Abstract
Background:
Changes in platelet indices have been reported in patients with panic disorder (PD). However, previous study findings are contradictory and inconclusive. The aim of this study was to evaluate and compare the platelet indices in patients with PD.
Materials and Methods:
Patients with PD (n = 123) and healthy controls (n = 133) were enrolled in this case control study. The platelet indices (mean platelet volume [MPV] and platelet distribution width [PDW]) along with red blood cell (RBC) indices (RBC count and red cell distribution width [RDW]) were compared between the two groups using the unpaired t-test.
Results:
Patients with PD had lower MPV (7.53 ± 0.93 fL vs. 8.91 ± 1.24 fL, P < 0.0001), higher PDW (16.96 ± 0.85 fL vs. 14.71 ± 2.07 fL, P < 0.0001), and higher platelet count (274.2 ± 80.66 × 109 L−1 vs. 243.1 ± 93.89 × 109 L−1, P < 0.005) than the healthy controls. Furthermore, there were significant differences between patients with PD and healthy controls in terms of their RBC count (4.32 ± 0.56 × 1012 L−1 vs. 4.08 ± 0.80 × 1012 L−1, P = 0.007) and RDW (16.48 ± 2.26 fL vs. 15.01 ± 2.25 fL, P < 0.0001).
Conclusion:
Patients with PD have increased PDW and RDW. The platelet and RBC indices may prove to be useful etiological and prognostic markers in patients with PD.
KEYWORDS: Mean platelet volume, panic disorder, platelet distribution width, red cell distribution width
INTRODUCTION
Panic disorder (PD) is a common and serious psychiatric illness, characterized by sudden, unexpected, and recurring panic attacks.[1] Panic attack symptoms include palpitations, sweating, shaking, shortness of breath, and fear of losing control.[2] These symptoms resemble those of serious medical conditions such as myocardial infarction and stroke.
Serotonergic system dysfunction and serotonin (5-hydroxytryptamine [5-HT]) have been postulated to play a role in the neurobiology of PD. Furthermore, this hypothesis was supported by clinical and experimental studies, brain imaging, genetic studies, and pharmacotherapy of PD.[3] Two opposing hypotheses, 5-HT excess or over activity[4] and 5-HT deficit or under activity,[5] have been proposed for explaining the panic phenomenon. Excess theory suggested that patients with PD had increased level of 5-HT release or hypersensitivity in postsynaptic 5-HT receptors. Deficit theory suggested that 5-HT had restraining effects on PD, particularly; in brain regions such as the dorsal periaqueductal, 5-HT deficit may facilitate panic episodes. The 5-HT system may have a dual role in the modulation of different forms of pathological anxiety; it not only inhibits panic responses but also contributes to anticipatory or generalized anxiety.[6]
5-HT is a neurotransmitter of the central nervous system (CNS), which plays a vital role in platelet aggregation and vascular tone regulation.[7] Platelet and serotonergic neurons use the same serotonin transporter to transport serotonin into their cells and ways to store it. These cells are major storage sites for serotonin.[8] Moreover, previous studies have indicated that serotonin levels in the cerebrospinal fluid and platelets are strongly correlated.[9,10] Platelet indices such as mean platelet volume (MPV) and platelet distribution width (PDW) were extensively studied in patients with depression, schizophrenia, and bipolar disorders for identifying the risk of developing cardiovascular diseases.[11,12,13] Furthermore, patients with PD are at increased risk of cardiovascular diseases.[14]
Until now, only few studies have been conducted to evaluate the MPV in patients with PD; however, their results were inconclusive and contradictory.[15,16,17] Furthermore, their findings were also limited because of small sample size, poor inclusion and exclusion criteria, and lack of use of standardized scales. To overcome these limitations, the present study primarily aimed to compare the MPV values in patients with PD and healthy controls. Furthermore, we aimed to compare the platelet and red blood cell (RBC) indices between patients with PD and healthy controls.
MATERIALS AND METHODS
This case-control study was conducted for 6 months (January 1, 2016 to June 30, 2016) at tertiary care hospital and was approved by the Institutional Ethical Committee.
Sample size
For sample size calculation, we predicted the MPV (mean ± standard deviation [SD]) of patients with PD (cases) and controls (8.8 ± 0.9 fL vs. 9.2 ± 0.8 fL) on the basis of previously conducted studies.[15,18] On the basis of the considerations (i.e. power of study, 95%; alpha risk, 5%; and equal number of cases and controls), a sample size of >118 in each group was considered adequate for the present study.
Patients
All patients attending emergency services of psychiatry department were evaluated by psychiatrist for the presence of PD. One hundred and twenty-three patients diagnosed as having PD (aged 18–45 years) with or without agoraphobia were included in the study following the criteria of the Diagnostic and Statistical Manual of Mental Disorders-fourth edition-text revision.[2] Patients with comorbid substance abuse, major depression, dysthymia, acute or chronic medical illness, psychotic disorder, diabetes mellitus, and cardiovascular diseases including hypertension, pregnancy, neurological/neurodevelopmental disorder, smoking, and generalized anxiety disorder were excluded from the study. Age- and sex-matched 133 participants from a community with negative history for PD and fulfilling the exclusion criteria of cases (mentioned above) were included in the control group.
Assessment
After explaining the aims and purpose of the study, a written informed consent was obtained from each participant. Subsequently, they were assessed for sociodemographic data, MPV, and other hematological parameters. Three milliliters venous blood of each patient/participant was collected under all aseptic precaution in ethylenediaminetetraacetic acid (EDTA)-containing sterile tubes, and the blood samples were shifted to the hematology laboratory of our hospital. The complete blood counts, platelet indices (MPV and PDW), and RBC indices (Red cell distribution width [RDW] and RBC count) were determined within 60 min using an automated hematology analyzer (Beckman coulter, AcT Diff 2/02020) to minimize the potential influence of EDTA on the MPV. Peripheral blood smear examinations were also performed to provide visual confirmation of the automated results. The reference ranges for MPV and PDW were 8.6–15.5 fL and 8.1–25.0 fL, respectively.[19] All the blood samples were analyzed daily to avoid systemic inconsistency at the same laboratory of our tertiary care teaching hospital.
Data analysis
Data analysis was conducted using SPSS Version. 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Armonk, NY). The categorical variables (e.g., gender, place of residence) were expressed in percentages and analyzed through independent Chi-square test and Fisher exact test. The quantitative data were expressed as mean ± SD and compared using the independent t-test. For each test, the significance level was set at P < 0.05.
RESULTS
Table 1 shows the sociodemographic characteristics of patients with PD and healthy controls. The two groups did not have statistically significant differences in terms of their age, gender, and place of residence. Table 2 demonstrates the clinical characteristics of patients with PD and healthy controls. The MPV of the patients with PD was significantly lower than that of healthy controls (7.53 ± 0.93 fL vs. 8.91 ± 1.24 fL, P < 0.0001). The PDW of patients with PD was higher than that of healthy controls (16.96 ± 0.85 fL vs. 14.71 ± 2.07 fL, P < 0.0001) [Figure 1]. Furthermore, patients with PD had significantly higher platelet count than did healthy controls (274.2 ± 80.66 × 1012 L−1vs. 243.1 ± 93.89 × 1012 L−1).
Table 1.
Sociodemographic characteristic patients with panic disorder and healthy controls
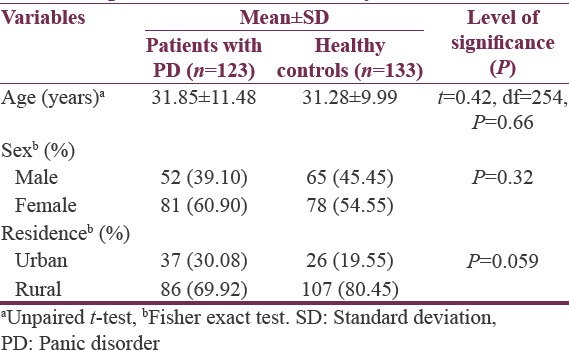
Table 2.
Clinical characteristic of patients with panic disorder and healthy controls
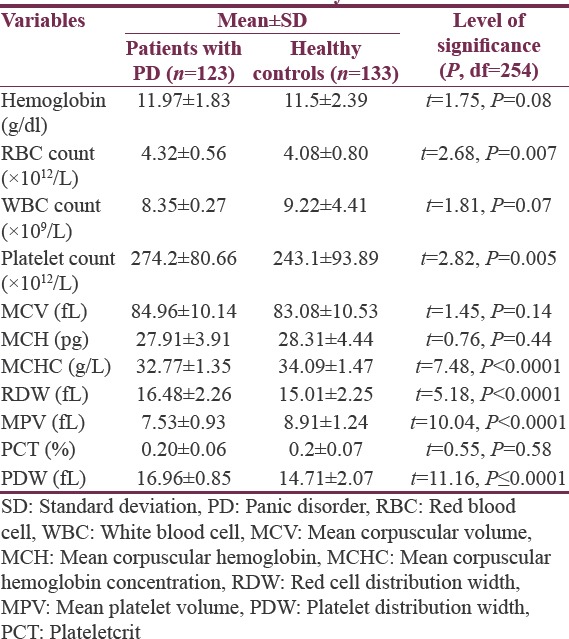
Figure 1.
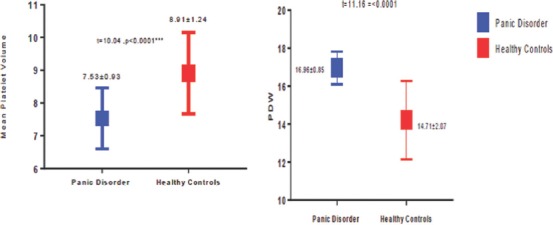
Comparison between patients with panic disorder and healthy controls in terms of mean platelet volume and platelet distribution width (mean ± standard deviation)
Among the RBC indices, the RBC count was higher in patients with PD than in healthy controls (4.32 ± 0.56 × 1012 L−1 vs. 4.08 ± 0.80 × 1012 L−1, P < 0.001), and the mean corpuscular hemoglobin concentration (MCHC) was significantly lower in patients with PD than in healthy controls (32.77 ± 1.35 g/L vs. 34.09 ± 1.47 g/L, P < 0.0001). The two groups did not significantly differ in terms of their hemoglobin concentration, mean corpuscular volume, MCH, and plateletcrit (PCT).
DISCUSSION
Platelet indices, such as MPV, PCT, and platelet counts, reflect the central serotonergic functions and are considered windows of brain serotonergic functions because the CNS is the most difficult system to assess.[20] The clinical utility of these platelet indices remains undetermined in psychiatry; despite, all modern hematological cell analyzers routinely produce platelet indices, and a clear evidence of the link between platelet indices and psychiatric disorders has been presented.[11,20,21]
In the present study, we observed lower MPV in patients with PD. This finding was consistent with the findings of Gögçegöz Gül et al.[15] and contradictory to those of Kokacya et al. and Asoglu et al.[16,17] In contrast to the study conducted by Gögçegöz Gül et al.,[15] patients included in our study did not have any comorbid illnesses, such as dysthymia and depression. In addition, adequate sample size was used in our study compared with that in a previously conducted study.[16] Moreover, this finding of the present study was contradictory to that of the postulated hypothesis, which suggested that increased sympathetic activity in cardiovascular diseases and depression can result in higher MPV values.[22,23] Furthermore, sympathoadrenal activation may stimulate platelets through 2-adrenoreceptor activation,[24] and consequently, platelet activation may cause shape change, thereby increasing MPV.[25] However, the mechanism of increased platelet activity and increased MPV in psychiatric population remains unclear.
In this study, lower values of MPV may be due to abnormal 5-HT transporter rate and its metabolism; this finding was consistent with those of a previously conducted study.[15] Disordered platelets and decreased platelet activity share a pathophysiology for some mental disorders, and PD may be one of them. Modification in serotonin levels and its activity and abnormalities in 5-HT1A receptor functioning in PD has been reported by many researchers.[26,27] In addition, this finding provides indirect evidence for the 5-HT deficit hypothesis postulated for PD instead of the 5-HT excess hypothesis.[4,5,6]
Platelet reactivity was measured in terms of MPV, PDW, and platelet count. PDW represents the variability in platelet size and is a more specific marker of platelet activation than MPV.[28,29]
In general, MPV and PDW are inversely related to each other;[30,31] therefore, patients with statistically low MPV value should have high PDW. In the present study, PDW was higher in patients with PD than in healthy controls. The findings of our study are in accordance with the MPV–PDW relationship and provide more marked clinical relevance. Kokacya et al.[16] did not report the status of PDW, and Gögçegöz Gül et al.[15] reported that the difference was insignificant. A substantial significant increase in MPV without any changes in PDW and platelet count is highly unlikely to occur; therefore, we tentatively believe that abnormal 5-HT metabolism reflects abnormal function of the platelets. This leads to decreased MPV and increased PDW, which are markers and the determinants of platelet functions. This abnormal function of platelets may be due to different variations in genetic composition of the Indian population. Increased PDW is one of the crucial prognostic factors in patients with myocardial infarction,[32] and patients with PD are at increased risk of cardiac diseases.[33] Therefore, increased PDW may be a useful predictor for cardiac diseases in patients with PD and may help in early detection of individuals at risk for cardiac diseases.
In the present study, patients with PD had increased RBC count and RDW, which support findings of previously conducted retrospective study.[17] An increased value of RDW in an otherwise normal complete blood count mostly represents early iron deficiency, other nutritional deficiencies (Vitamin B12, folic acid, zinc), chronic stress, and cardiovascular diseases.[34,35] In addition to RDW, several other factors related to RBCs (i.e., hemoglobin, hematocrit, and erythrocyte sedimentation rates) were identified as peripheral markers of inflammation and chronic stress and were associated with cardiovascular diseases.[36,37] Although the relationship between RBC count and cardiovascular diseases remains unclear, a strong relationship between RDW and cardiovascular diseases has been demonstrated in several studies.[34,38,39,40] In a previously conducted study, increased RDW and RBC count was reported in patients with depression, which improved after successful treatment with antidepressants.[41]
The findings of our study suggest that the governing hematological parameters (RDW, RBC count, MPV, and PDW) may have an etiological role and a prognostic significance in patients with PD, either independently or interdependently. Therefore, further research is warranted to study the combined effect of these parameters rather than that of individual parameters. One remarkable finding of our study was that variability in any of the RBC indices (RDW) or platelet indices (PDW) may be clinically important in identifying the risk of coronary artery diseases and may have a prognostic significance in patients with PD. Furthermore, Current study findings and previous literature suggest that the combination of PDW-RDW may have clinical significance than the MPV-RDW or MPV-PDW in patients with PD.[15,16,17]
In addition, MCHC was significantly lower in patients with PD than in healthy controls. MCHC is defined as the average hemoglobin level in RBC, and lower MCHC is associated with poorer outcomes in patients with myocardial infarction.[42] MCHC is an independent prognostic factor and an inflammatory marker similar to RDW.[43] The most novel and crucial finding of previous study is that the inflammatory process reduces MCHC,[44] which is supports the inflammatory or immunological origin of PD and risk to cardiovascular diseases. In addition, we reported increased RDW and PDW values.[45] All these hematological parameters can be documented in routine practice.
Strength and limitations
The present study was a case-control study with an adequate sample size. All other hematological parameters were also considered while interpreting the results. Though there are few studies which suggest the contradictory evidence of MPV-RDW or MPV-PDW in patients with PD. This study suggests that the elevated RDW and PDW may have better clinical significance rather than MPV. The results of this study certainly lead to strong, albeit, indirect conclusions, which will help clinicians and researchers in elucidating the possible role of serotonergic stress in the genesis of PD. In addition, the levels of inflammatory markers such as interleukin (IL)-6, IL-3, thyroid peroxidase, which were positively associated with platelet indices were not measured. Prospective, comprehensive, multicentric, and large-scale studies are warranted to explore the role of these indices in patients with PD and any possible link with cardiovascular diseases.
CONCLUSION
The MPV decreased in patients with PD, whereas the PDW, RDW, and RBC count increased; these findings were in accordance with the inflammatory origin of PD. The individual platelet indices may have little clinical significance; however, a combination of RBC and platelet indices more likely to have an etiological role and a prognostic significance in patients with PD. These factors may provide a link between PD and cardiac diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Freire RC, Zugliani MM, Garcia RF, Nardi AE. Treatment-resistant panic disorder: A systematic review. Expert Opin Pharmacother. 2016;17:159–68. doi: 10.1517/14656566.2016.1109628. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 3.Maron E, Shlik J. Serotonin function in panic disorder: Important, but why? Neuropsychopharmacology. 2006;31:1–11. doi: 10.1038/sj.npp.1300880. [DOI] [PubMed] [Google Scholar]
- 4.Iversen SD. 5-HT and anxiety. Neuropharmacology. 1984;23:1553–60. doi: 10.1016/0028-3908(84)90099-6. [DOI] [PubMed] [Google Scholar]
- 5.Bell CJ, Nutt DJ. Serotonin and panic. Br J Psychiatry. 1998;172:465–71. doi: 10.1192/bjp.172.6.465. [DOI] [PubMed] [Google Scholar]
- 6.Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–15. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 7.Vanhoutte PM. Platelet-derived serotonin, the endothelium, and cardiovascular disease. J Cardiovasc Pharmacol. 1991;17(Suppl 5):S6–12. [PubMed] [Google Scholar]
- 8.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: Regulation of plasma serotonin levels. Mol Interv. 2010;10:231–41. doi: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audhya T, Adams JB, Johansen L. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim Biophys Acta. 2012;1820:1496–501. doi: 10.1016/j.bbagen.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Yan D, Urano T, Pietraszek MH, Shimoyama I, Uemura K, Kojima Y, et al. Correlation between serotonergic measures in cerebrospinal fluid and blood of subhuman primate. Life Sci. 1993;52:745–9. doi: 10.1016/0024-3205(93)90237-w. [DOI] [PubMed] [Google Scholar]
- 11.Canan F, Dikici S, Kutlucan A, Celbek G, Coskun H, Gungor A, et al. Association of mean platelet volume with DSM-IV major depression in a large community-based population: The MELEN study. J Psychiatr Res. 2012;46:298–302. doi: 10.1016/j.jpsychires.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Wysokinski A, Szczepocka E. Platelet parameters (PLT, MPV, P-LCR) in patients with schizophrenia, unipolar depression and bipolar disorder. Psychiatry Res. 2016;237:238–45. doi: 10.1016/j.psychres.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Correll CU, Frederickson AM, Kane JM, Manu P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J Clin Psychiatry. 2006;67:575–83. doi: 10.4088/jcp.v67n0408. [DOI] [PubMed] [Google Scholar]
- 14.Katerndahl DA. The association between panic disorder and coronary artery disease among primary care patients presenting with chest pain: An updated literature review. Prim Care Companion J Clin Psychiatry. 2008;10:276–85. doi: 10.4088/pcc.v10n0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gögçegöz Gül I, Eryilmaz G, Ozten E, Hizli Sayar G. Decreased mean platelet volume in panic disorder. Neuropsychiatr Dis Treat. 2014;10:1665–9. doi: 10.2147/NDT.S69922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokacya MH, Copoglu US, Kivrak Y, Ari M, Sahpolat M, Ulutas KT. Increased mean platelet volume in patients with panic disorder. Neuropsychiatr Dis Treat. 2015;11:2629–33. doi: 10.2147/NDT.S94147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asoglu M, Aslan M, Imre O, Kivrak Y, Akil O, Savik E, et al. Mean platelet volume and red cell distribution width levels in initial evaluation of panic disorder. Neuropsychiatr Dis Treat. 2016;12:2435–8. doi: 10.2147/NDT.S111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. New York: Wiley and Sons; 1999. Hypothesis testing – Determining sample size to control type-II error. [Google Scholar]
- 19.Sachdev R, Tiwari AK, Goel S, Raina V, Sethi M. Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J Pathol Microbiol. 2014;57:231–5. doi: 10.4103/0377-4929.134676. [DOI] [PubMed] [Google Scholar]
- 20.Koudouovoh-Tripp P, Sperner-Unterweger B. Influence of mental stress on platelet bioactivity. World J Psychiatry. 2012;2:134–47. doi: 10.5498/wjp.v2.i6.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–62. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–15. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 23.Hausberg M, Hillebrand U, Kisters K. Addressing sympathetic overactivity in major depressive disorder. J Hypertens. 2007;25:2004–5. doi: 10.1097/HJH.0b013e3282ef9819. [DOI] [PubMed] [Google Scholar]
- 24.Hjemdahl P, Larsson PT, Wallén NH. Effects of stress and beta-blockade on platelet function. Circulation. 1991;84(6 Suppl):VI44–61. [PubMed] [Google Scholar]
- 25.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: Relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–19. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 26.Marazziti D, Rossi A, Dell'Osso L, Palego L, Placidi GP, Giannaccini G, et al. Decreased platelet 3H-paroxetine binding in untreated panic disorder patients. Life Sci. 1999;65:2735–41. doi: 10.1016/s0024-3205(99)00542-1. [DOI] [PubMed] [Google Scholar]
- 27.Lesch KP, Wolozin BL, Murphy DL, Reiderer P. Primary structure of the human platelet serotonin uptake site: Identity with the brain serotonin transporter. J Neurochem. 1993;60:2319–22. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 28.De Luca G, Venegoni L, Iorio S, Secco GG, Cassetti E, Verdoia M, et al. Platelet distribution width and the extent of coronary artery disease: Results from a large prospective study. Platelets. 2010;21:508–14. doi: 10.3109/09537104.2010.494743. [DOI] [PubMed] [Google Scholar]
- 29.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: A simple, practical and specific marker of activation of coagulation. Hippokratia. 2010;14:28–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Wendland AE, Farias MG, Manfroi WC. Mean platelet volume and cardiovascular disease. J Bras Patol Med Lab. 2009;45:371–8. [Google Scholar]
- 31.Shen J, Ran ZH, Zhang Y, Cai Q, Yin HM, Zhou XT, et al. Biomarkers of altered coagulation and fibrinolysis as measures of disease activity in active inflammatory bowel disease: A gender-stratified, cohort analysis. Thromb Res. 2009;123:604–11. doi: 10.1016/j.thromres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Cetin M, Bakirci EM, Baysal E, Tasolar H, Balli M, Cakici M, et al. Increased platelet distribution width is associated with ST-segment elevation myocardial infarction and thrombolysis failure. Angiology. 2014;65:737–43. doi: 10.1177/0003319713520068. [DOI] [PubMed] [Google Scholar]
- 33.Tully PJ, Wittert GA, Turnbull DA, Beltrame JF, Horowitz JD, et al. Panic disorder and incident coronary heart disease: A systematic review and meta-analysis protocol. Syst Rev. 2015;25(4):33. doi: 10.1186/s13643-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: In-depth review and update. Tex Heart Inst J. 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- 35.Yu C, Chen M, Chen Z, Lu G. Predictive and prognostic value of admission neutrophil-to-lymphocyte ratio in patients with CHD. Herz. 2016;41:605–13. doi: 10.1007/s00059-015-4399-8. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: Meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–20. doi: 10.1053/euhj.1999.1699. [DOI] [PubMed] [Google Scholar]
- 37.Gotoh S, Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, et al. Hematocrit and the risk of cardiovascular disease in a Japanese community: The Hisayama Study. Atherosclerosis. 2015;242:199–204. doi: 10.1016/j.atherosclerosis.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Skjelbakken T, Lappegård J, Ellingsen TS, Barrett-Connor E, Brox J, Løchen ML, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: The Tromsø Study. J Am Heart Assoc. 2014;3:pii: e001109. doi: 10.1161/JAHA.114.001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang P, Wang Y, Li H, Wu Y, Chen H. Relationship between the red blood cell distribution width and risk of acute myocardial infarction. J Atheroscler Thromb. 2015;22:21–6. doi: 10.5551/jat.23937. [DOI] [PubMed] [Google Scholar]
- 40.Khaki S, Mortazavi SH, Bozorgi A, Sadeghian S, Khoshnevis M, Mahmoodian M. Relationship between red blood cell distribution width and mortality of patients with acute myocardial infarction referring to Tehran Heart Center. Crit Pathw Cardiol. 2015;14:112–5. doi: 10.1097/HPC.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 41.Demircan F, Gözel N, Kilinç F, Ulu R, Atmaca M. The impact of red blood cell distribution width and neutrophil/lymphocyte ratio on the diagnosis of major depressive disorder. Neurol Ther. 2016;5:27–33. doi: 10.1007/s40120-015-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YL, Hu ZD. Lower mean corpuscular hemoglobin concentration is associated with poorer outcomes in intensive care unit admitted patients with acute myocardial infarction. Ann Transl Med. 2016;4:190. doi: 10.21037/atm.2016.03.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–93. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClung JP, Karl JP. Iron deficiency and obesity: The contribution of inflammation and diminished iron absorption. Nutr Rev. 2009;67:100–4. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 45.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


