarized result if a normal signal were to be used.
Fig. 2.
SABRE hyperpolarization of nicotinamide. (Left) Single-scan 1H NMR traces, with identical scaling, that illustrate nicotinamide (1) signals (as labeled) that result after SABRE (hyperpolarized) and when the polarization level matches that for thermodynamic equilibrium (normal). (Right) Similar single-scan 1H NMR traces using 1e (Table 1); the normal trace has a ×8 vertical expansion relative to the hyperpolarized trace (all measurements were performed in ethanol-d6).
Table 1.
Hyperpolarization and T1 data for nicotinamide (1) and the seven 2H-labeled analogs 1a–1g
 |
SABRE catalysis performed with 5 mM [IrCl(COD)(IMes)] [IMes is 1,3-bis(2,4,6-trimethylphenyl)-imidazol-2-ylidene], 4 eq of agent, under 3 bar p-H2 in methanol-d4 or ethanol-d6 at 298K. T1 measurements without catalyst were on a degassed sample containing 20 mM of agent. Errors are <5% unless otherwise specified.
As we have indicated, though, this signal gain must survive to the point of measurement. In methanol-d4 solution, the four distinct protons resonances of 1, H-2, H-4, H-5, and H-6, have T1 values of 16.3, 11.3, 6.9, and 9.8 s at 9.4 T, respectively (Table 1; errors are <5% unless specified). These T1 values change to 23.3 (50% increase), 6.7 (40% fall), 3.9 (45% fall), and 7.8 s (20% fall), respectively, in ethanol-d6 solution and range from 10.7 to 6.5 s in D2O solution. Hence, these values are highly solvent-dependent and not, at first glance, likely to be ideal for in vivo imaging; due to low SABRE efficiency in neat D2O solutions the corresponding polarization levels are not reported (34, 35).
Relaxation During SABRE Catalysis.
Examination of these NMR relaxation properties under SABRE conditions reveals that the observed T1 values for the four proton signals of 1 reduce to just 6.2, 6.3, 3.7, and 3.8 s, respectively, in methanol-d4. Furthermore, the H-2 T1 value in ethanol-d6 falls by 83% to 4.2 s, thereby implying even faster signal destruction. During SABRE, rapid polarization transfer is beneficial in leading to a large signal gain but at high field, or, without p-H2, the catalyst clearly destroys the contrast agents’ hyperpolarization by reducing T1 (28, 36).
Using 2H Labeling to Reduce Agent Relaxation.
Initially, we proposed overcoming these effects by using 2H labeling to extend the proton relaxation times of 1 (29). Hence, nicotinamide derivatives 1a–1g were prepared using the methods described in Fig. 3. These synthetic routes have been scaled to provide over 25 g of the desired isotopologue and thus provide a robust platform to underpin any potential clinical/preclinical studies. Table 1 presents their SABRE performance and proton T1 values, both with and without catalyst, at 9.4 T in methanol-d4, ethanol-d6, and D2O solutions, and clearly there is a beneficial extension in T1.
Fig. 3.
Synthetic methods used to prepare agents 1a–1g. Yields are isolated yields after column chromatography.
Because these structures all contain fewer protons than 1, they might be expected to achieve higher polarization levels under conditions when p-H2 is the limiting reagent. However, substrate 1a, whose ortho protons have been removed, gives low polarization under SABRE conditions. We conclude, therefore, that 1a has polarization acceptor sites that are too magnetically isolated from the hydride ligands of the catalyst for effective SABRE transfer. In contrast, forms 1b–1g all possess a proton adjacent to nitrogen, and based on the recent study by Eshuis et al. (37), the relative position of the ortho proton to the ring substituent is unlikely to affect its coupling with the hydride ligand in the catalyst. They should therefore all receive polarization at a similar rate and hence the poor performance of 1c under SABRE must be a consequence of rapid relaxation. Hence we conclude that locating one proton next to the N-binding site (for strong coupling to the hydride ligands) and a longer-range coupling to a second longer-lived storage site is optimal.
This is reflected in the fact that agents 1e and 1f have the largest average T1 values of the series and perform best under SABRE. Agent 1e possesses a 4J coupling between protons H-4 and H-6 that facilitates magnetization transfer between them and gives hyperpolarization levels of 2.7 and 3.2%, respectively, in methanol-d4 (Fig. 1). For 1f, the corresponding weaker 5J coupling between protons H-2 and H-5 now results in each exhibiting a 4.1% hyperpolarization level. Agent 1d, with a 4J, and 1b with a 3J coupling, and triply 2H-labeled 1g also perform less well than 1e and 1f, which can readily be explained by their poor T1 values.
Optimizing the SABRE Catalysis of Methyl Nicotinate.
Because the amide protons of nicotinamide are likely to play a role in these relaxation processes the series of related methyl nicotinates 2a–2e, shown in Table 2, were prepared and studied. The parent, protio-nicotinate 2, proved to be superior to 1 under SABRE catalysis, with its four aromatic proton resonances showing an average polarization level of 2.7% in methanol-d4 and 1.7% in ethanol-d6 under our test; transfer to the methyl group is negligible. Furthermore, proton H-2 now has a T1 value of 60 s in methanol-d4, 49 s in ethanol-d6, and 13.8 s in D2O. In contrast, doubly 2H-labeled 4,6-d2-nicotinate (2b) has proton T1 values in the absence of the catalyst for H-2 of 66 s and H-5 of 97.6 s in methanol-d4. These drop to 46.2 and 62.7 s, respectively, in ethanol-d6 but retain impressive values of 30.9 and 47.4 s in D2O. As a consequence of these changes we boost the delivered SABRE polarization levels to 10.1% and 9.5%, respectively, in methanol while retaining an average hyperpolarization level of ca. 8.8% in ethanol-d6. It proved possible to improve on these already impressive results by introducing a CD3 label such that d3-methyl 4,6-d2-nicotinate (2c) exhibits T1 values of 50.1 and 50.3 s in D2O while achieving 7.2% polarization in ethanol-d6.
Table 2.
Hyperpolarization and T1 data for methyl-nicotinate (2) and the four 2H-labeled analogs 2a–2d
 |
SABRE catalysis performed with 5 mM [IrCl(COD)(IMes)] [IMes is 1,3-bis(2,4,6-trimethylphenyl)-imidazol-2-ylidene], 4 eq of agent, under 3 bar p-H2 in methanol-d4 or ethanol-d6 at 298K. T1 measurements without catalyst were on a degassed sample containing 20 mM of agent. Errors are <5% unless otherwise specified.
Improving the SABRE Catalyst Through 2H Labeling.
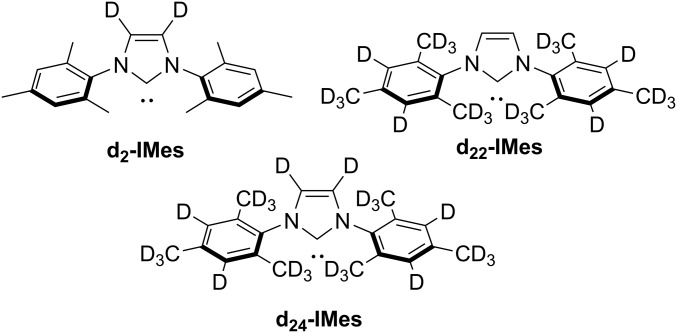
The presence of the catalyst acts to reduce the measured T1 values of the protons of the free substrate because the bound and free forms of the substrate are in dynamic exchange. Consequently, the recorded protons’ T1 values reflect their appropriately weighted average. Hence, the T1 value of a bound proton must be much smaller than the real value of the free material. We proved this by measuring the bound and free protons’ relaxation times at 263 K where ligand exchange is effectively quenched (SI Appendix, section 2.7). We hypothesized that the slow rate of productive polarization transfer (23) means that any increase in the bound protons’ relaxation times would be seen in an improved final agent polarization level. Conceptually, this could also be achieved by introducing 2H labeling into the N-heterocyclic carbene (NHC) ligand of the catalyst. Thus, we developed synthetic routes (SI Appendix, section 1.3) to produce d2-, d22-, and d24-IMes ligands, which were used to form the corresponding [IrCl(COD)(NHC)] catalyst (Fig. 4). The measured T1 values of the protons in 2b (bound and free) with these catalyst isotopomers, under 3 bar of H2 or D2, in methanol-d4 solution are detailed in Table 3.
Fig. 4.
The 2H-labeled NHC ligands used for SABRE polarization with catalysts of type [IrCl(COD)(NHC)].
Table 3.
Effective T1 values for protons H-2 and H-5 in methyl-4,6-d2-nicotinate 2b (20 mM loading) and the corresponding equatorial and axial ligands of the SABRE catalyst with [IrCl(COD)(NHC)]
| IMes T1(eff., with cat.)/s | d2-IMes T1(eff., with cat.)/s | d22-IMes T1(eff., with cat.)/s | d24-IMes T1(eff., with cat.)/s | |||||||||||||
| Site | H2 | D2 | H2 | D2 | H2 | D2 | H2 | D2 | ||||||||
| Free 2b | H-2: | 10.6 | H-2: | 10.9 | H-2: | 13.1 | H-2: | 13.8 | H-2: | 13.5 | H-2: | 20.1 | H-2: | 12.1 | H-2: | 14.1 |
| H-5: | 18.4 | H-5: | 18.3 | H-5: | 20.3 | H-5: | 25.3 | H-5: | 23.2 | H-5: | 23.7 | H-5: | 21.0 | H-5: | 30.9 | |
| Equatorial bound 2b | H-2: | 1.4 | H-2: | 1.9 | H-2: | 2.0 | H-2: | 1.5 | H-2: | 2.0 | H-2: | 2.6 | H-2: | 3.2 | H-2: | 2.3 |
| H-5: | 6.7 | H-5: | 5.6 | H-5: | 6.7 | H-5: | 6.5 | H-5: | 9.0 | H-5: | 7.3 | H-5: | 12.0 | H-5: | 10.5 | |
| Axially bound 2b | H-2: | 1.9 | H-2: | 4.1 | H-2: | 1.7 | H-2: | 3.6 | H-2: | 2.5 | H-2: | 4.8 | H-2: | 1.7 | H-2: | 4.0 |
| H-5: | 2.3 | H-5: | 2.4 | H-5: | 4.0 | H-5: | 2.7 | H-5: | 8.0 | H-5: | 3.8 | H-5: | 12.3 | H-5: | 10.1 | |
NHC is IMes, d2-, d22-, or d24-IMes, at 5 mM loading and H2 or D2 (3 bar) in methanol-d4 solution at 263 K.
These data show that as predicted the T1 values of the H-2 resonance in equatorially bound 2b is increased by 36% on moving from H2 to D2 at 263 K. This confirms that coupling to the hydride ligands provides a significant route to relaxation. Furthermore, on comparing the d2-, d22-, and d24-IMes systems, there is an increase in both the bound T1 and free substrate T1 values. More importantly, when moving to 298 K where SABRE catalysis is conducted (38) further increases in the protons’ T1 values free substrates are observed. Consequently we reexamined the SABRE efficiency of 2b with these catalysts (Table 4). Both the effective T1 relaxation times and polarization levels increased with the best-performing catalyst, [IrCl(COD)(d22-IMes)], giving average polarization levels of ca. 22.0% under 3 bar p-H2 in both methanol-d4 and ethanol-d6, which reflects a 250% improvement in the latter case.
Table 4.
Effect of 2H labeling the catalyst on the effective T1 values and polarization levels of H-2 and H-5 in 2b under SABRE catalysis by [IrCl(COD)(NHC)]
| Methanol-d4 | Ethanol-d6 | |||||||
| NHC | T1(eff., with cat.)/s | Polarization/% | T1(eff., with cat.)/s | Polarization/% | ||||
| d2-IMes | H-2: | 5.8 | H-2: | 10.1 | H-2: | 5.5 | H-2: | 9.3 |
| H-5: | 20.9 | H-5: | 9.5 | H-5: | 15.6 | H-5: | 8.2 | |
| d22-IMes | H-2: | 7.7 | H-2: | 24.1 | H-2: | 7.7 | H-2: | 26.8 |
| H-5: | 26.6 | H-5: | 19.9 | H-5: | 17.2 | H-5: | 16.6 | |
| d24-IMes | H-2: | 9.5 | H-2: | 18.6 | H-2: | 7.5 | H-2: | 13.3 |
| H-5: | 33.5 | H-5: | 16.5 | H-5: | 19.3 | H-5: | 8.2 | |
NHC is d2-, d22-, or d24-IMes with 3 bar p-H2 at 298 K.
Effect of p-H2 Pressure on the Level of SABRE.
Up to this point we have conducted all SABRE catalysis under 3 bar pressure of p-H2, which in our standard 5-mm J. Young tap NMR tubes reflects ca. 13-fold excess with respect to the substrate. Thus, if p-H2 is the limiting reagent in this catalytic process, the reaction will ultimately progress to a point where normal H2 (formed by conversion of p-H2) is the dominant form in solution. Hence the SABRE hyperpolarization of 2b was reexamined with [IrCl(COD)(d22-IMes)] in methanol-d4 and ethanol-d6 solution under 3.0–5.5 bar of p-H2 (Fig. 5). In methanol-d4 solution we see an increase in H-2 polarization to 26.4% and 28.5% at 4.0 and 5.5 bar, respectively. A more significant effect is observed in ethanol-d6 solution, where a 55% increase in enhancement is observed at 5.5 bar of p-H2 compared with 3.0 bar. This results in a 41.7% polarization being measured on the H-2 site of 2b under these conditions. These conditions are not used in the routine experiments we have described because they take the sample close to its safe pressure limit, and we would encourage anyone repeating this work to take appropriate precautions. We further note that these experiments use a 10-s p-H2 exposure time to produce their optimal hyperpolarization levels. If a larger excess of p-H2 were to be available, then increased exposure times could lead to even higher hyperpolarization levels.
Fig. 5.
Effect of p-H2 pressure on the SABRE hyperpolarization of 2b (4 eq) with [IrCl(COD)(d22-IMes)] (5 mM) in methanol-d4 and ethanol-d6.
Increasing SABRE Hyperpolarization by Using a Coligand.
Our final consideration in optimizing SABRE catalysis was to overcome the presence of multiple copies of the agent being attached simultaneously to a single iridium center and thus diluting any polarization coming from p-H2. This would require a catalyst redesign, but if a coligand is added that cannot itself receive polarization a suitable test scenario can be established. Thus, SABRE hyperpolarization was conducted using a single equivalent of 2b and three equivalents of methyl-2,4,5,6-d4-nicotinate, relative to [IrCl(COD)(d22-IMes)], under 5.5 bar p-H2 pressure. This led to 50% polarization’s being achieved in the H-2 site of 2b, with an average of 45% across the two sites. This is a truly remarkable result because it is delivered after just 10 s of SABRE. We validated this result on three samples that were each examined between 6 and 10 times. Hence, we believe that this strategy reflects a compelling route to achieve high levels of hyperpolarization that could be improved still further by the use of even higher pressures and a superior catalyst.
Solvent Effect on Relaxation Times.
The efficiency of SABRE catalysis has been shown to be highly dependent on the solvent, with low hyperpolarization levels resulting in 100% D2O solutions despite the development of water-soluble catalysts (34, 35, 39, 40). We suggest that hyperpolarization in an ethanol-d6 solution followed by sample dilution to obtain a biocompatible bolus for injection reflects a sensible route to obtain the required signal strength for in vivo study. The T1 values of 2b were measured in an ethanol-d6−D2O solvent mixtures (SI Appendix, section 2.8). Despite the reduction in T1 values for the H-2 and H-5 signals of 2b in the mixed solvent systems, in a biocompatible mixture of 10% ethanol-d6 in D2O the T1 value for H-5 remains above 30 s. Hence, very high levels of hyperpolarization are expected to survive the point of injection. We have measured the T1 values of H-2 and H-5 in 2b in water (containing 5% D2O) at 9.4 T, and they fall to 6.6 and 9.3 s, respectively. These values suggest that a signal will be visible in vivo for at least 20 s, although if these approaches were to be used in drug/urine screening, or in analytical NMR more generally, there would be no issue with the decay of signal before measurement.
Expanding the Range of Substrates.
We have also established the generality of this method by testing a range of additional substrates. To capture a suitable cross-section of targets, we have used materials bearing the nitrogen heterocyclic motifs pyridazine (3), pyrimidine (4), and pyrazine (5) alongside isonicotinamide (6). The best-performing isotopologues are presented in Fig. 6 and full data, including effective T1 relaxation times in the presence of the catalyst, can be found in SI Appendix, section 2.4. In all cases, the relaxation times and polarization levels are increased. For 3,5-d2-pyridazine (3a) the relaxation time is increased by 550% and the polarization level by 1630% relative to pyridazine (3). Similar effects were found for pyrimidine (4), where the deuterated isotopologue 4a yields 4.8% polarization and a signal with a T1 value of over 2 min. Comparison of pyrazine (5) to 2,5-d2-pyrazine (5a) shows a relaxation time increase from 32.3 s to 86.2 s and a polarization level improvement from 3.3 to 11.6%. Finally, 2,5-d2-isonicotinamide (6a) gives a ca. fivefold improvement in T1 and 80- and 6-fold improvements in polarization levels for H-3 and H-6, respectively, over 6.
Fig. 6.
T1(no cat) values (in blue, seconds) and SABRE polarization levels (in red, percentage) of a range of substrates in methanol-d4 solution to illustrate the general benefits of 2H labeling.
These data show, therefore, that the strategy we detail to optimize the SABRE technology is applicable to a broad range of structural motifs and we suggest that most agents that have a need to be studied using either analytical NMR or in a clinical setting will benefit from isotope labeling. SI Appendix, Table S5 details the corresponding T1 values in a 95% H2O, 5% D2O solution, with 2b, 2c, 3a, 4a, and 5a being the most likely to be detectable in vivo.
Assessing the Impact of These Changes for MRI Detection.
Images of the resultant SABRE-derived 1H magnetization of 1 and 2, and their better-performing deuterated analogs, have been collected at 9.4 T in conjunction with a 100 mM substrate concentration in ethanol-d6. Analogous results under reversible flow (41), where measurement repeat is possible, are also detailed in SI Appendix, section 3.3 and confirm that deuteration not only improves the resultant image intensity relative to their parents but also extends the duration over which visible signals can be detected. The long T1 values that these agents exhibit would normally inhibit standard pulse-recovery observations but are now expected to enable in vivo detection after transport to a region of clinical interest.
We have also been able to show that when high contrast is desired, hyperpolarized signals can be observed in the presence of a water phantom whose signal can initially be suppressed to further improve the dynamic range of the image. In the cases of 1f and 2b, the hyperpolarized agents are visible 30 s after completion of the polarization step with image intensity superior to that of the residual water signal that survives its suppression. Furthermore, when 2b was examined after polarization transfer by [IrCl(COD)(d22-IMes)] under 7 bar of p-H2 the corresponding rapid acquisition with relaxation enhancement (42) images of the same samples under normal conditions would take 9 and 40 d of continuous averaging in ethanol-d6 and methanol-d4, respectively, to reproduce the hyperpolarized results (Fig. 7) that are collected in less than 20 s after the start of hyperpolarization. Hence, we can conclude that the MRI assessment of these agents without hyperpolarization would be impractical. We also compared these data to those obtained for an excised spleen and noted a 30-fold signal-to-noise improvement relative to the tissue’s H2O signal.
Fig. 7.
Hyperpolarized images of methyl nicotinate using [IrCl(COD)(d22-IMes)] under 7 bar p-H2. (A) Control image of a tube of water. (B) SABRE hyperpolarized image of a sample of 2b (100 mM in methanol-d4). (C) Analogous SABRE hyperpolarized image of a sample of 2b (100 mM in ethanol-d6). Image signal-to-noise ratio values are 1,586, 4,085, and 2,955, respectively. Data were acquired under identical conditions and results are plotted on the same scale.
Conclusions
The results presented here suggest that placing a proton next to nitrogen is most desirable for efficient magnetization transfer via SABRE and having an isolated proton environment is desirable for a long signal lifetime. In the case of nicotinamide (1) and methyl nicotinate (2), the deuterated forms that hyperpolarize optimally correspond to 1f and 2b. This is because polarization now flows optimally from the hydride ligands of the catalyst into receptor protons of the bound substrates that relax more slowly. Consequently, when [IrCl(COD)(IMes)] is used as the catalyst precursor, 10% polarization in 2b is achieved for H-2 according to NMR spectroscopy measurement. However, because the catalyst also relaxes the hyperpolarization it creates 2H labeling of it is also highly beneficial. This simple change was found to double the level of delivered hyperpolarization to 22%. Increasing the p-H2 pressure from 3 to 5.5 bar boosts this value to 41%, and 50% H-2 polarization in 2b can be achieved when the coligand methyl-2,4,5,6-d4-nicotinate is also present. Collectively, these results reflect a strategy to create high levels of SABRE hyperpolarization that should be applicable to any target nucleus. Furthermore, by illustrating the success of this approach for a variety of agents we believe that we illustrate the generality of this strategy.
The resulting hyperpolarized and slowly relaxing agent’s magnetization was also detected in a series of imaging experiments that served to establish that these performance improvements were maintained in MRI. It must be borne in mind, though, that when measuring an MRI response signal to noise, not the level of signal enhancement, is critical. Hence, the interplay between 1H concentration and hyperpolarization level must be taken into account. An automated delivery system needs to be assembled if these results are to form the basis of future in vivo studies because rapid injection of these agents must follow sample dilution and catalyst removal steps. We are currently using this approach to optimally polarize the 13C and 15N nuclei of a range of substrates with long magnetization lifetimes. It should be noted that in recent reports hyperpolarized 15N and 1H NMR signals can be detected over 15 min after their creation by harnessing SABRE in conjunction with long-lived singlet states, which reflects a further route to extend this approach (43, 44).
At a molecular level we have developed and demonstrated a strategy to optimize the hyperpolarization levels of a biomarker and its relaxation characteristics. This has required the production of a range of 2H-labeled substrates and the selection of their optimal form based on their relaxation time and SABRE hyperpolarization level. We are also using this approach to improve NMR sensitivity for analytical purposes in a similar way.
Materials and Methods
Synthesis and Reactivity Studies.
All reactions using air- and moisture-sensitive reagents were performed in dried glassware under an atmosphere of dry nitrogen. Dry solvents (THF, toluene, and dichloromethane) were obtained from a Braun MB-SPS-800. For TLC analysis, Merck precoated plates (silica gel 60 F254, Art 5715, 0.25 mm) were used. Column chromatography was performed on Fluka silica gel (60 Å, 220–440 mesh). Deuterated solvents (methanol-d4, ethanol-d6, and D2O) were obtained from Sigma-Aldrich and used as supplied. Detailed synthetic procedures and characterization data can be found in SI Appendix.
Hyperpolarization Studies.
1H NMR spectra in addition to the relaxation data were measured on a Bruker 400-MHz spectrometer. SABRE hyperpolarization transfer experiments involved p-H2 that was produced by cooling H2 gas over Fe2O3 at 30 K. Samples contained 5 mM concentrations of the catalyst and between 5 and 20 equivalents of substrate relative to iridium in the specified solvent in a 5-mm NMR tube fitted with a J. Young tap. The resulting solutions were degassed before the introduction of p-H2 at a pressure of 3 bar unless otherwise stated. Samples were shaken for 7 s in a specified fringe field of a 9.4-T Bruker Avance (III) NMR spectrometer for hyperpolarization transfer before being rapidly transported into the main magnetic field of the instrument for subsequent probing by NMR or MRI methods. The associated polarization transfer experiment data can be found in SI Appendix.
Statistical Analysis.
Errors have been calculated using the SD formula, taking into account the limited number of population samples. Gaussian error propagation has been assumed (SI Appendix).
Supplementary Material
Acknowledgments
We thank Professor V. H. Perry for useful discussions. This work was supported by Wellcome Trust Grants 092506 and 098335, Bruker BioSpin, and the University of York.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data created during this research are available by request from the University of York Data Catalogue (dx.doi.org/10.15124/7ef7e98c-ef5f-47e3-8739-9ee1ae3bae68).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620457114/-/DCSupplemental.
References
- 1.Kurhanewicz J, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: Prospects for translation to clinical research. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutte H, et al. The use of dynamic nuclear polarization (13)C-pyruvate MRS in cancer. Am J Nucl Med Mol Imaging. 2015;5:548–560. [PMC free article] [PubMed] [Google Scholar]
- 3.Comment A, Merritt ME. Hyperpolarized magnetic resonance as a sensitive detector of metabolic function. Biochemistry. 2014;53:7333–7357. doi: 10.1021/bi501225t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardenkjaer-Larsen JH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y. Dissolution dynamic nuclear polarization-enhanced magnetic resonance spectroscopy and imaging: Chemical and biochemical reactions in nonequilibrium conditions. Appl Spectrosc Rev. 2016;51:190–206. [Google Scholar]
- 6.Nikolaou P, et al. Near-unity nuclear polarization with an open-source 129Xe hyperpolarizer for NMR and MRI. Proc Natl Acad Sci USA. 2013;110:14150–14155. doi: 10.1073/pnas.1306586110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaou P, et al. XeNA: An automated ‘open-source’ (129)Xe hyperpolarizer for clinical use. Magn Reson Imaging. 2014;32:541–550. doi: 10.1016/j.mri.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Miller GW, Tobias WA, Cates GD. A method for imaging and spectroscopy using γ-rays and magnetic resonance. Nature. 2016;537:652–655. doi: 10.1038/nature19775. [DOI] [PubMed] [Google Scholar]
- 9.Bowers CR, Weitekamp DP. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J Am Chem Soc. 1987;109:5541–5542. [Google Scholar]
- 10.Duckett SB, Mewis RE. Application of parahydrogen induced polarization techniques in NMR spectroscopy and imaging. Acc Chem Res. 2012;45:1247–1257. doi: 10.1021/ar2003094. [DOI] [PubMed] [Google Scholar]
- 11.Green RA, et al. The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Prog Nucl Magn Reson Spectrosc. 2012;67:1–48. doi: 10.1016/j.pnmrs.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Anwar MS, et al. Preparing high purity initial states for nuclear magnetic resonance quantum computing. Phys Rev Lett. 2004;93:040501. doi: 10.1103/PhysRevLett.93.040501. [DOI] [PubMed] [Google Scholar]
- 13.Beckonert O, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 14.Reile I, et al. NMR detection in biofluid extracts at sub-μM concentrations via para-H2 induced hyperpolarization. Analyst (Lond) 2016;141:4001–4005. doi: 10.1039/c6an00804f. [DOI] [PubMed] [Google Scholar]
- 15.Eshuis N, et al. Toward nanomolar detection by NMR through SABRE hyperpolarization. J Am Chem Soc. 2014;136:2695–2698. doi: 10.1021/ja412994k. [DOI] [PubMed] [Google Scholar]
- 16.Adams RW, et al. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science. 2009;323:1708–1711. doi: 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- 17.Frydman L. NMR spectroscopy: Chemistry awakens a silent giant. Nat Chem. 2009;1:176–178. doi: 10.1038/nchem.227. [DOI] [PubMed] [Google Scholar]
- 18.Shchepin RV, et al. (15)N hyperpolarization of imidazole-(15)N2 for magnetic resonance pH sensing via SABRE-SHEATH. ACS Sens. 2016;1:640–644. doi: 10.1021/acssensors.6b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan AWJ, Theis T, Colell JFP, Warren WS, Malcolmson SJ. Hyperpolarization of nitrogen-15 Schiff bases by reversible exchange catalysis with para-hydrogen. Chemistry. 2016;22:10777–10781. doi: 10.1002/chem.201602393. [DOI] [PubMed] [Google Scholar]
- 20.Truong ML, et al. (15)N hyperpolarization by reversible exchange using SABRE-SHEATH. J Phys Chem C Nanomater Interfaces. 2015;119:8786–8797. doi: 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, et al. Optimization of SABRE for polarization of the tuberculosis drugs pyrazinamide and isoniazid. J Magn Reson. 2013;237:73–78. doi: 10.1016/j.jmr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dücker EB, Kuhn LT, Münnemann K, Griesinger C. Similarity of SABRE field dependence in chemically different substrates. J Magn Reson. 2012;214:159–165. doi: 10.1016/j.jmr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Adams RW, Duckett SB, Green RA, Williamson DC, Green GGR. A theoretical basis for spontaneous polarization transfer in non-hydrogenative parahydrogen-induced polarization. J Chem Phys. 2009;131:194505. doi: 10.1063/1.3254386. [DOI] [PubMed] [Google Scholar]
- 24.Hövener J-B, et al. A hyperpolarized equilibrium for magnetic resonance. Nat Commun. 2013;4:2946. doi: 10.1038/ncomms3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovedo P, et al. Molecular MRI in the Earth’s magnetic field using continuous hyperpolarization of a biomolecule in water. J Phys Chem B. 2016;120:5670–5677. doi: 10.1021/acs.jpcb.6b02830. [DOI] [PubMed] [Google Scholar]
- 26.MacKay D, Hathcock J, Guarneri E. Niacin: Chemical forms, bioavailability, and health effects. Nutr Rev. 2012;70:357–366. doi: 10.1111/j.1753-4887.2012.00479.x. [DOI] [PubMed] [Google Scholar]
- 27.Allouche-Arnon H, Lerche MH, Karlsson M, Lenkinski RE, Katz-Brull R. Deuteration of a molecular probe for DNP hyperpolarization–A new approach and validation for choline chloride. Contrast Media Mol Imaging. 2011;6:499–506. doi: 10.1002/cmmi.452. [DOI] [PubMed] [Google Scholar]
- 28.Mewis RE, Fekete M, Green GGR, Whitwood AC, Duckett SB. Deactivation of signal amplification by reversible exchange catalysis, progress towards in vivo application. Chem Commun (Camb) 2015;51:9857–9859. doi: 10.1039/c5cc01896j. [DOI] [PubMed] [Google Scholar]
- 29.Mewis RE, et al. Probing signal amplification by reversible exchange using an NMR flow system. Magn Reson Chem. 2014;52:358–369. doi: 10.1002/mrc.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowley MJ, et al. Iridium N-heterocyclic carbene complexes as efficient catalysts for magnetization transfer from para-hydrogen. J Am Chem Soc. 2011;133:6134–6137. doi: 10.1021/ja200299u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W, et al. Hyperpolarized 15N-pyridine derivatives as pH-sensitive MRI agents. Sci Rep. 2015;5:9104. doi: 10.1038/srep09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theis T, et al. Microtesla SABRE enables 10% nitrogen-15 nuclear spin polarization. J Am Chem Soc. 2015;137:1404–1407. doi: 10.1021/ja512242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shchepin RV, Barskiy DA, Mikhaylov DM, Chekmenev EY. Efficient synthesis of nicotinamide-1-15N for ultrafast NMR hyperpolarization using parahydrogen. Bioconjug Chem. 2016;27:878–882. doi: 10.1021/acs.bioconjchem.6b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fekete M, et al. Utilisation of water soluble iridium catalysts for signal amplification by reversible exchange. Dalton Trans. 2015;44:7870–7880. doi: 10.1039/c5dt00311c. [DOI] [PubMed] [Google Scholar]
- 35.Spannring P, et al. A new Ir-NHC catalyst for signal amplification by reversible exchange in D2 O. Chemistry. 2016;22:9277–9282. doi: 10.1002/chem.201601211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barskiy DA, Pravdivtsev AN, Ivanov KL, Kovtunov KV, Koptyug IV. A simple analytical model for signal amplification by reversible exchange (SABRE) process. Phys Chem Chem Phys. 2016;18:89–93. doi: 10.1039/c5cp05134g. [DOI] [PubMed] [Google Scholar]
- 37.Eshuis N, et al. Determination of long-range scalar (1)H-(1)H coupling constants responsible for polarization transfer in SABRE. J Magn Reson. 2016;265:59–66. doi: 10.1016/j.jmr.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd LS, et al. Hyperpolarisation through reversible interactions with parahydrogen. Cat Sci Tech. 2014;4:3544–3554. [Google Scholar]
- 39.Shi F, et al. Aqueous NMR signal enhancement by reversible exchange in a single step using water-soluble catalysts. J Phys Chem C Nanomater Interfaces. 2016;120:12149–12156. doi: 10.1021/acs.jpcc.6b04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hövener J-B, et al. Toward biocompatible nuclear hyperpolarization using signal amplification by reversible exchange: Quantitative in situ spectroscopy and high-field imaging. Anal Chem. 2014;86:1767–1774. doi: 10.1021/ac403653q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mewis RE. Developments and advances concerning the hyperpolarisation technique SABRE. Magn Reson Chem. 2015;53:789–800. doi: 10.1002/mrc.4280. [DOI] [PubMed] [Google Scholar]
- 42.Hennig J, Nauerth A, Friedburg H. RARE imaging: A fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 43.Roy SS, Norcott P, Rayner PJ, Green GGR, Duckett SB. A hyperpolarizable (1) H magnetic resonance probe for signal detection 15 minutes after spin polarization storage. Angew Chem Int Ed Engl. 2016;55:15642–15645. doi: 10.1002/anie.201609186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theis T, et al. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal (15)N2-diazirine molecular tags. Sci Adv. 2016;2:e1501438. doi: 10.1126/sciadv.1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.