Abstract
Background
Exposures to extreme ambient temperature and air pollution are linked to adverse birth outcomes, but the associations with small for gestational age (SGA) and term low birthweight (tLBW) are unclear. We aimed to investigate exposures to site-specific temperature extremes and selected criteria air pollutants in relation to SGA and tLBW.
Methods
We linked medical records of 220,572 singleton births (2002–2008) from 12 US sites to local temperature estimated by the Weather Research and Forecasting model, and air pollution estimated by modified Community Multiscale Air Quality models. Exposures to hot (>95th percentile) and cold (<5th percentile) were defined using site-specific distributions of daily temperature over three-month preconception, each trimester, and whole-pregnancy. Average concentrations of five criteria air pollutants and six fine particulate matter constituents were also calculated for these pregnancy windows. Poisson regression with generalized estimating equations calculated the relative risks (RR) and 95% confidence intervals for SGA (weight <10th percentile conditional on gestational age and sex) and tLBW (≥37 weeks and <2,500 grams) associated with an interquartile range increment of air pollutants, and cold or hot compared to mild (5–95th percentile) temperature. Models were adjusted for maternal demographics, lifestyle, and clinical factors, season, and site.
Results
Compared to mild temperature, cold exposure during trimester 2 [RR: 1.21 (1.05–1.38)], trimester 3 [RR: 1.18 (1.03–1.36)], and whole-pregnancy [RR: 2.57 (2.27–2.91)]; and hot exposure during trimester 3 [RR: 1.31 (1.15–1.50)] and whole-pregnancy [RR: 2.49 (2.20–2.83)] increased tLBW risk. No consistent association was observed between temperature and SGA. Air pollutant analyses were generally null but preconception elemental carbon was associated with a 4% increase in SGA while dust particles increased tLBW by 10%. Particulate matter ≤10 microns in the second trimester and whole pregnancy also appeared related to tLBW. Conclusions: Our findings suggest prenatal exposures to extreme ambient temperature relative to usual environment may increase tLBW risk. Given concerns related to climate change, these findings merit further investigation.
Keywords: temperature, climate change, fetal growth, low birthweight, SGA
Introduction
Fetal growth restriction is a relatively common birth outcome that is due to a variety of conditions resulting in a fetus being unable to achieve its potential size (Stan et al., 2013). Small for gestational age (SGA), and term low birthweight (tLBW), two common indicators of fetal growth restriction, affects about 10% and 2% of live births in the US, respectively (ACOG, 2013; CDC, 2016). Fetal growth restriction is responsible for many adverse perinatal and childhood outcomes including perinatal mortality; complications related to immunologic, respiratory, and metabolic function; and impaired motor and neurobehavioral development during childhood (Arcangeli et al., 2012; Pallotto and Kilbride, 2006). The etiology of growth restriction may be influenced by many factors including exposure to environmental risk factors (Stan et al., 2013).
Environmental risk factors, such as prenatal exposure to tobacco smoke, have received considerable attention in the literature, and have been shown to have a deleterious impact on fetal growth (Janisse et al., 2014; Prabhu et al., 2010; Reeves and Bernstein, 2008). However, other potentially harmful and ubiquitous prenatal exposures, such as extreme ambient temperature have been relatively understudied with respect to their potential effects on fetal outcomes. Prenatal exposures to air pollution in relation to fetal growth have received more attention, but findings are generally inconsistent across pollutants and studies partially due to heterogeneity in study design and exposure distribution (Jacobs et al., 2016; Stieb et al., 2012). Previous research suggests that exposures to extreme temperature as well as high air pollution may increase oxidative stress and systemic inflammation in the general population (Ghio et al., 2012; Kahle et al., 2015; Moller et al., 2014). Although mechanistic studies in pregnant women are still scarce in relation to these environmental risk factors, exposures during pregnancy may decrease uterine blood flow, placental fetal exchange and ultimately slow fetal growth (Biberoglu et al., 2016; Browne et al., 2015; Prada and Tsang, 1998; Slama et al., 2008). Consistent with this potential biologic mechanism, extreme ambient temperature and air pollution exposure have been linked to adverse birth outcomes such as low birthweight and preterm birth, but their association with SGA and tLBW— is still unclear given the scarcity of literature on this topic and the inconsistent findings across pollutants and studies (Auger et al., 2014; Shah and Balkhair, 2011; Strand et al., 2011). This is an important knowledge gap given the expected increase in ambient temperature associated with global warming (Karl et al., 2015), and the increasing concerns due to air pollution emission from anthropogenic sources including transportation and industrial activities(EPA, 2016). We sought to investigate the potential association of extreme ambient temperature, five criteria air pollutants, and six particulate matter constituents with fetal growth restriction, indicated by SGA and tLBW, in a large nationwide US obstetric cohort.
Methods
Participants
We used data from the Air Quality and Reproductive Health Study, which linked local air pollution and meteorological data to participants in the Consortium on Safe Labor (CSL) in 2013. A detailed description of the CSL study design has been published elsewhere (Zhang et al., 2010). Briefly, CSL was an observational cohort study that collected detailed medical records from 228,438 deliveries at ≥23 weeks of gestation (2002–2008) from 12 clinical sites (15 hospital referral regions, 19 hospitals) across the US (eFigure 1). Maternal and infant data were abstracted from electronic delivery records and/or discharge summaries. After excluding multiple births (n=5,053) and those missing exposure information (n=10), birthweight (n=2,485), or infant sex (n=318), 220,572 births remained in the analyses. The CSL was approved by Institutional Review Boards from all participating clinical centers. Since data were de-identified, informed consent was not required.
Exposure assessment
Temperature
Due to the anonymity of CSL data, we did not have residential address to perform detailed interpolation of exposures. Alternatively, we estimated exposures for each woman based on the average concentrations in the 15 non-overlapping delivery hospital referral regions (area range: 415 – 312,644 km2) as a proxy for maternal residence and local mobility (i.e. short range spatial movements associated with daily activities such as work and errands). In other words, a woman’s exposure percentile during pregnancy was assigned based on the average temperature distribution within her hospital referral region. Hourly ambient temperature and relative humidity were obtained using the Weather Research and Forecasting Model (WRF). Modeling approach of the WRF and model evaluation has been described elsewhere (Chen et al., 2014; Zhang et al., 2014). Briefly, WRF is a next-generation weather prediction system designed for atmospheric research and forecasting, developed by research, governmental, and academic entities. Performance statistics comparing with observed records suggested that temperature estimates were acceptable and in agreement with other WRF modeling studies (Chen et al., 2014; Zhang et al., 2014). For each pregnancy, daily data for temperature and relative humidity were averaged across several potentially critical windows before and during pregnancy: three months preconception (91 days before last estimated menstrual period (eLMP)), first trimester (eLMP through 13 weeks), second trimester (weeks 14 to 28), third trimester (weeks 29 to delivery), and whole pregnancy (eLMP through delivery). eLMP was back calculated from date of delivery using best clinical estimate of gestational age recorded in delivery records. In obstetrical practice in the U.S., the best clinical estimate of gestational age is typically derived from either last menstrual period (LMP) or ultrasound measurements if they differ from the LMP based on specific criteria. Since temperature-related health risk is likely driven by temperature deviation from the usual environment, we accounted for regional acclimatization by categorizing our temperature exposure based on the local area temperature distributions for each of the perinatal time windows. Specifically, for each clinical site, we evaluated the temperature distributions for all perinatal time windows (preconception, trimesters and whole pregnancy) among all women from that site. For each site and time window, we defined cold exposure as the bottom (<5th percentile), hot exposure as the top (>95th percentile), and mild exposure as the middle (5–95th percentile) of that site- and window-specific temperature distribution (three-level exposure variable). In other words, cold/hot cut-offs varied for women from different sites to account for acclimatization.
Selected criteria air pollutants
Hourly concentrations were calculated for five criteria air pollutants including carbon monoxide (CO), nitrogen oxides (NOx), ozone (O3), particulate matter with diameter <2.5 or <10 microns (PM2.5 or PM10), and sulfur dioxide (SO2); and six PM2.5 constituents including elemental carbon, organic compound, ammonium ions, sulfate particles, nitrate particles, and dust particles. These pollutants were assessed using a modified Community Multiscale Air Quality (CMAQ) model (v4.7.1), which is a three-dimensional multipollutant air quality model developed by the US Environmental Protection Agency (Foley et al., 2010). A detailed description and model evaluation have been published (Chen et al., 2014). Briefly, CMAQ predicts air pollution concentrations at any location/time using inputs from several sources including local emission from the National Emission Inventory, local weather from the WRF, and photochemical properties of the pollutants. Outputs for criteria air pollutant concentrations were corrected for measurement errors between modelled and observed levels at local air monitors using inverse distance weighting (IDW). IDW is a commonly used technique to interpolate to unknown points based on measured data at known points. We corrected our model estimates to match the monitor at the point of measurement and then reduce the correction coefficient as the distance gets further away. Data were also weighted by population density to improve accuracy. Model evaluation showed that these methods significantly improved model performance for all pollutants (Chen et al., 2014; Zhang et al., 2014). Since PM2.5 constituents were rarely monitored by air quality monitors, only modelled concentrations were used. The concentration of each pollutant was averaged over the same exposure windows as previously described for temperature.
Outcome and covariates
Variables related to the main outcomes and a set of a priori covariates were obtained from electronic delivery records or discharge summaries for each participant. SGA was defined as any infant who weighed <10th percentile of infants with the same gestational age and sex using an internal reference (Mannisto et al., 2013). tLBW was defined as any infant born ≥37 weeks with a birthweight <2,500 grams. The best clinical estimate of gestational age, infant sex and birth weight were as recorded in the medical records. Covariates included infant sex (female, male), maternal race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islanders, Other, Unknown), maternal age (<20, 20–24, 25–29, 30–34, ≥35 years, Unknown), marital status (not married, married, unknown), parity (0, 1, ≥2), pre-pregnancy body mass index (BMI, <18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2, Unknown), smoking during pregnancy (yes/no), alcohol use during pregnancy (yes/no), gestational complications (i.e. hypertensive disorders of pregnancy or gestational diabetes, yes/no), chronic comorbidity (pre-gestational hypertension, diabetes, human immunodeficiency virus, asthma, or thyroid disorders, yes/no), insurance (private, not private, unknown), season of conception (spring, summer, fall, winter), relative humidity (continuous), and study site (12 sites). Gestational complications and chronic comorbidities were assessed by electronic medical records and/or discharge summaries using International Classification of Diseases, version 9 (ICD-9) codes and were evaluated in preliminary analyses but found not to be effect modifiers.
Statistical analysis
Basic statistics including Spearman correlation coefficients were calculated for selected pollutants and temperature. We used Poisson regression to obtain the relative risks (RR) and 95% confidence intervals (CI) for the associations of temperature as well as the selected air pollutants with SGA and tLBW. A total of 18,590 (8.4%) women had more than one singleton delivery during the study period, so we used robust standard errors from generalized estimating equations to adjust for possible clustering effects within women. Estimates were quantified for an interquartile range (IQR) increase in air pollution exposure during each exposure window (i.e., 3-month preconception, each of the three trimesters, and whole pregnancy) after adjusting for covariates, temperature and humidity. For temperature effect estimates, we compared the site-specific cold and hot groups to the mild temperature group to account for potential regional acclimation while adjusting for all covariates and O3 as well as PM2.5, two pollutants commonly correlated with temperature and frequently implicated pollutants for health in the literature (Salam et al., 2005). The reference group for the SGA analyses was births of appropriate weight for gestational age (10–90th percentile of infants at the same gestational age and sex); and in the tLBW analyses, the reference group was other term births ≥2500 grams. Post hoc multiple-comparison adjustments for p-values were performed using the false discovery rate (FDR) method (Benjamini and Hochberg, 1995) within each exposure window for criteria air pollutants and PM2.5 constituents. All missing observations were kept in the analyses as a separate category for all covariates, and categorical variables where dummy coded where appropriate.
Additional analyses
Given the seasonal trend of temperature, length of pregnancy may affect our results. Therefore, for the tLBW analyses where we compared tLBW to term non-LBW, we compared whole pregnancy exposures truncated to 37 weeks among term births to ensure equal length of exposures. For the SGA analyses, we restricted to only term births and repeated the same method. To adjust for temporal dependency of temperature (i.e., women who is exposed to ‘hot’ in the first trimester is not likely to be exposed to ‘hot’ again during the third trimester), we also adjusted our analyses for exposures during other time windows. In addition, we repeated our analyses restricting to only nulliparous women to avoid confounding by history of fetal growth restriction. To evaluate the impact of averaging exposure over a large geographic area, we repeated our analyses excluding Intermountain Health, the largest site. Lastly, we also stratified our analyses by season (warm: May–September; cold: October–April).
Results
Of the 220,572 births included in the analyses, there were 22,239 (11.2%) cases of SGA, and 4,322 (2.2%) cases of tLBW. Term LBW, but not SGA, was more common among female infants. In addition, both outcomes were more common among infants of mothers who were Black, younger, unmarried, underweight, smoked during pregnancy, had gestational complications or comorbidity, or had no private insurance (Table 1). On average, ambient temperature during the five exposure windows were slightly higher among the SGA and tLBW group compared to their counterparts (eTable 1). The sample distributions of temperature and air pollutants, temperature hot/cold cutoffs by site and pregnant windows, as well as their Pearson correlation matrix are provided in eTables 2, 3, and 4, respectively.
Table 1.
Characteristics of Study Participants from the Consortium on Safe Labor, 2002–2008.
| Characteristics | Fetal growth | Term births | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SGA (N=22,239) |
AGA (N=176,064) |
LBW (N=4,322) |
Not tLBW (N=190,850) |
|||||
| Infant sex | N | % | N | % | N | % | N | % |
| Female | 10,880 | 48.9 | 86,064 | 48.9 | 2,606 | 60.3 | 93,229 | 48.9 |
| Male | 11,359 | 51.1 | 90,000 | 51.1 | 1,716 | 39.7 | 97,621 | 51.2 |
| Race/ethnicity | ||||||||
| NH-White | 8,612 | 38.7 | 87,990 | 50.0 | 1,597 | 37.0 | 97,238 | 51.0 |
| NH-Black | 7,299 | 32.8 | 38,618 | 21.9 | 1,538 | 35.6 | 39,880 | 20.9 |
| Hispanic | 3,724 | 16.8 | 30,675 | 17.4 | 704 | 16.3 | 33,308 | 17.5 |
| Asian/Pacific Islander | 1,051 | 4.7 | 7,383 | 4.2 | 204 | 4.7 | 8,045 | 4.2 |
| Other | 617 | 2.8 | 4,031 | 2.3 | 119 | 2.8 | 4,317 | 2.3 |
| Unknown | 936 | 4.2 | 7,367 | 4.2 | 160 | 3.7 | 8,062 | 4.2 |
| Maternal age | ||||||||
| <20 | 3,191 | 14.4 | 16,195 | 9.2 | 640 | 14.8 | 16,903 | 8.9 |
| 20–24 | 6,654 | 29.9 | 44,894 | 25.5 | 1,260 | 29.2 | 48,094 | 25.2 |
| 25–29 | 5,725 | 25.7 | 49,415 | 28.1 | 1,067 | 24.7 | 54,037 | 28.3 |
| 30–34 | 3,865 | 17.4 | 39,623 | 22.5 | 752 | 17.4 | 43,399 | 22.7 |
| ≥35 | 2,782 | 12.5 | 25,700 | 14.6 | 597 | 13.8 | 28,157 | 14.8 |
| Unknown | 22 | 0.1 | 237 | 0.1 | 6 | 0.1 | 260 | 0.1 |
| Marital status | ||||||||
| Not Married | 11,155 | 50.2 | 66,031 | 37.5 | 2,283 | 52.8 | 69,627 | 36.5 |
| Married | 10,316 | 46.4 | 104,425 | 59.3 | 1,882 | 43.5 | 115,196 | 60.4 |
| Unknown | 768 | 3.5 | 5,608 | 3.2 | 157 | 3.6 | 6,027 | 3.2 |
| Parity | ||||||||
| 0 | 11,224 | 50.5 | 69,876 | 39.7 | 2,157 | 49.9 | 75,561 | 39.6 |
| 1 | 5,667 | 25.5 | 54,371 | 30.9 | 1,067 | 24.7 | 59,558 | 31.2 |
| ≥2 | 5,348 | 24.1 | 51,817 | 29.4 | 1,098 | 25.4 | 55,731 | 29.2 |
| Pre-pregnancy BMI | ||||||||
| <18.5 | 1,327 | 6.0 | 6,239 | 3.5 | 305 | 7.1 | 6,631 | 3.5 |
| 18.5–24.9 | 7,914 | 35.6 | 64,165 | 36.4 | 1,498 | 34.7 | 69,243 | 36.3 |
| 25–29.9 | 2,852 | 12.8 | 26,349 | 15.0 | 537 | 12.4 | 29,084 | 15.2 |
| ≥30 | 2,317 | 10.4 | 21,114 | 12.0 | 458 | 10.6 | 23,600 | 12.4 |
| Unknown | 7,829 | 35.2 | 58,197 | 33.1 | 1,524 | 35.3 | 62,292 | 32.6 |
| Smoking during pregnancy | 2,475 | 11.1 | 11,364 | 6.5 | 596 | 13.8 | 11,590 | 6.1 |
| Alcohol use during pregnancy | 525 | 2.4 | 3,176 | 1.8 | 127 | 2.9 | 3,270 | 1.7 |
| Gestational complications | 2,417 | 10.9 | 16,044 | 9.1 | 585 | 13.5 | 16,157 | 8.5 |
| Comorbidity | 3,246 | 14.6 | 22,733 | 12.9 | 696 | 16.1 | 23,929 | 12.5 |
| Insurance type | ||||||||
| Private insurance | 10,411 | 46.8 | 99,171 | 56.3 | 1,850 | 42.8 | 109,068 | 57.2 |
| No private insurance | 9,410 | 42.3 | 58,253 | 33.1 | 1,997 | 46.2 | 61,579 | 32.3 |
| Unknown | 2,418 | 10.9 | 18,640 | 10.6 | 475 | 11.0 | 20,203 | 10.6 |
| Season of conception | ||||||||
| Spring (March–May) | 5,276 | 23.7 | 41,450 | 23.5 | 1,023 | 23.7 | 45,230 | 23.7 |
| Summer (June–August) | 5,540 | 24.9 | 45,423 | 25.8 | 1,072 | 24.8 | 49,268 | 25.8 |
| Fall (September–November) | 6,163 | 27.7 | 48,551 | 27.6 | 1,191 | 27.6 | 52,850 | 27.7 |
| Winter (December–February) | 5,260 | 23.7 | 40,640 | 23.1 | 1,036 | 24.0 | 43,502 | 22.8 |
Abbreviations: AGA, appropriate for gestational age; BMI, body mass index; tLBW, term low birthweight; SGA, small for gestational age.
There was no clear evidence of association between site-specific extreme temperature and SGA during any of the exposure windows except a weak inverse association with third-trimester cold exposure (Table 2). When restricted to only term SGA, results remained consistent (Table 3). We observed increased tLBW risk associated with exposures to both hot and cold temperatures during trimesters 2 and 3 and the whole pregnancy (Figure 1, also see Table 2 for risk estimates). Specifically, compared to mild temperature, cold exposures during trimesters 2 and 3 and whole pregnancy were associated with 19%, 18% and 148% increase in risk, respectively. Similarly, hot exposures during trimester 3 and whole pregnancy were associated with 31% and 138% increased risk compared to mild temperature, respectively. No associations were observed in other windows. After additional adjustment for air pollution, the associations remained robust (Table 2). When we truncated whole-pregnancy exposures to 37 weeks to ensure equal length, the results remained consistent. Specifically, the associations between site-specific extreme temperature and tLBW were still strong (RRcold: 2.59, 95% CI: 2.28–2.93, RRhot: 2.32, 95% CI: 2.05–2.63). Results did not change when we accounted for temporal dependence by adjusting for exposures during other windows (data not shown). Analyses restricted to nulliparous women also produced similar findings, but the confidence intervals for tLBW were wide due to lower sample size (eTable 5). Analyses excluding the largest site resulted in the exclusion of 3,850 SGA and 705 tLBW cases but yielded consistent results (eTable 6). Stratification by season generally yielded similar results (eTable 7)
Table 2.
Association of site-specific extreme ambient temperature with SGA and tLBW for various pregnancy windows.
| Exposure windows | RR (95% CI) | ||||
|---|---|---|---|---|---|
| Model 1a | Model 2b | ||||
|
|
|||||
| Cold (<5th | Hot (>95 | Cold (<5th | Hot (>95 | ||
| SGA | Preconception | 0.99 (0.93, 1.05) | 0.99 (0.93, 1.05) | 0.98 (0.92, 1.04) | 1.01 (0.95, 1.07) |
| Trimester 1 | 1.04 (0.98, 1.10) | 1.02 (0.97, 1.09) | 1.05 (0.99, 1.11) | 1.02 (0.96, 1.08) | |
| Trimester 2 | 1.02 (0.96, 1.08) | 0.98 (0.92, 1.03) | 1.02 (0.97, 1.09) | 0.96 (0.91, 1.02) | |
| Trimester 3 | 0.91 (0.86, 0.97) | 0.99 (0.93, 1.05) | 0.91 (0.86, 0.97) | 0.99 (0.93, 1.05) | |
| Whole | 0.98 (0.92, 1.04) | 0.98 (0.93, 1.04) | 0.98 (0.93, 1.04) | 0.98 (0.92, 1.04) | |
|
| |||||
| tLBW | Preconception | 0.96 (0.83, 1.10) | 1.00 (0.87, 1.16) | 0.96 (0.83, 1.11) | 1.01 (0.88, 1.17) |
| Trimester 1 | 1.05 (0.92, 1.20) | 1.02 (0.89, 1.17) | 1.05 (0.92, 1.21) | 1.02 (0.88, 1.17) | |
| Trimester 2 | 1.19 (1.04, 1.36) | 1.04 (0.91, 1.20) | 1.21 (1.05, 1.38) | 1.06 (0.92, 1.22) | |
| Trimester 3 | 1.18 (1.03, 1.35) | 1.31 (1.15, 1.49) | 1.18 (1.03, 1.36) | 1.31 (1.15, 1.50) | |
| Whole | 2.48 (2.19, 2.81) | 2.38 (2.11, 2.69) | 2.57 (2.27, 2.91) | 2.49 (2.20, 2.83) | |
Abbreviations: CI, confidence interval; tLBW, term low birthweight; RR, relative risk; SGA, small for gestational age.
Models were adjusted for infant sex, maternal race/ethnicity, maternal age, marital status, parity, pre-pregnancy body mass index (BMI), smoking or alcohol use during pregnancy, gestational complications, chronic comorbidity, insurance, season of conception, study site, and humidity.
Models were additionally adjusted for O3 and PM2.5.
Table 3.
Association between site-specific extreme ambient temperature and SGA among term births for various pregnancy windows (n= 195,172).
| Exposure windows | Adjusted RRa (95% CI)
|
|
|---|---|---|
| Cold (<5th percentile) | Hot (>95 percentile) | |
| Preconception | 1.01 (0.95, 1.08) | 0.93 (0.88, 1.00) |
| Trimester 1 | 1.03 (0.97, 1.10) | 1.01 (0.95, 1.07) |
| Trimester 2 | 1.02 (0.96, 1.08) | 0.99 (0.93, 1.05) |
| Trimester 3 | 0.91 (0.85, 0.97) | 1.01 (0.94, 1.08) |
| Whole pregnancy | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.13) |
Abbreviations: CI, confidence interval; tLBW, term low birthweight; RR, relative risk; SGA, small for gestational age.
Models were adjusted for infant sex, maternal race/ethnicity, maternal age, marital status, parity, pre-pregnancy body mass index (BMI), smoking or alcohol use during pregnancy, gestational complications, chronic comorbidity, insurance, season of conception, study site, temperature, and humidity.
Figure 1.
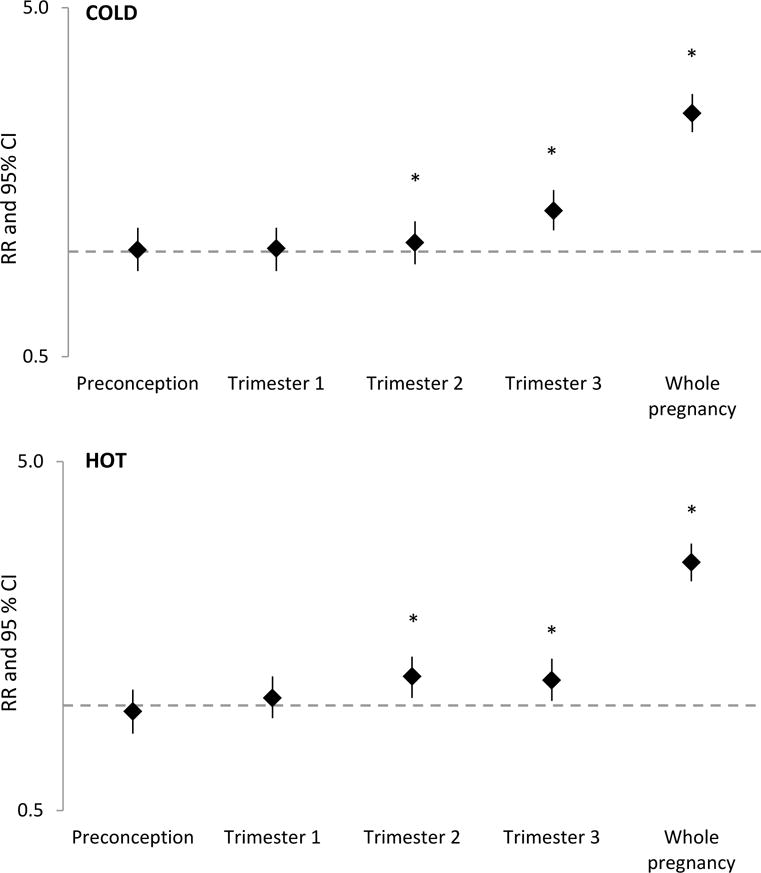
Adjusted relative risks (RR)a and 95% confidence intervals of tLBW associated with extreme cold (top) and hot (bottom) temperature. The y-axis is plotted on a logarithmic scale. Asterisks (*) indicates statistical significance at alpha<0.05.
aModels were adjusted for infant sex, race/ethnicity, maternal age, marital status, parity, pre-pregnancy BMI, smoking or alcohol use during pregnancy, gestational complications, comorbidity, insurance, season of conception, study site, particulate matter with diameter ≤2.5 microns, ozone, and humidity during the same exposure window.
There was no consistent evidence of associations between air pollutants and either measure of fetal growth restriction (eTable 8). We observed slightly elevated risk of SGA associated with exposure to elemental carbon during preconception (RR: 1.04, 95% CI: 1.01–1.07) and sulfate particles during the 2nd trimester (RR: 1.03, 95% CI: 1.00–1.07. Inverse associations with total PM2.5 (RR: 0.96, 95% CI: 0.92–0.99) and sulfate particles (RR: 0.96, 95% CI: 0.93–0.99) during this period as well as first trimester CO exposures (RR: 0.97, 95% CI: 0.94–1.00) were also observed. For tLBW, exposures to higher concentrations of dust particles during preconception (RR: 1.10, 95% CI: 1.03–1.17) and trimester 2 (RR: 1.06, 95% CI: 1.00–1.13), and PM10 exposures during trimester 2 (RR: 1.06, 95% CI: 1.00–1.13) and whole pregnancy (RR: 1.08, 95% CI: 1.01–1.16) were positively and significantly associated. No other exposure-outcome associations were observed. After FDR adjustment for multiple comparisons, no statistically significant association remained. Analyses stratified by season, excluding the largest site, or restricting to nulliparous women yielded consistent results (not shown).
Discussion
In this large nationwide US cohort, we found consistent evidence of positive associations between exposures to locally-defined extreme temperature and tLBW. No consistent evidence for air pollution exposures and either growth measure was observed. Although there are existing studies examining the associations of ambient temperature with birth size (e.g. length and weight) (Strand et al., 2011), this is the first geographically diverse cohort study to report ambient temperature in relation to fetal development indicated by SGA and tLBW.
Our results with respect to tLBW are consistent with a large cohort of over 1.4 million births in the Netherlands which also reported an association between high temperature and reduced birth weight (Poeran et al., 2016). In addition, studies in Marmara University Hospital, Turkey (Elter et al., 2004) and Northern Ireland (Murray et al., 2000) found associations between exposures to colder temperatures during the mid-trimester and lower birth weight, while studies in two German states (Wolf and Armstrong, 2012) and Uppsala, Sweden (Bruckner et al., 2014) did not find clear associations for any pregnancy windows. The discrepancy in findings with respect to cold temperature can partially be explained by differences in study design, study location, and geographical difference in temperature distributions. Of particular note is the definition of fetal growth. The aforementioned studies used birthweight or LBW (<2,500g) as outcomes, whereas we also accounted for preterm birth status and gestational age by excluding preterm births from term analyses and using SGA as an outcome. Our study also has more geographic diversity and an overall temperate setting.
Our findings for cold/hot exposures during the latter windows of pregnancy (trimesters 2 and 3) and whole pregnancy, but not early pregnancy or preconception are biologically plausible. The second and third trimesters are important times for fetal growth (Buck Louis et al., 2015). As extreme temperature may affect uterine blood flow and placental exchange necessary for fetal growth (Browne et al., 2015; Prada and Tsang, 1998), disruption to the mechanism needed for proper growth during these time windows would have the greatest impact. In addition, the fact that whole-pregnancy average was significantly associated with low birthweight suggests that chronic exposures to high/low temperature maybe more important with respect to adverse birth outcomes than previously thought.
The consistent lack of associations with site-specific extreme temperature and SGA (both all SGA births and term SGA births) is interesting in comparison with the significant findings for tLBW. It may be that the proportion of infants in the SGA group that were constitutionally small is somewhat larger. That is, some of the infants in the lower end of a normal distribution may be small for reasons unrelated to the clinical significance of a growth restricted fetus who was unable to achieve its growth potential (e.g., their parents are small) (Resnik, 2002). In fact, Hinkle et al. showed that the traditional definition of SGA does not accurately reflect health risk among obese women (Hinkle et al., 2016). This may have biased our results towards the null. In addition, while our SGA definition is conventional, using the 5th or 3rd percentile cut-offs may better help target those with more high-risk outcomes (Faraci et al., 2011; Pilliod et al., 2012). An additional analysis using the 5th percentile cutoff to define SGA showed generally consistent findings except for a positive association between first-trimester cold and SGA (RR: 1.13, 95% CI: 1.04–1.22), but we were unable to explore more extreme cutoffs.
With respect to air pollution, in Connecticut, USA, PM10 exposures during the second trimester and whole pregnancy period were associated with both SGA and tLBW, independent of residential mobility (Pereira et al., 2016). However, we found no evidence of association of any outcome with PM10. Although there were statistically significant associations for several pollutants, they were likely due to chance as suggested by the results after FDR adjustment for multiple comparisons. This discrepancy in findings is not clear and needs further investigation but we note that our results were likely biased towards the null given exposures were assessed at the area level.
This study has some limitations with respect to exposure assessment. We did not have women’s address or residential mobility, which may have caused some degree of exposure misclassification and does not allow us to account for small, local area micro-climates. Although up to 30% of pregnant women may move during pregnancy, most typically relocate within 10 km from their previous residence (Bell and Belanger, 2012). As a result, the use of hospital referral region as a surrogate may have helped by accounting for some local mobility both in day-to-day travels and residential moves which occurred within the same delivery hospital referral region. We note that another study on air pollution and fetal growth found that residential mobility did not substantively change effect estimates (Pereira et al., 2016). On the other hand, although we fused model-based exposures to observed monitor records and population-weighted our data to improve accuracy for air pollution measures, the use of average pollutant and temperature exposure across a geographic area as a proxy for residential exposure likely resulted in lower variability and biased our findings towards the null. This may explain some of the null findings observed for air pollutants. Reassuringly, removing the largest study site from the analyses did not change our findings. The lack of data on daily activities (e.g. time spent indoor/outdoor/at work, air conditioner/heater use) could also affect our results; however, we do not expect this to be differential by outcome status, so the lack of clarifying information is likely to lessen the precision of our estimates, making significant findings less likely. We also acknowledge that there are more ways to define SGA in the recent literature, and that our traditional definition may not reflect all health-related risk. Individual- and area-level socioeconomic status was not available in medical records; however, we adjusted for site, race, marital status, and insurance status, which helped account for some variability related to area- and individual-level socioeconomic indicators that may have influenced our findings (Thompson et al., 2005). The broad adjustment of site may have biased our results towards the null and cannot explain the significant findings we observed. Lastly, the best clinical estimate of gestational age available in medical records was derived from either ultrasound measurements or LMP; however, information on specific dating method was not systematically collected in the CSL. If air pollution is associated with SGA, then the use of ultrasound may have affected gestational age estimation (i.e., underestimate the prevalence of SGA), leading to an underestimation of risk. We recognize that LMP may be a reasonable alternative but almost 52% of our participants do not have LMP date for analysis.
The strengths of the study include its broad geographic coverage with outcomes based on medical record data. The rich clinical data allowed us to control for a variety of potential confounders that are unavailable to many other cohort studies. It is the first cohort study to investigate ambient temperature in relation to fetal growth indicated by SGA and tLBW. While measuring low birth weight overall results in mixing preterm infants who may be appropriately sized for their gestational age with growth restricted infants, examining SGA and tLBW provides an assessment of growth across the spectrum of gestational age as well as potential deficits in growth at term. In addition, we accounted for potential regional acclimation, a critical issue that previous studies often overlooked. This recognizes that women living a cooler region may experience heat stress at a lower temperature compared to those acclimatized to a warmer region, and vice versa. Our study is also one of the few that considered both meteorological factors and air pollution. Our large sample size obtained from multiple clinical sites ensured generalizability. Our consistent results after multiple sensitivity analyses ensured that findings were not affected by preterm status, length of gestation, temporal dependence of temperature, or history of fetal growth restriction. Lastly, our exposure assessment accounted for multiple factors including local emissions, observed data, meteorology, population density, and photochemical properties of pollutants to ensure high temporal and spatial accuracy.
Conclusion
In this large nationwide obstetric cohort, we observed associations between site-specific extreme ambient local area temperature and tLBW, especially for exposures in mid to late pregnancy and averaged across the whole pregnancy. No consistent evidence for air pollution was found. Given the global concerns related to the expected increase in frequency and intensity of extreme weather events, these results highlight the need for more research as well as public health awareness of the potential adverse effects of extreme local temperature during pregnancy.
Supplementary Material
Highlights.
Whole-pregnancy cold or hot temperature increased term low birthweight (tLBW) risk.
Cold exposures during trimesters 2 and 3 & hot during trimester 3 increased tLBW risk.
There was no association between temperature and small for gestational age (SGA).
Most associations between air pollution and SGA or tLBW were null.
Acknowledgments
The authors thank the study participants and participating clinical centers involved in the Consortium on Safe Labor: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Alllen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; Summa Health System, Akron, Ohio; and University of Texas Health Science Center at Houston, Houston, TX. We also thank the Emmes Corporation, Rockville, MD, which provided data coordination; and the Texas A&M Supercomputing Facility and the Texas Advanced Computing Center, which provided computing resources essential to completing exposure estimations in this study.
The authors also thank Drs. Candace Robledo and Tuija Männistö for their contributions on an earlier version of the manuscript, and Dr. Katherine L. Grantz for her expert advice with respect to the CSL data.
Funding source: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health; including Contract No. HHSN267200603425C (Consortium on Safe Labor), Contract No. HHSN275200800002I, and Task Order No. HHSN27500008 (Air Quality and Reproductive Health). Although the NICHD cleared this manuscript for publication, it had no role in the design, conduct, or writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethic statement: This project was approved by institutional review boards from all participating centers. Informed consent was not required because data were anonymous.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- ACOG. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet Gynecol. 2013;121:1122–33. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- Arcangeli T, et al. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol. 2012;40:267–75. doi: 10.1002/uog.11112. [DOI] [PubMed] [Google Scholar]
- Auger N, et al. Extreme heat and risk of early delivery among preterm and term pregnancies. Epidemiology. 2014;25:344–50. doi: 10.1097/EDE.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol. 2012;22:429–38. doi: 10.1038/jes.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Biberoglu E, et al. Circulating and myometrial markers of oxidative stress in pregnant women with fetal growth restriction. J Obstet Gynaecol Res. 2016;42:29–35. doi: 10.1111/jog.12857. [DOI] [PubMed] [Google Scholar]
- Browne VA, et al. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140068. doi: 10.1098/rstb.2014.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner TA, et al. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol. 2014;24:116–21. doi: 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449 e1–449 e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Natality public-use data 2007–2014. United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vital Statistics; Atlanta, GA: 2016. 12/21, 2016. https://wonder.cdc.gov/controller/datarequest/D66. [Google Scholar]
- Chen G, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ. 2014;485–486:563–74. doi: 10.1016/j.scitotenv.2014.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elter K, et al. Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Aust N Z J Obstet Gynaecol. 2004;44:553–7. doi: 10.1111/j.1479-828X.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- EPA. Air Pollution: Current and Future Challenges Environmental Protection Agency, Washington, DC. 2016 April 13, 2016. https://www.epa.gov/clean-air-act-overview/air-pollution-current-and-future-challenges.
- Faraci M, et al. Fetal growth restriction: current perspectives. J Prenat Med. 2011;5:31–3. [PMC free article] [PubMed] [Google Scholar]
- Foley K, et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. Geosci Model Develop. 2010;3:205–226. doi: 10.5194/gmd-10-1703-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, et al. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Hinkle SN, et al. Comparison of methods for identifying small-for-gestational-age infants at risk of perinatal mortality among obese mothers: a hospital-based cohort study. BJOG. 2016;123:1983–1988. doi: 10.1111/1471-0528.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, et al. The association between ambient air pollution and selected adverse pregnancy outcomes in China: A systematic review. Sci Total Environ. 2016 doi: 10.1016/j.scitotenv.2016.11.100. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janisse JJ, et al. Alcohol, tobacco, cocaine, and marijuana use: relative contributions to preterm delivery and fetal growth restriction. Subst Abus. 2014;35:60–7. doi: 10.1080/08897077.2013.804483. [DOI] [PubMed] [Google Scholar]
- Kahle JJ, et al. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: a crossover study of healthy young volunteers. Environ Health Perspect. 2015;123:310–6. doi: 10.1289/ehp.1307986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl TR, et al. Climate change. Possible artifacts of data biases in the recent global surface warming hiatus. Science. 2015;348:1469–72. doi: 10.1126/science.aaa5632. [DOI] [PubMed] [Google Scholar]
- Mannisto T, et al. Neonatal outcomes and birth weight in pregnancies complicated by maternal thyroid disease. Am J Epidemiol. 2013;178:731–40. doi: 10.1093/aje/kwt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller P, et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat Res Rev Mutat Res. 2014;762:133–66. doi: 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Murray LJ, et al. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol. 2000;96:689–95. doi: 10.1016/s0029-7844(00)01022-x. [DOI] [PubMed] [Google Scholar]
- Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. 2006;49:257–69. doi: 10.1097/00003081-200606000-00008. [DOI] [PubMed] [Google Scholar]
- Pereira G, et al. Particulate air pollution, fetal growth and gestational length: The influence of residential mobility in pregnancy. Environ Res. 2016;147:269–74. doi: 10.1016/j.envres.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilliod RA, et al. The risk of intrauterine fetal death in the small-for-gestational-age fetus. Am J Obstet Gynecol. 2012;207:318 e1–6. doi: 10.1016/j.ajog.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeran J, et al. The Impact of Extremes in Outdoor Temperature and Sunshine Exposure on Birth Weight. J Environ Health. 2016;78:92–100. [PubMed] [Google Scholar]
- Prabhu N, et al. First trimester maternal tobacco smoking habits and fetal growth. Thorax. 2010;65:235–40. doi: 10.1136/thx.2009.123232. [DOI] [PubMed] [Google Scholar]
- Prada JA, Tsang RC. Biological mechanisms of environmentally induced causes of IUGR. Eur J Clin Nutr. 1998;52(Suppl 1):S21–7. discussion S27–8. [PubMed] [Google Scholar]
- Reeves S, Bernstein I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstet Gynecol. 2008;3:719–730. doi: 10.1586/17474108.3.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–6. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- Salam MT, et al. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–44. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37:498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Slama R, et al. Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect. 2008;116:791–8. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan CM, et al. Hydration for treatment of preterm labour. Cochrane Database Syst Rev. 2013;11:CD003096. doi: 10.1002/14651858.CD003096.pub2. [DOI] [PubMed] [Google Scholar]
- Stieb DM, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Strand LB, et al. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res. 2011;111:451–62. doi: 10.1016/j.envres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Thompson LA, et al. Regional variation in rates of low birth weight. Pediatrics. 2005;116:1114–21. doi: 10.1542/peds.2004-1627. [DOI] [PubMed] [Google Scholar]
- Wolf J, Armstrong B. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One. 2012;7:e40228. doi: 10.1371/journal.pone.0040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Evaluation of a seven-year air quality simulation using the Weather Research and Forecasting (WRF)/Community Multiscale Air Quality (CMAQ) models in the eastern United States. Sci Total Environ. 2014;473–474:275–85. doi: 10.1016/j.scitotenv.2013.11.121. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326 e1–326 e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


