Abstract
After an injury to the central nervous system, a dramatic change in the astrocytes bordering the wound occurs. The most characteristic feature of this process, termed reactive gliosis, is the upregulation of the intermediate filament protein, glial fibrillary acidic protein. In the present study, we show that reactive astrocytes express high levels of microtubule-associated protein 2 (MAP-2), a protein normally found in the somatodendritic compartment of neurons. When sections of injured brain are double-stained with antibodies directed against MAP-2 and glial fibrillary protein, all of the reactive astrocytes are found to contain MAP-2. The high levels of this protein appear to represent a permanent change in reactive astrocytes. In parallel quantitative studies, an elevated level of MAP-2 in the injured brain is confirmed by an immunoblot analysis of injured and normal white matter. This report demonstrates the direct involvement of a microtubule protein in the process of reactive gliosis.
Full text
PDF


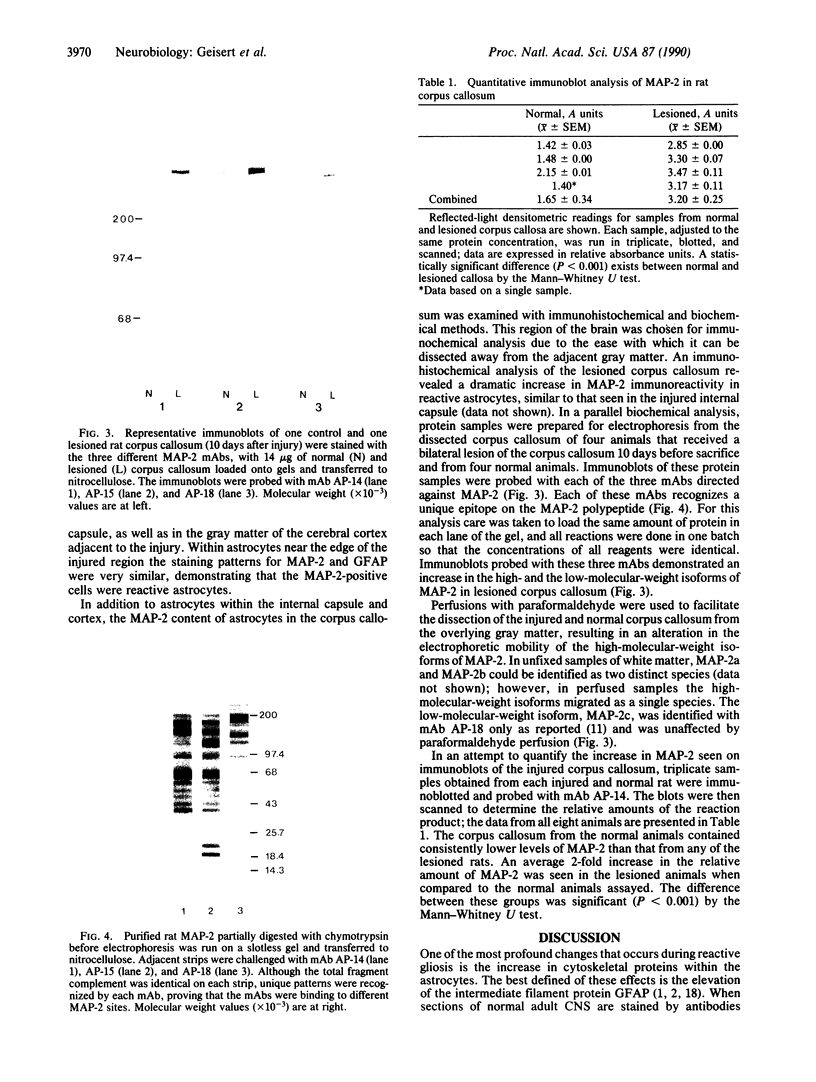

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M., Maxwell W. L., Logan A., Mathewson A., McConnell P., Ashhurst D. E., Thomas G. H. Deposition of scar tissue in the central nervous system. Acta Neurochir Suppl (Wien) 1983;32:31–53. doi: 10.1007/978-3-7091-4147-2_3. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann N Y Acad Sci. 1986;466:145–166. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Vallee R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983 Jun;96(6):1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A., Weber K., Osborn M. Filament proteins in rat optic nerves undergoing Wallerian degeneration: localization of vimentin, the fibroblastic 100-A filament protein, in normal and reactive astrocytes. Exp Neurol. 1981 Aug;73(2):496–506. doi: 10.1016/0014-4886(81)90283-1. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Fedoroff S., White R., Neal J., Subrahmanyan L., Kalnins V. I. Astrocyte cell lineage. II. Mouse fibrous astrocytes and reactive astrocytes in cultures have vimentin- and GFP-containing intermediate filaments. Brain Res. 1983 Apr;283(2-3):303–315. doi: 10.1016/0165-3806(83)90187-6. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Alley C. D. Antiserum-induced growth of axons across lesions of the adult rat brain. Brain Res Bull. 1985 Jul;15(1):19–28. doi: 10.1016/0361-9230(85)90056-5. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Jr, Frankfurter A. The neuronal response to injury as visualized by immunostaining of class III beta-tubulin in the rat. Neurosci Lett. 1989 Jul 31;102(2-3):137–141. doi: 10.1016/0304-3940(89)90068-2. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Interactions between neurofilaments and microtubule-associated proteins: a possible mechanism for intraorganellar bridging. J Cell Biol. 1982 Dec;95(3):982–986. doi: 10.1083/jcb.95.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Wong J., Liem R. K., Shelanski M. L. Promotion of microtubule assembly by neurofilament-associated microtubule-associated proteins. J Neurochem. 1984 Nov;43(5):1385–1391. doi: 10.1111/j.1471-4159.1984.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Moody S. A., Quigg M. S., Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J Comp Neurol. 1989 Jan 22;279(4):567–580. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Johnson K. A., Borisy G. G. Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol. 1977 Nov 25;117(1):33–52. doi: 10.1016/0022-2836(77)90021-3. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Roisen F. J. Ultrastructure of a new microtubule-neurofilament coupler in nerves. Brain Res. 1982 Dec 16;253(1-2):31–37. doi: 10.1016/0006-8993(82)90670-9. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I., Bender P. K., Payne M. R. Microtubule-associated protein 2 within axons of spinal motor neurons: associations with microtubules and neurofilaments in normal and beta,beta'-iminodipropionitrile-treated axons. J Cell Biol. 1985 Jan;100(1):74–85. doi: 10.1083/jcb.100.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Microtubule-associated protein 2 (MAP2) is present in astrocytes of the optic nerve but absent from astrocytes of the optic tract. J Neurosci. 1986 Jun;6(6):1748–1756. doi: 10.1523/JNEUROSCI.06-06-01748.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomura Y., Hirokawa N. Colocalization of microtubule-associated protein 1A and microtubule-associated protein 2 on neuronal microtubules in situ revealed with double-label immunoelectron microscopy. J Cell Biol. 1987 Jun;104(6):1575–1578. doi: 10.1083/jcb.104.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoff R. P. The fine structure of pulse labeled (3-H-thymidine cells) in degenerating rat optic nerve. J Comp Neurol. 1975 Jun 15;161(4):595–611. doi: 10.1002/cne.901610408. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Smith G. M., Miller R. H., Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1986 Sep 1;251(1):23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Binder L. I., Viereck C., Hemmings B. A., Matus A. I. The sequential appearance of low- and high-molecular-weight forms of MAP2 in the developing cerebellum. J Neurosci. 1988 Dec;8(12):4503–4512. doi: 10.1523/JNEUROSCI.08-12-04503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. E., Pease D. C. Electron microscopic studies of wallerian degeneration in rat optic nerves. II. Astrocytes, oligodendrocytes and adventitial cells. J Comp Neurol. 1970 Oct;140(2):207–226. doi: 10.1002/cne.901400205. [DOI] [PubMed] [Google Scholar]